Abstract
Objective
Radiotherapy is commonly used in induction regimens for non-small cell lung cancer (NSCLC) patients with operable mediastinal nodal disease, though evidence has not shown benefit over induction chemotherapy (IC) alone. We compared outcomes between IC and induction chemoradiation (ICR) using the National Cancer Data Base (NCDB).
Methods
Induction radiation use and survival of patients who had lobectomy or pneumonectomy after induction chemotherapy for clinical T1-3N2M0 NSCLC in the NCDB from 2003 to 2006 were assessed using logistic regression, general linear regression, Kaplan-Meier and Cox proportional hazard analysis.
Results
Of 1362 patients who met study criteria, 834 (61%) underwent ICR while 528 (39%) underwent IC. Lobectomy was performed in 82% (n=1111) of patients and pneumonectomy in 18% (n=251). Pneumonectomy was performed more often after ICR than after IC (20% versus 16%, p=0.04). Down-staging from N2 to N0/N1 was more common with ICR compared to IC (58% vs 46%, p<0.01), but 5-year survival of ICR and IC patients were similar in unadjusted analysis (41% vs 41%, p=0.41). In multivariable analysis, the addition of radiation to IC was also not associated with a survival benefit (Hazard Ratio [HR]: 1.03; 95% CI: 0.89-1.18; p=0.73).
Conclusions
ICR is used in the majority of NSCLC patients with N2 disease who undergo induction therapy prior to surgical resection but is not associated with improved survival compared to IC.
Keywords: Lung cancer surgery, neoadjuvant therapy
Introduction
The optimal induction strategy for patients with stage IIIA-N2 NSCLC patients who are selected for surgery is not well-established. Induction chemotherapy (IC) has been shown to definitively improve survival over the primary use of surgery [2, 3]. However, the benefit of using induction chemoradiotherapy (ICR) compared to IC alone is not as clear. Only a handful of studies have compared IC and ICR for stage IIIA-N2 NSCLC, and the two largest and most recent randomized controlled trials evaluating IC and ICR were both performed in Europe with induction regimens that likely do not reflect current U.S. practice [13, 14]. Of the limited available data, ICR has been found to be associated with a higher rate of mediastinal nodal down-staging and histopathologic response but also increased acute toxicity and cost [11, 13, 15]. More importantly, adding radiotherapy to IC regimens has not been shown to improve overall survival [16].
Considering the lack of definitive evidence that establishes an optimal preoperative regimen, clinical practice not surprisingly varies across the United States [10]. There is no consensus on the best strategy in the National Cancer Center Network (NCCN), as reflected by the fact that 50% of member institutions use ICR and 50% use IC alone [11]. The present study was undertaken to improve the level of evidence available to guide induction therapy choice for NSCLC patients with N2 disease, considering that a large randomized prospective trial that adequately compares ICR and IC may never happen. The specific purpose of this study was to test the hypothesis that ICR does not improve survival over IC alone in patients with operable stage IIIA-cN2 NSCLC using a national population-based oncology outcomes database.
Methods
National Cancer Database
The National Cancer Data Base (NCDB) is jointly managed by the American College of Surgeons Commission on Cancer (CoC) and the American Cancer Society; it captures approximately 70% of all newly diagnosed cases of cancer in the United States and Puerto Rico [17]. Clinical staging information is directly recorded in the NCDB using American Joint Committee on Cancer (AJCC) 6th edition TNM classifications for the years of study inclusion (2003-2006) [18].
Study design
This NCDB analysis was approved by the Duke University Institutional Review Board. From a de-identified NCDB participant user file, we selected all patients in the NCDB diagnosed with clinical T1-3 N2, M0 NSCLC from 1/1/2003 to 12/31/2006 were identified using International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) histology and topography codes. This study period was chosen for two primary reasons: 1) the Charlson/Deyo comorbidity score was not recorded prior to 2003 and 2) at the time of our analysis, long-term survival data was available for patients diagnosed up until the end of 2006. Within this population, we abstracted patients treated with either IC followed by surgery (IC group) or combination ICR, followed by surgery (ICR group). Exclusion criteria included non-malignant pathology, history of previous unrelated malignancy, N3 or metastatic disease, patients who received no induction chemotherapy or received only palliative treatment. The primary outcome was overall survival. Secondary outcomes included rates of nodal down-staging, 30-day mortality and readmission, hospital length of stay, regional lymph nodes examined. Of note, the NCDB does not have data regarding specific perioperative outcomes; however, 30-day readmission and hospital length of stay may be interpreted as surrogate markers for postoperative morbidity. We also examined the practice patterns of hospitals to determine the percentage of patients who undergo induction chemotherapy or induction chemoradiation within each hospital. A hospital that practices “primarily” induction chemotherapy or chemoradiation was defined as a hospital where 80 to 100% of their patients underwent induction chemotherapy or chemoradiation.
Statistical analysis
Patients were grouped according to induction therapy regimen (IC or ICR) and comparisons of baseline characteristics and unadjusted outcomes were performed using the Wilcoxon Rank Sum test for continuous variables and Pearson's chi-square test for discrete variables. A logistic regression model was developed to identify predictors of induction radiation use. Variables in the model included age, clinical T stage, Charlson/Deyo comorbidity condition (CDCC) score (which is recorded a “0”, “1” or “2”), sex, race, treatment facility type, insurance type, histology, and tumor location. Median survival and 5-year survival were estimated by the Kaplan-Meier product limit approach, both for the overall cohort as well as for the subgroup of patients treated with lobectomy.
A multivariable linear regression model was used to identify variables that were significantly associated with the number of lymph nodes examined. Variables in the model included age, tumor size, CDCC score, sex, race, facility type, histology and tumor location.
