Abstract
Background
The effect of surgical wait times on survival in patients with non–small cell lung cancer (NSCLC) remains largely unknown. Our objective was to determine the effect of surgical wait time on survival and incidence of upstaging in patients with stage I and II NSCLC.
Methods
All patients with clinical stage I and II NSCLC who underwent surgical resection in a single centre between January 2010 and December 2011 were reviewed. Analysis was stratified based on preoperative clinical stage. We assessed the effect of wait time on survival using a Cox proportional hazard model with wait time in months as a categorical variable. Incidence of upstaging at least 1 stage was assessed using logistic regression.
Results
We identified 222 patients: 180 were stage I and 42 were stage II. For stage I, wait times up to 4 months had no significant effect on survival or incidence of upstaging. For stage II, patients waiting between 2 and 3 months had significantly decreased survival (hazard ratio 3.6, p = 0.036) and increased incidence of upstaging (odds ratio 2.0, p = 0.020) than those waiting 0 to 1 month. For those waiting between 1 and 2 months, there was no significant difference in survival or upstaging.
Conclusion
We did not identify an effect of wait time up to 4 months on survival or upstaging for patients with stage I NSCLC. For patients with stage II disease, wait times greater than 2 months adversely affected survival and upstaging.
Abstract
Contexte
En chirurgie, l’effet des temps d’attente sur la survie des patients atteints d’un cancer du poumon non à petites cellules (CPNPC) demeure pour une bonne part inconnu. Notre objectif était de déterminer l’effet des temps d’attente sur la survie et sur l’incidence de la restadification à un niveau plus élevé chez les patients atteints d’un CPNPC de stade I et II.
Méthodes
Tous les patients présentant un CPNPC clinique de stade I et II ayant subi une résection chirurgicale dans un seul centre entre janvier 2010 et décembre 2011 ont été passés en revue. L’analyse a été stratifiée selon le stade clinique préopératoire. Nous avons évalué l’effet des temps d’attente sur la survie à l’aide d’un modèle de risques proportionnels de Cox, les temps d’attente en mois ayant servi de variable catégorielle. L’incidence de la restadification à la hausse d’au moins un stade a été évaluée par régression logistique.
Résultats
Nous avons recensé 222 patients : 180 de stade I et 42 de stade II. Pour le stade I, les temps d’attente allant jusqu’à 4 mois n’ont eu aucun effet significatif sur la survie ou sur l’incidence de la restadification. Pour les stades II, les patients ayant attendu de 2 à trois 3 mois ont présenté une réduction significative de la survie (risque relatif 3,6, p = 0,036) et une incidence accrue de restadification (rapport des cotes 2,0, p = 0,02) comparativement à ceux qui avaient attendu 1 mois et moins. Chez les patients ayant attendu 1 ou 2 mois, on n’a noté aucune différence significative sur la survie ou la restadification.
Conclusion
Nous n’avons observé aucun effet d’une attente allant jusqu’à 4 mois sur la survie ou la restadification chez les patients atteints d’un CPNPC de stade I. Pour les patients atteints d’une maladie de stade II, les temps d’attente de plus de 2 mois ont eu un impact négatif sur la survie et la restadification.
A new diagnosis of lung cancer can be very distressing for patients. Adding to this distress is the concern that prolonged surgical wait times may result in cancer progression and impact survival. A study by Visser and colleagues1 identified significantly impaired quality of life in cancer patients awaiting surgical treatment. They concluded that surgical wait times should be minimized to optimize patient well-being.
In the province of Ontario, Canada, a wait time target of 28 days has been set for the interval between the decision to operate and resection. This target was mandated by Cancer Care Ontario and was determined after a literature review2 that identified 57 studies assessing the effect of increased wait times on outcomes across a number of different malignancies, including non–small cell lung cancer (NSCLC). Of the 57 studies identified, 9 were specific to lung cancer. These studies generally failed to identify an impact of increased wait times on survival. Cancer Care Ontario concluded that there was very little evidence on the association between surgical wait times and outcomes. In the end, the recommendation of a maximum wait time of 28 days between the decision to operate and the surgical resection reflects consensus expert opinion.2 We performed a retrospective cohort study to better clarify the effect of increased surgical wait times on survival and the incidence of upstaging in patients with resectable NSCLC.
