Abstract
Background
Pancreatic resections have traditionally been associated with substantial morbidity and mortality. The robotic platform is believed to improve technical aspects of the procedure while offering minimally invasive benefits. We sought to determine the safety and feasibility of the first robotic pancreaticoduodenectomies performed at our institution.
Methods
We retrospectively reviewed data on all patients who underwent robotic-assisted pancreaticoduodenectomy (RAPD) between July 2010 and June 2014 and compared them to outcomes of patients undergoing hybrid laparoscopic pancreaticoduodenectomies (HLAPD) during the same time period.
Results
Fifteen patients were scheduled for RAPD; 2 were converted to an open approach and 1 to a mini-laparotomy during the laparoscopic portion of the procedure. Patients who had RAPD (n = 12) had a median duration of surgery of 596.6 (range 509–799) minutes, estimated blood loss of 275 (range 50–1000) mL and median length of stay of 7.5 (range 5–57) days. Mean total opioid use up to postoperative day 7 was 142.599 ± 68.2 versus 176.9 ± 112.7 mg equivalents of intravenous morphine for RAPD and HLAPD, respectively. There was no significant difference between RAPD and HLAPD in any parameters, highlighting the safety and feasibility of a step-wise minimally invasive learning platform. Most patients in the RAPD group had malignant pathology (88.2%). Oncologic outcomes were maintained with no significant difference in ability to resect lymph nodes or achieve negative margins. There were 4 (28.5%) Clavien I–II complications and 3 (29.4%) Clavien III–IV complications, 2 of which required readmission. There were no reported deaths at 90 days. Complication, pancreatic leak and mortality rates did not differ significantly from our laparoscopic experience.
Conclusion
Outcomes of RAPD and HLAPD were comparable at our centre, even during the early stages of our learning curve. These results also highlight the safety, feasibility and patient benefits of a step-wise transition from open to hybrid to fully robotic pancreaticoduodenectomies in a high-volume academic centre.
Abstract
Contexte
L’ablation du pancréas a de tout temps été associée à une morbidité et une mortalité importantes. Le recours à une plateforme assistée par robot devrait vraisemblablement améliorer les aspects techniques de l’intervention et offrir en même temps les avantages d’une intervention minimalement effractive. Nous avons voulu déterminer l’innocuité et la faisabilité des premières pancréatoduodénectomies assistées par robot effectuées dans notre établissement.
Méthodes
Nous avons passé en revue de manière rétrospective les données concernant tous les patients ayant subi une pancréatoduodénectomie assistée par robot (PDAR) entre juillet 2010 et juin 2014 et nous les avons comparées aux résultats enregistrés chez les patients ayant subi une pancréatoduodénectomie laparoscopique hybride (PDLH) au cours de la même période.
Résultats
Quinze patients ont été pressentis pour une PDAR; 2 ont plutôt subi une intervention ouverte et 1 a subi une mini-laparotomie durant la portion laparoscopique de l’intervention. Chez les patients soumis à la PDAR (n = 12), la durée médiane de la chirurgie a été de 596,6 (plage de 509 à 799) minutes, les pertes sanguines estimées ont été de 275 (plage de 50 à 1000) mL et la durée médiane du séjour hospitalier a été de 7,5 (plage de 5 à 57) jours. L’utilisation totale moyenne d’opioïdes jusqu’au septième jour postopératoire a été de 142,599 ± 68,2 mg équivalents de morphine intraveineuse contre 176,9 ± 112,7 pour la PDAR et la PDLH, respectivement. On n’a noté aucune différence significative entre la PDAR et la PDLH au plan des paramètres, ce qui souligne l’innocuité et la faisabilité d’une plateforme d’apprentissage séquentielle minimalement effractive. La plupart des patients du groupe soumis à la PDAR étaient atteints d’un cancer (88,2 %). Les paramètres oncologiques se sont maintenus, sans différence significative quant à la capacité de réséquer les ganglions lymphatiques ou d’obtenir des marges négatives. On a dénombré 4 (28,5 %) complications de stade I–II et 3 (29,4 %) de stade III–IV selon la classification de Clavien; 2 de ces dernières ont nécessité une réadmission. On n’a déploré aucun décès à 90 jours. Les taux de complications, de fuite pancréatique et de mortalité n’ont pas différé significativement par rapport à nos interventions laparoscopiques.
Conclusion
Les résultats de la PDAR et de la PDLH ont été comparables dans notre établissement, même aux premières étapes de notre courbe d’apprentissage. De tels résultats soulignent l’innocuité, la faisabilité et les bienfaits pour les patients d’une transition graduelle des pancréatoduodénectomies ouvertes, hybrides puis entièrement assistées par robot dans un centre universitaire traitant de forts volumes de patients.