A Cox proportional hazards model was used to compare overall survival between groups, adjusting for induction radiation use, type of operation (lobectomy versus pneumonectomy), insurance type (private, Medicare/Medicaid, other government, uninsured and unknown), age, gender, race, CDCC score, clinical T stage, facility type, histology (adenocarcinoma, squamous cell carcinoma, and others) and tumor location.
Subgroup analyses were performed to examine the impact of the extent of surgical resection on outcomes, and the survival analysis described above was repeated on the subgroup of patients whose surgical resection was lobectomy and on the subgroup of patients whose surgical resection was pneumonectomy. Further subgroup analyses were performed to examine the impact of downstaging on survival. We also performed subgroup analyses of patients who had clinical T1N2, T3N2 and pathologic T0N0 who underwent lobectomy.
In another subgroup analyses, we examined the long-term survival of ICR patients using a Kaplan-Meier analysis, stratified by dose of induction radiation given (<4000, 4001-5000, 5001-6000 and > 6000 cGy). We performed a multivariable Cox proportional hazards analysis of only the ICR cohort of patients, adjusting for the covariates of induction radiation dose, type of operation (lobectomy versus pneumonectomy), insurance type (private, Medicare/Medicaid, other government, uninsured and unknown), age, gender, race, CDCC score and clinical T status, facility type, histology, tumor location
All statistical analyses were performed using SAS for Windows, Version 9.3; SAS Institute Inc.; Cary, NC. A 2-sided p-value of 0.05 was used to define significance.
Results
Between 2003 and 2006, 1362 patients were diagnosed with clinical T1-T3N2M0 NSCLC and had underwent lobectomy or pneumonectomy (Figure 1). Of these, 834 (61%) were treated using ICR and surgery and 528 (39%) were treated using IC and surgery. Approximately 77% of hospitals used primarily induction chemotherapy or primarily induction chemoradiation as their induction therapy strategy.
Figure 1. Consort Diagram Showing Schema of Study Subject Selection.
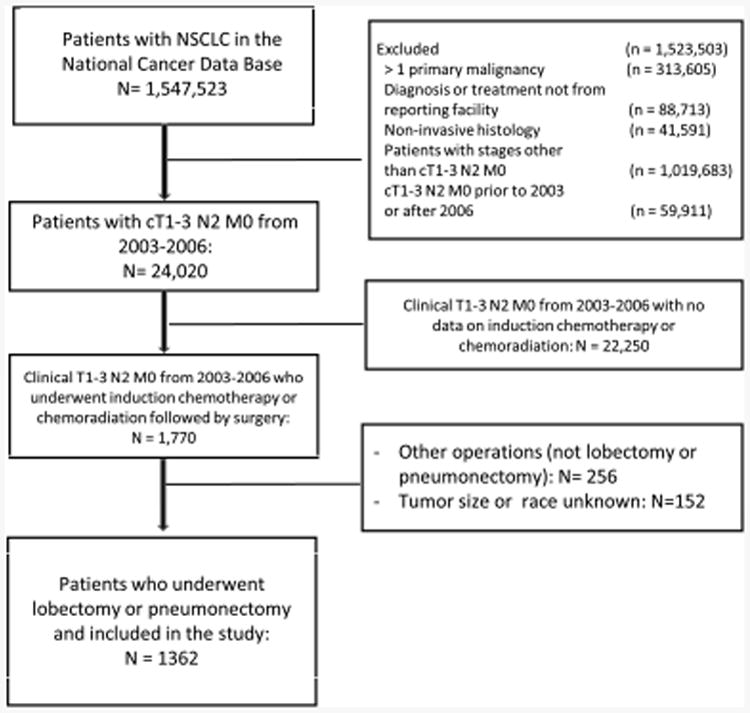
Table 1A and 1B shows the pre-operative, demographic, peri- and post-operative data of the two groups. The patients who received ICR were younger than the IC patients, but there were no statistically significant differences between the two groups in regards to sex, race, CDCC score, and insurance status. The ICR group had a larger tumor size and a higher clinical T stage.
Table 1A. Preoperative and Demographic Characteristics.
| IC (N=528) | ICR (N=834) | p-value* | |
|---|---|---|---|
| Patient Age (year) | <0.01 | ||
| Mean (SD) | 62.2 (10.0) | 59.9 (9.8) | |
| Median, IQR | 63 (55.0, 70.0) | 61 (53.0, 67.0) | |
| Sex, n (%) | 0.23 | ||
| Male | 250 (47.3%) | 423 (50.7%) | |
| Female | 278 (52.7%) | 411 (49.3%) | |
| Race, n (%) | 0.80 | ||
| White | 475 (90.0%) | 750 (89.9%) | |
| Black | 35 (6.6%) | 60 (7.2%) | |
| Other | 18 (3.4%) | 24 (2.9%) | |
| CDCC Score, n (%) | 0.83 | ||
| 0 | 371 (70.3%) | 596 (71.5%) | |
| 1 | 129 (24.4%) | 199 (23.9%) | |
| 2 | 28 (5.3%) | 39 (4.7%) | |
| Insurance Type, n (%) | 0.55 | ||
| Uninsured | 15 (2.8%) | 17 (2.0%) | |
| Private | 261 (49.4%) | 449 (53.8%) | |
| Medicare/aid | 232 (43.9%) | 340 (40.8%) | |
| Other Government | 9 (1.7%) | 13 (1.6%) | |
| Unknown | 11 (2.1%) | 15 (1.8%) | |
| Clinical T Stage, n (%) | <0.01 | ||
| T1 | 155 (29.4%) | 176 (21.1%) | |
| T2 | 318 (60.2%) | 512 (61.4%) | |
| T3 | 55 (10.4%) | 146 (17.5%) | |
| Facility Type, n (%) | <0.01 | ||
| Community Cancer Program | 29 (5.5%) | 67 (8.0%) | |
| Comprehensive Community Cancer Program | 202 (38.3%) | 406 (48.7%) | |
| Academic/Research Program | 295 (55.9%) | 355 (42.6%) | |
| Other specified types of cancer programs | 2 (0.4%) | 6 (0.7%) | |
| Tumor Location, n (%) | 0.11 | ||
| RLL | 87 (16.5%) | 100 (12.0%) | |
| LLL | 33 (6.3%) | 74 (8.9%) | |
| RML | 18 (3.4%) | 20 (2.4%) | |
| RUL | 233 (44.1%) | 374 (44.8%) | |
| LUL | 131 (24.8%) | 212 (25.4%) | |
| Main Bronchus | 6 (1.1%) | 15 (1.8%) | |
| Overlapping Lesion | 11 (2.1%) | 15 (1.8%) | |
| Other | 9 (1.7%) | 24 (2.9%) |
P values provided are from Wilcoxon Rank Sum test on continuous variables and from Chi-square test on categorical variables.
