Abstract
Objective
The impact of prolonged episodes of atrial fibrillation on atrial and ventricular function has been incompletely characterized. The purpose of this study was to investigate the influence of atrial fibrillation on left atrial and ventricular function in a rapid paced porcine model of atrial fibrillation.
Methods
A control group of pigs (group 1, n = 8) underwent left atrial and left ventricular conductance catheter studies and fibrosis analysis. A second group (group 2, n = 8) received a baseline cardiac magnetic resonance imaging to characterize left atrial and left ventricular function. The atria were rapidly paced into atrial fibrillation for 6 weeks followed by cardioversion and cardiac magnetic resonance imaging.
Results
After 6 weeks of atrial fibrillation, left atrial contractility defined by atrial end-systolic pressure-volume relationship slope was significantly lower in group 2 than in group 1 (1.1 ± 0.5 vs 1.7 ± 1.0; P = .041), whereas compliance from the end-diastolic pressure-volume relationship was unchanged (1.5 ± 0.9 vs 1.6 ± 1.3; P = .733). Compared with baseline, atrial fibrillation resulted in a significantly higher contribution of left atrial reservoir volume to stroke volume (32% vs 17%; P = .005) and lower left atrial booster pump volume contribution to stroke volume (19% vs 28%; P = .029). Atrial fibrillation also significantly increased maximum left atrial volume (206 ± 41 mL vs 90 ± 21 mL; P < .001). Left atrial fibrosis in group 2 was significantly higher than in group 1. Atrial fibrillation decreased left ventricular ejection fraction (29% ± 9% vs 58 ± 8%; P < .001), but left ventricular stroke volume was unchanged.
Conclusions
In a chronic model of atrial fibrillation, the left atrium demonstrated significant structural remodeling and decreased contractility. These data suggest that early intervention in patients with persistent atrial fibrillation might mitigate against adverse atrial and ventricular structural remodeling.
Keywords: atrial fibrillation, atrial function, Cox-Maze
Preoperative atrial fibrillation (AF) occurs in 11.5% of patients who are undergoing cardiac surgery.1 To understand the impact of cardiac surgery on atrial physiology, it is first important to understand the effect of AF on the atrium. The impact of prolonged AF on left atrial (LA) and left ventricular (LV) function has been incompletely characterized. Although there have been reports on the effect of paroxysmal atrial fibrillation (PAF) on LA and LV function by echocardiography2 and reports on the effect of acute AF on LA and LV function by conductance catheter in an animal model,3 there are few reports describing the influence of AF on LA and LV function and volume, and the severity of fibrosis induced by a defined period of AF.
There is clinical evidence that PAF has a negative impact. Compared with patients in normal sinus rhythm (NSR), those with PAF have normal LA booster pump function when in NSR, but have reduced reservoir function and increased conduit function.4 In patients with persistent AF, it is not possible to preoperatively assess atrial booster pump function. As an example, when patients with persistent AF who have undergone the Cox-Maze procedure and postoperatively are in NSR are compared with patients with postoperative PAF in NSR, they have only 50% of the booster pump function of the PAF group. These findings suggest that although NSR has been achieved in both groups, the long history of AF results in a significant and possibly irreversible impairment of atrial pump function.
Motivated by our studies in patients with persistent AF who have undergone the Cox-Maze procedure, the present study was designed to investigate the hemodynamic and histologic impact of persistent AF on LA and LV function, independently of any surgical intervention, in a 6-week rapid atrial pacing porcine model of AF.
MATERIALS AND METHODS
Domestic (n = 16) pigs weighing 56 ± 19 kg were divided into a control group (group 1, n = 8) and a paced group (group 2, n = 8). Because conductance catheters cannot be used noninvasively, the control (group 1) pigs were used to obtain normal LA and LV pressure volume (PV) data and normal histology. In the paced group (group 2), all pigs received a preoperative cardiac magnetic resonance imaging (cMRI) while in NSR and had a pacemaker subsequently implanted. They were then rapidly atrial paced to maintain AF for 6 weeks. After this, the animals were cardioverted to NSR and had a repeat cMRI. The pigs then had a terminal study to obtain LA and LV PV data and tissue for histology. All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (revised 2011). The study was also approved by the Washington University School of Medicine Animal Studies Committee.
Pigs were premedicated (tiletamine/zolazepam [Telazol; Zoetis, Florham Park, NJ] 4.4 mg/kg, ketamine 2.5 mg/kg, and xylazine 2.2 mg/kg), intubated, and anesthetized with isoflurane. Heart rate, arterial blood pressure, blood gases, and electrolytes were monitored and normalized during the procedure.