Surgical procedures of the pancreas have traditionally been associated with substantial morbidity and mortality.1 Minimizing morbidity has been one of the major factors initiating a shift toward minimally invasive (MIS) approaches in pancreatic resections. While still accounting for a minority of all pancreatic resections, MIS techniques now account for 1 of every 13 pancreatic resections.2 Three recent meta-analyses, including 1 using data from the Nationwide Inpatient Sample database, report that minimally invasive hepatobiliary surgery has consistently been associated with decreased blood loss and intraoperative complications as well as decreased length of stay in hospital (LOS) and increased lymph node harvest. These benefits have been achieved while maintaining comparable complication and leak rates to open resections despite increased duration of surgery.2,3
Beyond the traditional laparoscopic techniques, robotics offer additional advantages with increased magnification, depth, range of motion and dexterity.4 Giulianotti and colleagues5 first reported the outcomes of 8 robotic-assisted pancreaticoduodenectomies (RAPD) in 2003, demonstrating safety and feasibility, with morbidity and mortality comparable to open surgery.5 Because of the robotic platform’s versatility and relatively rapid learning curve, several groups worldwide have gained ease in the procedure and have unanimously reported decreased conversion rates, decreased blood loss and ability to maintain oncologic outcomes.6 In 2011, a meta-analysis reported that more than 1 in 5 of all MIS cases were performed using a robotic approach, with the majority of robotic hepatopancreatobiliary (HPB) cases (75%) being performed on the pancreas, with increasing pancreaticoduodenectomies performed over time.2 Despite this progress, robotic pancreatic surgery continues to account for a very small proportion of overall cases performed worldwide.
In Canada, the experience with the robotic platform in pancreatic resections is forthcoming, and to our knowledge no cases have yet been reported in the literature. Certain high-volume Canadian institutions have begun to transition into laparoscopic techniques with encouraging results. We previously reported outcomes for hybrid laparoscopic pancreaticoduodenectomies (HLAPD), which showed significantly lower intraoperative blood loss and shorter LOS compared with open surgery while maintaining comparable results for oncologic outcomes and complications. This experience has provided us with the tools needed to adopt an MIS approach for nearly all pancreatic surgery at our centre within well-established inclusion criteria.7 Furthermore, the experience with the hybrid approach, where the pancreaticoduodenectomy resection is completed laparoscopically and the reconstruction completed through a mini-laparotomy, provided our group with the necessary preparation to transition from an open reconstruction to a robotic reconstruction, as previously described by Zureikat and colleagues.6
The purpose of the present study was to determine the safety and feasibility of robotic pancreatic surgeries during the initial phase of our institutional learning curve and to compare these results to our previously validated outcomes for HLAPD.
Methods
This is a retrospective study of patients who underwent RAPD between July 2010 and June 2014. We compared the results with the outcomes for patients undergoing HLAPD. All procedures were performed by the same 2 attending staff surgeons (T.V. and S.B.).
Patient selection
All patients underwent preoperative high-resolution imaging (either computed tomography [CT], magnetic resonance imaging [MRI], or both). Patients were selected for a minimally invasive approach (RAPD or HLAPD) based primarily on tumour characteristics: localized tumours with no vascular invasion. A clear, fat plane had to be present around all arterial and venous structures, including the superior mesenteric artery (SMA), superior mesenteric vein (SMV) and common hepatic artery on preoperative high-resolution CT imaging. Any patient with suspected vascular invasion underwent an open pancreaticoduodenectomy (OPD). In cases of equivocal vascular invasion, our group erred on the side of attempting a laparoscopic resection and then converting to an open resection rather than opting for an upfront open approach. This was not an intention to treat protocol, therefore all patients who were converted to open or mini-laparotomy before the docking of the robot for the reconstruction were not included in the RAPD group, as no robotic reconstruction was undertaken to justify inclusion into this group. We previously reported that patients in the HLAPD group who were converted to the OPD group did not contribute to worse outcomes for this group or favour outcomes of the minimally invasive group.7 Thus, any patient in whom a conversion to an open operation was required was included in the open group. All patients for whom a mini-laparotomy was used for reconstruction following a totally laparoscopic resection were included in the HLAPD group. Of note the choice of HLAPD versus RAPD during the study period was purely dictated by robot availability.
Data collection
Data were abstracted from the medical records and the surgical clinic notes and limited by the patient chart reporting. A blood loss reported as “nil” in the patient chart was estimated to be 50 mL.
We recorded the following demographic and clinical characteristics: age, sex and American Society of Anaesthesiologists (ASA) class. We also collected the following operative factors: duration of surgery, estimated blood loss, positive margin rate, positive lymph node rate, number of lymph nodes harvested, intraoperative transfusion rate and tumour size. We reported complications up to postoperative day 90 based on the Clavien Classification System;8 pancreatic fistula rates as per the postoperative pancreatic fistula international study group (ISPGF) criteria outlined in Appendix 1, available at canjsurg.ca;9 and delayed gastric emptying, as defined by the grading scheme outlined in Appendix 1.10
Readmission, reoperation and mortality at 90 days were also reported.
