Abstract
Objective
Older adults with obesity are at risk for osteoarthritis(OA) and are predisposed to functional decline and disability. We examined the association of obesity on disability, physical activity and quality of life at six years.
Methods
Using data from the longitudinal Osteoarthritis Initiative, we analyzed older adults (age≥60years) with a body mass index at baseline (BMI)≥18.5kg/m2 (n=2,378) using standard BMI categories. Outcomes were assessed at 6year follow-up and included:Late-life Disability Index (LLDI), Short Form-12 (SF-12) questionnaire and the Physical Activity Scale for the Elderly (PASE). Linear regression predicted outcomes based on BMI category, adjusting for age, sex, race, education, smoking, cohort status, radiographic knee osteoarthritis, co-morbidity scores and baseline scores when available.
Results
Follow-up data were available on 1,727(71.9%) participants. Mean age was 67.9±5.3years, and 61.6% were female. At baseline, obese subjects compared to overweight and normal, were on a greater number of medications(4.28 vs. 3.63 vs. 3.32), had lower gait speeds(1.22 vs. 1.32 vs. 1.36m/s), higher Charlson (0.59 vs 0.37 vs. 0.30) and higher WOMAC scores (right: 14.8 vs. 10.3 vs. 7.5; left: 14.4 vs. 9.9 vs. 7.5). SF-12 scores at 6-years were lower in obese patients than overweight or normal(99.5 [98.7–100.4] vs. 101.1 [100.4–101.8] vs. 102.8 [101.8–103.8]), as were PASE scores (115.1 [110.3–119.8] vs. 126.2 [122.2–130.2] vs. 131.4[125.8–137.0]).The LLDI-limitation component demonstrated differences in obese compared to overweight or normal (78.6 [77.4–79.9] vs. 81.2[80.2–82.3] vs. 82.5[81.1–84.0].
Conclusion
Obesity was associated with worse physical activity scores, lower quality of life, and higher risk of 6-year disability.
Keywords: Obesity, Elderly, Osteoarthritis, Physical Function
INTRODUCTION
Osteoarthritis (OA) is a leading cause of functional impairment[1] and is increasingly observed in an aging population[2]. Aging leads to a number of physiological changes characterized by a reduced ability to participate in one’s activities of daily living, leading to further disability, institutionalization and death[3]. Epidemiologic surveys demonstrate rises in the prevalence of knee osteoarthritis (OA) in the elderly[4]. Parallel trends have been observed in the prevalence of obesity as measured by body mass index (BMI)[5]. While obesity impacts cardiometabolic factors[6], it importantly leads to impairment in quality of life[7] by decreasing functional status and leading to disability[8]. Additionally, increased fat is associated with institutionalization and premature death[9, 10].
The interplay of muscle and function is likely a modulating factor in incident disability[11]. Subjects with knee osteoarthritis often decline in their ability to function due to muscle weakness, pain and a reduced ability to engage in physical activity[12, 13]. Obesity can exacerbate the development of knee OA[14, 15] which, left untreated or unmanaged, can place patients at risk for of worsening pain leading to chronic musculoskeletal disorders[16].
Few longitudinal studies have examined the impact of obesity on physical activity, quality of life, and disability in older adults with obesity at risk for knee OA, and none to our knowledge have examined functional capabilities over a six year period of time. We examined the impact of baseline obesity measured using body mass index (BMI) on long-term (six year) outcomes in a population at risk for progression and development of OA. We hypothesized that older adults with obesity, as compared to overweight or normal weight patients had worse functional outcomes at six-years.
MATERIALS AND METHODS
The Osteoarthritis Initiative (OAI) is a multi-center, longitudinal, prospective observational study of knee OA which began in 2004. The primary purpose of this cohort study was to evaluate biomarkers as potential endpoints for disease onset and progression. The dataset is prospectively gathered and includes questionnaires, examination data, imaging and biological specimens. The four clinical recruitment sites included Baltimore, MD, Pawtuckett, RI, Pittsburgh, PA, and Columbus, OH. Recruitment and enrollment at baseline involved an initial contact to reach persons in an intended targeted population through mailings, advertisements, and community meetings. An initial eligibility interview occurred by telephone to determine if interested individuals qualified for the study and those qualifying attended a screening clinic visit where additional assessments were performed. An enrollment clinic visit where the majority of baseline data were collected was then performed. All visits took place within a six-week time frame. Data and study protocols were obtained from the OAI database, which is available for public access at http://www.oai.ucsf.edu/. Specific datasets used in this analysis were at baseline and at six years. The study analysis was exempt from local review due to the de-identified nature of the data.
Study Population
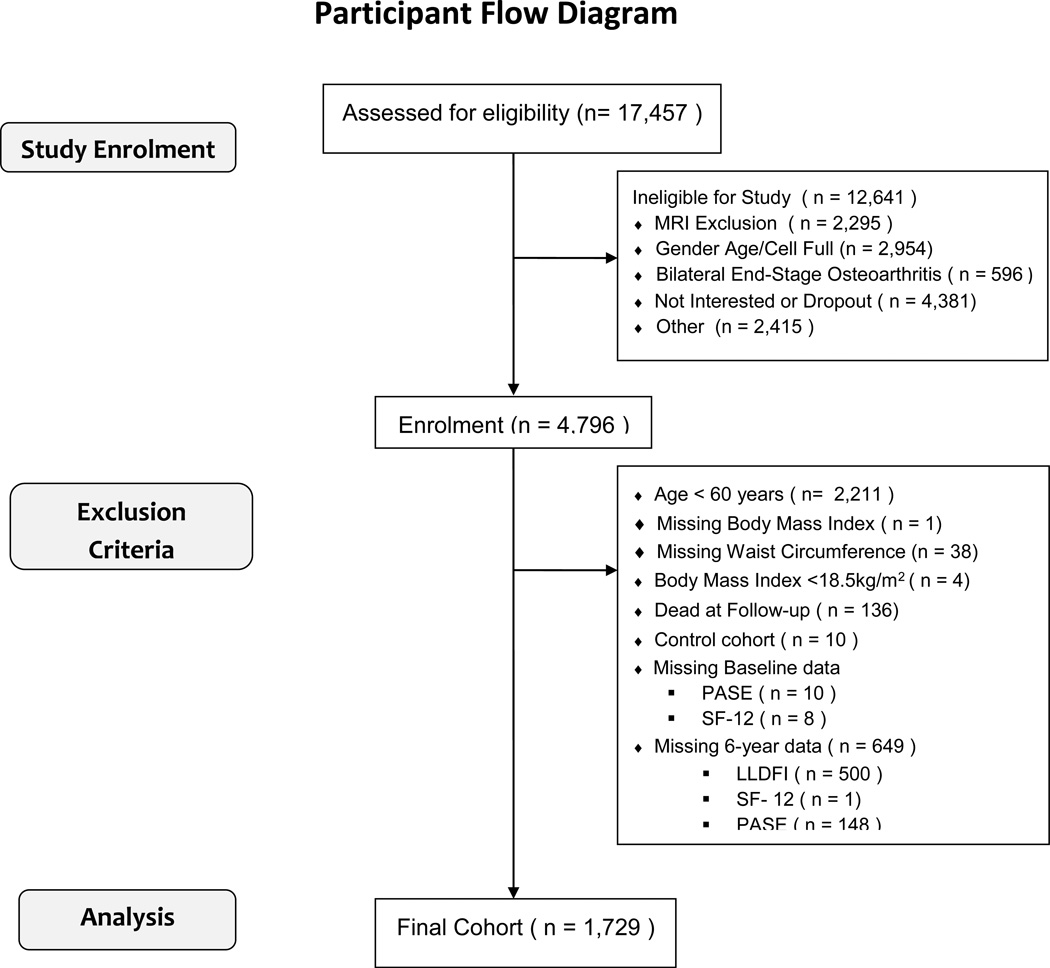
The study consists of three distinct subgroups: participants with clinically significant knee OA who are at risk of disease progression; those at high risk of developing clinically significant knee OA; and a control group. The initial sample included an ethnically diverse sample of both sexes aged 45–79 years. Following the eligibility interview, subjects were classified according to subcohort based on data collected. The progression cohort consisted of subjects with frequent knee symptoms and radiographic tibiofemoral knee OA in at least one native knee. The incidence cohort did not have symptomatic knee OA in either knee at baseline but had established risk factors: presence of heberden’s node in both hands; weight defined by using gender and age-specific cutpoints; previous knee operation; previous knee injury; family history; and pain in knee on most days of the preceding month. The nonexposed subcohort had no pain, radiographic findings or risk factors. Exclusion criteria were: rheumatoid arthritis; severe joint space narrowing; bilateral total knee replacements; inability to undergo an MRI; unable to provide a blood sample; co-morbid conditions interfering with participation in the study; unlikely to reside in the area for at least 3 years; other research participation; or unwilling to sign informed consent. Participant flow is shown in Figure 1.