In group 1, a median sternotomy was performed. The animals were heparinized (350 U/kg intravenously), and the activated clotting time was maintained for more than 250 seconds; 10- to 12-mm ultrasonic flow probes (T206 Flowmeter; Transonic Systems, Ithaca, NY) were placed around the superior vena cava and inferior vena cava approximately 1 cm from the caval-atrial junction to measure right atrial (RA) inflow. Through a purse-string suture in the ascending aorta, a 5F PV conductance catheter (Millar SPR-766; Millar Instruments, Inc, Houston, Tex) was introduced and positioned along the long axis of the LV, with the tip of the catheter resting in the LVapex.3 The pericardium was kept intact in both the control and paced animals. A second conductance catheter was inserted through a small pericardial incision into the LA through a purse-string suture at the base of the appendage. The conductance catheter was positioned along the long axis of the LA, with the tip directed toward the orifice of the right pulmonary veins. Pressures and chamber conductance were measured by using dual-field technology with 10 electrodes set 4 mm apart. After data collection, the animals were killed using saturated potassium chloride, and the heart was fixed, sectioned, and stained with Masson’s Trichrome for subsequent analysis of atrial and ventricular fibrosis.
Anesthetized animals in group 2 underwent a baseline cMRI. The animals were given at least 2 days to recover before undergoing pacemaker implantation. The cMRI protocol has been described in detail.5
Pacemaker implantation also was performed under general anesthesia (Medtronic InSync Maximo; Medtronic Inc, Minneapolis, Minn). The right internal jugular vein was dissected, and the pacing lead (Medtronic 5076 65 cm; Medtronic Inc) was placed under fluoroscopic guidance into the RA appendage.
Antibiotics were given preoperatively and postoperatively for 7 days postoperatively. Daily oral digoxin 0.25 mg was started on the operative day to control the ventricular rate during high rate pacing. Complete blood count, liver function, and renal function were checked preoperatively and on postoperative day 7. The digoxin dosage was determined on the basis of the serum digoxin concentration level and heart rate. High rate pacing (400 beats/min) was initiated after the serum digoxin level reached 0.5 to 2 ng/mL. Continuous high-rate pacing started on postoperative day 14 for 6 weeks. In this well-documented pig model of AF, animals usually developed AF after 1 week of pacing.6 After 6 weeks, blood was drawn to determine brain natriuretic peptide (BNP) levels. The high rate pacing was then discontinued, and the animals were placed under general anesthesia. All animals were in AF and were electrically cardioverted with 200 J. The pacemaker was explanted, and the animals were allowed to recover in NSR for 1 day and then underwent a cMRI followed by a conductance catheter study. Although the pacemaker was magnetic resonance imaging (MRI) compatible, we removed it to prevent artifacts on the image. At the conclusion of the study, the animals were euthanized and the hearts were removed en bloc for histologic assessment.
Data Analysis
Instantaneous LA volume versus cardiac cycle and LV volume versus cardiac cycle curves were generated by using the short-axis stack cMRI images as previously reported (Figure 1).5 All volumes were normalized to body surface area (BSA).7 In a previous study, we demonstrated in sham pigs that normalized atrial and ventricular volumes remained unchanged with increased body weight.5 Global LA function was characterized by calculating the reservoir, conduit, and booster pump volumes and their percentage of contribution to left ventricular stroke volume (LVSV) (Figure 1). Parameters for the reservoir, conduit, and booster pump functions of the LA were also derived, as previously described by Jarvinen and colleagues8 and Spencer and colleagues.9 Atrial elastance (contractility) was assessed by using the LA or LV end-systolic PV relationship derived from the data recorded with the PV catheters as previously described. Details of the MRI and PV catheter data acquisition and analysis are provided in the Online Methods.
Figure 1.