Operative technique
The following outlines the operative steps for the laparoscopic portion of the RAPD.
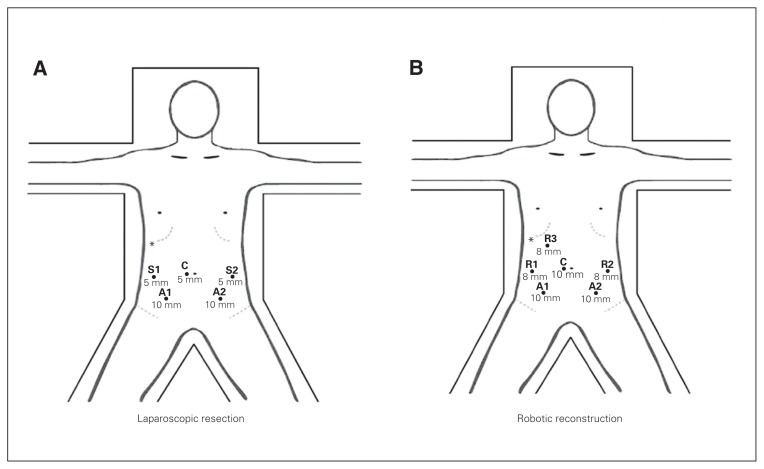
The patient is prepared and draped in a sterile fashion and positioned in a dorsal lithotomy postion. Placement of the trocars are illustrated in Figure 1.
Diagnostic laparascopy is performed.
The gastrocolic ligament is divided from the midportion of the greater curvature to the right side, identifying the plane between the gastroepiploic omentum and the transverse mesocolon. The gastroepiploic vein is identified and traced back to the level of the infrapancreatic SMV and then divided.
The right colon is mobilized and the duodenum kocherized until the ligament of Treitz is released.
The jejunum is brought back toward the right side of the abdomen and divided using a linear stapler. The mesentery of the proximal jejunum and duodenum is divided.
The distal stomach is divided using a linear stapler.
The common hepatic node is identified and sampled.
The gastroduodenal artery is divided using a vascular linear stapler.
The retropancreatic tunnel is developed above the SMV/portal vein (PV), and a Penrose drain is used to encircle the pancreas.
A complete hepatic hilar lymphadenectomy is performed, including all retropancreatic and periportal nodes.
A retrograde cholecystectomy is performed.
The common bile duct is transected above the junction with the cystic duct using a linear stapler.
The pancreas is then divided using bipolar energy.
The uncinate process dissection is performed by dividing the venous branches coming off the SMV as well as the first jejunal branches with clips or bipolar energy. The SMA is identified inferiorly, a subadventitial plane is developed, and the uncinate process is divided along this plane in a cephalad direction.
The specimen is extracted through a Pfanenstiel incision. This incision is closed and pneumoperitoneum is reobtained.
The proximal jejunum, onto which all reconstructions are performed, is brought up in the right upper quadrant. A side-to-side antecolic retrogastric loop gastrojejunostomy is created.
Fig. 1.
Patient and trocar position for (A) laparoscopic resection and (B) robotic reconstruction. *Liver retractor; A1 and A2 = assistant laparoscopic port; C = camera port (10 mm); R1, R2 and R3 = robotic port (8 mm); S1 and S2 = surgeon laparoscopic port (5 mm).
The robotic portion is performed as outlined below. Patient and trocar positioning are outlined in Figure 1B.
The robotic trocars are placed and the robot is then docked.
A duct-to-mucosa pancreaticojejunostomy is performed in a Blumgart fashion.11 Using a 3–0 silk, several stitches are placed through the pancreas and back through the jejunum. Three duct-to-mucosa stitches using 4–0 suture are placed along the 9, 6 and 3 o’clock positions.
A pediatric feeding tube is placed in the pancreatic duct as a stent. The posterior duct-to-mucosa stiches and Blumgart stitches are tied. The anterior ductal mucosa stitches are completed using 4–0 suture. The needles left on the Blumgart stitches are then used to dunk the pancreaticojejunostomy.
The hepaticojejunostomy is performed in an interrupted fashion using 4–0 suture.
Two drains are left deep and superficial to the pancreatic and biliary anastomoses.
Statistical analysis
We used SPSS software version 22.0 to perform all data analyses. We chose to report medians over means for all parameters except for postoperative opioid use, given non-normally distributed numbers in a population with outliers. For reported medians, we used a Mann–Whitney U test to determine significance between RAPD and HLAPD. We performed a χ2 test to determine significance of categorical values. We used Microsoft Excel to generate a graph of postoperative opioid use.
