Abstract
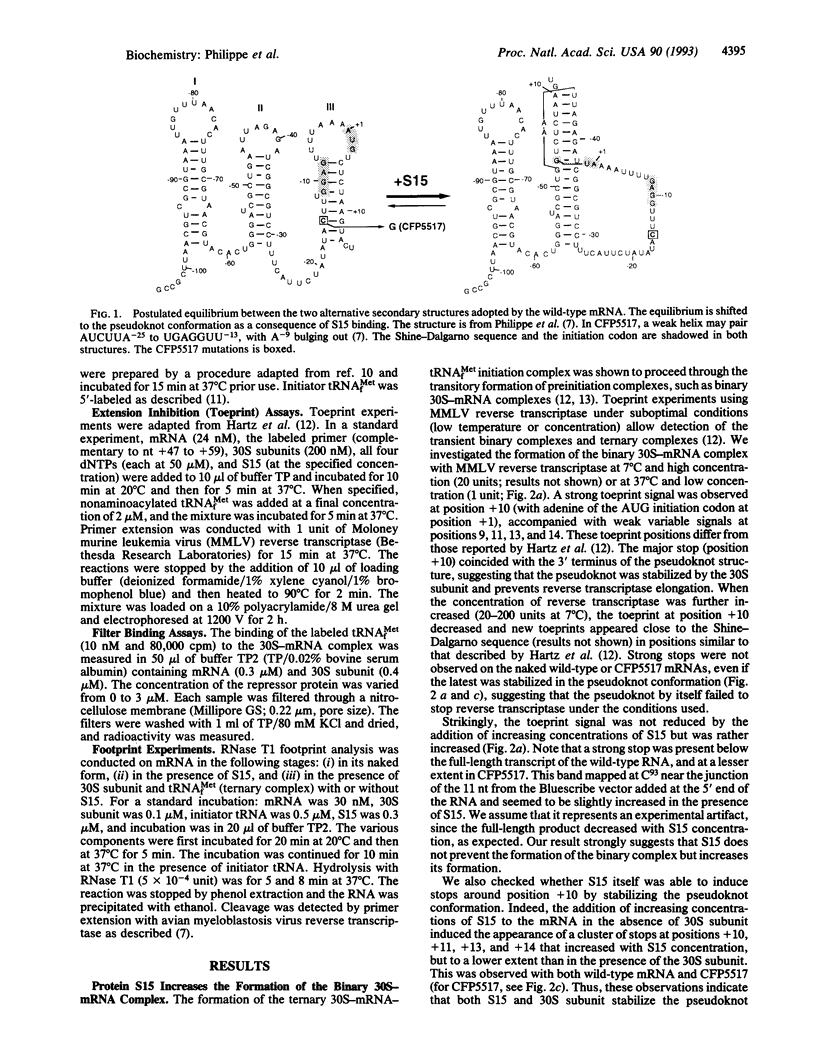
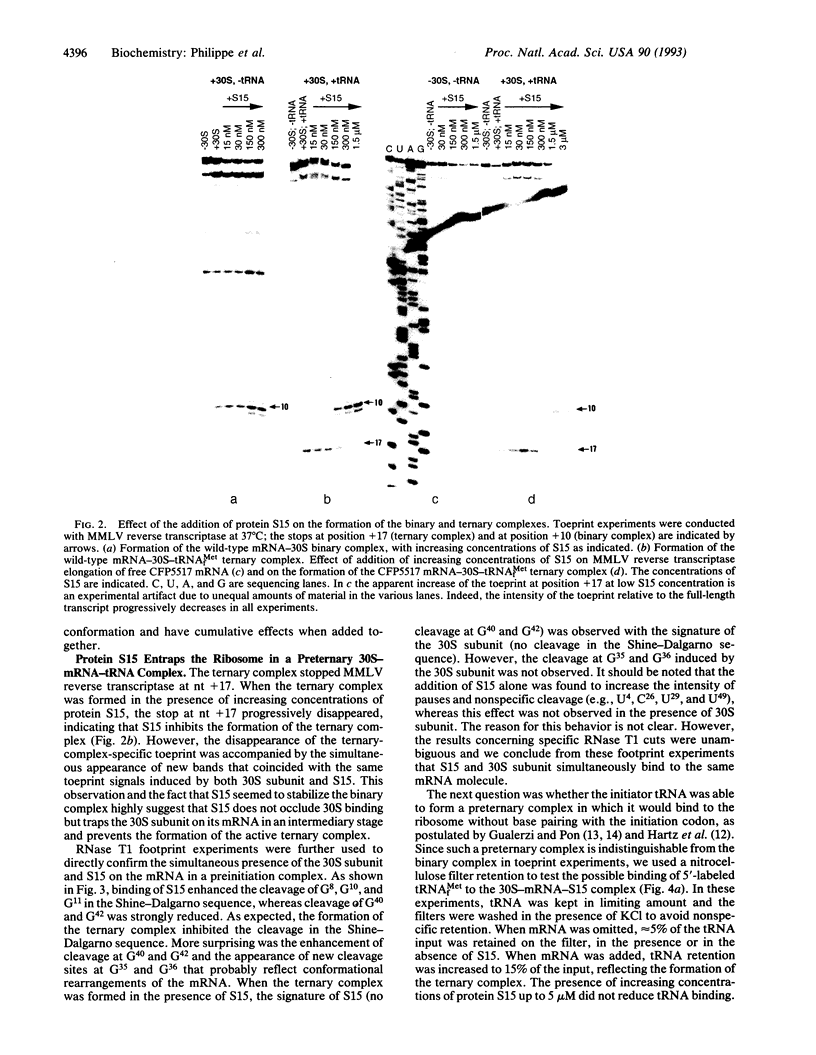
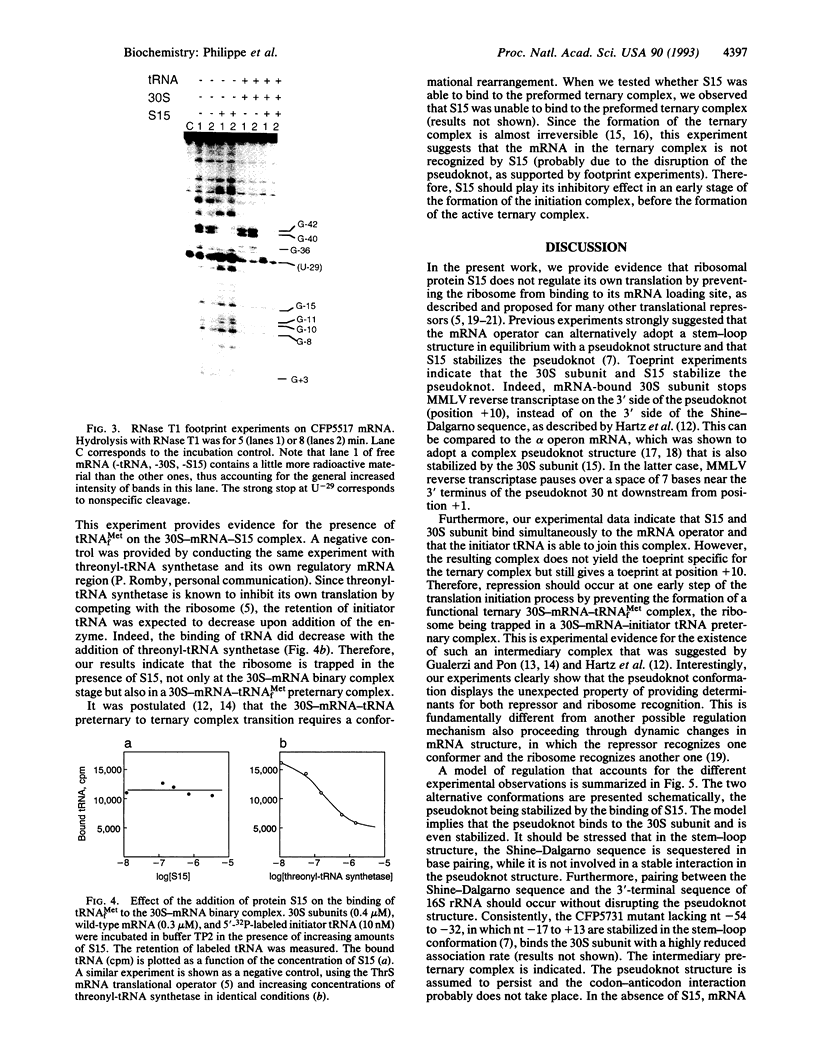
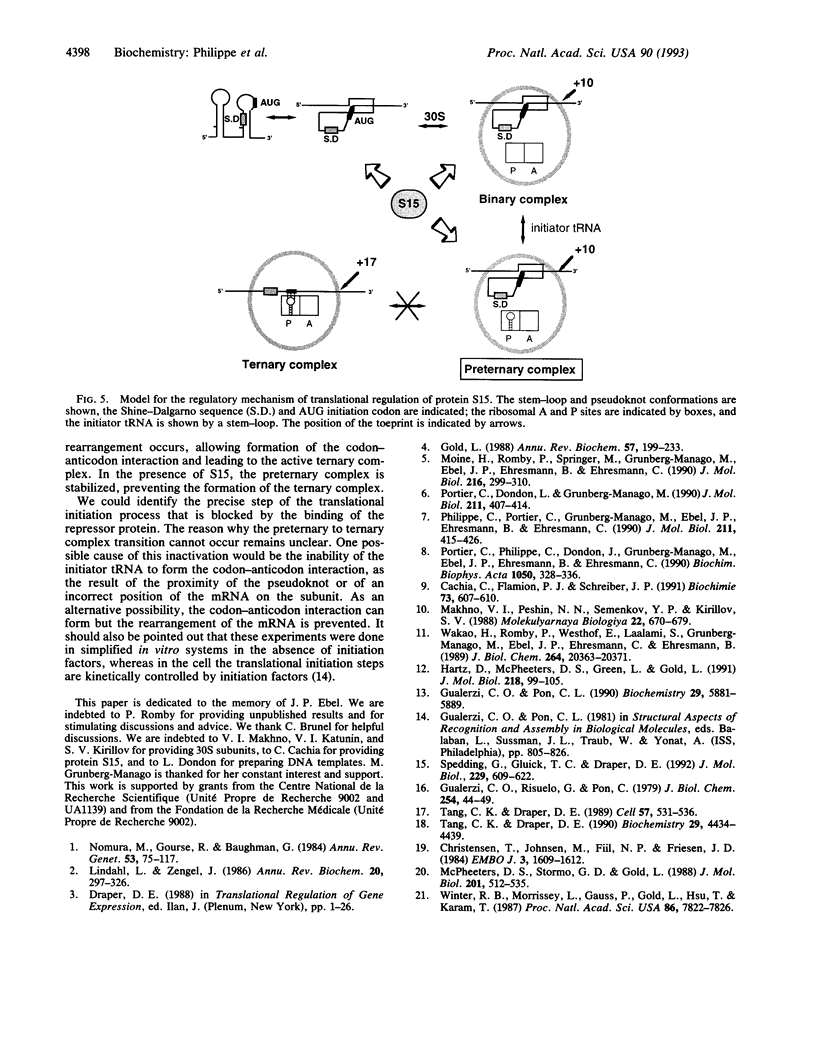
From genetic and biochemical evidence, we previously proposed that S15 inhibits its own translation by binding to its mRNA in a region overlapping the ribosome loading site. This binding was postulated to stabilize a pseudoknot structure that exists in equilibrium with two stem-loops. Here, we use "toeprint" experiments with Moloney murine leukemia virus reverse transcriptase to analyze the effect of S15 on the formation of the ternary mRNA-30S-tRNA(fMet) complex. We show that the binding of the 30S subunit on the mRNA stops reverse transcriptase near position +10, corresponding to the 3' terminus of the pseudoknot, most likely by stabilizing the pseudoknot conformation. Furthermore, S15 is found to stabilize the binary 30S-mRNA complex. When the ternary 30S-mRNA-tRNA(fMet) complex is formed, a toeprint is observed at position +17. This toeprint progressively disappears when the ternary complex is formed in the presence of increasing concentrations of S15, while a shift from position +17 to position +10 is observed. Beside, RNase T1 footprinting experiments reveal the simultaneous binding of S15 and 30S subunit on the mRNA. Otherwise, we show by filter binding assays that initiator tRNA remains bound to the 30S subunit even in the presence of S15. Our results indicate that S15 prevents the formation of a functional ternary 30S-mRNA-tRNA(fMet) complex, the ribosome being trapped in a preternary 30S-mRNA-tRNA(fMet) complex.