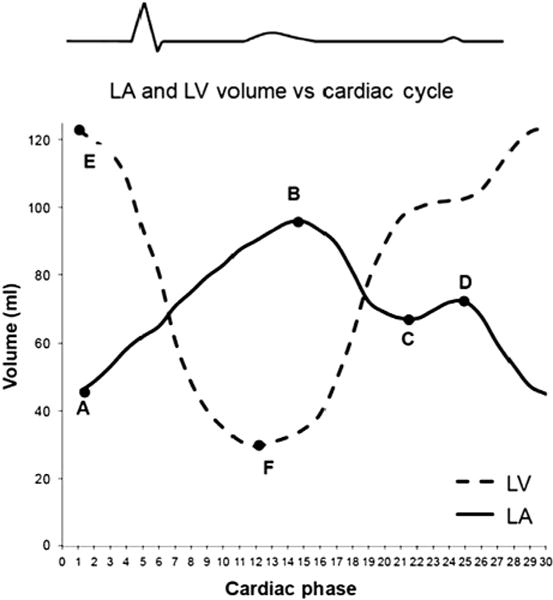
LA and LV volume versus cardiac cycle curves. The curve with A, B, C, and D represents a normal LA volume curve. Point A = minimal LA volume (LAmin); point B = maximal LA volume (LAmax); point C = relative minimal LA volume (LArel max); point D = relative maximal LA volume (LArel min). The curve with E and F represents a normal LV volume curve. Point E = LV end-diastolic volume; point F = LV end-systolic volume. LVSV = E−F; LA reservoir volume = B−C; LA booster pump volume = D−A; LA conduit volume = LVSV−LA reservoir volume−LA booster pump volume. LA, Left atrium; LV, left ventricular.
The hearts were removed and fixed in formalin. Ten tissue blocks (10×5 mm) from the LA (appendage, free wall, roof, left upper pulmonary vein, left lower pulmonary vein, right upper pulmonary vein, right lower pulmonary vein, and posterior wall) and 2 sections from the RA (appendage and free wall) and LV free wall were excised. Six equally distributed slices from each block were sent for Masson’s trichrome staining. Each slice was examined microscopically to assess the amount of fibrosis. The amount of fibrosis was calculated by the amount of fibrosis/total tissue using Image J (Version 1.46r, National Institutes of Health, Bethesda, Md).10
Statistical Analysis
All data were reported as mean ± standard deviation. Hemodynamic data obtained during conductance catheter recordings were reported as the average of 3 consecutive beats. Fibrosis analysis was reported as the average amount of fibrosis in 3 slices from 1 block. Data were compared between the group 1 and group 2 conductance studies using an unpaired t test with Dunn-Sidak correction for multiple test, baseline cMRI and after 6 weeks pacing cMRI, and group 1 and group 2 fibrosis analysis by using repeated-measures analysis of variance with 1 factor. All statistical analyses were performed with Systat version 13 (Systat Inc, Chicago, Ill).
RESULTS
The baseline body weight of the paced animals was 58 ± 7.3 kg (BSA = 1.27 ± 0.10 m2), and the body weight after 6 weeks of pacing was 109 ± 5.9 kg (BSA = 1.89 ± 0.06 m2). Baseline NSR heart rate was 107 ± 16 beats/min, and at 6 weeks after conversion to NSR, it was 74 ± 8 beats/min (P < .001). During rapid pacing, the mean heart rate was 133 ± 24 beats/min (P = .015 compared with baseline heart rate). BNP levels before the terminal study were less than 10 pg/mL for all pigs, and none of the animals exhibited clinical signs of heart failure.
Cardiac Magnetic Resonance Imaging Data
Because body weight increased over the course of the study, LV end-diastolic and end-systolic volumes were scaled in accordance with change in BSA (Table 1). Corrected LV end-systolic and LV end-diastolic volumes were significantly larger after 6 weeks of pacing. Left ventricular ejection fraction (LVEF) decreased (29% ± 9% vs 58% ± 8%; P < .01); however, there was no significant difference in LVSV (58 ± 16 mL vs 59 ± 12 mL; P = .932).
TABLE 1.