Results
Between July 2010 and June 2014, 19 patients were scheduled to undergo robotic pancreaticoduodenectomies; 4 were found to have metastatic disease upon diagnostic laparoscopy and thus were excluded from the present analysis. Among the 15 remaining patients, 2 were converted to an open approach before the docking of the robot: 1 owing to adherence between the tumour and the common bile duct and right hepatic artery and 1 owing to a puckered mesocolon and tumour invasion into the SMV. A third patient, who had familial polyposis, was converted to a mini-laparotomy owing to adhesions precluding mobilization at the level of the ligament of Treitz. These 3 patients were not included in the present analysis, as their conversions were not related to any aspect of the robotic reconstruction but rather to intraoperative findings that could not be predicted preoperatively and for which a minimally invasive approach was deemed unsafe.
Twelve patients successfully underwent RAPD and were included in the present analysis. The median age of patients was 71 (range 26–80) years, and the median duration of surgery was 596.6 (509–799) min. There was a net improvement in the learning curve; patients in the second half of the study period had a median duration of surgery of 567 (range 509–650) min compared with 668.5 (range 555–799) min for those in the first half of the study period. The median estimated blood loss was 275 (300–1000) mL. There was 1 intraoperative complication with injury to the SMV during the laparoscopic portion of the procedure, which was immediately repaired laparoscopically. Demographic and operative findings are summarized in Table 1. Fourteen patients underwent HLAPD during the same timeframe. The choice of HLAPD versus RAPD was mostly dictated by robot availability. There was no significant difference between the RAPD and the HLAPD groups.
Table 1.
Patient demographic and perioperative characteristics
| Characteristic | Group; mean (range)* | p value† | |
|---|---|---|---|
| RAPD | HLAPD | ||
| Patient, no. | 12 | 14 | |
| Age, yr | 71 (26–80) | 69 (49–88) | 0.95 |
| Sex, male:female, % | 50:50 | 78.6:21.4 | 0.31 |
| ASA score | 2 (2–3) | 2 (2–3) | 0.07 |
| Duration of surgery, min | 596.5 (509–799) | 592.5 (407–779) | 0.78 |
| Estimated blood loss, mL | 275 (50–1000) | 400 (100–4000) | 0.26 |
| Intraoperative blood transfusion, no. (%) | 1 (8.3) | 5 (35.7) | 0.05 |
| Total 7-day analgesic use, mg IV, mean ± SD | 142.599 ± 68.2 | 176.9 ± 112.7 | 0.35 |
| LOS, d | 7.5 (5–57) | 8 (6–14) | 0.78 |
| 90-day mortality, % | 0% | 7.1% | 0.14 |
ASA = American Society of Anaesthesiologists; HLAPD = hybrid laparoscopy-assisted pancreaticoduodenectomy; IV = intravenous; LOS = length of stay; RAPD = robotic-assisted pancreaticoduodenectomy; SD = standard deviation.
Unless indicated otherwise.
Mann–Whitney U test.
There was no significant difference in oncologic outcomes between the RAPD and HLAPD groups (Table 2). The majority of patients (88.5%) had malignant pathology. The most common pathology for RAPD was pancreatic ductal adenocarcinoma, accounting for 58.3% of cases, followed by pancreatic neuroendocrine tumours (16.7%), cholangiocarcinoma (8.3%), duodenal gastrointestinal stromal tumour (GIST; 8.3%) and intraepithelial neoplasia (8.3%). The R0 resection rate was 91.7%, and the median number of harvested lymph nodes was 22.5 (range 4–44), 58.3% of which were positive. The median tumour size was 2.85 cm. There was no recurrence at 90 days.
Table 2.
Pathologic and oncologic outcomes
| Outcome | Group; mean [range] or no. (%) | p value* | |
|---|---|---|---|
| RAPD (n = 12) | HLAPD (n = 16) | ||
| Tumour size, cm | 2.85 [1.2–7] | 3.4 [1.8–4.2] | 0.98 |
| Positive resection margin | 1 (8.3) | 3 (18.8) | 0.66 |
| Lymph node harvest | 22.5 [4–44] | 22 [13–56] | 0.55 |
| Positive lymph nodes | 7 (58.3) | 9 (56.3) | 0.40 |
| Malignant | 11 (91.7) | 12 (75.0) | 0.23 |
| Pancreatic adenocarcinoma | 6 (50.0) | 10 (62.5) | — |
| Ampullary adenocarcinoma | 1 (8.3) | 1 (6.3) | — |
| Neuroendocrine tumour | 2 (16.7) | 1 (6.3) | — |
| Cholangeocarcinoma | 1 (8.3) | 0 | — |
| Duodenal GIST | 1 (8.3) | 0 | — |
| Benign | 1 (8.3) | 2 (12.5) | 0.13 |
| IPMN | 0 | 1 (6.3) | — |
| Pan-IN | 1 (8.3) | 0 | — |
| Duodenal polyp | 0 | 1 (6.3) | — |
| Perforated gastric ulcer | 0 | 0 | — |
| Familial polyposis related tubular adenomas | 0 | 0 | — |
GIST = gastrointestinal stromal tumour; HLAPD = hybrid laparoscopy-assisted pancreaticoduodenectomy; IPMN = intraductal papillary mucinous neoplasm; Pan-IN = pancreatic intraepithelial neoplasia; RAPD = robotic-assisted pancreaticoduodenectomy.