Table 1B. Perioperative and Postoperative Data.
| IC (N=528) | ICR (N=834) | p-value * | |
|---|---|---|---|
| Type of Surgery, n (%) | 0.04 | ||
| Pneumonectomy | 83 (15.7%) | 168 (20.1%) | |
| Lobectomy | 445 (84.3%) | 666 (79.9%) | |
| Time from Induction Therapy to Surgery, days | |||
| Mean (SD) | 97 (43) | 99 (41) | 0.68 |
| Median, IQR | 89 (71, 113) | 88 (72, 113) | |
| Regional Lymph Nodes (LN) Examined | <0.01 | ||
| No. of patients with LN examined | 416 | 702 | |
| Median, IQR | 10 (4.5, 16.0) | 7 (4.5, 16.0) | |
| Size of Tumor (cm) | <0.01 | ||
| Mean (SD) | 4.0 (2.2) | 4.5 (3.3) | |
| Median, IQR | 3.5, (2.5, 5.0) | 4 (2.6, 6.0) | |
| Downstaging | |||
| T stage downstaging** | 99 (24.2) | 214 (37.7) | <0.01 |
| N2 to N0 down-staging | 136 (32.5) | 267 (45.4) | <0.01 |
| N2 to N0/N1 downstaging*** | 190 (45.5) | 338 (57.5) | <0.01 |
| Margin Status, n (%) | |||
| Negative | 469 (88.8%) | 741 (87.6%) | 0.55 |
| Positive | 42 (8.0) | 58 (7.0) | |
| Unknown | 17 (3.2) | 35 (4.2) | |
| Histology, n (%) | 0.14 | ||
| Adenocarcinoma | 240 (45.5%) | 332 (39.8%) | |
| Squamous | 137 (25.9%) | 219 (26.3%) | |
| Large cell | 22 (4.2%) | 38 (4.6%) | |
| Other | 129 (24.4%) | 245 (29.4%) | |
| Re-admission in 30 days, n (%) | 29 (5.7%) | 54 (7.0%) | 0.38 |
| Surgical Inpatient Stay, Days from Surgery | 0.37 | ||
| No. of patients with available data | 466 | 705 | |
| Mean (SD) | 7.0 (6.7) | 7.4 (7.4) | |
| Median, IQR | 6 (4, 8) | 6 (4, 8) | |
| Perioperative Mortality, n (%) | |||
| Lobectomy | 6 (1.4) | 18 (2.7) | 0.14 |
| Pneumonectomy | 7 (8.4) | 10 (5.9) | 0.59 |
P values provided are from Wilcoxon Rank Sum test on continuous variables and from Chi-square test on categorical variables.
Includes cases of T1 to T0, T2 to T0/T1, and T3 to T0/T1/T2. Excludes 385 unknown cases of pathologic T stage.
Excludes 356 unknown cases of pathologic N stage.
More patients in the ICR group underwent pneumonectomy compared to the IC group (20.1% vs 15.7%, p=0.04). Adjuvant radiation was used in 31% of patients in the IC group. Of the patients who underwent an operation for pT0N0, 48 patients underwent a lobectomy and 8 underwent a pneumonectomy.
Patient characteristics that were associated with induction radiation included younger age (adjusted odds ratio [AOR]: 0.97/year; 95% CI: 0.96-0.99; p<0.0001), T stage (AOR, 1.47; 95% CI: 1.12-1.92; p=0.005 and AOR: 2.25; 95% CI: 1.52-3.35; p<0.0001 for T2 and T3 tumors respectively, compared to T1), and treatment at a non-academic community cancer program (AOR, 2.00; 95% CI: 1.25-3.21; p=0.004) and comprehensive cancer program (AOR, 1.72; 95% CI: 1.36-2.18; p<0.0001).
After multivariable adjustment, use of induction radiation was found to be significantly associated with fewer lymph nodes examined (regression coefficient [SE]: -1.9 (0.54); p=0.001).
Overall T and N down-staging was more common with ICR when compared to IC. Although pathologic M1 disease was uncommon overall, there was a higher number of patients with pathologic M1 disease in the IC group compared to the ICR group (11 [2.1%] vs 4 [0.5%], p<0.01). There were no further details in the NCDB regarding the site of distant metastases or type of M1 disease found. Hospital readmission in 30 days and hospital length of stay was similar in both groups (Table 1B). There were no significant differences in perioperative deaths between IC and ICR groups (Table 1B).