Figure 1. Participant Flow: Participant Flow among 17,457 screened in the Osteoarthritis Initiative Protocol.
Patient flow is demonstrated from initial telephone screen to cohort included in this study. Abbreviations: LLDFI – Late-Life Function and Disability Instrument; MRI – magnetic resonance imaging; PASE – Physical Activity Scale for the Elderly; SF – Short Form;
Study Measures
All data were obtained from either self-report or measurements. Age was determined at initial visit. Marital status was defined as married or single, where the latter consisted of widowed, divorced, separated or never married. Education status was re-classified in four categories: attended high school (with or without graduation), attended college, college graduate, graduate level. Any person who smoked >100 cigarettes in their lifetime was considered an ever smoker. Self-reported knee pain was measured using the Western Ontario and McMaster University OA Index (WOMAC) using a 5-point Likert scale asking questions about both knees separately in the past 7 days. Pain scores ranged from 0–20 with higher scores representing worse symptoms. Hip pain was assessed by self-report. Subjects with documented knee osteoarthritis OA on X-ray were considered to have radiographic knee OA. Co-morbidity score was calculated based on the Charlson index[17]. Death was identified based on reporting to the Osteoarthritis Initiative Coordinating Center and confirmed through a formal adjudication process.
Measurements
Standing height was measured with a wall-mounted stadiometer. Weight was measured without participant’s shoes or heavy jewelry and in light weight clothing, using a calibrated standard balance beam scale. We calculated BMI as weight (in kilograms) divided by height (in meters) squared, and categorized as underweight (<18.5kg/m2), normal (18.5–24.9kg/m2), overweight (25–29.9kg/m2), and obese (>30kg/m2). Waist circumference was measured at the level between the lower rib and the iliac crest, normally at the level of the umbilicus in centimeters.
Mobility Measures
The chair stand test is a measure of balance and measure of strength of knee extensor and back muscles. It is a test of overall physical performance[18]. Subjects are seated with their arms folded across their chest and stand up and sit down five times. Chair stands were administered by a certified examiner and timed (measured in seconds). This test has excellent test-retest reliability (0.81–0.92) and responsiveness to change[19, 20]. The 20meter walk test (gait speed) is a standard outcome measure of subjects with osteoarthritis and a measure of functional performance[21], consisting of a course length of 20m, in an unobstructed, dedicated corridor. The participant is asked to walk at their usual speed in a 20m cooridor turns around walks in the opposite direction. Both the chair stand and 20m walk test have excellent reliability and intraclass correlations ranging from 0.93 to 0.98 within and between testers in patients with moderate to severe knee OA[22].
Outcome Measures
The Late-Life Function and Disability Instrument (LLDI)[23] parallels Nagi’s disablement framework on disability in community dwelling adults[24]. This self-reported instrument has two main domains each scored on a scale of 0–100, with higher scores indicating higher levels of function: functional limitations characterized by the inability to perform daily activities, and frequency limitations reflecting the inability to engage in social environments and major life tasks. The scale correlates well with the physical functioning subscale of the Medical Outcomes Study 36-item Short Form (SF) Health Survey[25] and the London Handicap Scale[26].
Quality of life was ascertained using the SF-12, a self-reported measure of a person’s perceived health status and a reasonable alternative to the lengthier SF-36[25]. The shorter form accounts for >90% of the statistical variance of the SF-36 and is reliable, valid, and easily self-administered[25]. The SF-12 uses Likert scales divided into both physical (PCS) and mental (MCS) components scores, both standardized and weighted to a mean of 50±10 in the general population[27, 28] with higher scores signify better health. The overall SF-12 score combines the PCS and MCS scoring.
The Physical Activity Scale for the Elderly (PASE) is a 26-item instrument measuring occupational, household and leisure activities during a one-week period in elderly (≥65year old) persons. It can be administered by telephone, mail or in-person and is reliable and valid. Scores increase with greater intensity of activity time. Population-based means are available with the general population having a mean score of 103±64.1. No known minimally clinically important differences are available[29].
Statistical Analysis
All baseline six year data were downloaded in March 2013 and merged into a single dataset for analysis. Continuous variables are represented as means ± standard deviations, and categorical values as counts (percent). A one-way ANOVA compared baseline and follow-up values across all three BMI categories. An ANOVA was also used to compare within-person differences from baseline across BMI categories. Post-hoc Bonferroni adjustments were performed for unadjusted mean variables between BMI-categories. Standard statistical tests compared subjects included vs. those excluded for the purposes of the analysis. Our primary six-year outcomes of interest included SF-12, PASE, and LLDI scores (frequency and limitation domains). As LLDI frequency and limitations scores only had follow-up data, we tested significances across all BMI categories. Within each BMI category, paired t-tests were used to compare baseline and follow-up values within-person
Using our primary outcomes as the dependent variable, we determined the mean follow-up scores in each baseline BMI category (referent=normal BMI), after adjusting for age, sex, education level, race, presence of radiographic knee OA, hip pain, cohort type (incidence, progression), Charlson co-morbidity score and current smoking status. To account for differences in baseline measures between BMI categories, we adjusted for baseline scores where data was available. As disability advances with age[3], we stratified our cohort by age group (60–70 and ≥70years). We explored similar models in those with and without radiographic knee OA. A secondary analysis determined the impact of clinically significant weight loss/gain of 5%[30] on our primary outcomes, and stratified both by age and OA status. Due to the extent of missing data over the six-year time period, we performed a sensitivity analysis using inverse probability weighting. Adjusted mean differences with 95% confidence intervals were calculated. All estimates were calculated using STATA version 12 (STATACorp, College Station, TX). A p-value <0.05 was considered statistically significant.