Mann–Whitney U test.
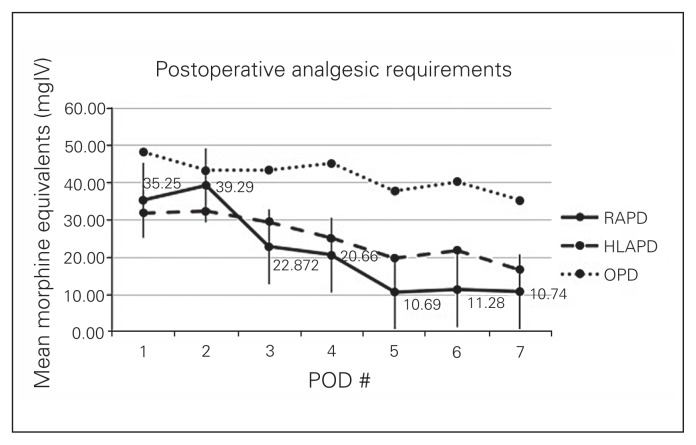
The median LOS was 7.5 (range 5–57) days in the RAPD group, as compared to 8 (range 6–14) days in the HLAPD group (p = 0.78), and mean total 7-day opioid use was 142.599 ± 68.2 mg of intravenous morphine equivalents in the RAPD group versus 176.9 ± 112.7 mg of intravenous morphine equivalents in the HLAPD group (Fig. 2). There was no significant difference between 90-day complication and pancreatic leak rates. Four patients had Clavien I–II complications that were conservatively managed (Table 3). Three patients had Clavien III–IV complications, 2 of which required admission to the intensive care unit; there was 1 reoperation for transverse colon perforation and peritonitis and 1 case of sepsis from pancreatic leak requiring intubation and CT-guided drainage. The third patient was readmitted with delirium secondary to a biliary leak and intra-abdominal abscess and required percutaneous drainage. There were no deaths within 90 days.
Fig. 2.
Postoperative analgesic requirements. Data points from hybrid laparoscopy-assisted pancreaticoduodenectomy (HLAPD) and open pancreaticoduodenectomy (OPD) patients extracted from the study by Wang and colleagues.7 POD = postoperative day; RAPD = robotic-assisted pancreaticoduodenectomy.
Table 3.
Ninety-day complication rate
| Complication | Group; no. (%) | p value* | |
|---|---|---|---|
| RAPD (n = 12) | HLAPD (n = 16) | ||
| Clavien I–II† | 4 (33) | 4 (25.0) | 0.63 |
| Intra-abdominal abscess | 0 | 1 (6.3) | — |
| Wound infection | 1 (8.3) | 0 | — |
| Delayed gastric emptying | 2 (16.7) | 2 (12.5) | — |
| Pneumonia | 0 | 0 | — |
| Hypotension | 0 | 1 (6.3) | — |
| Wound dehiscence | 1 (8.3) | 0 | — |
| Clavien III–IV‡ | 3 (25) | 4 (25.0) | 0.07 |
| Intra-abdominal abscess | 2 (16.7) | 2 (12.5) | — |
| Anastomotic breakdown | 0 | 1 (6.3) | — |
| Portal vein thrombosis | 0 | 0 | — |
| Postoperative hemorrhage | 0 | 0 | — |
| Peritonitis/colon perforation | 1 (8.3) | 0 | — |
| Acute myocardial infarction | 0 | 0 | — |
| Bowel ischemia | 0 | 1 (6.3) | — |
| Pancreatic fistula | 4 (33.0) | 5 (31.3) | 0.12 |
| Grade A | 1 (8.3) | 3 (18.8) | — |
| Grade B | 3 (25.0) | 2 (12.5) | — |
HLAPD = hybrid laparoscopy-assisted pancreaticoduodenectomy; RAPD = robotic-assisted pancreaticoduodenectomy.
Calculated using the χ2 test.
Not necessitating radiological, endoscopic or operative intervention and not causing organ failure
Necessitating radiological, endoscopic or operative intervention and/or causing organ failure.
Discussion
The versatility of the robotic platform to successfully perform well-selected pancreatic procedures with a low conversion rate is a major advantage when transitioning from an open to a minimally invasive approach.6 In doing so, fundamental surgical principles must be maintained: safe dissection, ability to control hemorrhage, achieving negative margins and focusing on meticulous reconstruction.6 To date, Zureikat and colleagues6 have provided the largest body of evidence for robotic pancreatic resections performed in a single centre (n = 250), including 132 pancreaticoduodenectomies, 83 distal pancreatectomies, 13 central pancreatectomies, 10 enucleations, 5 total pancreatectomies, 4 Appleby procedures, and 3 Frey procedures. In regards to RAPD, their findings support low complication rates (14% and 6% for Clavien III and Clavien IV complications, respectively) and mortality (2% at 90 days), with minimal conversion (6%). Their findings emphasize the ability to successfully complete complex pancreatic resections and reconstructions in a minimally invasive fashion, provided a minimal learning curve of 60–80 cases.6 They demonstrated that with increasing experience robotics has replaced open as the most common approach to pancreatic resections at their institution since the transition in 2008.