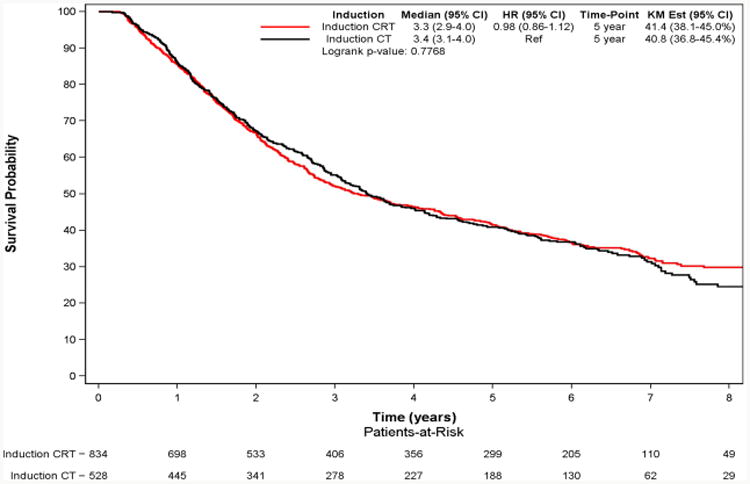
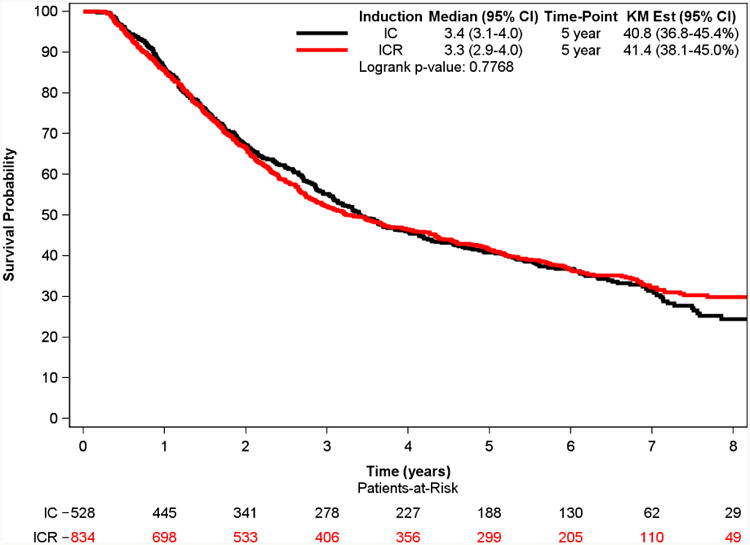
The median follow-up was 6.6 years (IQR 5.3 to 7.3 years). Kaplan–Meier analysis demonstrated a 5-year survival of 40.8% for the IC group and 41.4% for ICR group (p=0.41). The multivariable adjusted survival analyses demonstrated that induction radiation use was not associated with improved survival (HR: 1.03; 95% CI: 0.89-1.18; p=0.73) (Table 2).
Table 2. Independent predictors of survival following Cox proportional hazards adjustment for patients with stage IIIA-N2 NSCLC who have undergone induction chemotherapy or chemoradiation followed by lobectomy or pneumonectomy.
| Hazard Ratio | 95% CI | p-value | |
|---|---|---|---|
| ICR vs IC | 1.03 | 0.89, 1.18 | 0.73 |
| Lobectomy vs Pneumonectomy | 0.66 | 0.55, 0.79 | <.001 |
| Insurance Type (ref=Private) | |||
| Medicare/aid | 0.99 | 0.84, 1.16 | 0.87 |
| Other government | 1.70 | 1.07, 2.71 | 0.02 |
| Uninsured | 2.15 | 1.44, 3.20 | <.001 |
| Unknown | 1.27 | 0.77, 2.11 | 0.35 |
| Age (year) | 1.02 | 1.01, 1.03 | <.001 |
| Female vs Male | 0.94 | 0.82, 1.08 | 0.37 |
| Race (ref=White) | |||
| Black | 0.92 | 0.70, 1.20 | 0.53 |
| Other | 0.91 | 0.62, 1.34 | 0.64 |
| CDCC Score (ref=0) | |||
| 1 | 1.12 | 0.96, 1.31 | 0.16 |
| 2+ | 1.11 | 0.81, 1.52 | 0.52 |
| Clinical T stage (ref=T1) | |||
| T2 | 1.07 | 0.91, 1.27 | 0.40 |
| T3 | 1.05 | 0.83, 1.33 | 0.68 |
| Facility Type (ref=Academic) | |||
| Community | 1.22 | 0.94, 1.58 | 0.13 |
| Comprehensive | 0.98 | 0.85, 1.13 | 0.76 |
| Histology (ref=Adenocarcinoma) | |||
| Squamous | 0.92 | 0.77, 1.10 | 0.34 |
| Other | 1.01 | 0.87, 1.19 | 0.87 |
| Tumor Location (ref=RUL) | |||
| LLL | 1.43 | 1.12, 1.84 | <.01 |
| LUL | 1.17 | 0.99, 1.39 | 0.08 |
| RLL | 1.45 | 1.18, 1.77 | <.001 |
| RML | 1.09 | 0.71, 1.67 | 0.71 |
| Other | 1.11 | 0.82, 1.50 | 0.50 |
Subgroup Analyses
In a subgroup analysis of only those patients who underwent lobectomy, use of induction radiation was again not associated with a significant difference in long-term survival (5-year survival: 42.3% vs 44.0% for the IC and ICR groups respectively, p=0.54) (Figure 3). Following adjustment, this lack of significant survival benefit was maintained (HR: 1.01; 95% CI: 0.86-1.18).