RESULTS
We identified 1,742 subjects (72.6%) with complete follow-up data at six years on our primary outcomes and included them in our final analysis. Obese subjects at baseline were younger and had lower education and socioeconomic status than overweight or normal subjects (Table 1). Baseline WOMAC scores were higher in the obese category as compared to the overweight or normal. A higher proportion of obese subjects had radiographic OA. We compared included and excluded subjects and found that the excluded cohort were less educated, were less likely to be Caucasian, had higher WOMAC and Charlson scores and had lower gait speed, PASE scores and SF-12 scores (Appendix 1).
Table 1.
Baseline Characteristics of all subjects ≥60 years old (n=1,729)
| BMI Category | ||||
|---|---|---|---|---|
| Normal 18.5–25kg/m2 |
Overweight 25–29.9kg/m2 |
Obese ≥30kg/m2 |
p-value | |
| Variable | N=408 | N=747 | N=574 | |
| Age, years | 68.3 ± 5.6 | 68.4 ± 5.2 | 67.1 ± 5.2 | <0.001 |
| Female Sex, % | 303 (74.3) | 405 (54.2) | 357 (62.2) | <0.001 |
| Education Status | ||||
| < High School | 57 (14.0) | 136 (18.2) | 113 (19.7) | |
| Some College | 77 (19.1) | 162 (21.7) | 164 (28.5) | |
| College | 89 (21.8) | 150 (20.1) | 105 (18.3) | <0.001 |
| >College | 185 (45.4) | 299 (40.0) | 192 (33.5) | |
| Yearly Income | ||||
| >$50,000 | 239 (61.1) | 406 (56.6) | 274 (49.9) | 0.002 |
| Marital Status | ||||
| Married | 287 (70.3) | 525 (70.3) | 356 (62.1) | 0.003 |
| Race | ||||
| White | 377 (92.4) | 649 (86.9) | 419 (73.0) | |
| Black | 20 (4.9) | 83 (11.1) | 144 (25.1) | <0.001 |
| Other | 11 (2.7) | 15 (2.0) | 11 (1.9) | |
| Charlson Score | 0.30 ± 0.66 | 0.37 ± 0.80 | 0.59 ± 1.0 | <0.001 |
| Baseline WOMAC Right | 7.5 ± 9.4 | 10.3 ± 12.0 | 14.8 ± 16.3 | <0.001 |
| Baseline WOMAC Left | 7.5 ± 11.8 | 9.9 ± 13.5 | 14.4 ± 16.9 | <0.001 |
| Ever Smoker (y/n) | 186 (45.8) | 387 (52.1) | 285 (50.1) | 0.13 |
| # Medications | 3.32 ± 2.23 | 3.63 ± 2.34 | 4.28± 2.57 | <0.001 |
| Radiographic Knee Osteoarthritis | 197 (48.3) | 456 (61.0) | 412 (71.8) | <0.001 |
| Hip Pain | 32 (7.8) | 83 (11.1) | 72 (12.5) | 0.06 |
| Waist circumference, cm | 90.6 ± 7.7 | 101.4 ± 7.6 | 113.7±10.1 | <0.001 |
| Cohort Allocation* | ||||
| Progression | 63 (15.4) | 205 (27.4) | 214 (37.3) | |
| Incidence | 345 (84.6) | 542 (72.6) | 360 (62.7) | <0.001 |
All values represent mean ± SD, or count (%)
P-value represents the ANOVA across all body mass index categories
OA – defined as having radiographic knee osteoarthritis on either knee or both knees
Some fields may not add up to overall cohort totals due to missing values
WOMAC – Western Ontario and McMaster University Arthritis Index
controls were not included in this analysis
Table 2 presents our univariate baseline and follow-up outcome measures. Table 3 demonstrates our modeling representing mean adjusted scores in each BMI category. Specifically, obese subjects had worse overall and physical function SF-12 and PASE scores over time. Self-reported function, PASE, gait speed, and chair stands were all lower in the 70+ age group as compared to the 60–70year age group. The limitation component of the LLDI scores were markedly lower in the obese category, as compared to the overweight or referent categories, in the 60–70 year age group but not in other groups. We present in Appendix 2 the analysis stratified by osteoarthritis. Patients with obesity and OA consistently had lower scores in all domains as compared to those without OA. Appendix 3 and 4 represent the exploratory analysis demonstrating the impact of weight change on the primary outcomes. A 5% weight gain is associated with lower SF-12, PASE and LLDI-limitation scores, as compared to those who lost weight or those with no change in weight.
Table 2.
Primary and Follow-up outcome Measures - Unadjusted
| BMI Category | |||||
|---|---|---|---|---|---|
| Overall Cohort |
Normal 18.5–25kg/m2 |
Overweight 25–29.9kg/m2 |
Obese ≥30kg/m2 |
p-value | |
| Variable | N=408 | N=747 | N=574 | ||
| Short Form12 Score | |||||
| Total | |||||
| Baseline | 104.4 ± 10.1 | 106.6 ± 8.3NS | 105.2 ± 9.5## | 101.8 ± 11.4## | <0.001* |
| Follow-up | 101.0 ± 12.3 | 104.3 ± 10.2## | 101.6 ± 12.2## | 97.7 ± 13.2## | <0.001* |
| p-value | <0.001† | <0.001† | <0.001† | <0.001† | 0.022# |
| % of Difference | −3.04 ± 10.3 | −1.89 ±9.2NS | −3.23 ± 9.9NS | −3.61 ± 11.5## | 0.029†† |
| Physical | |||||
| Baseline | 49.3 ± 8.3 | 51.9 ± 6.9## | 49.7 ± 7.9## | 47.1 ± 9.3## | <0.001* |
| Follow-up | 46.2 ± 9.8 | 49.2 ± 8.5## | 46.6 ± 9.6## | 43.3 ± 10.2## | <0.001* |
| p-value | <0.001† | <0.001† | <0.001† | <0.001† | 0.20# |
| % of Difference | −5.29 ± 19.9 | −4.2 ± 17.9NS | −5.0 ± 20.3NS | −6.3 ± 20.7NS | 0.24†† |
| Mental | |||||
| Baseline | 55.1 ± 7.1 | 54.7 ± 6.9NS | 55.5 ± 6.9NS | 54.7 ± 7.6NS | 0.06* |
| Follow-up | 54.8 ± 8.0 | 55.1 ± 7.2NS | 55.0 ± 7.7NS | 54.3 ± 8.8NS | 0.20* |
| p-value | 0.20† | 0.31† | 0.07† | 0.28† | 0.16# |
| % of Difference | 0.66 ± 17.4 | 2.3 ± 19.9NS | 0.05 ± 16.3NS | 0.29 ± 16.9NS | 0.09†† |
| Physical Activity Score for Elderly | |||||
| Baseline | 141.5 ± 67.1 | 149.3 ± 67.7NS | 143.3 ± 65.5| | 133.6 ± 68.2## | <0.001* |
| Follow-up | 123.8 ± 63.4 | 132.3 ± 63.6NS | 127.0 ± 62.4## | 113.8 ± 63.3## | <0.001* |
| p-value | <0.001† | <0.001† | <0.001† | <0.001† | 0.64# |
| % of Difference | −5.18 ± 91.9 | −6.0 ± 83.4NS | −5.5 ± 93.8NS | −4.2 ± 95.1NS | 0.95†† |
| Late-life Disability Index | |||||
| Frequency | 55.3 ± 6.3 | 56.5 ± 6.3## | 55.1 ±6.2NS | 54.7 ± 6.4## | <0.001* |
| Limitation | 80.6 ± 15.1 | 82.9 ± 14.3NS | 81.3 ± 15.2## | 78.1 ± 15.1## | <0.001* |
| Gait Speed, m/s | |||||
| Baseline | 1.30 ± 0.21 | 1.36 ± 0.20| | 1.32 ± 0.20| | 1.22 ± 0.20## | <0.001* |
| Follow-up | 1.23 ± 0.21 | 1.30 ± 0.10## | 1.25 ± .20## | 1.16 ± 0.21## | <0.001* |
| p-value | <0.001† | <0.001† | <0.001† | <0.001† | 0.16# |
| % of Difference | −4.51 ± 15.9 | −3.2 ± 12.5NS | −4.74 ± 15.0NS | −5.2 ±18.9NS | 0.19†† |
| Chair Stand Pace | |||||
| Baseline | 0.48 ± 0.13 | 0.52 ± 0.13## | 0.48 ± 0.13## | 0.45 ± 0.13## | <0.001* |
| Follow-up | 0.50 ± 0.15 | 0.54 ± 0.15## | 0.50 ± 0.14| | 0.47 ± 0.14## | <0.001* |
| p-value | <0.001† | 0.03† | 0.008† | 0.008† | 0.99# |
| % of Difference | 6.18 ± 30.2 | 1.5 ± 12.7NS | 1.4 ± 13.0NS | 1.6 ± 12.3NS | 0.45†† |
All values represent mean ± standard deviation, or count (percent)
A drop (negative change) in Short-form 12 score (Total, physical and mental) represents a reduction in self-reported health status. A decrease in Physical Activity for the Elderly Score and Gait Speed, represent reductions in physical activity and mobility speeds. An increase in chair stand pace represents a slower speed. Higher scores of Late-life function and disability scores represent better function (or less disability).
