Abstract
Background
An infected total knee arthroplasty (TKA) can be treated with irrigation and débridement with polyethylene exchange (IDPE) or a 2-staged revision (2SR). Although research has examined infection eradication rates of both treatments, patient outcomes have not been reported. We examined patient-reported outcomes following treatment compared with matched, noninfected controls.
Methods
We retrospectively identified patients with infected TKAs who had undergone the index procedure between May 1991 and November 2011. Patient-reported outcomes included the 12-item Short Form Health Survey, Western Ontario and McMaster Universities Arthritis Index, and Knee Society Scores as well as range of motion. Patients with noninfected primary TKAs matched by age and age-adjusted Charlson Comorbidity Index score were used as controls. Intention-to-treat groups of 2SR and IDPE were used, with the IDPE group subdivided into successful and unsuccessful groups.
Results
We included 145 patients with infected TKAs with mean follow-up of 64.2 months and 145 controls with a mean follow-up of 35.4 months in our analysis. Outcomes of the controls and the successful IDPE groups were equivalent. The 2SR cohort had lower scores in all categories than controls. There was a 39% success rate in eradicating infection with IDPE. Patients in whom IDPE failed had lower scores in all categories than controls. There was no difference between the failed IDPE group and the 2SR group.
Conclusion
Controversy regarding treatment options for acutely infected TKA has been focused on infection eradication. However, functional outcomes following treatment need to be taken into consideration. Patients whose infections were successfully treated with IDPE had equivalent outcomes to controls.
Abstract
Contexte
Il est possible de traiter une arthroplastie totale du genou (ATG) infectée par irrigation et débridement avec changement du polyéthylène (IDCP) ou par une révision en 2 étapes. Même si la recherche a examiné les taux d’éradication de l’infection au moyen des 2 traitements, les résultats chez les patients n’ont pas fait l’objet de rapports. Nous avons comparé les résultats enregistrés chez les patients traités à ceux de témoins assortis non infectés.
Méthodes
Nous avons recensé de manière rétrospective les patients qui ont présenté une infection de leur ATG et qui avaient initialement subi leur intervention entre mai 1991 et novembre 2011. Les résultats rapportés par les patients incluaient le questionnaire SF (Short Form) sur la santé en 12 points, l’indice WOMAC (établi par les universités Western Ontario et McMaster), le score de la Knee Society, de même que l’amplitude de mouvement. Des patients soumis à une ATG primaire non infectée assortis selon l’âge et le score de comorbidités de Charlson ajusté selon l’âge ont servi de participants témoins. On a réparti les groupes selon l’intention de traiter par révision en 2 étapes ou par IDCP; le groupe IDCP a été subdivisé selon que l’intervention avait réussi ou non.
Résultats
Notre analyse a regroupé 145 patients dont l’ATG s’était infectée et qui ont été suivis en moyenne pendant 64,2 mois, et 145 témoins suivis en moyenne pendant 35,4 mois. Les résultats ont été équivalents chez les témoins et les groupes dont l’IDCP avait réussi. La cohorte soumise à la révision en 2 étapes a obtenu des scores moindres dans toutes les catégories, comparativement aux témoins. On a noté un taux de succès de 39 % pour l’éradication de l’infection avec l’IDCP. Les patients chez qui l’IDCP a échoué présentaient des scores moindres dans toutes les catégories comparativement aux témoins. On n’a noté aucune différence entre le groupe chez qui l’IDCP avait échoué et le groupe soumis à la révision en 2 étapes.
Conclusion
La controverse quant aux options thérapeutiques pour les infections aiguës d’ATG portait sur l’éradication de l’infection. Or, les résultats fonctionnels après le traitement devraient aussi entrer en ligne de compte. Chez les patients dont les infections ont été traitées avec succès par IDCP, les résultats ont été équivalents à ceux des témoins.