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cachia C., Flamion P. J., Schreiber J. P. Fast preparative separation of 'native' core E coli 30S ribosomal proteins. Biochimie. 1991 May;73(5):607–610. doi: 10.1016/0300-9084(91)90029-z. [DOI] [PubMed] [Google Scholar]
- Christensen T., Johnsen M., Fiil N. P., Friesen J. D. RNA secondary structure and translation inhibition: analysis of mutants in the rplJ leader. EMBO J. 1984 Jul;3(7):1609–1612. doi: 10.1002/j.1460-2075.1984.tb02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. Posttranscriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- Gualerzi C. O., Pon C. L. Initiation of mRNA translation in prokaryotes. Biochemistry. 1990 Jun 26;29(25):5881–5889. doi: 10.1021/bi00477a001. [DOI] [PubMed] [Google Scholar]
- Gualerzi C., Risuleo G., Pon C. Mechanism of the spontaneous and initiation factor 3-induced dissociation of 30 S.aminoacyl-tRNA.polynucleotide ternary complexes. J Biol Chem. 1979 Jan 10;254(1):44–49. [PubMed] [Google Scholar]
- Hartz D., McPheeters D. S., Green L., Gold L. Detection of Escherichia coli ribosome binding at translation initiation sites in the absence of tRNA. J Mol Biol. 1991 Mar 5;218(1):99–105. doi: 10.1016/0022-2836(91)90876-8. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Zengel J. M. Ribosomal genes in Escherichia coli. Annu Rev Genet. 1986;20:297–326. doi: 10.1146/annurev.ge.20.120186.001501. [DOI] [PubMed] [Google Scholar]