represents an analysis of variance across each BMI category within a specified time period (baseline or follow-up);
represents a paired t-test within each BMI category between baseline and follow-up;
represents the difference across all BMI categories between baseline and follow-up.
represents an analysis of variance across all BMI categories comparing the percent change in scores between baseline and follow-up of a measure
Post-hoc Bonferroni adjustments are presented within each BMI category and the mean score of each outcome represented by symbols
p<0.001;
p<0.05;
NS – non-signifcant. Those represented in the normal BMI column represent values comparing normal BMI vs. obese BMI; symbols in the overweight column represent values comparing normal BMI vs. overweight BMI; symbols in the obese column represent overweight BMI vs. normal BMI.
Table 3.
Multivariable Analysis of Follow-up Primary Outcome Measures
| BMI Category | |||||||
|---|---|---|---|---|---|---|---|
| Normal | 95% CI | Overweight | 95% CI | Obese | 95% CI | ||
| (18.5–24.9kg/m2) | (25.0–29.9kg/m2) | (≥30kg/m2) | |||||
| Short-Form 12 Score | |||||||
| Overall Score | Overall | 102.8 | 101.8–103.8 | 101.1 | 100.4–101.8 | 99.5 | 98.7 – 100.4 |
| 60–70 | 103.2 | 102.0–104.5 | 102.1 | 101.2–103.0 | 100.1 | 99.1–101.0 | |
| 70+ | 102.3 | 100.7–103.8 | 99.6 | 98.4–100.7 | 98.7 | 97.1–100.2 | |
| Physical Overall | 47.8 | 46.9–48.6 | 46.4 | 45.8–47.0 | 44.7 | 44.0–45.3 | |
| 60–70 | 48.1 | 47.0–49.3 | 47.2 | 46.4–48.0 | 45.2 | 44.3–46.0 | |
| 70+ | 47.3 | 46.1–48.6 | 45.2 | 44.3–46.1 | 43.7 | 42.5–45.0 | |
| Mental Overall | 55.2 | 54.5–55.9 | 54.8 | 54.3 – 55.3 | 54.6 | 54.0–55.2 | |
| 60–70 | 55.2 | 54.2–56.2 | 55.0 | 54.3–55.7 | 54.7 | 54.0–55.4 | |
| 70+ | 55.1 | 54.1–56.2 | 54.5 | 53.7–55.2 | 54.6 | 53.5–55.6 | |
| Physical Activity Scale for Elderly | Overall | 131.4 | 125.8–137.0 | 126.2 | 122.2–130.2 | 115.1 | 110.3–119.8 |
| 60–70 | 140.6 | 132.5–148.7 | 136.4 | 130.7–142.1 | 124.4 | 118.3–130.6 | |
| 70+ | 118.3 | 110.9–126.1 | 110.7 | 105.2–116.2 | 100.8 | 93.3–108.3 | |
| Late-Life Disability Index | |||||||
| Frequency Overall | 55.8 | 55.2–56.4 | 55.3 | 54.9–55.7 | 55.0 | 54.5–55.5 | |
| 60–70 | 56.3 | 55.5–57.1 | 55.7 | 55.2–56.3 | 55.4 | 54.8–56.0 | |
| 70+ | 55.1 | 54.2–56.0 | 54.6 | 53.9–55.3 | 54.3 | 53.4–55.2 | |
| Limitation Overall | 82.5 | 81.1–84.0 | 81.2 | 80.2–82.3 | 78.6 | 77.4–79.9 | |
| 60–70 | 84.1 | 82.1–86.1 | 82.6 | 81.2–84.0 | 79.9 | 78.4–81.4 | |
| 70+ | 80.9 | 78.7–83.1 | 79.0 | 77.4–80.6 | 76.4 | 74.2–78.6 | |
| Gait Speed | Overall | 1.26 | 1.25–1.28 | 1.23 | 1.22–1.24 | 1.20 | 1.19–1.22 |
| 60–70 | 1.29 | 1.27–1.31 | 1.27 | 1.25–1.28 | 1.24 | 1.23–1.26 | |
| 70+ | 1.22 | 1.20–1.24 | 1.18 | 1.16–1.20 | 1.14 | 1.12–1.17 | |
| Chair Stand | Overall | 0.52 | 0.50–0.53 | 0.50 | 0.49–0.51 | 0.49 | 0.48–0.50 |
| 60–70 | 0.53 | 0.51–0.55 | 0.52 | 0.50–0.53 | 0.51 | 0.49–0.52 | |
| 70+ | 0.50 | 0.48–0.52 | 0.47 | 0.46–0.49 | 0.46 | 0.44–0.48 | |
Values represent mean score (95% confidence interval) of the indicated metric adjusted for: Age, sex, education level, race, Charlson co-morbidity index, smoking status, radiographic knee OA, hip pain and cohort type (incidence, progressionl), and baseline scoring (Short Form 12, Physical Activity Scale for the Elderly) where available. Models stratified by age adjust for the similar co-variates other than age.
Cohort included in the modeling includes all eligible subjects with follow-up data on outcomes of Short Form 12, Physical Activity Scale for the Elderly, and Late-Life Function and Disability Instrument (n=1,729)
BMI – Body Mass Index
DISCUSSION
In an older adult population at risk for developing and progressing to osteoarthritis, obesity places subjects at risk for lower physical activity scores, quality of life and disability indices than overweight or normal BMI. These results provide estimates of the degree of functional impairment elderly persons with obesity may expect over time.