Figure 3. Overall Survival of Patients with Stage IIIA-N2 NSCLC who Underwent Induction Chemotherapy (CT) vs Induction Chemoradiation (CRT) Followed by Lobectomy.
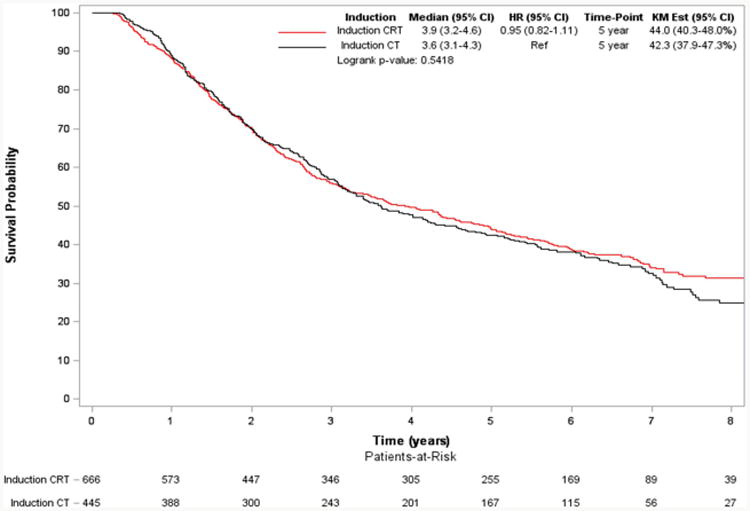
In a subgroup analysis of only those patients who underwent pneumonectomy, use of induction radiation was again not associated with a significant difference in long-term survival (5-year survival: 32% vs 22% for the IC and ICR groups, respectively, p=0.99) (Figure 4). Following adjustment, this lack of significant survival benefit was maintained (HR: 1.15; 95% CI: 0.80-1.65).
Figure 4. Overall Survival of Patients with Stage IIIA-N2 NSCLC who Underwent Induction Chemotherapy (CT) vs Induction Chemoradiation (CRT) Followed by Pneumonectomy.
Patients who had T-stage down-staging had improved median (4.3 years [95% CI: 3.6-5.4]) and 5-year survival (46.0% [95% CI: 40.5-52.3%]) when compared to patients who did not have T-stage down-staging (median survival of 2.9 years [95% CI: 2.7-3.3] and 5-year survival of 36.4% [95% CI: 32.9-40.3]). Patients with nodal down-staging (cN2 to pN1/N0) had improved median (4.0 years [95% CI: 3.3-4.9]) and 5-year survival (44.8% [95% CI: 40.6-49.3%]) when compared to patients who did not have nodal down-staging (median survival 3.0 [95% CI: 2.7-3.4] and 5-year survival of 34.4% [95% CI: 30.2-39.0%]).
There was no significant difference in survival between patients in the IC group who had T-stage down-staging versus patients in the ICR group who had T-stage down-staging (median survival 4.6 years (95% CI: 3.6-6.2) vs 4.3 years (95% CI: 3.2-5.7); 5-year survival 46.6% (95% CI: 37.4-58.1%) vs 45.8% [95% CI: 39.1-53.5%]). For patients with nodal down-staging, there was no significant difference in survival between IC and ICR groups (median survival 4.0 years (95% CI: 3.3-5.7) vs 4.0 years (95% CI: 2.8.-5.2); 5-year survival 44.6% (95% CI: 37.9-52.4%) vs 44.9% (95% CI: 39.8-50.7%)).
In a subgroup analysis of 310 patients who had cT1N2 NSCLC, there were no significant differences in long-term survival between the groups (5-year survival: 48.9 (95% CI: 41.3-57.8) vs 40.6% (95% CI: 33.4-49.2) in the IC and ICR groups, respectively; log-rank, p=0.41). In a subgroup analysis of 46 patients who had pT1N0 NSCLC, there were no significant differences in median survival between the groups (7.6 (95% CI: 4.6-NE) vs 8.2 (95% CI: 4.1-NE) years in the IC and ICR groups, respectively; log-rank, p=0.70).
Because the induction chemoradiation cohort had a significantly higher proportion of T3N2 patients, which has been shown to have worse prognosis than T1-T2 N2 patients, we performed a subset analysis of patients with cT3 cN2 who underwent induction therapy followed by lobectomy and found no significant differences in overall survival between the induction chemotherapy and induction chemoradiation groups (5-year survival 45.3 (95% CI: 31.9-64.3%) vs 41.5 (95% CI: 32.6-52.9%), p=0.89).
In addition, we examined the type of radiation that ICR patients received. Of the 810 ICR patients with available data, 97% of patients underwent concurrent chemoradiation. 745 out of 834 patients had radiation dose data available. 200 (26.9%) patients had a radiation dose ≤ 4000 cGy, 359 (48.2%) patients had a dose of 4001-5000 cGy, 124 (16.6%) patients had a dose from 5001-6000 cGy and 62 (8.3%) patients had a dose greater than 6000 cGy. The 5-year survival was 45.8 (95% CI: 40.8-51.4), 41.5 (95% CI: 33.5-51.4), 37.6 (95% CI: 31.4-45.1) and 35.2 (95% CI: 28.0-44.3%) for ICR patients undergoing induction radiation doses of 4001-5000 cGy, 5001-6000 cGy, <4000 cGy, and >6000 cGy respectively (p=0.058). In multivariable analysis, there were no categories of induction radiation dose that were significantly associated with increased survival.