Periprosthetic joint infection is a devastating complication after total knee arthroplasty (TKA). Between 2005 and 2006, 25% of revisions were to manage infection.1 Demand for primary TKA in the United States is projected to grow by 673% to 3.48 million procedures by 2030.2 This would translate into a huge number of patients experiencing periprosthetic joint infections, with the care of these patients representing a substantial financial burden to society. The surgical options for treatment of periprosthetic infection include irrigation and débridement with polyethylene exchange (IDPE), single-stage revision, or 2-stage revision (2SR).
Irrigation and débridement with polyethylene exchange is an attractive alternative for both patient and surgeon. Compared with a 2SR, benefits of an IDPE include retention of implants, preservation of bone stock, shorter procedure duration, less chance of intraoperative fracture from removal of components and implantation of cement spacers, and faster postoperative rehabilitation.3–5 However, the reported success rate of IDPE is variable, with reports ranging from 29% to 83%.6–10 By comparison, 2SR is considered the gold standard, with success rates reported in the range of 75%–100%.11–17 In addition, it has been reported that failure rates of 2SR for TKA infections are higher in patients treated with previous IDPE than in patients who did not receive IDPE.18 Therefore, surgeons considering IDPE need to balance potential benefits of the procedure with the lower eradication rate and potentially decreased chance of eradication should the patient ultimately receive 2SR.
There may be a role for IDPE in certain situations, such as the treatment of acute postoperative and acute hematogenous infections.19 An acute postoperative infection has been defined as one that occurs within the first 4 weeks after index TKA.20 Other studies are more reserved in their recommendations and state that IDPE should be considered only in immunologically optimized patients with acute non-Staphylococcal infections.21 Although there is an abundance of literature studying the successful eradication rates with IDPE and 2SR, there is a paucity of data reporting on the patient experience or patient satisfaction associated with these revision procedures. Understanding patient-reported satisfaction is important to the treatment decision process. Therefore, the purpose of this study was to examine patient-reported outcomes in patients with infected TKAs based on whether the patients were treated with initial 2SR, successful IDPE, or failed IDPE with subsequent 2SR; to compare each of the above cohorts to a matched control group of patients with noninfected TKAs; and to determine the success rates of 2SR and IDPE in our study population.
Methods
After obtaining institutional review board approval, we performed a database query to identify patients whose index TKAs, performed between May 1991 and November 2011, were acutely infected. Inclusion criteria for our retrospective review were a minimum 1-year follow-up after surgical treatment of infection.
All procedures were performed by 1 of 7 surgeons at our institution. All 7 are high-volume, arthroplasty fellowship–trained surgeons. Implant type for the index procedures included varying levels of constraint, including posterior stabilized, varus-valgus constrained non-hinged, and hinged knees.
In 2011, The Musculoskeletal Infection Society created guidelines for the diagnosis of periprosthetic joint infection (PJI). A definite diagnosis of PJI can be made when the following conditions are met.22
Sinus tract communicating with the prosthesis.
Pathogen isolated by culture from 2 separate tissue or fluid samples obtained from the affected prosthetic joint.
Presence of at least 4 of the following: elevated serum erythrocyte sedimentation rate (ESR) or serum C-reactive protein (CRP) concentration, elevated synovial white blood cell count, elevated synovial neutrophil percentage, presence of purulence in the affected joint, isolation of a microorganism in 1 culture of periprosthetic tissue or fluid, and more than 5 neutrophils per high-power field in 5 high-power fields observed from histologic analysis of periprosthetic tissue at ×400 magnification.
At our institution, diagnosis of infection follows these criteria, with the whole clinical picture used to guide treatment. Threshold values for ESR and CRP in the present study were are 30 mm/hr and 10 mg/L, respectively. We excluded patients with less than 1 year of complete follow-up.