Previous studies have demonstrated the impact of obesity on pain scores[31] and progression of osteoarthritis[32], all of which may can impact our outcomes. Cross-sectional data have consistently demonstrated associations between obesity and quality of life, physical function and exercise[33–36]. Very few studies have examined the impact of obesity in a longitudinal manner on physical activity, quality of life, and disability, and none have utilized the PASE and LLDI. A study utilizing National Health and Nutrition Examination Surveys demonstrated that obesity predicts long-term disability in knee OA[1]. The three scales used in OAI are well validated in the elderly suggesting that osteoarthritis will inevitably impact one’s ability to engage in physical activity, and may lead to compromised limitation scales of the LLDI in addition to lower quality of life scores. Our findings taken together with those of previous studies suggest the importance of preventing weight gain in knee OA [37–40].
Knee OA impacts quality of life, physical activity, and disability [41]. Our results suggest that as compared to normal BMI, subjects with obesity differed across measures with lower self-reported physical function, PASE scores and gait speeds. We explored a number of secondary outcomes of function, such as gait speed and chair stand. Notably, these dropped with increasing age consistent with other studies that demonstrate gradual decline with aging [42]. Interestingly, SF-12 and LLDI both had subscales that are related to mental health. Our data suggest that any impact of obesity was solely due to physical limitations and not psychological. While controversy exists with respect to obesity impacting mental health[43], our longitudinal data analysis suggests that there may not be any association.
While our results provide some additional credence to the relationship between obesity and important geriatric outcomes on a longitudinal basis, we caution that our results indeed may underestimate the natural history of the demonstrated decline. Our cohort excluded 677 subjects (28.1%) due to missing outcome data, a group noted in our sensitivity analysis to be associated with increased comorbidity, lower socioeconomic class, and higher WOMAC scores, all of which negatively impact our outcomes of choice. One may argue that the magnitude of the changes, while statistically significant, may not necessarily be clinically significant. While a 10-point difference in SF-12 is considered clinically significant[28], to our knowledge there are no clinically meaningful differences in PASE or the LLDI. One potential explanation for lower magnitude in change could be the baseline characteristics of our study population, which may be considered healthier and/or have lower degrees of co-morbidity. Disability is known to increase in prevalence as one ages[3]; our population’s mean age was 68years and in part, this ‘young’ population may not fully reflect the degree of potential disability people with obesity are subject to. A six-year follow-up may be too short for this phenomenon considering the examined cohort included those both with knee OA but also those with risk factors for OA (and no OA). Stratifying by age further demonstrated that age in fact plays a large role in functional decline. As increased co-morbidity may lead to frailty and disability, it often is preceded by many years of preserved or minimally declining function. The cohort enrolled in this observational cohort may not be typically representative of older adults in the community. Future studies should focus on cohorts with varied medical and social demographics to correctly ascertain the natural history of overweight and obesity on quality of life, physical activity and functional limitations.
CONCLUSION
Obesity measured by BMI leads to reduced physical function, physical activity and disability in a six year period in subjects with or at risk for OA.
ACKNOWLEDGEMENT
The authorship team thanks Emily Scherer, PhD, for her oversight and input on the statistical methods of the manuscript.
The Osteoarthritis Initiative is funded in part by the National Institute of Arthritis, Musculoskeletal and Skin Diseases through a public/private collaboration.
FUNDING:
This work was supported in part from the Dartmouth Centers for Health and Aging, and the Junior Faculty Development Award from the Department of Medicine, Dartmouth-Hitchcock Medical Center.
The Osteoarthritis Initiative is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratoires; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners.
ABBREVIATIONS
- BMI
body mass index
- LLDI
Late Life Function and Disability Instrument
- MCS
mental component scale
- OA
osteoarthritis
- OAI
Osteoarthritis Initiative
- PASE
Physical Activity Score for the Elderly
- PCS
physical component score
- SF
Short form
- WC
Waist circumference
- WOMAC
Western Ontario and McMaster University OA Index
Appendix 1: Baseline Characteristics of Subjects Included for Outcome Analysis Compared to those Excluded
| Variable | Included 1,729 (71.9) |
Excluded 677 (28.1) |
p-value |
|---|---|---|---|
| Age | 67.9 ± 5.3 | 69.0 ± 5.4 | <0.001 |
| Female Sex (%) | 1,065 (61.6) | 438 (64.7) | 0.17 |
| Education | |||
| < High School | 306 (17.7) | 167 (25.0) | |
| Some College | 403 (23.3) | 177 (26.5) | |
| College | 344 (19.9) | 113 (17.1) | <0.001 |
| >College | 676 (39.1) | 207 (31.4) | |
| Yearly Income >$50,000 | 919 (55.5) | 303 (48.6) | 0.003 |
| Marital Status | 1,168 (67.6) | 425 (63.6) | 0.07 |
| Race | |||
| White | 1,445 (83.6) | 533 (79.0) | |
| Black | 247 (14.3) | 115 (17.0) | 0.005 |
| Other | 37 (2.1) | 27 (4.0) | |
| WOMAC Right | 11.1 ±13.4 | 14.2±16.0 | <0.001 |
| WOMAC Left | 10.8 ± 14.6 | 14.2 ± 16.6 | <0.001 |
| Charlson Comorbidity | 0.43 ± 0.86 | 0.55 ± 1.0 | 0.004 |
| Medication Number | 3.78 ± 2.43 | 4.03 ± 2.57 | 0.04 |
| Smoker (yes/no) | 858 (49.9) | 332 (50.2) | 0.93 |
| Radiographic Knee OA Present/Absent |
447 (29.6) | 230 (25.7) | 0.04 |
| Hip Pain (yes/no) | 187 (10.8) | 89 (13.2) | 0.14 |
| 20M Gait Speed, m/s | 1.30 ± 0.21 | 1.25 ± 0.22 | <0.001 |
| PASE | 141.5 ± 67.1 | 127.4 ± 68.3 | <0.001 |
| SF-12 | |||
