Abstract
Background
Whipple surgery (pancreaticodeudenectomy) has a high complication rate. We aimed to evaluate whether adding Braun jejunojejunostomy (side-to-side anastomosis of afferent and efferent loops distal to the gastrojejunostomy site) to a standard Whipple procedure would reduce postoperative complications.
Methods
We conducted a randomized clinical trial comparing patients who underwent standard Whipple surgery (standard group) and patients who underwent standard Whipple surgery with Braun jejunojejunostomy (Braun group). Patients were followed for 1 month after the procedure and postoperative complications were recorded.
Results
Our study included 30 patients: 15 in the Braun and 15 in the standard group. In the Braun group, 4 (26.7%) patients experienced 6 complications, whereas in the standard group, 7 (46.7%) patients experienced 11 complications (p = 0.14). Complications in the Braun group were gastrointestinal bleeding and wound infection (n = 1 each) and delayed gastric emptying and pulmonary infection (n = 2 each). Complications in the standard group were death, pancreatic anastomosis leak and biliary anastomosis leak (n = 1 each); gastrointestinal bleeding (n = 2); and afferent loop syndrome and delayed gastric emptying (n = 3 each). There was no significant difference between groups in the subtypes of complications.
Conclusion
Our results showed that adding Braun jejunojejunostomy to standard Whipple procedure was associated with lower rates of afferent loop syndrome and delayed gastric emptying. However, more studies are needed to define the role of Braun jejunojejunostomy in this regard.
Trial registration
IRCT2014020316473N1 (www.irct.ir)
Abstract
Contexte
La chirurgie de Whipple (pancréatoduodénectomie) s’accompagne de taux de complications élevés. Nous avons voulu vérifier si l’ajout d’une jéjunojéjunostomie de Braun (anastomose latérolatérale des anses afférente et efférente à la partie distale de la gastrojéjunostomie) à une chirurgie de Whipple standard permet de réduire les complications postopératoires.
Méthodes
Nous avons procédé à un essai clinique randomisé pour comparer des patients soumis à une chirurgie de Whipple standard (groupe standard) à des patients soumis à une chirugie de Whipple standard avec jéjunojéjunostomie de Braun (groupe Braun). Les patients ont été suivis pendant 1 mois après l’intervention et les complications postopératoires ont été notées.
Résultats
Notre étude a regroupé 30 patients : 15 dans le groupe Braun et 15 dans le groupe standard. Dans le groupe Braun, 4 patients (26,7 %) ont présenté 6 complications, tandis que dans le groupe standard, 7 patients (46,7 %) ont présenté 11 complications (p = 0,14). Les complications dans le groupe Braun ont été saignements gastro-intestinaux et infection de plaie (n = 1 chacun) et retard de la vidange gastrique et infection pulmonaire (n = 2 chacun). Les complications dans le groupe standard ont été décès, fuite de l’anastomose pancréatique et fuite de l’anastomose biliaire (n = 1 chacun); saignement gastro-intestinal (n = 2); et syndrome de l’anse afférente et retard de la vidange gastrique (n = 3 chacun). On n’a noté aucune différence significative entre les groupes pour ce qui est des sous-types de complications.
Conclusion
Nos résultats ont montré que l’ajout de la jéjunojéjunostomie de Braun à une chirurgie de Whipple standard a été associé à des taux moindres de syndrome de l’anse afférente et de retard de la vidange gastrique. Il faudra toutefois procéder à d’autres études pour définir le rôle de la jéjunojéjunostomie de Braun à cet égard.