Similarly, our centre began performing robotic-assisted pancreatic resections in July 2010. We have adopted a more diversified robotic-assisted laparoscopic approach, in contrast to a purely robotic approach as described by Zureikat and colleagues.6 We complete the entire resection laparoscopically and also undertake the gastrojejunostomy laparoscopically. We use the robotic platform only for the pancreaticojejunostomy and hepatojejunostomy — steps for which we believe the robot adds the most value relative to a purely laparoscopic or hybrid approach. Our experience previously gained performing HLAPD has facilitated a step-wise introduction of minimally invasive techniques and eased our transition from laparoscopic to robotic techniques. In our previous study comparing 13 HLAPD and 20 open cases, with similar demographic parameters, we found significantly decreased blood loss (450 mL v. 1000 mL, p = 0.023) and postoperative LOS (8 v. 12 d, p = 0.025), with no difference in complication rates, pancreatic leak rates or mortality.7 This initial experience with a hybrid approach allowed us not only to master the laparoscopic resection, which is identical to the resection performed in RAPD, but also to focus on the reconstruction phase and maximize the robotic platform’s advantages for magnification, stability and increased ability for challenging suturing of small-calibre ducts.6 As such, the present study compares outcomes of RAPD with HLAPD to truly determine if there is any difference in the robotic reconstruction as compared with a hybrid approach and if it is safe and feasible to incorporate it in a centre with previous laparoscopic experience.
Though we initially expected to see an impact of robotics when performing enteric anastomosis, there was no significant difference in the pancreatic leak rate as compared with HLAPD; 25% of the RAPD patients experienced a high-grade (B or C) leak as compared with 12.5% in the HLAPD group. This is consistent with the range for RAPD reported in the literature (4%–38%; Table 4).6 Our operative approach to the pancreatic remnant involved a duct-to-mucosa pancreaticojejunostomy in a Blumgart fashion with placement of a pediatric feeding tube as a stent in an attempt to reduce pancreatic leaks.18 Zureikat and colleagues6 used the same approach and reported a 21% leak rate among all patients undergoing robotic duct-to-mucosa pancreaticoje-junostomies, which is less than other groups that opted for alternative measures, such as sclerosis with fibrin glue.11 Giulianotti and colleagues1 reported a 36.5% pancreatic fistula rate in the RAPD patients who underwent sclerosis compared with those who underwent anastomosis (21%). Given multiple failed attempts to decrease pancreatic leak rates reported in the literature and in light of the present findings, there may be an inherent pancreatic leak rate that must be accepted and managed early, despite minimally invasive approaches. That being said, our leak rate is well within the norms reported in the literature and is in fact below that reported by the largest centre performing RAPD despite the early phase of our learning curve.6
Table 4.
Early comparative experience with robotic pancreaticoduodenectomy, June 2010–July 2014
| Study | Country | n | Procedure | LOS | Duration, mean (range) min | Pancreatic leak | Complications | Mortality |
|---|---|---|---|---|---|---|---|---|
| Giulianotti et al.5 | Italy | 8 | PD | 20 | 490 | NA | 37.5% | 12.5% |
| Narula12 | USA | 5 | PD | 9.6 | 420 | 0 | 0 | 0 |
| Horiguchi13 | Japan | 3 | PD | 26 | 703 | 33% | NA | NA |
| Zeh14 | USA | 50 | PD | 10 | 568 | 22% | Clavien I–II (26%) Clavien III–IV (30%) |
2% |
| Zureikat et al.15 | USA | 24 | PD | 9 | 512 (327–848) | 21% | Clavien III–IV (25%) Clavien I–II (27%) |
3.3% |
| Chalikonda16 | USA | 30 | PD | 9.8 | 476 | 6.7% | 30% | 4% |
| Zhou17 | China | 8 | PD | 16.4 | 718 | 50% | 25% | 0 |
| Zureikat et al.6 | USA | 132 | PD | 10 | 527 | 17% | Clavien III: 14% Clavien IV: 6% |
1.5 |
LOS = length of stay; NA = not available; PD = pancreaticoduodenectomy.