| Total | 104.4 ± 10.1 | 100.2 ±12.9 | <0.001 |
| Physical | 49.3 ± 8.3 | 45.8 ± 10.3 | <0.001 |
| Mental | 55.1 ± 7.1 | 54.4 ± 8.4 | 0.06 |
All values represent mean ± SD, or count (%)
P-value represents the difference between the excluded and included analytical cohort
PASE - Physical Activity Scale for the Elderly; SF - Short Form 12; WOMAC – Western Ontario and McMaster University Arthritis Index
Appendix 2: Multivariable Analysis of Follow-up Primary Outcome Measures Stratified by Osteoarthritis
| BMI | |||||||
|---|---|---|---|---|---|---|---|
| Normal (18.5–24.9kg/m2) |
95% CI | Overweight (25.0–29.9kg/m2) |
95% CI | Obese (≥30kg/m2) |
95% CI | ||
| Short-Form 12 Score | |||||||
| Overall Score | OA + | 101.7 | 100.2–103.1 | 100.3 | 99.4–101.3 | 98.5 | 97.5–99.6 |
| OA − | 104.5 | 103.2–105.8 | 102.3 | 101.3–103.4 | 101.4 | 99.9–103.0 | |
| Physical | OA + | 46.4 | 45.2–47.7 | 45.5 | 44.7–46.3 | 43.5 | 42.7–44.4 |
| OA − | 49.8 | 48.6–50.8 | 47.8 | 46.9–48.7 | 46.4 | 45.1–47.6 | |
| Mental | OA + | 55.4 | 54.5–56.4 | 55.0 | 54.3–55.6 | 54.8 | 54.0–55.5 |
| OA − | 54.9 | 53.9–55.9 | 54.5 | 53.7–55.4 | 54.3 | 53.2–55.4 | |
| Physical Activity Scale for Elderly | OA + | 128.4 | 120.5–136.3 | 126.2 | 121.1–131.2 | 112.6 | 107.1–118.1 |
| OA − | 135.4 | 127.4–143.4 | 125.9 | 119.2–132.6 | 120.3 | 111.1–129.5 | |
| Late-Life Disability Index | |||||||
| Frequency | OA + | 55.64 | 54.6–56.3 | 55.2 | 54.7–55.8 | 54.7 | 54.1–55.2 |
| OA − | 56.2 | 55.3–57.0 | 55.5 | 54.8–56.2 | 55.5 | 54.5–56.4 | |
| Limitation | OA + | 80.4 | 78.3–82.5 | 81.5 | 80.1–82.9 | 77.9 | 76.4–79.4 |
| OA − | 84.8 | 82.8–86.9 | 80.9 | 79.2–82.6 | 79.8 | 77.5–82.1 | |
| Gait Speed | OA + | 1.24 | 1.22–1.27 | 1.22 | 1.21–1.24 | 1.18 | 1.17–1.20 |
| OA − | 1.29 | 1.27–1.31 | 1.25 | 1.23–1.27 | 1.24 | 1.22–1.27 | |
| Chair Stand | OA + | 0.50 | 0.48–0.52 | 0.50 | 0.48–0.51 | 0.48 | 0.47–0.49 |
| OA − | 0.55 | 0.53–0.57 | 0.52 | 0.50–0.53 | 0.51 | 0.49–0.53 | |
OA + : radiographic knee osteoarthritis present; OA − : radiographic knee osteoarthritis absent
Values represent mean score (95% confidence interval) of the indicated metric adjusted for: Age, sex, education level, race, Charlson co-morbidity index, smoking status, hip pain and cohort type (incidence, progression), and baseline scoring (Short Form 12, Physical Activity Scale for the Elderly) where available. Models stratified by age adjust for the similar co-variates other than age.
Cohort included in the modeling includes all eligible subjects with follow-up data on outcomes of Short Form 12, Physical Activity Scale for the Elderly and Late-Life Function and Disability Instrument (n=1,729)
BMI – Body mass index
Appendix 3: Impact of Weight Change on Primary Outcomes and Age
| 5 % Weight Loss N=598 |
95% CI | No Change in Weight N=916 |
95% CI | 5 % Weight Gain N=215 |
95% CI | ||
|---|---|---|---|---|---|---|---|
| Short-Form 12 Score | |||||||
| Overall Score | Overall | 100.5 | 99.7–101.3 | 101.7 | 101.1–102.3 | 99.0 | 97.7–100.3 |
| 60–70 | 101.3 | 100.2–102.3 | 102.5 | 101.7–103.3 | 99.2 | 97.7–100.7 | |
| 70+ | 99.4 | 98.1–100.6 | 100.6 | 99.5–101.7 | 99.4 | 96.7–102.1 | |
| Physical | Overall | 46.2 | 45.5–46.8 | 46.5 | 45.9–47.0 | 44.5 | 43.4–45.6 |
| 60–70 | 46.8 | 45.9–47.7 | 7.1 | 46.4–47.8 | 44.7 | 43.4–46.0 | |
| 70+ | 45.2 | 44.2–46.3 | 45.6 | 44.7–46.4 | 44.6 | 42.4–46.8 | |
| Mental | Overall | 54.3 | 53.7–54.9 | 55.2 | 54.8–55.7 | 54.4 | 53.4–55.4 |
| 60–70 | 54.4 | 53.6–55.2 | 55.4 | 54.8–56.0 | 54.4 | 53.3–55.5 | |
| 70+ | 54.2 | 53.3–55.0 | 55.0 | 54.3–55.8 | 54.6 | 52.7–56.5 | |
| Physical Activity Scale for Elderly | Overall | 122.3 | 117.7–126.8 | 126.7 | 123.0–130.3 | 115.4 | 107.8–123.0 |
| 60–70 | 127.7 | 121.3–134.1 | 137.7 | 132.7–142.8 | 127.0 | 117.6–136.3 | |
| 70+ | 112.9 | 106.8–119.0 | 110.3 | 105.1–115.5 | 95.3 | 82.0–108.6 | |
| Late-Life Disability Index | |||||||
| Frequency | Overall | 54.8 | 54.3–55.2 | 55.8 | 55.4–56.1 | 54.9 | 54.0–55.7 |
| 60–70 | 55.3 | 54.6–55.9 | 56.1 | 55.6–56.6 | 55.5 | 54.5–56.5 | |
| 70+ | 54.1 | 53.4–54.8 | 55.2 | 54.6–55.8 | 53.6 | 52.0–55.2 | |
| Limitation | Overall | 80.1 | 79.0–81.3 | 81.6 | 80.6–82.5 | 78.2 | 76.2–80.2 |
| 60–70 | 81.8 | 80.3–83.4 | 82.6 | 81.3–83.8 | 79.9 | 77.6–82.2 | |
| 70+ | 77.9 | 76.1–79.7 | 79.9 | 78.4–81.4 | 75.5 | 71.7–79.4 | |
| Gait Speed | Overall | 1.23 | 1.22–1.24 | 1.24 | 1.23–1.25 | 1.19 | 1.17–1.21 |
| 60–70 | 1.26 | 1.24–1.28 | 1.27 | 1.26–1.28 | 1.23 | 1.21–1.25 | |
| 70+ | 1.19 | 1.16–1.21 | 1.19 | 1.17–1.20 | 1.13 | 1.09–1.17 | |
| Chair Stand | Overall | 0.51 | 0.49–0.52 | 0.50 | 0.50–0.51 | 0.49 | 0.47–0.51 |
| 60–70 | 0.52 | 0.51–0.53 | 0.51 | 0.49–0.53 | 0.52 | 0.51–0.54 | |
| 70+ | 0.48 | 0.46–0.49 | 0.48 | 0.47–0.49 | 0.47 | 0.44–0.50 | |
Values represent mean score (95% confidence interval) of the indicated metric adjusted for: Age, sex, education level, race, Charlson co-morbidity index, smoking status, radiographic knee pain, hip pain and cohort type (incidence, progression), and baseline scoring (Short Form 12, Physical Activity Scale for the Elderly) where available. Models stratified by age adjust for the similar co-variates other than age. Cohort included in the modeling includes all eligible subjects with follow-up data on outcomes of Short Form 12, Physical Activity Scale for the Elderly and Late-Life Function and Disability Instrument (n=1,729)
Appendix 4: Impact of Weight Change on Primary Outcome Measures Stratified by Osteoarthritis status
| 5 % Weight Loss N=598 |
95% CI | No Change in Weight N=916 |
95% CI | 5 % Weight Gain N=215 |
95% CI | ||
|---|---|---|---|---|---|---|---|
| Short-Form 12 Score | |||||||
| Overall Score | OA+ | 99.7 | 98.7–100.7 | 100.5 | 99.7–101.4 | 97.7 | 95.9–99.4 |
| OA− | 103.5 | 102.5–104.5 | 101.2 | 99.2–103.2 | 101.8 | 100.6–103.1 | |
| Physical | OA+ | 45.1 | 44.2–45.9 | 45.2 | 44.5–45.9 | 43.2 | 41.8–44.8 |
| OA− | 48.0 | 46.9–49.0 | 48.5 | 47.6–49.3 | 46.4 | 44.8–48.2 | |
| Mental | OA+ | 54.6 | 53.8–55.3 | 55.4 | 54.8–56.0 | 54.4 | 53.1–55.6 |
| OA− | 53.9 | 52.9–54.8 | 55.1 | 54.4–55.8 | 54.5 | 52.9–56.0 | |
| Physical Activity Scale for Elderly | OA+ | 119.1 | 113.5–124.7 | 125.8 | 121.3–130.4 | 108.6 | 99.1–118.2 |