Enregistrement de l’essai
IRCT2014020316473N1 (www.irct.ir)
Pancreaticoduodenectomy, as first described by Allen Whipple in 1935, is a well-established operation and standard treatment for operable pancreatic head and other periampullary tumours.1–3 Although this operation has a great impact on treatment and patient survival, up to 60% of patients experience various complications, including postoperative bleeding, delayed gastric emptying, afferent loop syndrome, and systemic complications, such as sepsis, deep vein thrombosis and infection.4
In order to reduce these complications, particularly delayed gastric emptying and afferent loop syndrome, various methods for anastomosis, including pyloric preservation, Roux-en-Y, or different methods of intestinal loop resection to separate pancreatic anastomosis from other anastomoses, are introduced to use with or replace the standard Whipple procedure. The Roux-en-Y method has been reported to reduce postoperative complications of Whipple surgery, especially pancreatic fistula and delayed gastric emptying.5 Braun jejunojejunostomy (side-to-side anastomosis of afferent and efferent loops distal to the gastrojejunostomy site) is functionally equal to Roux-en-Y, but is performed in less time and could replace Roux-en-Y in Whipple surgery. However, there are few reports demonstrating the efficacy of Braun jejunojejunostomy in reducing postoperative delayed gastric emptying or afferent loop syndrome.6 We aimed to evaluate the efficacy of adding Braun jejunojejunostomy to the standard Whipple surgery in reducing these type of postoperative complications.
Methods
Study design and participants
Between June and December 2013, patients aged 18–75 years with confirmed operable pancreatic head, duodenal or common bile duct tumours who were scheduled for a Whipple procedure at Tabriz Imam Reza hospital, Tabriz University of Medical Sciences, were recruited for this study. Patients with previous upper abdominal surgery, preoperative signs of inoperability according to imaging studies (e.g., mesenteric or portal vein or superior mesenteric artery involvement, distant metastases, nearby organ involvement) or general conditions not suitable for a Whipple procedure (e.g., heart or renal failure) were excluded from the study. We also excluded patients whose tumours were found to be inoperable according to intraoperative findings and patients who died during the operation.
The study was a prospective, randomized clinical trial designed by the surgery department and approved by the Institutional Review Board and Ethics Committee of Tabriz University of Medical Sciences. The trial was registered in the Iranian Registry of Clinical Trials (IRCT2014020316473N1; www.irct.ir). We obtained written informed consent from all patients.
Sample size
The trial was powered to detect an effect size of d ≥ 0.70 as statistically significant in a 2-tailed test with α = 0.05 and a power of 0.90 with 14 participants per condition. As there was a possibility that some patients would not complete the study, we recruited 15 participants per group.
Randomization and blinding
Randomization of patients into 2 equal groups was performed using Randlist software (DatInf). The control group underwent the standard Whipple procedure (standard group). In the intervention group (Braun group), a Braun jejunojejunostomy was added at the end of the standard Whipple procedure. The attending surgeon received a sealed envelope in the operating room indicating the type of operation to be performed. The envelopes were unsealed only after complete resection of the pancreatico-duodenal complex. All the preoperative and postoperative clinical and paraclinical data were collected, and the follow-up was done by another member of the team who was completely blind to the type of surgery.
Pancreaticoduodenectomy procedure
All patients underwent the standard Whipple procedure, including 20%–40% distal gastrectomy, cholecystectomy, pancreaticoduodenectomy and then pancreaticojejunostomy, choledochojejunostomy and antecolic gastrojejunostomy. All anastomoses were performed using the appropriate size of PDS II polydioxanone sutures ( Ethicon, Johnson & Johnson). In the intervention group, Braun jejunojejunostomy was added to other anastomoses at the end of the operation. In both groups, pancreaticojejunostomy was performed using the duct-to-mucosa method in 2 layers. Jejunojejunostomy was performed using a standard, manual 2-layer method (side-to-side anastomosis of afferent and efferent loops distal to the gastrojejunostomy site). The jejunojejunostomy site was performed between the proximal and distal jejunal loops about 30 cm distal to the choledochojejunostomy and 45 cm distal to the gastrojejunostomy sites, respectively. At the end of operation, 2 corrugated drains were inserted bilaterally in the liver and pancreas bed. Drainage fluid was collected by sterile colostomy-type bags every 6 hours until the removal of the drains.