Discussion
This is the first population-based study and largest observational study to demonstrate that the addition of radiation to induction chemotherapy for operable stage IIIA-cN2 NSCLC is not associated with a significant improvement in overall survival. Although there was a slight predilection for pneumonectomy in the ICR group, this finding remained significant after limiting the analysis to patients who underwent lobectomy. In addition, we found that there was no difference in length of stay and hospital readmission between induction chemotherapy and induction chemoradiation groups suggesting that perioperative complication rates are similar between the groups. Our primary finding, that induction chemoradiation is not associated with improved survival when compared to induction chemotherapy, is consistent with findings by previous studies limited by smaller sample sizes [13, 16, 19, 21-26], and adds to the current literature by reporting the results of a contemporary “real-world” practice in the U.S.
Although there was no difference in long-term survival, induction radiation was associated with a significant increase in mediastinal down-staging. This finding confirms existing studies and is thought to represent the key advantage of adding radiation to induction regimens [13, 19]. We also measured the number of lymph nodes collected and found that induction radiation was associated with a significant decrease in the number of lymph nodes examined. It is possible that the decrease in mediastinal down-staging associated with induction radiation was due to fewer overall lymph nodes and stations examined—as induction radiation could lead to obliterated planes and more difficult mediastinal lymph node dissections. There may also be the possibility that some surgeons did not perform a complete mediastinal lymph node dissection because they felt the mediastinum was already treated with radiation. An additional consideration is that depending on the institution, multi-disciplinary tumor boards may decide against preoperative radiation treatment if there is a high probability that the surgeon can remove all affected nodes at the time of surgery, which could have also resulted in higher numbers of nodes harvested in the induction chemotherapy cohort and a more definitive oncologic operation. However, as reported by previous restaging studies, it is also possible that radiation obliterated more nodal tissue resulting in fewer viable lymph nodes that could be collected [20].
In the present study, the outcomes of patients who underwent pneumonectomy following induction therapy were significantly better than that reported by the Intergroup 0139 trial, which described a treatment-related mortality of 26% for pneumonectomy patients [9]. It is unclear why there is this difference in outcomes but it is important to note that the Intergroup 0139 trial had an accrual period (1994-2001) that was older than the present study time period (2003-2006). Since the 1990's, changes by institutions may have occurred regarding the specific selection criteria for pneumonectomy, extent of evaluation of comorbidity, strategies to protect the bronchial stump, perioperative fluid administration, use of elective postoperative mechanical ventilation and use of 3-dimensional radiation planning [28-32]. These changes or variations in practice patterns noted above may explain the better outcomes reported in the present study.
Our results can be used to aid the decision process when patients and providers are choosing the optimal strategy for treatment of stage IIIA-N2 NSCLC. First, the results of our study demonstrate that careful selection of patients with N2 disease for induction therapy followed by surgery can lead to excellent outcomes. Of note, the 5-year survival of 41% found in our study for both the IC and ICR groups is better than the 15.7% and 27% 5-year survival of the surgery arms for trials EORTC 8941 and INT0139, (phase III randomized controlled trials evaluating the role of surgery after induction therapy in the treatment of N2 NSCLC) respectively [8, 9]. Second, because we showed no difference in overall survival between treatment groups, there may be a number of potential advantages to using chemotherapy alone in induction regimens: 1) higher delivery of preoperative chemotherapy which may contribute to improved survival, 2) a more accurate assessment of the tumor's response to chemotherapy and 3) a lower perioperative complication rate [16]. Of note, adding radiation to induction chemotherapy is associated with higher rates of acute toxicity and increased cost [11].
There are several limitations to this study that are inherent to analyses of stage IIIA patients in the NCDB, as previously reported [17], which include its retrospective nature and possible incompleteness of data. The NCDB also does not have data on the extent of mediastinal lymph node dissection, on the number of N2 stations dissected, on details regarding radiation type and technique, on local and distant recurrence and disease-free survival, on performance status of patients at different time points prior to neoadjuvant or adjuvant therapies. This NCDB analysis also had missing data on pathologic T and N status for a significant number of patients.
In addition, it is unclear how many patients in our cohort had biopsy-proven N2 disease because the NCDB does not provide detailed information regarding invasive mediastinal staging prior to induction therapy or surgery. Because of this limitation of the NCDB, there is a possibility that the patients included in the present study were overstaged (although there is no particular reason to suspect a difference in overstaging between the groups).
Due to the possibility of overstaging, the downstaging rates reported in the present study should be interpreted with caution, although the rates we found are comparable to those reported by other randomized trials [9, 14]. In addition, there is a possibility that overstaging contributed to the higher survival seen in the present study when compared with survival reported from previous randomized trials (e.g., EORTC 8941 and Intergroup 0139), although the accrual period of those trials was much older than the present study time period and differences in patient selection, staging/restaging and operative strategies between previous trials and the present study could have accounted at least in part for differences in survival seen between the present study and previous trials. We can only speculate and infer from recent survey results [11], but presumably the present study reflects a more contemporary practice pattern where surgeons mostly only operate on single station, microscopic N2 disease, where patients undergo brain imaging as part of initial preoperative assessment, where staging and restaging with PET/CT is more common, and where use of pneumonectomy is more selective and infrequent.
The intrinsic limitations of the NCDB data also do not allow for us to accurately determine the number of patients who were intended to go on to surgery following induction therapy but who did not. This may have biased our results if there was a difference in the likelihood of completing induction therapy and subsequently undergoing surgery between the chemotherapy and chemoradiation groups.