| OA− | 127.7 | 120.0–135.4 | 127.9 | 121.8–134.0 | 125.7 | 113.3–138.2 | |
| Late-Life Disability Index | |||||||
| Frequency | OA+ | 54.6 | 54.0–55.2 | 55.4 | 54.9–55.9 | 54.6 | 53.6–55.7 |
| OA− | 55.0 | 54.2–55.8 | 56.3 | 55.6–56.9 | 55.1 | 53.8–56.4 | |
| Limitation | OA+ | 79.4 | 77.9–80.9 | 80.9 | 79.7–82.1 | 77.1 | 74.5–79.6 |
| OA− | 81.3 | 79.3–83.2 | 82.7 | 81.1–84.3 | 80.2 | 77.0–83.4 | |
| Gait Speed | OA+ | 1.21 | 1.19–1.23 | 1.22 | 1.21–1.23 | 1.16 | 1.14–1.19 |
| OA− | 1.26 | 1.24–1.29 | 1.27 | 1.25–1.28 | 1.24 | 1.21–1.27 | |
| Chair Stand | OA+ | 0.49 | 0.48–0.51 | 0.49 | 0.48–0.50 | 0.48 | 0.46–0.50 |
| OA− | 0.52 | 0.50–0.54 | 0.52 | 0.51–0.54 | 0.51 | 0.48–0.54 | |
OA + : radiographic knee osteoarthritis present; OA − : radiographic knee osteoarthritis absent
Values represent mean score (95% confidence interval) of the indicated metric adjusted for: Age, sex, education level, race, Charlson co-morbidity index, smoking status, hip pain and cohort type (incidence, progression), and baseline scoring (Short Form 12, Physical Activity Scale for the Elderly) where available. Models stratified by age adjust for the similar co-variates other than age.
Cohort included in the modeling includes all eligible subjects with follow-up data on outcomes of Short Form 12, Physical Activity Scale for the Elderly and Late-Life Function and Disability Instrument (n=1,729)
Appendix 5 – Impact of Weight Change on Primary Outcome Measures Stratified by Osteoarthritis status
| Normal (18.5–24.9kg/m2) |
95% CI | Overweight (25.0–29.9kg/m2) |
95% CI | Obese (≥30kg/m2) |
95% CI | ||
|---|---|---|---|---|---|---|---|
| Short-Form 12 Score | |||||||
| Overall Score | Overall | 101.9 | 100.4–103.3 | 100.9 | 100.2–101.7 | 99.6 | 98.6–100.6 |
| Physical | Overall | 47.2 | 46.2–48.2 | 46.3 | 45.6–46.9 | 44.9 | 44.2–45.7 |
| Mental | Overall | 55.2 | 54.4–55.9 | 54.7 | 54.2–55.3 | 54.5 | 53.8–55.1 |
| Physical Activity Scale for Elderly | Overall | 128.7 | 121.3–136.0 | 125.8 | 121.5–130.0 | 116.9 | 111.6–122.1 |
| Late-Life Disability Index | |||||||
| Frequency | Overall | 55.4 | 54.8–56.1 | 55.2 | 54.8–55.7 | 54.9 | 54.4–55.4 |
| Limitation | Overall | 81.9 | 80.3–83.4 | 80.9 | 79.9–82.0 | 78.8 | 77.5–80.0 |
| Gait Speed, m/s | Overall | 1.26 | 1.23–1.28 | 1.23 | 1.22–1.25 | 1.20 | 1.18–1.22 |
Values represent mean score (95% confidence interval) of the indicated metric adjusted for: Age, sex, education level, race, Charlson co-morbidity index, smoking status, radiographic knee osteoarthritis, hip pain and cohort type (incidence, progression), and baseline scoring (Short Form 12, Physical Activity Scale for the Elderly) where available. All subjects included in the modeling with missing values accounted for using Inverse probability weighting
Footnotes
Work was presented in part at the American Geriatrics Society’s Annual Meeting, May 2013, Grapevine, Texas
JA Batsis: formulated the research question, designed the study, carried it out, analyzed the data and drafted and approved the final version
A Zbehlik: formulated the research question, designed the study, carried it out, assisted with analyzing the data and approved the final version
LK Barre: formulated the research question, designed the study, carried it out, assisted with analyzing the data and approved the final version
JPW Bynum: formulated the research question, designed the study, assisted with analyzing the data and approved the final version
D Pidgeon: provided input on designing the study, interpreting the data and approved the final version
SJ Bartels: formulated the research question, designed the study, carried it out, assisted with interpreting the data and approved the final version
CONFLICTS OF INTEREST: NONE
REFERENCES
- 1.Ettinger WH, Davis MA, Neuhaus JM, Mallon KP. Long-term physical functioning in persons with knee osteoarthritis from NHANES. I: Effects of comorbid medical conditions. J Clin Epidemiol. 1994;47(7):809–815. doi: 10.1016/0895-4356(94)90178-3. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunlop DD, Hughes SL, Manheim LM. Disability in activities of daily living: patterns of change and a hierarchy of disability. Am J Public Health. 1997;87(3):378–383. doi: 10.2105/ajph.87.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suri P, Morgenroth DC, Hunter DJ. Epidemiology of osteoarthritis and associated comorbidities. PM R. 2012;4(5 Suppl):S10–S19. doi: 10.1016/j.pmrj.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 6.Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293(15):1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 7.Chambers BA, Guo SS, Siervogel R, Hall G, Chumlea WC. Cumulative effects of cardiovascular disease risk factors on quality of life. J Nutr Health Aging. 2002;6(3):179–184. [PubMed] [Google Scholar]
- 8.Alley DE, Chang VW. The changing relationship of obesity and disability, 1988–2004. JAMA. 2007;298(17):2020–2027. doi: 10.1001/jama.298.17.2020. [DOI] [PubMed] [Google Scholar]
- 9.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293(15):1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 10.Zizza CA, Herring A, Stevens J, Popkin BM. Obesity affects nursing-care facility admission among whites but not blacks. Obes Res. 2002;10(8):816–823. doi: 10.1038/oby.2002.110. [DOI] [PubMed] [Google Scholar]
- 11.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Cur Opin Clin Nutr Metab Care. 2008;11(6):693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segal NA, Glass NA. Is quadriceps muscle weakness a risk factor for incident or progressive knee osteoarthritis? Phys Sportsmed. 2011;39(4):44–50. doi: 10.3810/psm.2011.11.1938. [DOI] [PubMed] [Google Scholar]
- 13.Slemenda C, Heilman DK, Brandt KD, Katz BP, Mazzuca SA, Braunstein EM, et al. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998;41(11):1951–1959. doi: 10.1002/1529-0131(199811)41:11<1951::AID-ART9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Coriolano K, Aiken A, Pukall C, Harrison M. Changes in self-reported disability after performance-based tests in obese and non-obese individuals diagnosed with osteoarthritis of the knee. Disabil Rehabil. 2014:1–10. doi: 10.3109/09638288.2014.956813. [DOI] [PubMed] [Google Scholar]