Chamber volumes and function
| Baseline | Chronic paced | P value | |
|---|---|---|---|
| Left ventricle | |||
| LVEDV (mL) | 103 ± 22 | 203 ± 25 | <.001 |
| LVESV (mL) | 44 ± 14 | 145 ± 32 | <.001 |
| LVSV (mL) | 59 ± 12 | 58 ± 16 | .932 |
| LVEF (%) | 58 ± 8 | 29 ± 9 | <.001 |
| Left atrium | |||
| Volumes | |||
| LAV MAX (mL) | 90 ± 21 | 206 ± 41 | <.001 |
| LAV REL MAX (mL) | 81 ± 19 | 198 ± 41 | <.001 |
| LAV MIN (mL) | 65 ± 20 | 187 ± 40 | <.001 |
| LAV REL MIN (mL) | 79 ± 19 | 187 ± 40 | <.001 |
| Reservoir | |||
| LACC (mL) | 25 ± 8 | 19 ± 6 | .01 |
| LAPTE (%) | 28 ± 9 | 9 ± 3 | <.001 |
| LAEI (%) | 42 ± 20 | 11 ± 3 | .002 |
| LARV (mL) | 11 ± 6 | 19 ± 7 | .003 |
| Conduit | |||
| LAPE (%) | 35 ± 14 | 42 ± 20 | .191 |
| LAPEI (%) | 9 ± 5 | 4 ± 3 | .002 |
| LACV (mL) | 33 ± 11 | 29 ± 9 | .48 |
| Booster pump | |||
| LAAE (%) | 65 ± 14 | 57 ± 20 | .191 |
| LAAEI (%) | 20 ± 9 | 6 ± 3 | .002 |
| LAEF (%) | 19 ± 8 | 5 ± 3 | .001 |
| LABPV (mL) | 16 ± 5 | 11 ± 5 | .006 |
| LA segmental Shortening | |||
| Medial (%) | 31 ± 17 | 8 ± 5 | .001 |
| Lateral (%) | 34 ± 12 | 10 ± 4 | .003 |
| Anterior (%) | 26 ± 11 | 7 ± 5 | .006 |
| Posterior (%) | 42 ± 6 | 13 ± 11 | .001 |
All volumes are adjusted to BSA. BSAwas calculated by using the following formula: BSA = 0.097 × (kg body weight)0.633.7 LVEDV, Left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVSV, left ventricular stroke volume; LVEF, left ventricular ejection fraction; LAV MAX, maximal left atrial volume; LAV REL MAX, relative maximal left atrial volume; LAV MIN, minimum left atrial volume; LA REL MIN, relative minimal left atrial volume; LACC, left atrial volume change; LAPTE, left atrial percentage total emptying; LAEI, left atrial expansion index; LARV, left atrial reservoir volume; LAPE, left atrial passive percentage of total emptying; LAPEI, left atrial passive emptying index; LACV, left atrial conduit volume; LAAE, left atrial active emptying percentage of total emptying; LAAEI, left atrial active emptying index; LAEF, left atrial active ejection fraction; LABPV, left atrial booster pump volume; LA, left atrial.
The LA reservoir, conduit, and booster pump functional parameters are summarized in Table 2. At 6 weeks after high rate atrial pacing, the LA reservoir function indices of LA percentage total emptying (P < .001) and LA expansion index (P = .02) were significantly lower than baseline. The LA conduit function indices of LA passive percentage of total emptying (P = .19) and LA passive emptying index (P = .002) were significantly decreased after 6 weeks of pacing; however, the LA conduit volume was not significantly different (P = .48). The booster pump function indices of LA active emptying index (P = .001) and LA active ejection fraction (P = .001) were significantly decreased. In addition to these findings, 6 weeks of AF had a significant effect on regional LA wall motion. All 4 segmental LA walls (anterior, posterior, medial, and lateral wall segments) evaluated had significant shortening between atrial systole and diastole in group 2 (Figure 2). The relative contribution of booster function decreased from 28% ± 11% to 19% ± 16% (P = .029). The reservoir function increased from 17% ± 7% to 32% ± 8% (P = .005). The conduit function did not show a significant change: 54% ± 13% versus 49% ± 10% (P = .298).
TABLE 2.
Chamber pressure, elastance, and compliance
| Control | Chronic Paced | P Value | |
|---|---|---|---|
| Left atrium | |||
| min LAP | 8.7 ± 3.0 | 10.8 ± 3.7 | .113 |
| max LAP | 15.0 ± 3.4 | 16.0 ± 5.8 | .561 |
| mean LAP | 11.2 ± 2.8 | 13.5 ± 12.4 | .121 |
| ESPVR slope | 1.7 ± 1 | 1.1 ± 0.5 | .041 |
| dP/dT slope | 19 ± 13 | 5.7 ± 7.3 | .004 |
| EDPVR slope | 1.6 ± 1.3 | 1.5 ± 0.9 | .733 |
| Left ventricle | |||
| Min LVP | 8 ± 3 | 8 ± 3 | .725 |
| Max LVP | 92 ± 18 | 88 ± 18 | .644 |
| max LV dp/dt | 969 ± 458 | 964 ±183 | .994 |
| mean LVP | 41 ± 7 | 31 ±9 | .013 |
| ESPVR slope | 1.9 ± 1.9 | 1.4 ± 1 | .339 |
| EDPVR slope | 1.9 ± 2 | 1.6 ± 1.5 | .968 |
| mean AOP | 61.3 ± 6.4 | 67.6 ± 12.4 | .098 |
min LAP, Minimum left atrial pressure; max LAP, maximum left atrial pressure; mean LAP, mean left atrial pressure; ESPVR, end-systolic pressure-volume relations; EDPVR, end-diastolic pressure-volume relations; min LVP, minimum left ventricular pressure; max LVP, maximum left ventricular pressure; max LV dp/dt, maximum left ventricular dp/dt; mean LVP, mean left ventricular pressure; mean AOP, mean aortic pressure.