Early management of these leaks, including drain removal, would contribute to decreasing progression to intra-abdominal abscesses which accounted for 2 of the 3 major (Clavien III–IV) complications encountered in our study. One patient, who had ileus in the immediate postoperative course, was readmitted 2 days postdischarge with an intra-abdominal abscess requiring admission to the intensive care unit. The patient was subsequently stabilized but remained admitted for a pre-existing comorbidity unrelated to the surgical procedure. Similarly, the second patient was readmitted 2 days postdischarge with delirium secondary to intra-abdominal collection, which resolved with ultrasound-guided drainage. The third severe complication was in a patient with cholangiocarcinoma who required admission to the intensive care unit for sepsis and peritonitis secondary to transverse colon perforation. This patient underwent reoperation on postoperative day 7 and subsequently experienced recurrent bowel obstructions, potentially due to recurrent cholangiocarcinoma, and was eventually discharged on postoperative day 57 with no further complications. Zureikat and colleagues6 reported a drop in severe (grade III–IV) complications in the later half of their learning curve (30.7% among the first 80 patients v. 13.7% for the late group of patients undergoing RAPD). While our centre has yet to reach this learning curve of 80 patients, our severe complication rate is comparable with that of Zureikat and colleagues during their initial experience with RAPD and is predicted to improve with experience.
Furthermore, there was no significant difference in estimated blood loss, duration of surgery, postoperative opioid use or postoperative LOS between the RAPD and HLAPD groups in the present study. The benefit of the robotic arms to gain access into the retroperitoneal space and provide adequate hemostasis is demonstrated by our median estimated blood loss of 275 mL, which is less than the rate reported in our laparoscopic cases (400 mL) and consistent with the significant difference reported in the literature.4 This also reflects our ability to control bleeding during both phases of the procedure and highlights the benefits of experience with both laparoscopy and robotics, so as to avoid severe complications related to hypovolemia.3 Because of less damage to surrounding vasculature, we achieved a low rate of severe complications (25% Clavien III–IV), and none was related to hypovolemia or disseminated intravascular coagulation.
In addition, owing to the precision and care needed to perform these intricate resections and reconstructions with minimal complications, duration of surgery in the RAPD group (596 min) was longer than that reported for open resections3,19 but virtually identical to that in the HLAPD group (592 min) and is in the range reported in the literature for RAPD (431–718 min).6,7,17 These long operations, despite our centre’s experience with the hybrid laparoscopic approach, may be in part attributed to the added complexity of malignant pathology in most of our patients (91.7%) and to the increased care in executing an adequate resection to preserve oncologic outcomes comparable to the open experience. Similar lymphadenectomy rates (22.5 nodes, range 4–44) were achieved as with HLAPD and in the range described by Zureikat and colleagues18 using a completely robotic approach (17 nodes, range 5–37). In addition, both attending surgeons are still within their learning curve, which is 60–80 procedures.6 In the second half of our study, we observed a median improvement of 101.5 minutes, and we predict future improvement in operative duration with increasing experience, as was reported by Zureikat and colleagues, 6 who experienced a significant decrease in operative duration in their last 60 cases.6
Although long-term and quality of life data are not available in the present study, we quantified the level of immediate postoperative pain based on daily analgesic requirements up to postoperative day 7. We noted minimal use of opioids with a mean of 142.599 ± 68.2 mg of intravenous morphine equivalents in the week following RAPD compared with 176.9 ± 112.7 mg of intravenous morphine equivalents in the HLAPD group, tapering to close to zero by postoperative day 7 in both groups; 41% of RAPD patients were weaned off by postoperative day 5. To our knowledge, we are the first to report postoperative analgesic use as an indicator of postoperative pain, but conclude decreased need for opioids in patients undergoing minimally invasive than in those undergoing open pancreaticoduodenectomy as previously reported.7 Comparative trends up to postoperative day 7 are illustrated in Figure 2. We believe that the decreased need for opioids may have directly contributed to a decreased LOS in the RAPD group (7.5 d) compared with historic open data, but there was no significant difference between the RAPD and HLAPD groups.
In light of our findings, patients at our institution continue to be selected for a minimally invasive approach based solely on tumour characteristics on preoperative imaging. These criteria have not changed over time, even with increasing experience. Including a patient in the RAPD or HLAPD group is not based on superiority or preference of one procedure over another but rather on robot availability on the scheduled procedure date, as this resource limitation is a reality we must face in a Canadian institution. This resource limitation also restricts our ability to overcome our learning curve in a more timely fashion. Despite this constraint, our centre nonetheless completed 28 minimally invasive pancreaticoduodenectomies (12 RAPD and 16 HLAPD) during the study period. What has evolved over time, however, is our willingness and confidence to begin a procedure by laparoscopy and then convert to hybrid or open if needed, so that the patient may benefit from as much of a minimally invasive procedure as possible. We have noted no deaths or significant differences in major complications and thus plan to pursue RAPD at our institution in all patients amenable to a minimally invasive approach according to our aforementioned inclusion criteria.
Conclusion
In our early experience, RAPD is safe and feasible and can ensure adequate oncologic resections and lymphadenectomies with acceptable complication rates compared with HLAPD. The importance of minimal blood loss, lower levels of postoperative opioids and shorter LOS emphasize the ability to perform robotic reconstructions safely in a highly specialized centre with previous experience in laparoscopic pancreatic resections. Our initial results with RAPD are positive and encouraging compared with both HLAPD and an open procedure, further providing evidence that the robotic platform provides meaningful patient benefit and should be considered in centres with access to a robot and trained surgeons.