- 15.Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109(1):18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 16.Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez-Jimenez F, Bartels SJ. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999–2004. J Am Geriatr Soc. 2013;61(6):974–980. doi: 10.1111/jgs.12260. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Wicherts IS, van Schoor NM, Boeke AJ, Visser M, Deeg DJ, Smit J, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92(6):2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 19.Schaubert KL, Bohannon RW. Reliability and validity of three strength measures obtained from community-dwelling elderly persons. J Strength Cond Res. 2005;19(3):717–720. doi: 10.1519/R-15954.1. [DOI] [PubMed] [Google Scholar]
- 20.Sherrington C, Lord SR. Reliability of simple portable tests of physical performance in older people after hip fracture. Clin Rehabil. 2005;19(5):496–504. doi: 10.1191/0269215505cr833oa. [DOI] [PubMed] [Google Scholar]
- 21.Altman R, Brandt K, Hochberg M, Moskowitz R, Bellamy N, Bloch DA, et al. Design and conduct of clinical trials in patients with osteoarthritis: recommendations from a task force of the Osteoarthritis Research Society. Results from a workshop. Osteoarthritis Cartilage. 1996;4(4):217–243. doi: 10.1016/s1063-4584(05)80101-3. [DOI] [PubMed] [Google Scholar]
- 22.Gill S, McBurney H. Reliability of performance-based measures in people awaiting joint replacement surgery of the hip or knee. Physiother Res Int. 2008;13(3):141–152. doi: 10.1002/pri.411. [DOI] [PubMed] [Google Scholar]
- 23.Sayers SP, Jette AM, Haley SM, Heeren TC, Guralnik JM, Fielding RA. Validation of the Late-Life Function and Disability Instrument. J Am Geriatr Soc. 2004;52(9):1554–1559. doi: 10.1111/j.1532-5415.2004.52422.x. [DOI] [PubMed] [Google Scholar]
- 24.Nagi SZ. A Study in the Evaluation of Disability and Rehabilitation Potential: Concepts, Methods, and Procedures. Am J Public Health Nations Health. 1964;54:1568–1579. doi: 10.2105/ajph.54.9.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ware J, Kosinski M, Bjorner J, Turner-Bowker D, Gandek B, Maruish M. User's manual for the SF-36v2 Health Survey. Lincoln, RI: QualityMetric Incorporated; 2007. [Google Scholar]
- 26.Harwood RH, Rogers A, Dickinson E, Ebrahim S. Measuring handicap: the London Handicap Scale, a new outcome measure for chronic disease. Qual Health Care. 1994;3(1):11–16. doi: 10.1136/qshc.3.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosinski M. Scoring the SF-12 physical and mental health summary measures. Med Outcomes Trust Bull. 1997;5:3S–4S. [Google Scholar]
- 28.Ware J, Kosinski M, Keller S. How to Score the SF-12 Physical. The Mental Health Summary Scales. Boston, MA: The Health Institute, New England Medical Center; 1995. [Google Scholar]
- 29.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 30.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2013 [Google Scholar]
- 31.Goulston LM, Kiran A, Javaid MK, Soni A, White KM, Hart DJ, et al. Does obesity predict knee pain over fourteen years in women, independently of radiographic changes? Arthritis Care Res (Hoboken) 2011;63(10):1398–1406. doi: 10.1002/acr.20546. [DOI] [PubMed] [Google Scholar]
- 32.Mork PJ, Holtermann A, Nilsen TI. Effect of body mass index and physical exercise on risk of knee and hip osteoarthritis: longitudinal data from the Norwegian HUNT Study. J Epidemiol Community Health. 2012;66(8):678–683. doi: 10.1136/jech-2011-200834. [DOI] [PubMed] [Google Scholar]
- 33.Ambrose NL, Keogan F, O'Callaghan JP, O'Connell PG. Obesity and disability in the symptomatic Irish knee osteoarthritis population. Ir J Med Sci. 2010;179(2):265–268. doi: 10.1007/s11845-009-0459-5. [DOI] [PubMed] [Google Scholar]
- 34.Kauppila AM, Kyllonen E, Mikkonen P, Ohtonen P, Laine V, Siira P, et al. Disability in end-stage knee osteoarthritis. Disabil Rehabil. 2009;31(5):370–380. doi: 10.1080/09638280801976159. [DOI] [PubMed] [Google Scholar]
- 35.Reichmann WM, Katz JN, Kessler CL, Jordan JM, Losina E. Determinants of self-reported health status in a population-based sample of persons with radiographic knee osteoarthritis. Arthritis Rheum. 2009;61(8):1046–1053. doi: 10.1002/art.24839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosemann T, Grol R, Herman K, Wensing M, Szecsenyi J. Association between obesity, quality of life, physical activity and health service utilization in primary care patients with osteoarthritis. Int J Behav Nutr Phys Act. 2008;5:4. doi: 10.1186/1479-5868-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brosseau L, Wells GA, Tugwell P, Egan M, Dubouloz CJ, Casimiro L, et al. Ottawa Panel evidence-based clinical practice guidelines for the management of osteoarthritis in adults who are obese or overweight. Phys Ther. 2011;91(6):843–861. doi: 10.2522/ptj.20100104. [DOI] [PubMed] [Google Scholar]
- 38.Foy CG, Lewis CE, Hairston KG, Miller GD, Lang W, Jakicic JM, et al. Intensive lifestyle intervention improves physical function among obese adults with knee pain: findings from the Look AHEAD trial. Obesity (Silver Spring) 2011;19(1):83–93. doi: 10.1038/oby.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rejeski WJ, Focht BC, Messier SP, Morgan T, Pahor M, Penninx B. Obese, older adults with knee osteoarthritis: weight loss, exercise, and quality of life. Health Psychol. 2002;21(5):419–426. doi: 10.1037//0278-6133.21.5.419. [DOI] [PubMed] [Google Scholar]
- 40.Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364(13):1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin KR, Kuh D, Harris TB, Guralnik JM, Coggon D, Wills AK. Body mass index, occupational activity, and leisure-time physical activity: an exploration of risk factors and modifiers for knee osteoarthritis in the 1946 British birth cohort. BMC Musculoskelet Disord. 2013;14:219. doi: 10.1186/1471-2474-14-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Preiss K, Brennan L, Clarke D. A systematic review of variables associated with the relationship between obesity and depression. Obes Rev. 2013;14(11):906–918. doi: 10.1111/obr.12052. [DOI] [PubMed] [Google Scholar]