Identified patients were matched to a control cohort of patients with noninfected primary TKAs based on age and the age-adjusted Charlson Comorbidity Index (CCI) score. The CCI score is a validated method of estimating risk of death from comorbid disease and has also been found to correlate well with major complications in revision surgery.23,24 Patients with a surgically managed infected TKA were then divided into either the 2SR or IDPE group based on intention to treat. The type of treatment performed was at the discretion of the treating surgeon. The IDPE group was then further subdivided based on whether the IDPE was successful or unsuccessful at eradicating infection; patients in whom IDPE was not successful required subsequent 2SR. Both acute hematogenous and acute postoperative infections were defined as those presenting within 4 weeks of onset of symptoms. We considered the infection to be eradicated when the inflammatory markers had normalized, the clinical symptoms had improved and the surgical wound had healed.
Functional outcomes and reoperations associated with unsuccessful eradication of infection were reviewed. We used the most recent patient-reported scores and range of motion (ROM) for analysis. For patients who had unsuccessful IDPE and required subsequent 2SR, clinical outcomes were measured at their most recent follow-up (i.e., after their 2SR). We calculated CCI scores based on a review of patient charts. At each clinic visit, ROM was recorded using a goniometer; ROM at the initial visit and at latest review was used in this study. Western Ontario and McMaster Universities Arthritis Index (WOMAC), Knee Society Clinical Rating System (KSS) and the 12-item Short-Form Health Survey (SF12) scores were recorded from standardized forms that are routinely used for all arthroplasty patients at our institution. All 3 scores have been validated for use in quantifying knee pain and function.25–27
Statistical analysis
We used the Statistical Package for the Social Sciences (SPSS Inc.) for the statistical analysis. We used the Student t test for parametric comparisons and the Mann–Whitney U test for nonparametric comparisons between the groups. The Mann–Whitney U test was used when data for a particular variable did not meet the distribution assumptions required by their parametric counterpart.
Results
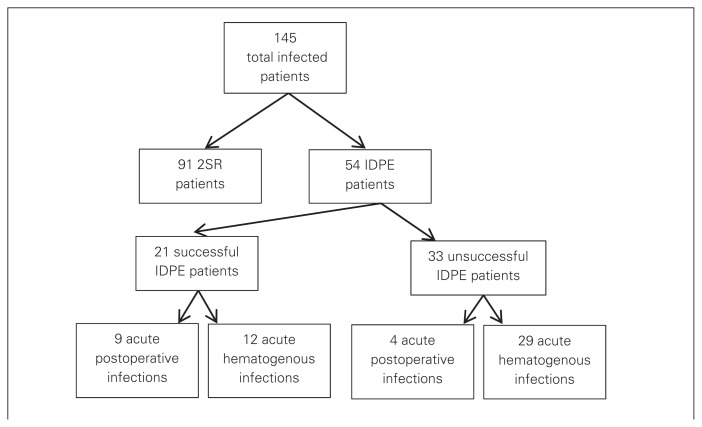
During our study period, 1857 knee revisions were performed at our institution. Review of our database identified 145 infected TKAs in 145 patients. Of the 145 patients with infected TKAs, 91 were treated initially with 2SR and 54 were treated with IDPE. Of the 91 patients treated with 2SR, 79 had successful eradication of infection and 12 had reoperations for infection. Of the 54 patients treated with IDPE, 21 had successful infection eradication and 33 had a persistent infection and required 2SR (Fig. 1). All of the patients in our cohort in whom IDPE failed received a subsequent 2SR. Of the 21 patients in whom IDPE was successful, 9 had their infections diagnosed during the acute postoperative period and 12 had diagnoses of acute hematogenous infection. Of the 33 patients in whom IDPE was unsuccessful, 4 had their infections diagnosed during the acute postoperative period and 29 patients had diagnoses of acute hematogenous infection (Fig. 1). In other words, acute postoperative infection in our patient cohort represented 43% of successful IDPE and 12% of failed IDPE.
Fig. 1.
Distribution of patients based on treatment algorithm. 2SR = 2-staged revision; IDPE = irrigation and débridement with polyethylene exchange.
There was no difference in age, CCI scores or body mass index between controls and patients with infected TKAs (Table 1). Mean clinical follow-up for patients with infected TKAs was 64.2 (range 12–237) months compared with 35.4 (range 24–120) months in the control group (p < 0.001; Table 1).