Methods
We identified all patients who underwent surgical resection for preoperative clinical stage I or II NSCLC at a single centre between January 2010 and December 2011. Patients were staged according to the seventh edition of the American Joint Committee on Cancer (AJCC) lung cancer staging system. We calculated the surgical wait time as the interval between the decision to operate and the date of surgery. Clinical staging was determined using preoperative computed tomography (CT) of the chest and head as well as positron emission tomography (PET) and invasive mediastinal staging (in the form of mediastinoscopy, endobronchial ultrasonography or endoscopic ultrasonography-guided biospies) when performed. Lymph nodes with a standardized uptake value (SUV) of 2.5 or greater were considered positive unless pathological evaluation from preoperative invasive mediastinal staging showed them to be negative. For patients who did not undergo PET, we considered lymph nodes greater than 1.0 cm in the short axis to be positive unless mediastinal staging proved otherwise.
Statistical analysis
The primary outcome in this study was survival, which we assessed using a Cox proportional hazard model with wait time in months as a categorical variable. Using logistic regression, we also assessed the secondary outcome of incidence of upstaging. We considered patients to have been upstaged if the pathological stage increased by at least 1 stage compared with the clinical stage. Analysis was stratified based on clinical stage based on the hypothesis that wait times may affect stage I and II NSCLC differently. The stage I analysis was adjusted for presence of a complete resection with pathologically negative margins (R0), histology (adenocarcinoma v. nonadenocarcinoma NSCLC) and type of resection (lobar v. sublobar). These potential confounders were selected a priori and were adjusted for in the analysis regardless of statistical significance. A small sample size precluded any adjustments in the analysis for stage II patients. We considered results to be significant at p < 0.05.
Results
We identified 222 patients who underwent resection for NSCLC during the study period: 180 patients were stage I based on preoperative clinical staging and 42 were stage II.
Stage I
Patient characteristics
Of the clinical stage I patients, 39 had wait times less than 1 month, 79 waited 1 to less than 2 months, 36 waited 2 to less than 3 months, and 26 waited 3 to less than 4 months. No patients had a wait time longer than 4 months. Age and sex were similar among the patients with various wait times (Table 1). There was a trend of decreased tumour size with increased wait times. Adenocarcinoma was the most frequent histology for tumours in all wait time categories. Most patients underwent lobar resections, with a minority of patients receiving sublobar resections. Most patients received R0 resections (Table 1). All patients underwent CT as part of the staging workup, and 83% of those with clinical stage I NSCLC underwent PET. Only 9% of the stage I patients underwent mediastinal staging. Most prolonged wait times were owing to waits for operating room access. Of the patients waiting longer than 3 months, 5 (19%) were owing to patient request, 1 (4%) was owing to preoperative medical optimization and the remainder (77%) were owing to operating room access.
Table 1.
Stage I patient (n = 180) characteristics
| Characteristic | Wait time group; mean ± SD or no. (%) | |||
|---|---|---|---|---|
| 0 to < 1 mo | 1 to < 2 mo | 2 to < 3 mo | 3 to < 4 mo | |
| No. of patients | 39 | 79 | 36 | 26 |
| Age, yr | 69 ± 8.0 | 68 ± 10 | 68 ± 10 | 64 ± 13 |
| Female sex | 21 (53) | 30 (38) | 21 (58) | 16 (62) |
| Tumour size, cm | 3.09 ± 1.03 | 2.74 ± 1.10 | 2.42 ± 0.94 | 2.38 ± 0.83 |
| Adenocarcinoma | 24 (62) | 44 (56) | 22 (61) | 17 (65) |
| Sublobar resection | 5 (13) | 15 (19) | 5 (14) | 3 (12) |
| R0 resection | 36 (92) | 76 (96) | 34 (94) | 26 (100) |
SD = standard deviation.
Survival
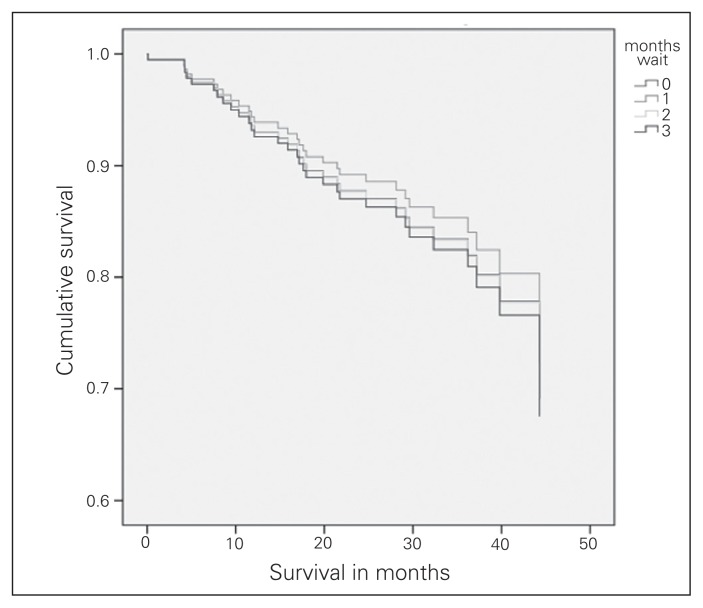
At total of 32 patients with clinical stage I NSCLC died within a mean follow up of 30 ± 11 months. There was no difference in survival among patients waiting 1 to less than 2 months, 2 to less than 3 months, or more than 3 months compared with those who waited less than 1 month (Table 2). The adjusted survival curves for all 4 wait time groups are displayed in Figure 1. The presence of an R0 resection was significantly associated with improved survival (hazard ratio [HR] 4.96, p = 0.013). Neither the type of resection nor the histology of the tumour was significantly associated with survival.