Figure 2.
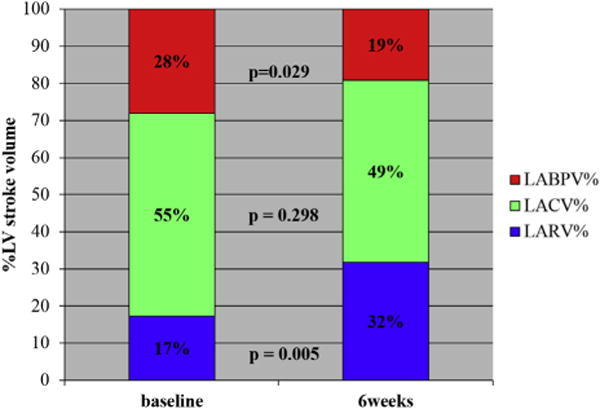
Before and after pacing LA contribution to LVSV. The graph shows the relative contribution of LA booster pump volume (%), LA conduit volume (%), and LA reservoir volume (%) to LVSV in the paced animals. LABPV, Left atrial booster pump volume; LACV, left atrial conduit volume; LARV, left atrial reservoir volume; LV, left ventricular.
Conductance Catheter Data
There were no significant LA, LV, or aortic pressure differences between groups 1 and 2. Minimum LA pressures were 8.7 ± 3.0 mm Hg and 10.8 ± 3.7 mm Hg, respectively (P = .113). The LA elastance, as measured by the end-systolic PV relationship, decreased in group 2 (1.7 ± 1.0 vs 1.1 ± 0.5; P = .041) (Table 3), but there were no differences in LV end-systolic PV relationship between groups 1 and 2 (1.9 ± 1.9 vs 1.4 ± 1.0; P = .339). There were no significant differences between groups 1 and 2 in LA and LV stiffness, as measured by the end-diastolic PV relationship (1.6 ± 1.3 vs 1.5 ± 0.9; P = .733). LA contractility, as measured by the dp/dt slope, was significantly decreased in group 2 (19 ± 13 vs 5.7 ± 7.3; P = .004), although there were no significant LV dp/dt differences between groups 1 and 2 (969 ±458 vs 964 ± 183; P = .994).
TABLE 3.
Myocardial fibrosis and connective tissue thickness
| Baseline | Chronic paced | P value | |
|---|---|---|---|
| Left atrium | |||
| Myocardial fibrosis | |||
| LAA (%) | 14.4 ± 5.4 | 20.7 ± 3.6 | .017 |
| LAFW (%) | 14.5 ± 6.2 | 20.6 ±2.1 | .020 |
| LAPW (%) | 17.1 ± 5.9 | 25.6 ± 2.0 | .013 |
| LAR (%) | 18.9 ± 7.4 | 25.8 ± 2.0 | .024 |
| LIPV (%) | 15.7 ± 4.7 | 29.6 ± 4.6 | <.001 |
| LSPV (%) | 15.9 ± 4.1 | 29.2 ± 4.6 | <.001 |
| RIPV (%) | 14.4 ± 5.0 | 25.6 ± 4.3 | <.001 |
| RSPV (%) | 16.2 ± 5.3 | 25.6 ± 3.7 | .001 |
| Epicardial thickness | |||
| LAA (mm) | 0.150 ± 0.089 | 0.150 ± 0.067 | .991 |
| LAFW (mm) | 0.110 ± 0.029 | 0.161 ± 0.051 | .028 |
| LAPW (mm) | 0.128 ± 0.142 | 0.139 ± 0.104 | .880 |
| LAR (mm) | 0.149 ± 0.093 | 0.176 ± 0.126 | .632 |
| LIPV (mm) | 0.187 ± 0.156 | 0.164 ±0.119 | .472 |
| LSPV (mm) | 0.169 ± 0.153 | 0.119 ± 0.090 | .472 |
| RIPV (mm) | 0.152 ± 0.136 | 0.130 ± 0.086 | .717 |
| RSPV (mm) | 0.135 ± 0.072 | 0.960 ± 0.069 | .302 |
| Endocardial Thickness | |||
| LAA (mm) | 0.048 ± 0.019 | 0.054 ± 0.023 | .564 |
| LAFW (mm) | 0.052 ± 0.024 | 0.055 ± 0.024 | .803 |
| LAPW (mm) | 0.091 ± 0.053 | 0.164 ± 0.080 | .049 |
| LAR (mm) | 0.160 ± 0.126 | 0.191 ± 0.066 | .542 |
| LIPV (mm) | 0.097 ± 0.062 | 0.127 ± 0.054 | .112 |
| LSPV (mm) | 0.72 ± 0.042 | 0.105 ± 0.035 | .112 |
| RIPV (mm) | 0.080 ± 0.038 | 0.153 ± 0.046 | .005 |
| RSPV (mm) | 0.083 ± 0.052 | 0.126 ± 0.050 | .104 |
| Right atrium | |||
| Myocardial fibrosis | |||
| RAA (%) | 11.7 ± 2.3 | 24.0 ± 4.5 | <.001 |
| RAFW (%) | 12.2 ± 4.8 | 22.6 ± 5.3 | .001 |
| Epicardial thickness | |||
| RAA (mm) | 0.206 ± 1.119 | 0.187 ± 0.119 | .757 |
| RAFW (mm) | 0.146 ±0.114 | 0.174 ± 0.088 | .59 |
| Endocardial Thickness | |||
| RAA (mm) | 0.024 ± 0.008 | 0.041 ± 0.011 | .003 |
| RAFW (mm) | 0.020 ± 0.007 | 0.027 ± 0.007 | .173 |
| Left ventricle | |||
| LV (%) | 9.3 ± 4.8 | 8.8 ± 3.18 | .799 |
| LV epi thickness (mm) | 0.135 ± 0.070 | 0.102 ± 0.047 | .299 |
| LV endo thickness (mm) | 0.068 ± 0.043 | 0.094 ± 0.035 | .208 |