Table 1.
Patient demographic and clinical characteristics
| Characteristic | Group; mean | p value | |
|---|---|---|---|
| Control | Infection | ||
| Age, yr | 67.7 | 68.9 | 0.21 |
| CCI score | 1.51 | 1.65 | 0.49 |
| BMI | 32.8 | 33.1 | 0.84 |
| Follow-up, mo | 35.4 | 64.2 | < 0.001 |
BMI = body mass index; CCI = Charlson Comorbidity Index.
For the successful IDPE cohort, 6.7% of patients had a hinged prosthesis and 93.3% had posterior stabilized prostheses. All patients in the failed IDPE group had posterior stabilized prostheses. In the 2SR group, 6.1% had a hinged prosthesis, 57.3% had varus-valgus constrained prostheses, and 36.6% had posterior stabilized prostheses. The mean duration from initial arthroplasty surgery to the 2SR was 31.7 (range 2–180) months. The mean duration from initial arthroplasty to successful IDPE was 15.3 (range 1–89) months. Finally, the mean duration from initial arthroplasty to failed IDPE was 23.8 (range 1–120) months.
Compared with the 2SR group, the control group performed better on all measures, with better SF12 mental composite scale (p = 0.005), SF12 physical composite scale (p = 0.002), WOMAC (p < 0.001) and KSS (p < 0.001) scores and improved ROM (p < 0.001) at latest review (Table 2). When the 2SR group was divided into successful and failed 2SR, the control group performed better than both on all measured outcomes (all p < 0.05). Similarly, the control group performed better on all measures than the failed IDPE group (all p < 0.05; Table 3). Comparing the failed IDPE group with the 2SR group revealed no difference in any outcome (all p > 0.05; Table 4). Comparing the control group with the successful IDPE group demonstrated no difference in any measured outcome (all p > 0.05; Table 5). The success rate with IDPE was 39% and the success rate with 2SR was 87% in our cohorts.
Table 2.
Outcome scores comparing controls with patients with infected TKAs who received a 2SR
| Outcome measure | Group; mean | p value | |
|---|---|---|---|
| Control | 2SR | ||
| SF12 mental component score | 53.4 | 49.7 | 0.045 |
| SF12 physical component score | 38.9 | 33.8 | 0.002 |
| KSS | 169.3 | 135.3 | < 0.001 |
| WOMAC | 78.1 | 62.8 | < 0.001 |
| ROM, arc | 116.9 | 91.1 | < 0.001 |
2SR = 2-staged revision; KSS = Knee Society score; ROM = range of motion; SF12 = 12-item Short Form Health Survey; TKA = total knee arthroplasty; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Table 3.
Outcome scores comparing controls with patients with infected TKAs in whom IDPE failed
| Outcome measure | Group; mean | p value | |
|---|---|---|---|
| Control | Failed IDPE | ||
| SF12 mental component score | 53 | 46.1 | 0.026 |
| SF12 physical component score | 42.5 | 37.3 | 0.045 |
| KSS | 170.4 | 142.1 | 0.004 |
| WOMAC | 76.2 | 63.9 | 0.036 |
| ROM, arc | 116.6 | 93.6 | 0.003 |
IDPE = irrigation and débridement with polyethylene exchange; KSS = Knee Society score; ROM = range of motion; SF12 = 12-item Short Form Health Survey; TKA = total knee arthroplasty; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Table 4.
Outcome scores comparing failed IDPE with 2SR
| Outcome measure | Group; mean | p value | |
|---|---|---|---|
| Failed IDPE | 2SR | ||
| SF12 mental component score | 45.2 | 49.7 | 0.08 |
| SF12 physical component score | 37.2 | 33.8 | 0.12 |
| KSS | 141.8 | 135.3 | 0.54 |
| WOMAC | 63.9 | 62.8 | 0.93 |
| ROM, arc | 93.4 | 91.1 | 0.47 |
2SR = 2-staged revision; IDPE = irrigation and débridement with polyethylene exchange; KSS = Knee Society score; ROM = range of motion; SF12 = 12-item Short Form Health Survey; TKA = total knee arthroplasty; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Table 5.