Table 2.
Stage I survival analysis
| Factor | p value | Hazard ratio |
|---|---|---|
| Wait 0 to < 1 mo | — | 1.0 |
| Wait 1 to < 2 mo | 0.77 | 0.875 |
| Wait 2 to < 3 mo | 0.99 | 1.007 |
| Wait > 3 mo | 0.92 | 1.064 |
| R0 resection | 0.013 | 4.959 (favours R0 resection) |
| Sublobar v. lobectomy | 0.28 | 2.228 (favours sublobar) |
| Histology | 0.21 | 1.262 (favours adenocarcinoma) |
Fig. 1.
Clinical stage I survival.
Incidence of upstaging
Thirty-four (19%) patients with clinical stage I disease were upstaged at least 1 stage. An increase in wait time of up to 4 months was not associated with an increased incidence of upstaging (Table 3). There was a trend toward a decreased incidence of upstaging with longer wait times; however, this finding was not significant. Patients undergoing sublobar resection had a decreased incidence of upstaging (odds ratio [OR] 7.82, 95% confidence interval [CI] 1.00–61.01, p = 0.05). Neither the histology of the tumour nor the presence of an R0 resection was associated with the incidence of upstaging. Fifty-nine percent of upstaged patients had positive N2 lymph nodes on final pathology, while 41% had positive N1 nodes. No patients were upstaged based on T stage.
Table 3.
Stage I incidence of upstaging
| Factor | p value | Odds ratio |
|---|---|---|
| Wait 0 to < 1 mo | — | 1.0 |
| Wait 1 to < 2 mo | 0.45 | 0.704 |
| Wait 2 to < 3 mo | 0.14 | 0.403 |
| Wait 3 to < 4 mo | 0.07 | 0.216 |
| Histology | 0.93 | 1.04 |
| R0 resection | 0.19 | 0.42 |
| Sublobar resection | 0.05 | 7.81 (favours sublobar) |
Stage II
Patient characteristics
Of the 42 patients with clinical stage II NSCLC, 16 waited less than 1 month, 19 waited 1–2 months and 7 waited 2 to less than 3 months. No patients waited more than 3 months for surgery. Age was similarly distributed among the wait time groups. There was a slight increase in the proportion of women in the group waiting 1–2 months compared with the other wait time groups (Table 4). Nonadeoncarcinoma was seen more frequently in those waiting up to 1 month and those waiting 2–3 months, whereas adenocarcinoma was more common in those waiting 1–2 months (Table 4). Tumour size was similar among the groups, and most patients received R0 resections. One patient who waited 1–2 months had a sublobar resection, whereas all other patients had lobectomies or pneumonectomies. All patients underwent CT as part of the staging workup, and 98% of those with clinical stage II NSCLC underwent PET. Thirty-eight percent of patients with stage II disease underwent mediastinal staging. Most prolonged wait times in patients with stage II disease were owing to waits for operating room access. For patients waiting 2–3 months, 1 (14%) was owing to patient request, 2 (28%) were owing to preoperative medical optimization and the remainder (57%) were owing to operating room access.
Table 4.
Stage II patient (n = 42) characteristics
| Characteristic | Wait time group; mean ± SD or no. (%) | ||
|---|---|---|---|
| 0 to < 1 mo | 1 to < 2 mo | 2 to < 3 mo | |
| No. of patients | 16 | 19 | 7 |
| Age, yr | 71 ± 7.2 | 67 ± 12 | 66 ± 8.0 |
| Female sex | 5 (29) | 9 (47) | 2 (29) |
| Tumour size, cm | 5.47 ± 2.16 | 4.90 ± 1.77 | 5.39 ± 1.92 |
| Adenocrcinoma | 4 (24) | 10 (56) | 3 (43) |
| R0 resection | 14 (88) | 17 (94) | 6 (85) |
SD = standard deviation.