Postoperative management
Postoperative treatment included prophylactic antibiotics (cefazolin and metronidazole), intravenous pantoprazole (as the prophylaxis against stress ulceration) for 1 week and 100 μg of subcutaneous octreotide 3 times per day for 48 hours. Antibiotics were continued for any infectious complications or changed according to the cultures of body fluids when needed. Patients spent at least 24 hours in the intensive care unit (ICU) and were transferred to the general ward when their hemodynamic and respiratory conditions were stable. Nasogastric tubes were removed after 48 hours if the bowel sounds returned, and patients were started on a soft diet usually on the fifth postoperative day.
Patient characteristics and outcome measures
We collected the following data using a checklist to compare the 2 groups: age, sex, body mass index, time from the first symptoms until the operation, cause of the disease (pancreatic head, duodenal, or common bile duct tumour), duration of the operation, intraoperative bleeding and total volume of intraoperative blood product transfusion. We also collected the following laboratory data: white blood cell count, hemoglobin level, alanine aminotransferase, aspartate aminotransferase, and total and direct bilirubin both preoperatively and postoperatively on days 1, 3, 5, 7 and 14. We recorded the volume of nasogastric tube evacuations for 48 hours after the operation as well as the number of times per day that patients vomited after the nasogastric tubes were extracted. We noted postoperative complications, including death and its cause, pancreatic anastomosis leakage, gastric anastomosis leakage, biliary anastomosis leakage, postoperative bleeding requiring another operation, postoperative gastrointestinal bleeding requiring another operation, afferent loop syndrome, delayed gastric emptying, surgical site infection, pulmonary infection and deep vein thrombosis. Patients were followed for 1 month after the operation or until in-hospital death.
Primary outcome measures were afferent loop syndrome, defined as sudden, bulky bile-stained vomit unrelated to eating; volume of nasogastric tube evacuations in the 48 hours after the operation; frequency of vomiting after extraction of nasogastric tubes; and delayed gastric emptying, defined as gastric stasis requiring nasogastric intubation for 10 days or more or the inability to tolerate a regular diet 14 days after the operation.
Secondary outcome measures were biliary leakage, defined as persistent secretion of bilirubin-rich drainage fluid of more 50 mL/d from drains; postoperative intra-abdominal bleeding, defined as the need for more than 2 units of red blood cells more than 24 hours after surgery or repeat laparotomy because of bloody discharge from the drains; and gastrointestinal bleeding, defined as the need for more than 2 units of red blood cells more than 24 hours after surgery or need for any surgical or endoscopic intervention because of bloody discharge from the nasogastric tube or lower gastrointestinal bleeding (melena or hematochezia).
Statistical analysis
All data were analyzed using the Statistical Package for Social Sciences, version 17.0 (SPSS). Baseline data are reported as means ± standard deviations for continuous data or as percentages for categorical data. In order to analyze the differences between the groups in the quantitative variables, we used the Student t test for normally distributed data and the Mann–Whitney U test for non-normally distributed data. We studied the association between qualitative variables using the χ2 test or Fisher exact test. We considered results to be significant at p < 0.05.