LAA, Left atrial appendage; LAFW, left atrial free wall; LAPW, left atrial posterior wall; LAR, left atrial roof; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; RAA, right atrial appendage; RAFW, right atrial free wall; LV, left ventricle; LV Epi, left ventricular epicardial; LV Endo, left ventricular endocardial.
Histology
There were significant differences in LA and RA percentages of fibrosis between groups 1 and 2, but no significant differences in the LV percentages of fibrosis (Table 3). The increased LA fibrosis in the paced animals was greatest around the pulmonary veins and posterior LA. The thickness of the epicardial connective tissue layer was not different except at 1 site (LA posterior wall). The connective tissue layer on the endocardium showed some increase in 2 LA sites (right inferior pulmonary vein and LA posterior wall) and 1 RA site (RA appendage), but the remainder of the sites had no significant changes.
DISCUSSION
Multiple factors affect the mechanical function of the LA and LV mechanical function after cardiac surgery. These include opening the chest and pericardium, the lesion created by incisions and ablation, and the preexisting effects of AF. In a previous study, we examined the effects of opening the chest and pericardium and the effects of lesion set.5 In this study, the effects of 6 weeks of simulated persistent AF on LA and LV structure and function were measured. AF was associated with remodeling of atrial and ventricular chamber sizes and increased LA fibrosis. Physiologically, these structural changes resulted in a decreased LA booster pump function.
Both the LA and the LV developed dilated cardiomyopathy due to the excessive work on the heart created by the tachyarrhythmia. The BSA-adjusted LA volumes more than doubled, with a decrease in all the indices of atrial contraction including a decrease in the LA active ejection fraction from 28% to 19%. Consistent with the decreased atrial pump function, there was a decrease in the atrial elastance as measured by the end-systolic pressure-volume relationship slope and the dp/dt slope.
The amount of fibrosis in the LA increased 50% to 80% with AF and was not homogeneous but was dependent on location within the LA, with the greatest increases occurring in the posterior LA in the vicinity of the pulmonary veins. AF also increased LV end-diastolic volume and end-systolic volume, but did not change LVSV, resulting in a 50% reduction in the LVEF. However, unlike the LA, the amount of LV fibrosis and the thickness of the epicardial and endocardial connective tissue layers were unchanged. There was no decrease in the LVelastance or compliance. Despite the decrease in LVEF, none of the animals developed clinical signs of heart failure and all had normal BNP levels. These differential effects of AF on the LV compared with the LA may be a consequence of the digitalis, which slowed the ventricular response to the AF. The atrial rate was approximately 300 beats/min compared with the ventricular rate of 133 beats/min. The LV was still able to compensate for the decrease in LA pump function with an increased reservoir volume because the LVSV was unchanged by the pacing. Conduit function remained unchanged.