- Makhno V. I., Peshin N. N., Semenkov Iu P., Kirillov S. V. Modifitsirovannyi sposob polucheniia "tight" 70S ribosom iz Escherichia coli, vysokoaktivnykh v otdel'nykh stadiiakh tsikla élongatsii. Mol Biol (Mosk) 1988 May-Jun;22(3):670–679. [PubMed] [Google Scholar]
- McPheeters D. S., Stormo G. D., Gold L. Autogenous regulatory site on the bacteriophage T4 gene 32 messenger RNA. J Mol Biol. 1988 Jun 5;201(3):517–535. doi: 10.1016/0022-2836(88)90634-1. [DOI] [PubMed] [Google Scholar]
- Moine H., Romby P., Springer M., Grunberg-Manago M., Ebel J. P., Ehresmann B., Ehresmann C. Escherichia coli threonyl-tRNA synthetase and tRNA(Thr) modulate the binding of the ribosome to the translational initiation site of the thrS mRNA. J Mol Biol. 1990 Nov 20;216(2):299–310. doi: 10.1016/S0022-2836(05)80321-3. [DOI] [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Philippe C., Portier C., Mougel M., Grunberg-Manago M., Ebel J. P., Ehresmann B., Ehresmann C. Target site of Escherichia coli ribosomal protein S15 on its messenger RNA. Conformation and interaction with the protein. J Mol Biol. 1990 Jan 20;211(2):415–426. doi: 10.1016/0022-2836(90)90362-P. [DOI] [PubMed] [Google Scholar]
- Portier C., Dondon L., Grunberg-Manago M. Translational autocontrol of the Escherichia coli ribosomal protein S15. J Mol Biol. 1990 Jan 20;211(2):407–414. doi: 10.1016/0022-2836(90)90361-O. [DOI] [PubMed] [Google Scholar]