Results
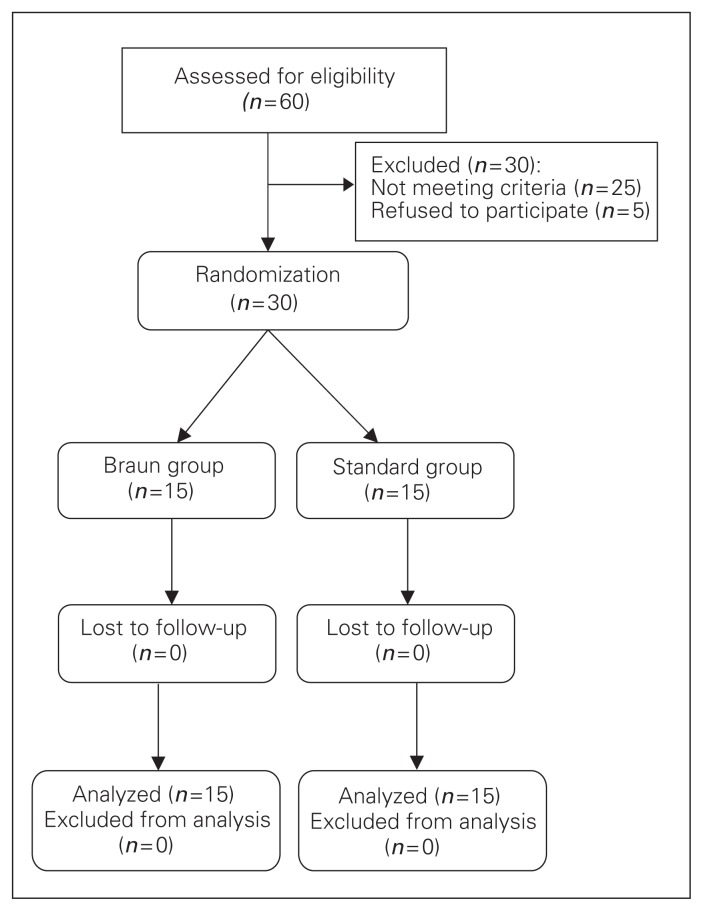
We included 30 patients in our analysis: 15 who underwent a standard Whipple procedure alone and 15 who underwent a standard Whipple procedure combined with Braun jejunojejunostomy. No patients were lost to follow-up (Fig. 1). There were no significant differences in baseline demographic and clinical characteristics between the groups (Table 1). The most common cause for surgery in both groups was pancreatic head tumour (n = 19), followed by obstructive jaundice with probable periampullary tumour (n = 5), duodenal tumour (n = 3), bile duct cancer (n = 2) and other disease (n = 1).
Fig. 1.
Flow of patients through the trial.
Table 1.
Baseline demographic and clinical characteristics of participants
| Characteristic | Group; no. (%) or mean ± SD | p value | |
|---|---|---|---|
| Braun | Standard | ||
| Age | 57.26 ± 13.80 | 55.26 ± 13.15 | 0.82 |
| Sex | |||
| Male | 10 (66.7%) | 10 (66.7%) | — |
| Female | 5 (33.3%) | 5 (33.3%) | — |
| Body mass index | |||
| < 20 | 5 (33.3%) | 3 (20%) | — |
| 20–25 | 8 (53.3%) | 10 (66.7%) | — |
| 26–30 | 1 (6.7%) | 1 (6.7%) | — |
| > 30 | 1 (6.7%) | 1 (6.7%) | — |
| Symptom duration, d | 159.33 ± 34.34 | 173.20 ± 52.28 | 0.68 |
| Reason for surgery | |||
| Pancreatic head tumour | 8 (53.3%) | 11 (73.3%) | — |
| Duodenum tumour | 3 (20%) | 0 | — |
| Tumour of distal coledoc | 1 (6.7%) | 1 (6.7%) | — |
| Obstructive jaundice with possible tumour of ampul water | 2 (13.3%) | 3 (20%) | — |
| Others | 1 (6.7%) | 0 | — |
SD = standard deviation.
Perioperative findings
Mean duration of surgery was 6.23 ± 1.83 hours in the Braun group compared with 6.08 ± 1.55 hours in the standard group (p = 0.81). Mean bleeding volume during surgery was 500.00 ± 142.00 mL in the Braun group and 626.66 ± 145.23 mL in the standard group (p = 0.53). There was no significant difference between the Braun and standard groups in mean packed red blood cells (1.60 ± 1.35 v. 1.33 ± 1.17 units, p = 0.56) or fresh frozen plasma (0.40 ± 0.28 v. 1.06 ± 0.54 units, p = 0.29) used during surgery.