Survival
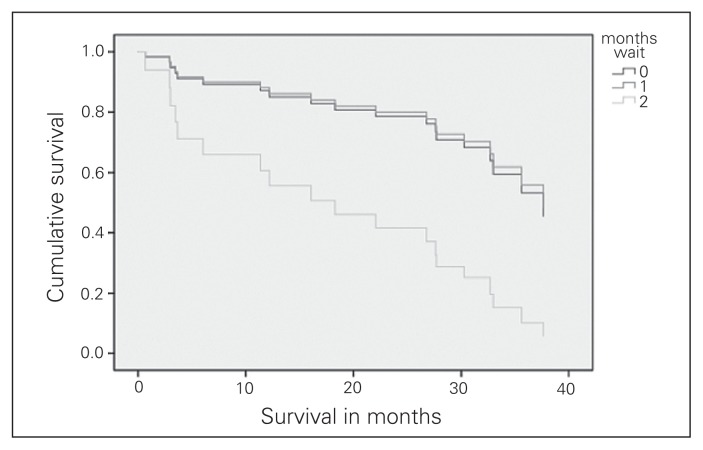
A total of 19 patients with clinical stage II NSCLC died within a mean follow up of 26 ± 13 months. Survival did not differ significantly between patients waiting 1–2 months and those waiting up to 1 month (HR 0.925, 95% CI 0.32–2.64, p = 0.89). Those who waited 2–3 months, however, had significantly decreased survival than those waiting up to 1 month (HR 3.6, 95% CI, 1.09–12.09, p = 0.036). Survival curves for the 3 wait time groups are shown in Figure 2.
Fig. 2.
Clinical stage II survival.
Incidence of upstaging
Nine (21%) patients with clinical stage II disease were upstaged at least 1 stage. Patients waiting 1–2 months did not have a significantly increased incidence of upstaging compared with those who waited less than 1 month (OR 4.0, 95% CI 0.40–40.08, p = 0.24). Those waiting 2–3 months, however, had a significantly increased incidence of upstaging (OR 20.0, 95% CI 1.61–248.3, p = 0.020). Fifty percent of upstaged patients had T3 N1 disease on final pathology, while 33% had positive N2 lymph nodes. Eleven percent had T4 disease, and another 11% were found to have metastatic disease at the time of resection.
Sensitivity analysis
In order to determine if the relatively low incidence of mediastinal staging may have altered the results, we performed a sensitivity analysis by eliminating all patients who had positive N2 lymph nodes on postoperative pathology, on the assumption that they may have been positive before resection. For the patients with clinical stage I disease, this had no effect on survival analysis. For those with clinical stage II disease, there was a similar trend, with decreased survival in those waiting more than 2 months; however, the results were no longer clinically significant.
Discussion
This retrospective cohort study examined the effect of surgical wait times in patients with clinical stage I and II NSCLC. For patients with clinical stage I disease, we identified no effect of wait times up to 4 months on survival or incidence of upstaging. For patients with clinical stage II disease, those waiting longer than 2 months had an increased incidence of upstaging as well as decreased survival compared with those waiting less than 1 month.
A number of studies have previously been performed to assess the effect of surgical wait times on survival in patients with NSCLC. However, these studies combined patients across multiple stages, and some included patients treated both operatively and nonoperatively. Myrdal and colleagues3 investigated the association between treatment delay and prognosis in 466 patients with NSCLC. Paradoxically, they found that shorter delay was associated with poorer prognosis. They speculated that this finding was due to selection bias, as patients with more severe signs and symptoms and perhaps more aggressive disease may have received more prompt treatment. Myrdal and colleagues3 included patients across all pathological stages, the majority of whom were stage III or IV. In a study by Buccheri and colleagues,4 delays in time between the presentation of first symptoms and consultation with a specialist were examined in 1277 patients with stage I–IV NSCLC. They found a small but statistically significant decrease in survival in patients with delays greater than 2 months compared with those who waited less than 2 months. This finding is contrary to those of other studies that found no correlation between delays in diagnosis or treatment of lung cancer and clinical outcome.5–8 In one of the largest studies, Aragoneses and colleagues2 failed to show any impact of therapeutic delay (defined as the interval from diagnosis to surgical resection) on survival in patients with NSCLC. All of these studies combined patients with various stages of NSCLC. To our knowledge, the present study is the only one to assess the effect of wait times on these outcomes with the analysis stratified based on stage.
