Abstract
Background:
Brain tissue analysis is necessary to confirm prion diseases. Clinically unsuspected cases may be identified through neuropathologic testing.
Methods:
National Alzheimer’s Coordinating Center (NACC) Minimum and Neuropathologic Data Set for 1984 to 2005 were reviewed. Eligible patients had dementia, underwent autopsy, had available neuropathologic data, belonged to a currently funded Alzheimer’s Disease Center (ADC), and were coded as having an Alzheimer’s disease clinical diagnosis or a nonprion disease etiology. For the eligible patients with neuropathology indicating prion disease, further clinical information, collected from the reporting ADC, determined whether prion disease was considered before autopsy.
Results:
Of 6000 eligible patients in the NACC database, 7 (0.12%) were clinically unsuspected but autopsy-confirmed prion disease cases.
Conclusion:
The proportion of patients with dementia with clinically unrecognized but autopsy-confirmed prion disease was small. Besides confirming clinically suspected cases, neuropathology is useful to identify unsuspected clinically atypical cases of prion disease.
Keywords: prion disease, Creutzfeldt–Jakob disease, Alzheimer’s disease, dementia, diagnosis
Introduction
Prion diseases are rare, fatal, neurodegenerative disorders. The most common prion disease, Creutzfeldt–Jakob disease (CJD), is characterized by rapidly progressive dementia and has a reported incidence of approximately 1 case per million population per year. 1 The illness often worsens rapidly, leading to death within 1 year of onset in the majority of affected patients. 1,2 Although short disease duration may be a distinguishing characteristic of CJD, 3 clinical signs, including dementia and movement disorders, may overlap with much more common diagnoses such as Alzheimer’s disease (AD).
The Centers for Disease Control and Prevention (CDC) conducts surveillance for CJD and other prion diseases through several mechanisms 2 ; however, this surveillance may not identify patients with prion disease misdiagnosed with other neurologic diseases such as AD. Furthermore, barriers to autopsy exist that limit the number of suspected prion disease cases with neuropathologic testing, although such analyses are necessary to confirm the diagnosis. 1 To assess the frequency of occurrence of clinically unrecognized prion disease, data sets from the National Alzheimer’s Coordinating Center (NACC) were analyzed. The NACC was established in 1999 by the National Institute on Aging (NIA) to facilitate collaborative research among NIA-funded Alzheimer’s Disease Centers (ADCs) across the United States. The center developed and maintains a large relational database of standardized clinical and neuropathologic research data collected from the ADCs. 4
Methods
The NACC Minimum Data Set (MDS) and Neuropathologic Data Set (NPDS) for 1984 to 2005 were extracted and reviewed. The MDS includes demographic data of patients reported to NACC as well as their clinical and neuropathological diagnoses. The NPDS contains 1 record for each autopsied patient in the MDS with available autopsy data, and detailed information is provided about neuropathologic findings and resultant diagnoses. 4 For patients with contradictory values between the MDS and NPDS, values from the NPDS, which relies on more specific criteria, were selected based on NACC’s previous experience with the data sets.
To be included in the study, patients had to meet eligibility criteria, and patients meeting these criteria had dementia, were coded as having either a primary clinical diagnosis of AD or a known suspected etiology other than a prion disease, underwent autopsy, had available neuropathologic data, and belonged to a currently funded ADC. For eligible patients who had neuropathology indicative of prion disease but were coded as having a nonprion disease clinical diagnosis, further information was collected via a short form sent to the appropriate ADC asking (1) whether a clinical diagnosis of prion disease was mentioned in the patient’s medical records (excluding neuropathologic findings) and the type of prion disease mentioned, if applicable and (2) what the neuropathologically confirmed diagnosis was for the patient. Space was also allotted for additional comments. Cases of clinically unrecognized prion disease were defined as cases with a neuropathology-confirmed prion disease in the absence of a clinical diagnosis of prion disease indicated in the patient’s medical records. The percentages of these cases in the database were calculated, with their 95% Wilson-corrected confidence intervals (CIs) determined using Stata version 13 (StataCorp, College Station, Texas).
Results
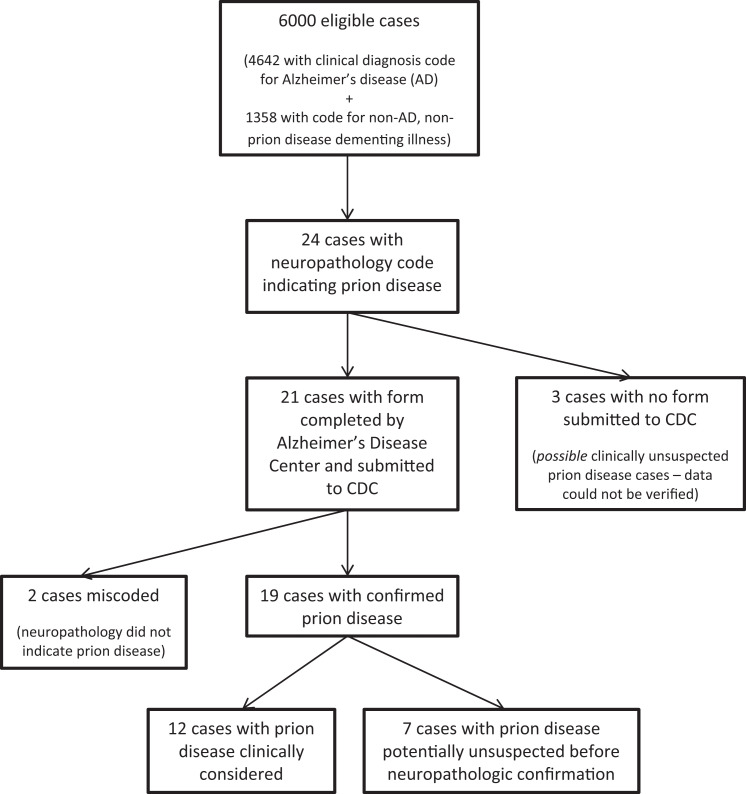
During 1984 to 2005, 6000 patients, representing 30 ADCs, met the eligibility criteria (Figure 1). The median age of the patients was 80 years (interquartile range: 73-86 years), and 53.3% were female. Of the 6000 patients, 4642 (77.4%) patients had a clinical diagnosis of AD. The remaining patients had clinical diagnoses of other nonprion disease dementing illnesses including frontal lobe dementia (5.1%), vascular dementia (3.6%), Parkinson’s disease dementia (2.2%), non-Parkinson’s dementia with Lewy bodies (1.9%), progressive supranuclear palsy (1.2%), and other dementing illnesses or conditions.