Postoperative findings
Mean nasogastric tube drainage was 468.00 ± 414.88 mL in the Braun group and 760.71 ± 503.51 mL in the standard group (p = 0.09). The standard group had significantly more periods of vomiting after nasogastric tube removal than the Braun group (1.54 ± 1.50 v. 0.53 ± 0.51 times/d, p = 0.032). We observed 6 complications in 4 (26.70%) patients in the Braun group compared with 11 complications in 7 (46.7%) patients in the standard group. Although the rate of complications in the Braun group was lower than in the standard group, the difference was not significant (p = 0.14). There was no significant difference between groups in the subtypes of postoperative complications (Table 1). There was 1 death in the standard group, which occurred due to pulmonary embolism. One patient in the Braun group had pancreatitis, which was managed conservatively, and 1 patient experienced biliary leak, which was managed conservatively. Three patients in the standard group had recurrent nausea and vomiting as well as afferent loop syndrome and underwent Braun jejunojejunostomy. One case of afferent loop syndrome occurred with postoperative bleeding. All cases of postoperative bleeding occurred at the gastrojejunostomy site and were controlled and managed conservatively. Table 2 summarizes the complications in our patients.
Table 2.
Postoperative complications between groups
| Complication | Group; no. (%) | p value | |
|---|---|---|---|
| Braun | Standard | ||
| Death after operation | 0 | 1 (6.7%) | 0.90 |
| Anastomosis leak | |||
| Pancreas | 0 | 1 (6.7%) | 0.90 |
| Gastric | 0 | 0 | — |
| Biliary | 0 | 1 (6.7%) | 0.90 |
| Postoperative bleeding | 1 (6.7%) | 2 (13.3%) | 0.90 |
| Postoperative bleeding requiring surgery | 0 | 0 | — |
| Afferent loop syndrome | 0 | 3 (20%) | 0.22 |
| Delayed gastric emptying | 2 (13.3%) | 3 (20%) | 0.90 |
| Wound infection | 1 (6.7%) | 0 | 0.90 |
| Pulmonary infection | 2 (13.3%) | 0 | 0.48 |
| Deep vein thrombosis | 0 | 0 | — |
Discussion
Braun jejunojejunostomy has been recommended as an adjacent method to a standard Whipple procedure to reduce postoperative delayed gastric emptying and afferent loop syndrome, and it is shorter than other methods such as Roux-en-Y diversion.7–9 Vogel and colleagues7 reported that postoperative complications, including enterogastric reflux and biliary reflux, were significantly less frequent in patients undergoing Braun jejunojejunostomy than in those undergoing any types of gastrojejunostomy without adding Braun jejunojejunostomy. Another study reported that adding Braun jejunojejunostomy after gastroenterostomy in patients with biliary reflux significantly reduced the reflux.8 Wang and colleagues9 documented a lower complication rate after adding Braun anastomosis to a standard Whipple procedure. In the present clinical trial, we evaluated the postoperative complications after standard Whipple surgery with or without Braun jejunojejunostomy. There was no significant difference between groups before or during the operation. Six complications were seen in 4 patients in the Braun group, and 11 complications were seen in 7 patients in the standard group, but the difference was not significant. We also observed no difference between groups in nasogastric tube volume; however, vomiting after the tube removal occurred significantly more frequently in the standard group. Hochwald and colleagues10 documented earlier nasogastric tube removal in patients undergoing Braun anastomosis than in those undergoing a standard Whipple procedure; however, they reported no significant difference in vomiting rate or reinsertion of the nasogastric tube between groups.
Various complications have been reported following standard Whipple surgery. In one study, anastomosis leak, delayed gastric emptying and bleeding were the prevalent complications of Whipple surgery.11 In another study, postoperative bleeding was the most common complication after surgery.12 In yet another study,13 fever, pancreatic fistula and increase in bilirubin levels were the prevalent complications.13 In our study, complications in the standard group were death, pancreatic and biliary anastomosis leakage, gastrointestinal bleeding, afferent loop syndrome and delayed gastric emptying. Complications in the Braun group were gastrointestinal bleeding, wound infection, delayed gastric emptying and pulmonary infection. There was no significant difference between the groups in the rate of complications observed. We observed pancreatic anastomosis and biliary anastomosis leak only in the standard group, whereas Hochwald and colleagues10 reported similar failure of pancreatic anastomosis between the groups.