Finally, the NCDB does not distinguish between “bulky” versus “non-bulky” N2 disease and single-station versus multi-station N2 disease. It is possible ICR actually improves survival but that we did not see a difference in survival between ICR and IC because the ICR group had worse N2 disease. To explore this possibility, we evaluated practice patterns of hospitals across the U.S., and we found that for stage IIIA-N2 disease, approximately 76% of hospitals either used primarily induction chemotherapy or primarily induction chemoradiation. This finding suggests that the decision to treat a patient with induction chemotherapy or chemoradiation may be based more on institution philosophy and less on individual patient factors (such as whether a patient had microscopic vs bulky N2 disease). We also evaluated the survival of patients with cT1N2 and pT0N0 (post-induction therapy) who underwent lobectomy with the assumption that these patients would more likely have single station or microscopic N2 disease and that in these subgroups there would be a fairly even distribution of single station or microscopic N2 disease between IC and ICR groups. We acknowledge that there are flaws with this assumption, but there have been some data from early stage NSCLC suggesting that the frequency of lymph node metastases increases as the tumor size increases [33]. In these subset analyses, we found no differences in survival between IC and ICR groups. The results of these two exploratory analyses should be interpreted with caution, but do provide some evidence suggesting that our primary finding that there is no significant difference in overall survival between IC and ICR is at least not entirely due to patients in the ICR group having significantly worse N2 disease when compared to the IC group.
Conclusion
In this NCDB analysis, induction chemoradiotherapy was used in the majority of patients who had induction therapy prior to surgical resection of clinical stage IIIA-N2 NSCLC. The addition of induction radiation was not associated with improved survival when compared to induction chemotherapy. Given the key limitations of this present study noted above, the use of induction chemoradiation for operable stage IIIA-N2 NSCLC should be further reexamined in the context of randomized trials and future prospective and retrospective studies should focus on identifying characteristics (e.g., single station vs multi-station N2 disease) that can be used to indicate if and when radiation is needed in addition to chemotherapy. Multi-disciplinary evaluation and discussion of treatment options for patients with stage IIIA-N2 NSCLC is essential prior to operative intervention.
Figure 2. Overall Survival of Patients with Stage IIIA-N2 NSCLC who Underwent Induction Chemotherapy (CT) vs Induction Chemoradiation (CRT) Followed by Major Lung Resection.
Central Picture Legend: Overall Survival After Induction Therapy Followed by Surgery for Stage IIIA-N2 NSCLC.
Central Message.
Induction chemoradiation is not associated with improved survival when compared to induction chemotherapy for operable cN2 NSCLC.
Perspective
Radiation is commonly used in induction regimens for non-small cell lung cancer (NSCLC) patients with operable mediastinal nodal disease, though evidence has not shown benefit over induction chemotherapy (IC) alone. We compared outcomes between IC and induction chemoradiation (ICR) using the National Cancer Data Base. ICR is not associated with improved survival compared to IC for operable cN2 NSCLC.
Acknowledgments
The data used in this study are derived from a de-identified National Cancer Data Base file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators.
Disclosures: This work was supported by the NIH funded Cardiothoracic Surgery Trials Network (M.G.H and M.F.B), 5U01HL088953-05 and by the American College of Surgeons Resident Research Scholarship (C.J.Y.). P.J.S., B.C.G., L.G., X.W. have no disclosures to report. One of the authors (T.A.D.) serves as a consultant for Scanlan International, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robinson LA, Ruckdeschel JC, Wagner H, Jr et al. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:243S–265S. doi: 10.1378/chest.07-1379. [DOI] [PubMed] [Google Scholar]
- 2.Roth JA, Atkinson EN, Fossella F, et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer. 1998;21:1–6. doi: 10.1016/s0169-5002(98)00046-4. [DOI] [PubMed] [Google Scholar]
- 3.Rosell R, Gomez-Codina J, Camps C, et al. Preresectional chemotherapy in stage IIIA non-small-cell lung cancer: a 7-year assessment of a randomized controlled trial. Lung Cancer. 1999;26:7–14. doi: 10.1016/s0169-5002(99)00045-8. [DOI] [PubMed] [Google Scholar]
- 4.Douillard JY, Tribodet H, Aubert D, et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. J Thorac Oncol. 2010;5:220–228. doi: 10.1097/JTO.0b013e3181c814e7. [DOI] [PubMed] [Google Scholar]
- 5.Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 6.Johnstone DW, Byhardt RW, Ettinger D, Scott CB. Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non-small-cell lung cancer with spread to mediastinal lymph nodes (N2); final report of RTOG 89-01. Radiation Therapy Oncology Group Int J Radiat Oncol Biol Phys. 2002;54:365–369. doi: 10.1016/s0360-3016(02)02943-7. [DOI] [PubMed] [Google Scholar]
- 7.Taylor NA, Liao ZX, Cox JD, et al. Equivalent outcome of patients with clinical Stage IIIA non-small-cell lung cancer treated with concurrent chemoradiation compared with induction chemotherapy followed by surgical resection. Int J Radiat Oncol Biol Phys. 2004;58:204–212. doi: 10.1016/s0360-3016(03)01575-x. [DOI] [PubMed] [Google Scholar]
- 8.van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007;99:442–450. doi: 10.1093/jnci/djk093. [DOI] [PubMed] [Google Scholar]