The basic physiologic principle that governs characterization of atrial function is the constant-volume attribute of the 4-chambered heart.11 This means that atrial and ventricular volumes reciprocate so the volumetric content of the pericardial sac remains (essentially) invariant throughout the cardiac cycle. This makes characterization of LA function in terms of reservoir, conduit, and booster pump phases intuitive and clarifies that reservoir and conduit function, when the atrium is passive and relaxed, is a direct consequence of ventricular systolic and diastolic function, respectively. More specifically, during ventricular systole, longitudinal downward displacement of the mitral annulus (closed mitral valve) and anterior and downward displacement of the aortic root jointly act like a piston and aspirate blood from the PV into the (relaxed) atrium generating the reservoir volume of the LA. Upward displacement of the annulus during the ventricular suction initiates early rapid flow with simultaneous pulmonary vein flow into the LA generating the conduit function. Thus, both reservoir and conduit features of the LA are directly generated by LV systolic and diastolic function, respectively, whereas the LA is passive. Only LA booster pump function can be called an intrinsic feature of the LA, modulated by the load the contracting atrium encounters in the form of LV distensibility (chamber stiffness) and pulmonary compliance.12
With respect to LA and LV functional recovery after cardiac surgery that results in NSR, there is a high potential for recovery. Cameli and colleagues10 showed a strong correlation between the LA strain, which is one of the indices of contractility and fibrosis, and the thickness of the endocardial connective tissue layer; however, the degrees of fibrosis and the thickening of the connective tissue layers they observed were over a larger range (10%–90%) than those observed in the present study.10 On the basis of their data and the degree of fibrosis observed in the present study, which was less than 30% in the paced group, the change from control should not have a large effect. This suggests that the reduction in booster pump function and contractility is functional and not based on fibrotic changes in the atria, implying that termination of the AF may lead to reversal of the AF-induced changes. This is particularly relevant in patients undergoing surgery to ablate AF. Stulak and colleagues13 showed that LV function after the Cox-Maze procedure improved with restoration of sinus rhythm. Likewise, Pozzoli and colleagues14 also showed that in patients with persistent lone AF, a Cox-Maze procedure normalized LVEF, even in patients with severely reduced preoperative LVEF.
In patients who had been in PAF for several years, Robertson and colleagues4 showed that the LA booster pump function was preserved, but reservoir function was decreased and conduit function increased. After a Cox-Maze IV procedure, LA booster pump function and reservoir function were both decreased with increasing conduit function. Because sternotomy and pericardiotomy alone negatively influence LA function, this suggested that the AF disease process played a role in generating these changes. In patients with persistent AF, after a Cox-Maze IV, the LA booster pump function was half that of a patients who had PAF and the procedure. This suggested that the persistent AF caused an additional significant decrease in booster pump function that did not recover after ablation of the AF.
Study Limitations
In the volumetric analysis using MRI, the data do not take into account the hemodynamic loading, although the PV data suggest that the LA and LV pressures were not different in the paced and nonpaced animals. Although studies show that most of the mechanical effects of cardioversion are normalized within 20 minutes, there may be some small residual effects in the chronically paced animals. This study only addressed the changes in atrial and ventricular mechanics and did not examine how the changes in atrial size and fibrosis, as well as any molecular remodeling, might have affected electrophysiology. The study also was not designed to determine when the changes in the LA and LV induced by persistent AF become irreversible. Also, there is always the issue of using an animal model, and therefore the results need to be replicated in humans. Further studies are needed to determine the timing and mechanistic features of electrical atrial remodeling and their reversibility. The results of this work and further studies will advance our mechanistic understanding of AF and contribute to the development of a rational basis for the timing of surgery for patients with AF.
CONCLUSIONS
This study shows that after 6 weeks of rapid pacing and persistent AF, the LA booster function and contractility were significantly decreased. LVSV was preserved despite an increase in LV end-diastolic volume and a decrease in LVEF.
Supplementary Material
Central Message.
Persistent AF causes increased atrial fibrosis and decreased atrial contractility and atrial hypertrophy.
Perspective.
Early intervention in patients with AF increases the chances of restoring normal atrial function.
Acknowledgments
Supported by National Institutes of Health Grants R01 HL032257 and T32 HL007776 and the Barnes-Jewish Hospital Foundation.
Abbreviations and Acronyms
- AF
atrial fibrillation
- BNP
brain natriuretic peptide
- BSA
body surface area
- cMRI
cardiac magnetic resonance imagin
- LA
left atrium, left atrial
- LV
left ventricle, left ventricular
- LVEF
left ventricular ejection fraction
- LVSV
left ventricular stroke volume
- MRI
magnetic resonance imaging
- NSR
normal sinus rhythm
- PAF
paroxysmal atrial fibrillation
- PV
pressure volume
- RA
right atrium, right atrial
Footnotes
Conflict of Interest Statement
Authors have nothing to disclose with regard to commercial support.