In our study, delayed gastric emptying was observed in 13.3% cases in the Braun group and 20% of cases of the standard group. Similarly, Hochwald and colleagues10 and Nikfarjam and colleagues14 observed that delayed gastric emptying was significantly reduced after Braun anastomosis. Conversely, Jurgens and colleagues8 reported a gastric emptying complication rate of 81% after Braun anastomosis. However, 57% of these patients had gastroparesis before the operation; 42% of of those with delayed gastric emptying responded to medical therapy and the symptom improved.
Afferent loop syndrome is an unusual complication with a reported incidence of 0.2%–20%. It usually occurs after gastrojejunostomy with Billroth II reconstruction and partial gastrectomy. However, this complication could occur even after surgical resection and anastomosis between gastric and other parts of the foregut, such as in a pancreaticoduodenectomy procedure.15,16 In our study study, afferent loop syndrome was observed in 3 (20%) patients in the standard group, whereas no patients in the Braun group experienced this complication. Considering our findings, it is possible that Braun jejunojejunostomy was associated with the decrease in this complication rate.
Braun anastomosis has some potential advantages. It tends to stabilize the afferent and efferent limbs of the gastrojejunostomy, so the gastrojejunostomy has a low tendency to twist and angulate. Braun anastomosis prevents any increase in pressure in the biliopancreatic limb if an obstruction occurs at the level of the gastroenterostomy. The Braun anastomosis also allows gastric content to pass distally unimpeded into either the efferent or afferent limb of the gastroenterostomy. Kinking at either of these limbs alone theoretically would not alter gastric emptying in these circumstances.14,17 These advantages could prevent the occurrence of afferent loop syndrome.
Limitations
Although the occurrence of afferent loop syndrome did not differ significantly between the standard group and the Braun jejunojejunostomy group, the difference was clinically important, Braun jejunojejunostomy was associated with a reduced rate of complications. However, observing significant findings in previous studies and no significant differences in our study could be related to the smaller number of patients included in our study. Overall findings indicate the efficacy of adding Braun jejunojejunostomy to a standard Whipple procedure to reduce postoperative complications, especially the incidence of afferent loop syndrome and delayed gastric emptying.
Conclusion
Adding Braun jejunojejunostomy to a standard Whipple procedure may be associated with lower rates of afferent loop syndrome and delayed gastric emptying. More studies are necessary to define the role of Braun jejunojejunostomy in this regard.
Acknowledgments
This research was financially supported by the Vice Chancellor for Research, Tabriz University of Medical Sciences, Iran.
Footnotes
Competing interests: None declared.
Contributors: F. Kakaei, S. Beheshtirouy, S.M.R. Nejatollahi and A. Habibzadeh designed the study. F. Kakaei, I. Rashidi, T. Asvadi and A. Habibzadeh acquired the data, which I. Rashidi, A. Habibzadeh and M. Oliaei-Motlagh analyzed. F. Kakaei, I. Rashidi, T. Asvadi and A. Habibzadeh wrote the article, which all authors reviewed and approved for publication.