- 9.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry MF, Worni M, Pietrobon R, et al. Variability in the treatment of elderly patients with stage IIIA (N2) non-small-cell lung cancer. J Thorac Oncol. 2013;8:744–752. doi: 10.1097/JTO.0b013e31828916aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2012;10:1236–1271. doi: 10.6004/jnccn.2012.0130. [DOI] [PubMed] [Google Scholar]
- 12.Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e314S–340S. doi: 10.1378/chest.12-2360. [DOI] [PubMed] [Google Scholar]
- 13.Thomas M, Rube C, Hoffknecht P, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol. 2008;9:636–648. doi: 10.1016/S1470-2045(08)70156-6. [DOI] [PubMed] [Google Scholar]
- 14.Pless MSR, Hans-Beat R, et al. Neoadjuvant chemotherapy with or without preoperative irradiation in stage IIIA/N2 non-small cell lung cancer (NSCLC): A randomized phase III trial by the Swiss Group for Clinical Cancer Research (SAKK trial 16/00) J Clin Oncol. 2013;31 suppl; abstr 7503. [Google Scholar]
- 15.Ripley RT, Rusch VW. Role of induction therapy: surgical resection of non-small cell lung cancer after induction therapy. Thorac Surg Clin. 2013;23:273–285. doi: 10.1016/j.thorsurg.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Shah AA, Berry MF, Tzao C, et al. Induction chemoradiation is not superior to induction chemotherapy alone in stage IIIA lung cancer. Ann Thorac Surg. 2012;93:1807–1812. doi: 10.1016/j.athoracsur.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Sher DJ, Liptay MJ, Fidler MJ. Prevalence and predictors of neoadjuvant therapy for stage IIIA non-small cell lung cancer in the National Cancer Database: importance of socioeconomic status and treating institution. Int J Radiat Oncol Biol Phys. 2014;89:303–312. doi: 10.1016/j.ijrobp.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 18.Greene FL. AJCC cancer staging manual. New York: Springer; 2002. American Joint Committee on Cancer., American Cancer Society. [Google Scholar]
- 19.Higgins K, Chino JP, Marks LB, et al. Preoperative chemotherapy versus preoperative chemoradiotherapy for stage III (N2) non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;75:1462–1467. doi: 10.1016/j.ijrobp.2009.01.069. [DOI] [PubMed] [Google Scholar]
- 20.Jaklitsch MT, Gu L, Demmy T, et al. Prospective phase II trial of preresection thoracoscopic mediastinal restaging after neoadjuvant therapy for IIIA (N2) non-small cell lung cancer: results of CALGB Protocol 39803. J Thorac Cardiovasc Surg. 2013;146:9–16. doi: 10.1016/j.jtcvs.2012.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauvaget JRJ, Vannetzel J. Phase III study of neo-adjuvant MVP versus MVP plus chemoradiotherapy in stage III NSCLC. Proc Am Soc Clin Oncol. 2000;19 abstract 1935. [Google Scholar]
- 22.Fleck JCJ, Godoy D, et al. Chemoradiation therapy (CRT) versus chemotherapy (CT) alone as a neoadjuvant treatment for stage III non-small cell lung cancer (NSCLC): Preliminary report of a phase III prospective randomized trial. Proc Am Soc Clin Oncol. 1993;12 abstract 1108. [Google Scholar]
- 23.Girard N, Mornex F, Douillard JY, et al. Is neoadjuvant chemoradiotherapy a feasible strategy for stage IIIA-N2 non-small cell lung cancer? Mature results of the randomized IFCT-0101 phase II trial. Lung Cancer. 2010;69:86–93. doi: 10.1016/j.lungcan.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Dai CH, Shi SB, et al. Prognostic factors and long term results of neo adjuvant therapy followed by surgery in stage IIIA N2 non-small cell lung cancer patients. Ann Thorac Med. 2009;4:201–207. doi: 10.4103/1817-1737.56010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pezzetta E, Stupp R, Zouhair A, et al. Comparison of neoadjuvant cisplatin-based chemotherapy versus radiochemotherapy followed by resection for stage III (N2) NSCLC. Eur J Cardiothorac Surg. 2005;27:1092–1098. doi: 10.1016/j.ejcts.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 26.Mordant P, Fabre E, Gibault L, et al. Impact of induction therapies on pathology and outcome after surgical resection of non-small lung cancer: a 30-year experience of 859 patients. Rev Pneumol Clin. 2014;70:9–15. doi: 10.1016/j.pneumo.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Martins RG, D'Amico TA, Loo BW, Jr, et al. The management of patients with stage IIIA non-small cell lung cancer with N2 mediastinal node involvement J Natl Compr Canc Netw. 2012;10:599–613. doi: 10.6004/jnccn.2012.0062. [DOI] [PubMed] [Google Scholar]
- 28.Daly BD, Fernando HC, Ketchedjian A, et al. Pneumonectomy after high-dose radiation and concurrent chemotherapy for nonsmall cell lung cancer. The Annals of thoracic surgery. 2006;82:227–231. doi: 10.1016/j.athoracsur.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 29.Donington JS, Pass HI. Surgical resection of non-small cell lung cancer with N2 disease. Thorac Surg Clin. 2014;24:449–456. doi: 10.1016/j.thorsurg.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Gaissert HA, Keum DY, Wright CD, et al. POINT: Operative risk of pneumonectomy—Influence of preoperative induction therapy. The Journal of thoracic and cardiovascular surgery. 2009;138:289–294. doi: 10.1016/j.jtcvs.2008.11.069. [DOI] [PubMed] [Google Scholar]
- 31.Weder W, Collaud S, Eberhardt WE, et al. Pneumonectomy is a valuable treatment option after neoadjuvant therapy for stage III non-small-cell lung cancer. J Thorac Cardiovasc Surg. 2010;139:1424–1430. doi: 10.1016/j.jtcvs.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 32.Mansour Z, Kochetkova EA, Ducrocq X, et al. Induction chemotherapy does not increase the operative risk of pneumonectomy! Eur J Cardiothorac Surg. 2007;31:181–185. doi: 10.1016/j.ejcts.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Seok Y, Yang HC, Kim TJ, et al. Frequency of lymph node metastasis according to the size of tumors in resected pulmonary adenocarcinoma with a size of 30 mm or smaller. J Thorac Oncol. 2014;9:818–824. doi: 10.1097/JTO.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]