Footnotes
This paper was presented as a poster presentation at the SAGES Annual Meeting, Baltimore MD, Apr. 17–20, 2013, and as a poster presentation at the Canadian Surgery Forum, Ottawa, ON, Sept. 19–21, 2013.
Competing interests: None declared.
Contributors: S. Piedimonte, S. Bergman and T. Vanounou designed the study. S. Piedimonte and Y. Wang acquired and analyzed the data, which T. Vanounou also analyzed. S. Piedimonte and T. Vanounou wrote the article, which all authors reviewed and approved for publication.
References
- 1.Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc. 2010;24:1646–57. doi: 10.1007/s00464-009-0825-4. [DOI] [PubMed] [Google Scholar]
- 2.Ejaz A, Sachs T, He J, et al. A comparison of open and minimally invasive surgery for hepatic and pancreatic resections using the Nationwide Inpatient Sample. Surgery. 2014;156:538–47. doi: 10.1016/j.surg.2014.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correa-Gallego C, Dinkelspiel HE, Sulimanoff I, et al. Minimally-invasive vs open pancreaticoduodenectomy: systematic review and meta-analysis. J Am Coll Surg. 2014;218:129–39. doi: 10.1016/j.jamcollsurg.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Lei P, Wei B, Guo W, et al. Minimally invasive surgical approach compared with open pancreaticoduodenectomy: a systematic review and meta-analysis on the feasibility and safety. Surg Laparosc Endosc Percutan Tech. 2014;24:296–305. doi: 10.1097/SLE.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 5.Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch surg. 2003;138:777–84. doi: 10.1001/archsurg.138.7.777. [DOI] [PubMed] [Google Scholar]
- 6.Zureikat AH, Hogg ME, Zeh HJ., III The utility of the robot in pancreatic resections. Adv Surg. 2014;48:77–95. doi: 10.1016/j.yasu.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Bergman S, Piedimonte S, Vanounou T. Bridging the gap between open and minimally invasive pancreaticoduodenectomy: the hybrid approach. Can J Surg. 57:263–70. doi: 10.1503/cjs.026713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeOliveira ML, Winter JM, Schafer M, et al. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931–7. doi: 10.1097/01.sla.0000246856.03918.9a. discussion 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–8. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Kleespies A, Rentsch M, Seeliger H, et al. Blumgart anastomosis for pancreaticojejunostomy minimizes severe complications after pancreatic head resection. Br J Surg. 2009;96:741–50. doi: 10.1002/bjs.6634. [DOI] [PubMed] [Google Scholar]
- 12.Narula VK, Mikami DJ, Melvin WS. Robotic and laparoscopic pancreaticoduodenectomy: a hybrid approach. Pancreas. 2010;39:160–4. doi: 10.1097/MPA.0b013e3181bd604e. [DOI] [PubMed] [Google Scholar]
- 13.Horiguchi A, Uyama I, Miyakawa S. Robot-assisted laparoscopic pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2011;18:287–91. doi: 10.1007/s00534-010-0325-x. [DOI] [PubMed] [Google Scholar]
- 14.Zeh HJ, Zureikat AH, Secrest A, et al. Outcomes after robot-assisted pancreaticoduodenectomy for periampullary lesions. Ann Surg Oncol. 2012;19:864–70. doi: 10.1245/s10434-011-2045-0. [DOI] [PubMed] [Google Scholar]
- 15.Zureikat AH, Breaux JA, Steel JL, et al. Can laparoscopic pancreaticoduodenectomy be safely implemented? J Gastrointest Surg. 2011;15:1151–7. doi: 10.1007/s11605-011-1530-x. [DOI] [PubMed] [Google Scholar]
- 16.Chalikonda S, Aguilar-Saavedra JR, Walsh RM. Laparoscopic robotic-assisted pancreaticoduodenectomy: a case-matched comparison with open resection. Surg Endosc. 2012;26:2397–402. doi: 10.1007/s00464-012-2207-6. [DOI] [PubMed] [Google Scholar]
- 17.Zhou NX, Chen JZ, Liu Q, et al. Outcomes of pancreatoduodenectomy with robotic surgery versus open surgery. Int J Med Robot. 2011;7:131–7. doi: 10.1002/rcs.380. [DOI] [PubMed] [Google Scholar]
- 18.Zureikat AH, Nguyen KT, Bartlett DL, Zeh HJ, Moser AJ. Robotic-assisted major pancreatic resection and reconstruction. Arch Surg. 2011;146:256–61. doi: 10.1001/archsurg.2010.246. [DOI] [PubMed] [Google Scholar]
- 19.Nigri G, Petrucciani N, La Torre M, et al. Duodenopancreatectomy: open or minimally invasive approach? Surgeon. 2014;12:227–34. doi: 10.1016/j.surge.2014.01.006. [DOI] [PubMed] [Google Scholar]