References
- 1.Distler M, Rückert F, Hunger M, et al. Evaluation of survival in patients after pancreatic head resection for ductal adenocarcinoma. BMC Surg. 2013;13:12. doi: 10.1186/1471-2482-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Büchler MW, Wagner M, Schmied BM, et al. Changes in mortality after pancreatic resection: towards the end of completion pancreatectomy. Arch Surg. 2003;138:1310–4. doi: 10.1001/archsurg.138.12.1310. [DOI] [PubMed] [Google Scholar]
- 3.Balcom JH, Rattner DW, Warshaw AL, et al. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391–8. doi: 10.1001/archsurg.136.4.391. [DOI] [PubMed] [Google Scholar]
- 4.Sohn TA, Campbell KA, Pitt HA, et al. Quality of life and long-term survival after surgery for chronic pancreatitis. J Gastrointest Surg. 2000;4:355–65. doi: 10.1016/s1091-255x(00)80013-x. [DOI] [PubMed] [Google Scholar]
- 5.Shimoda M, Kubota K, Katoh M, et al. Effect of Billroth II or Roux-en-Y reconstruction for the gastrojejunostomy on delayed gastric emptying after pancreaticoduodenectomy: a randomized controlled study. Ann Surg. 2013;257:938–42. doi: 10.1097/SLA.0b013e31826c3f90. [DOI] [PubMed] [Google Scholar]
- 6.Qu H, Sun GR, Zhou SQ, et al. Clinical risk factors of delayed gastric emptying in patients after pancreaticoduodenectomy: a systematic review and meta-analysis. Eur J Surg Oncol. 2013;39:213–23. doi: 10.1016/j.ejso.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Vogel SB, Drane WE, Woodward ER. Clinical and radionuclide evaluation of bile diversion by Braun enteroenterostomy: prevention and treatment of alkaline reflux gastritis. An alternative to Roux-en-Y diversion. Ann Surg. 1994;219:458–65. doi: 10.1097/00000658-199405000-00003. discussion 465–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jurgens MJ, Drane WE, Vogel SB. Dual-radionuclide simultaneous biliary and gastric scintigraphy to depict surgical treatment of bile reflux. Radiology. 2003;229:283–7. doi: 10.1148/radiol.2291020661. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Su AP, Zhang Y, et al. Reduction of alkaline reflux gastritis and marginal ulcer by modified Braun enteroenterostomy in gastroenterologic reconstruction after pancreaticoduodenectomy. J Surg Res. 2014;189:41–7. doi: 10.1016/j.jss.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Hochwald SN, Grobmyer SR, Hemming AW, et al. Braun enteroenterostomy is associated with reduced delayed gastric emptying and early resumption of oral feeding following pancreaticoduodenectomy. J Surg Oncol. 2010;101:351–5. doi: 10.1002/jso.21490. [DOI] [PubMed] [Google Scholar]
- 11.Choon KH, Kleeff J, Friess H, et al. Complications of pancreatic surgery. HPB. 2005;7:99–108. doi: 10.1080/13651820510028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puppala S, Patel J, McPherson S, et al. Hemorrhagic complications after Whipple surgery: imaging and radiologic intervention. AJR Am J Roentgenol. 2011;196:192–7. doi: 10.2214/AJR.10.4727. [DOI] [PubMed] [Google Scholar]
- 13.Gervais DA, Fernandez-del Castillo C, O’Neill MJ, et al. Complications after pancreatoduodenectomy: imaging and imaging-guided interventional procedures. Radiographics. 2001;21:673–90. doi: 10.1148/radiographics.21.3.g01ma16673. [DOI] [PubMed] [Google Scholar]
- 14.Nikfarjam M, Houli N, Tufail F, et al. Reduction in delayed gastric emptying following non-pylorus preserving pancreaticoduodenectomy by addition of a Braun enteroenterostomy. JOP. 2012;13:488–96. doi: 10.6092/1590-8577/800. [DOI] [PubMed] [Google Scholar]
- 15.Aimoto T, Uchida E, Nakamura Y, et al. Malignant afferent loop obstruction following pancreaticoduodenectomy: report of two cases. J Nippon Med Sch. 2006;73:226–30. doi: 10.1272/jnms.73.226. [DOI] [PubMed] [Google Scholar]
- 16.Woodfield CA, Levine MS. The postoperative stomach. Eur J Radiol. 2005;53:341–52. doi: 10.1016/j.ejrad.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Tu BN, Sarr MG, Kelly KA. Early clinical results with the uncut Roux reconstruction after gastrectomy: limitations of the stapling technique. Am J Surg. 1995;170:262–4. doi: 10.1016/s0002-9610(05)80011-x. [DOI] [PubMed] [Google Scholar]