Limitations
There are a number of limitations to our study. The small sample size leaves the possibility of a type I error in the analysis. For patients with clinical stage I disease, it is possible that with a larger sample size, a subtle difference in survival or upstaging with wait times may have been identified. The small sample size with the stage II analysis also precluded any adjustments of possible confounders. The retrospective nature of the study was also a limitation. Patient comorbidities were not captured in this retrospective data set. For the findings in patients with stage II disease, it is conceivable that patients with more severe comorbidities may have had longer delays in order to optimize them for resection. This may have contributed to the findings of decreased survival in patients with stage II disease. If this were the case, however, we would have also expected to see decreased survival with increased wait times in patients with stage I disease. Had the effect of increased wait times on survival been confounded by patient comorbidities, adjustment for this bias would have eliminated the finding of decreased survival with wait times longer than 2 months. This would therefore lend weight to the argument that wait times in this interval have no significant effect on survival.
The patients in our study had a relatively low incidence of invasive mediastinal staging. One could argue that the results of this study could be biased by the potential inappropriate inclusion of patients who may have had positive mediastinal lymph nodes that were not identified on preoperative PET scans. As mentioned, we performed a sensitivity analysis to assess for this, with no significant impact on our findings. Had every patient undergone invasive mediastinal staging, it is unlikely that our conclusions would have been altered. Furthermore, elimination of this potential bias would be more likely to eliminate any effect of wait times on survival.
Conclusion
In patients with clinical stage I NSCLC, we failed to identify an effect of surgical wait times of up to 4 months on survival. In those with clinical stage II disease, patients waiting longer than 2 months had significantly worse survival and increased incidence of upstaging than those who waited less than 2 months. Consideration should be given to prioritizing patients with clinical stage II NSCLC for more timely resection, while those with clinical stage I disease may be able to tolerate longer waits to achieve this goal. Broad recommendations for surgical wait times across all stages therefore may not be appropriate.
Footnotes
Competing interests: None declared.
Contributors: S. Coughlin, M. Plourde, D. Fortin, E. Frechette and R. Inculet designed the study. S. Coughlin and K. Guidolin acquired the data, which S. Coughlin, M. Plourde, R. Malthaner and R. Inculet analyzed. S. Coughlin wrote the article, which all authors reviewed and approved for publication.
References
- 1.Visser MRM, van Lanschot JJ, van der Velden J, et al. Quality of life in newly diagnosed cancer patients waiting for surgery is seriously impaired. J Surg Oncol. 2006;93:571–7. doi: 10.1002/jso.20552. [DOI] [PubMed] [Google Scholar]
- 2.Aragoneses FG, Moreno N, Leon P, et al. Bronchogenic Carcinoma Cooperative Group of the Spanish Society of Pneumology and Thoracic Surgery (GCCB-S) Lung Cancer. 2002;36:59–63. doi: 10.1016/s0169-5002(01)00458-5. [DOI] [PubMed] [Google Scholar]
- 3.Myrdal G, Lambe M, Hillerdal G, et al. Effect of delays on prognosis in patients with non-small cell lung cancer. Thorax. 2004;59:45–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Buccheri G, Ferrigno D. Lung cancer: clinical presentation and specialist referral time. Eur Respir J. 2004;24:898–904. doi: 10.1183/09031936.04.00113603. [DOI] [PubMed] [Google Scholar]
- 5.Pita-Fernández S, Montero-Martinez C, Pertega-Diaz S, et al. Relationship between delayed diagnosis and the degree of invasion and survival in lung cancer. J Clin Epidemiol. 2003;56:820–5. doi: 10.1016/s0895-4356(03)00166-5. [DOI] [PubMed] [Google Scholar]
- 6.Bozcuk H, Martin C. Does treatment delay affect survival in non-small cell lung cancer? A retrospective analysis from a single UK centre. Lung Cancer. 2001;34:243–52. doi: 10.1016/s0169-5002(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 7.Quarterman RL, McMillan A, Ratcliffe MB, et al. Effect of preoperative delay on prognosis for patients with early stage non-small cell lung cancer. J Thorac Cardiovasc Surg. 2003;125:108–13. doi: 10.1067/mtc.2003.93. [DOI] [PubMed] [Google Scholar]
- 8.Billing JS, Wells FC. Delays in the diagnosis and surgical treatment of lung cancer. Thorax. 1996;51:903–6. doi: 10.1136/thx.51.9.903. [DOI] [PMC free article] [PubMed] [Google Scholar]