Supplemental material is available online.
References
- 1.Gammie JS, Haddad M, Milford-Beland S, Welke KF, Ferguson TB, Jr, O’Brien SM, et al. Atrial fibrillation correction surgery: lessons from the Society of Thoracic Surgeons National Cardiac Database. Ann Thorac Surg. 2008;85:909–14. doi: 10.1016/j.athoracsur.2007.10.097. [DOI] [PubMed] [Google Scholar]
- 2.Vieira MJ, Teixeira R, Goncalves L, Gersh BJ. Left atrial mechanics: echocardiographic assessment and clinical implications. J Am Soc Echocardiogr. 2014;27:463–78. doi: 10.1016/j.echo.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Weimar T, Watanabe Y, Kazui T, Lee US, Moon MR, Schuessler RB, et al. Differential impact of short periods of rapid atrial pacing on left and right atrial mechanical function. Am J Physiol Heart Circ Physiol. 2012;302:H2583–91. doi: 10.1152/ajpheart.01170.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson JO, Lee AM, Voeller RK, Damiano MS, Schuessler RB, Damiano RJ. Quantification of the functional consequences of atrial fibrillation and surgical ablation on the left atrium using cardiac magnetic resonance imaging. Eur J Cardiothorac Surg. 2014;46:720–8. doi: 10.1093/ejcts/ezt656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voeller RK, Zierer A, Lall SC, Sakamoto S, Chang NL, Schuessler RB, et al. The effects of the Cox maze procedure on atrial function. J Thorac Cardiovasc Surg. 2008;136:1257–64. 64.e1–3. doi: 10.1016/j.jtcvs.2008.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai H, Li Z, Goette A, Mera F, Honeycutt C, Feterik K, et al. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: potential mechanisms for atrial thrombosis and stroke. Circulation. 2002;106:2854–8. doi: 10.1161/01.cir.0000039327.11661.16. [DOI] [PubMed] [Google Scholar]
- 7.Brody SC, Comfort JE, Matthews JS. Growth and development of domestic animals. Mo Ag Exp Sta Res Bull. 1928;115:3–59. [Google Scholar]
- 8.Jarvinen VM, Kupari MM, Poutanen VP, Hekali PE. A simplified method for the determination of left atrial size and function using cine magnetic resonance imaging. Magn Reson Imaging. 1996;14:215–26. doi: 10.1016/0730-725x(95)02098-e. [DOI] [PubMed] [Google Scholar]
- 9.Spencer KT, Mor-Avi V, Gorcsan J, III, DeMaria AN, Kimball TR, Monaghan MJ, et al. Effects of aging on left atrial reservoir, conduit, and booster pump function: a multi-institution acoustic quantification study. Heart. 2001;85:272–7. doi: 10.1136/heart.85.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cameli M, Lisi M, Righini FM, Massoni A, Natali BM, Focardi M, et al. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol. 2013;111:595–601. doi: 10.1016/j.amjcard.2012.10.049. [DOI] [PubMed] [Google Scholar]
- 11.Bowman AW, Kovacs SJ. Assessment and consequences of the constant-volume attribute of the four-chambered heart. Am J Physiol Heart Circ Physiol. 2003;285:H2027–33. doi: 10.1152/ajpheart.00249.2003. [DOI] [PubMed] [Google Scholar]
- 12.Appleton CP, Kovacs SJ. The role of left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2:6–9. doi: 10.1161/CIRCIMAGING.108.845503. [DOI] [PubMed] [Google Scholar]
- 13.Stulak JM, Dearani JA, Daly RC, Zehr KJ, Sundt TM, III, Schaff HV. Left ventricular dysfunction in atrial fibrillation: restoration of sinus rhythm by the Cox-maze procedure significantly improves systolic function and functional status. Ann Thorac Surg. 2006;82:494–501. doi: 10.1016/j.athoracsur.2006.03.075. [DOI] [PubMed] [Google Scholar]
- 14.Pozzoli A, Taramasso M, Coppola G, Kamami M, La Canna G, Della Bella P, et al. Maze surgery normalizes left ventricular function in patients with persistent lone atrial fibrillation. Eur J Cardiothorac Surg. 2014;46:871–6. doi: 10.1093/ejcts/ezu034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