- Portier C., Philippe C., Dondon L., Grunberg-Manago M., Ebel J. P., Ehresmann B., Ehresmann C. Translational control of ribosomal protein S15. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):328–336. doi: 10.1016/0167-4781(90)90190-d. [DOI] [PubMed] [Google Scholar]
- Spedding G., Gluick T. C., Draper D. E. Ribosome initiation complex formation with the pseudoknotted alpha operon messenger RNA. J Mol Biol. 1993 Feb 5;229(3):609–622. doi: 10.1006/jmbi.1993.1067. [DOI] [PubMed] [Google Scholar]
- Tang C. K., Draper D. E. Evidence for allosteric coupling between the ribosome and repressor binding sites of a translationally regulated mRNA. Biochemistry. 1990 May 8;29(18):4434–4439. doi: 10.1021/bi00470a025. [DOI] [PubMed] [Google Scholar]
- Tang C. K., Draper D. E. Unusual mRNA pseudoknot structure is recognized by a protein translational repressor. Cell. 1989 May 19;57(4):531–536. doi: 10.1016/0092-8674(89)90123-2. [DOI] [PubMed] [Google Scholar]
- Wakao H., Romby P., Westhof E., Laalami S., Grunberg-Manago M., Ebel J. P., Ehresmann C., Ehresmann B. The solution structure of the Escherichia coli initiator tRNA and its interactions with initiation factor 2 and the ribosomal 30 S subunit. J Biol Chem. 1989 Dec 5;264(34):20363–20371. [PubMed] [Google Scholar]
- Winter R. B., Morrissey L., Gauss P., Gold L., Hsu T., Karam J. Bacteriophage T4 regA protein binds to mRNAs and prevents translation initiation. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7822–7826. doi: 10.1073/pnas.84.22.7822. [DOI] [PMC free article] [PubMed] [Google Scholar]