Outcomes comparing controls with patients with infected TKAs in whom IDPE was successful
| Outcome measure | Group; mean | p value | |
|---|---|---|---|
| Control | Successful IDPE | ||
| SF12 mental component score | 49.4 | 50.1 | 0.96 |
| SF12 physical component score | 37.2 | 37.7 | 0.93 |
| KSS | 160.8 | 150.1 | 0.48 |
| WOMAC | 75.6 | 72.1 | 0.67 |
| ROM, arc | 109 | 110.9 | 0.56 |
2SR = 2-staged revision; IDPE = irrigation and débridement with polyethylene exchange; KSS = Knee Society score; ROM = range of motion; SF12 = 12-item Short Form Health Survey; TKA = total knee arthroplasty; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Discussion
Periprosthetic joint infection continues to be a challenge in TKA for both patients and surgeons. Twenty-fice percent of revisions are done as a result of infection,1 with an incidence rate of 1% for TKA.28 The optimal treatment for patients with infected TKAs is controversial. While 2SR remains the gold standard in treatment with an eradication rate ranging from 75% to 100%,11–17 there is no clear consensus on the role of IDPE in the treatment of periprosthetic infection. Compared with 2SR, the benefits of IDPE include retention of implants with preservation of bone stock, shorter procedure durations, decreased chance of intraoperative fracture from removal of components with implantation of cement spacers, and faster postoperative rehabilitation.3–5 The main arguments against the use of IDPE as a treatment option have centered on its low success rate at eradicating infection and on the possibility that IDPE may reduce the success rate of a subsequent 2SR.18 There is an abundance of literature on the treatment of an infected TKA with success rates of IDPE reported to range from 29% to 83%.6–10 Therefore, we elected not to focus on success or failure rates of eradication. Instead, the aim of the present study was to add to the body of literature by being, to our knowledge, the first study to focus on patient-reported outcomes based on treatment provided.
Our results demonstrate that there is no difference in patient-reported clinical outcomes when comparing unsuccessful IDPE and 2SR. Most interestingly, we found no difference in any outcome when comparing the control group with the successful IDPE group. These findings are important when counselling a patient on the treatment options available for an infected TKA. The improved satisfaction of a successful IDPE must be weighed against the lower rates of successful eradication, and these issues need to be discussed with the patient.
In our cohort, IDPE resulted in an eradication rate of 39%, which is consistent with rates reported in the literature.6–10 Similarly, the eradication rate after 2SR in our cohort was 87%, which is also consistent with published rates.11–17 Treatment with IDPE is more likely to be successful in cases of acute postoperative and acute hematogenous infections.19 The failed IDPE group in our study had a greater proportion of acute hematogenous infection than the successful IDPE group. It is possibile that some of our patients in whom IPDE failed actually had misdiagnosed chronic infections. However, the 39% eradication rate in our study is consistent with that reported in previous studies evaluating IDPE for infected TKAs.6–10 Furthermore, the main purpose of the present study was to report on outcomes based on the treatment patients received rather than the success or failure of eradication.
Limitations
The study limitations were as follows. First, this study involved a retrospective review and was therefore subject to all the biases associated with this type of study design. Second, some patients in whom IDPE failed had been referred from other hospitals. As the referring surgeons followed IDPE treatment protocols similar to those at our tertiary care centre, these patients were included in the current study to maximize cohort size. Third, it should be noted that there is a difference between the infected and control cohorts with regards to mean duration of follow-up (64.2 mo in the infected cohorts v. 35.4 mo in the control cohort). The control cohort was selected by matching patients with noninfected primary TKAs with patients in the infected cohort based on age and age-adjusted CCI scores. This process resulted in a comparable control cohort in terms of patient number, age, age-adjusted CCI and body mass index. As a result of the matching process, there was a difference in mean duration of follow-up between the cohorts. However, since previous literature has demonstrated that clinical outcome scores do not change significantly beyond 18 months after surgery,29,30 the comparison of clinical outcomes in the cohorts is still relevant despite the differential follow-up. Finally, the data included in the current study depend on the quality of the data recorded in the medical records and are therefore subject to the limitations faced by many retrospective cohort designs. In some cases the onset of symptoms were not well recorded in terms of hours and/or days. Therefore, although we could definitively identify that patients fit our definition of acute symptoms (< 4 wk), in some cases we were unable to reliably calculate an hour or day value for onset of symptoms. As a result we have not presented these data in our study.
The main strength of this study is that it offers a unique look at a large patient cohort experiencing a difficult complication after TKA. It also examines how different treatment algorithms affect patient-reported outcomes and ROM after treatment of infection. To our knowledge, patient-reported outcomes have previously not been published in the literature or been considered as part of the controversy regarding the appropriate management of the infected TKA.
Conclusion
There may be a role for IDPE in the treatment of periprosthetic infections owing to the potential for greater patient satisfaction with IDPE than with 2SR. The improved satisfaction associated with a successful IDPE must be weighed against its lower rate of successful eradication of infection. By attempting to identify the patients in whom IDPE is most likely to succeed, a surgeon can maximize patient outcomes when dealing with periprosthetic infection.
Footnotes
Competing interests: J. Howard declares consultancy fees from DePuy, Smith and Nephew and Stryker; grants from DePuy; speaker fees from DePuy and Smith and Nephew; and institutional research support from DePuy, Smith and Nephew, Stryker, Zimmer and Micro-port. B. Lanting declares travel assistance from Smith and Nephew. No other competing interests declared.
Contributors: I. Dzaja, J. Howard and B. Lanting designed the study. I. Dzaja, L. Somerville and B. lanting acquired the data, which all authors analyzed. I. Dzaja and L. Somerville wrote the article, which all authors reviewed and approved for publication.
References
- 1.Bozic KJ, Kurtz SM, Lau EL, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468:45–51. doi: 10.1007/s11999-009-0945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 3.Choi HR. Can implant retention be recommended for treatment of infected TKA? Clin Orthop Relat Res. 2011;469:961–9. doi: 10.1007/s11999-010-1679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pulido L. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–5. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisman DN. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32:419–30. doi: 10.1086/318502. [DOI] [PubMed] [Google Scholar]
- 6.Rand JA. Alternatives to reimplantation for salvage of the total knee arthroplasty complicated by infection. Instr Course Lect. 1993;42:341–7. [PubMed] [Google Scholar]
- 7.Mont MA, Waldman B, Banerjee C, et al. Multiple irrigation, debridement, and retention of components in infected total knee arthroplasty. J Arthroplasty. 1997;12:426–33. doi: 10.1016/s0883-5403(97)90199-6. [DOI] [PubMed] [Google Scholar]
- 8.Silva M, Tharani R, Schmalzried TP. Results of direct exchange or debridement of the infected total knee arthroplasty. Clin Orthop Relat Res. 2002;404:125–31. doi: 10.1097/00003086-200211000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Marculescu CE, Berbari EF, Hanssen AD, et al. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin Infect Dis. 2006;42:471–8. doi: 10.1086/499234. [DOI] [PubMed] [Google Scholar]
- 10.Fehring TK, Odum SM, Berend KR, et al. Failure of irrigation and debridement for early postoperative periprosthetic infection. Clin Orthop Relat Res. 2013;471:250–7. doi: 10.1007/s11999-012-2373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirakawa K, Stulberg BN, Wilde AH, et al. Results of 2-stage reimplantation for infected total knee arthroplasty. J Arthroplasty. 1998;13:22–8. doi: 10.1016/s0883-5403(98)90071-7. [DOI] [PubMed] [Google Scholar]
- 12.Wilson MG, Kelley K, Thornhill TS. Infection as a complication of total knee-replacement arthroplasty. J Bone Joint Surg Am. 1990;72:878–83. [PubMed] [Google Scholar]
- 13.Hofmann AA, Goldberg T, Tanner AM, et al. Treatment of infected total knee arthroplasty using an articulating spacer: 2–12 year experience. Clin Orthop Relat Res. 2005;430:125–31. doi: 10.1097/01.blo.0000149241.77924.01. [DOI] [PubMed] [Google Scholar]
- 14.Fehring TK, Odum S, Calton TF, et al. Articulating versus statis spacers in revision total knee arthroplasty for sepsis. The Ranawat Award. Clin Orthop Relat Res. 2000;380:9–16. doi: 10.1097/00003086-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Haleem AA, Berry DJ, Hanssen AD. Mid-term to long-term follow-up of two-stage reimplantation for infected total knee arthroplasty. Clin Orthop Relat Res. 2004;428:35–9. doi: 10.1097/01.blo.0000147713.64235.73. [DOI] [PubMed] [Google Scholar]
- 16.Hart WJ, Jones RS. Two-stage revision of infected total knee replacements uring articulating cement spacers and short-term antibiotic therapy. J Bone Joint Surg Br. 2006;88:1011–5. doi: 10.1302/0301-620X.88B8.17445. [DOI] [PubMed] [Google Scholar]
- 17.Teeny SM, Dorr L, Murata G, et al. Treatment of infected total knee arthroplasty. J Arthroplasty. 1990;5:35–9. doi: 10.1016/s0883-5403(06)80007-0. [DOI] [PubMed] [Google Scholar]
- 18.Sherrell JC, Fehring TK, Odum S, et al. The Chitranjan Ranawat Award: fate of two-stage reimplantation after failed irrigation and debridement for periprosthetic knee infection. Clin Orthop Relat Res. 2011;469:18–25. doi: 10.1007/s11999-010-1434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu FY, Chen CM. Surgical debridement and parenteral antibiotics in infected revision total knee arthroplasty. Clin Orthop Relat Res. 2007;461:130–5. doi: 10.1097/BLO.0b013e318063e7f3. [DOI] [PubMed] [Google Scholar]
- 20.Segawa H, Tsukayama DT, Kyle RF, et al. Infection after total knee arthroplasty. a retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81:1434–45. doi: 10.2106/00004623-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Koyonos L, Zmistowski B, Della Valle CJ, et al. Infection control rate of irrigation and debridement for periprosthetic joint infection. Clin Orthop Relat Res. 2011;469:3043–8. doi: 10.1007/s11999-011-1910-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parvizi J, Zmistowski B, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–4. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Koenig K, Huddleston JI, 3rd, Huddleston H, et al. Advanced age and comorbidity increase the risk for adverse events after revision total hip arthroplasty. J Arthoplasty. 2012;27:1402–7. doi: 10.1016/j.arth.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 26.Lingard EA, Katz JN, Wright RJ, et al. Validity and responsiveness of the Knee Society clinical rating system in comparison with the SF-36 and WOMAC. J Bone Joint Surg Am. 2001;83-A:1856–64. doi: 10.2106/00004623-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Resnick B, Nahm ES. Reliability and validity testing of the revised 12-item Short Form Health Survey in older adults. J Nurs Meas. 2001;9:151–61. [PubMed] [Google Scholar]
- 28.Jämsen E, Varonen M, Huhtala H, et al. Incidence of prosthetic joint infections after primary knee arthroplasty. J Arthroplasty. 2010;25:87–92. doi: 10.1016/j.arth.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Fortin PR, Penrod JR, Clarke AE, et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46:3327–30. doi: 10.1002/art.10631. [DOI] [PubMed] [Google Scholar]
- 30.Bachmeier CJ, March LM, Cross MJ, et al. A comparison of outcomes in osteoarthritis patients undergoing total hip and knee replacement surgery. Osteoarthritis Cartilage. 2001;9:137–46. doi: 10.1053/joca.2000.0369. [DOI] [PubMed] [Google Scholar]