Figure 1.
Flow chart of the evaluation of clinically unsuspected prion disease among patients with dementia diagnoses in the National Alzheimer’s Coordinating Center database, 1984 to 2005.
Twenty-four (0.4%) patients were identified with a neuropathology code of prion disease. For these 24 patients, further clinical and/or neuropathological information was collected for 21, and attempts to gather information on the remaining 3 patients were unsuccessful. Two of the 21 patients were subsequently reclassified as not autopsy-confirmed prion disease cases based on information provided by the reporting ADCs indicating that the prion disease diagnosis had been miscoded due to misinterpretation of neuropathologic information. In the remaining 19 patients, representing 7 ADCs, 12 patients were included for whom a premortem prion disease diagnosis based on clinical assessment had been considered. No mention of a prion disease diagnosis was found for the other 7 patients (Table 1). The percentage of clinically unrecognized prion disease cases in the NACC database was therefore 0.12% (95% CI: 0.06-0.24, 7 cases of the 6000 patients); among patients with a clinical diagnosis of AD, the percentage was 0.11% (95% CI: 0.05-0.25, 5 cases of the 4642 patients).
Table 1.
Neuropathology-Confirmed Prion Disease Cases in the National Alzheimer’s Coordinating Center Database With No Mention of a Clinical Diagnosis of Prion Disease in Medical Records, 1984 to 2005.
| Year of Death | Age | Sex | Clinical Diagnosis | Final Diagnosis | Illness Duration | |
|---|---|---|---|---|---|---|
| Case 1 | 1999 | 47 | F | AD | GSS | NA |
| Case 2 | 2000 | 47 | F | Dementia NOS | CJD | 15+ years |
| Case 3 5 | 1994 | 49 | M | AD | fCJD | 8 years |
| Case 4 | 2000 | 72 | M | PSP | CJD | NA |
| Case 5 | 1996 | 78 | F | AD | CJD/AD/Wernicke–Korsakoff syndrome | NA |
| Case 6 | 1995 | 81 | M | AD | GSS; or CJD with concomitant AD | 7 years |
| Case 7 | 2000 | 88 | M | AD | CJD/AD | NA |
Abbreviations: AD, Alzheimer’s disease; CJD, Creutzfeldt–Jakob disease; fCJD, familial CJD; GSS, Gerstmann–Sträussler–Scheinker syndrome; NA, not available; NOS, not otherwise specified; PSP, progressive supranuclear palsy.
Discussion
Generally, CJD and other prion diseases can be recognized by typical clinical manifestations and a rapid progression from illness onset to death. 1,2 Although details on clinical course were not collected in the present study, at least 3 of the 7 patients with prion disease who were not recognized clinically had an illness duration exceeding 2 years, and 3 patients were younger than 50 years; these characteristics, along with other atypical manifestations, may have made correct diagnosis more difficult. The lack of a reliable antemortem clinical test for prion diseases may have also contributed to the difficulty in diagnosis. 6
The authors of a 1989 study reported that 13% of patients with clinically diagnosed AD were found upon autopsy to actually have CJD. 7 Although the sample was small (n = 46) and the proportion of these patients for whom clinical consideration of a prion disease diagnosis may have led to an autopsy was not reported, this finding has repeatedly fueled speculation that a substantial proportion of CJD cases are potentially being missed. By comparison, in the present study, the proportion of patients with dementia with clinically unsuspected but autopsy-confirmed prion disease was much less; for most years, no such prion disease cases were identified. Even if the 3 cases with a neuropathology code of prion disease for whom further information could not be obtained were assumed to be additional unrecognized prion disease cases, the percentage of such cases in the database would only increase minimally, from 0.12% to 0.17% (95% CI: 0.09-0.31, 10 cases of the 6000 patients); among patients with a clinical diagnosis of AD, the percentage would also increase to 0.17% (95% CI: 0.09-0.34, 8 cases of the 4642 patients).
The findings of this study are consistent with a much smaller study (n = 22) that did not find any prion disease cases upon neuropathological analysis of patients initially diagnosed with other disorders. 3 On the other hand, authors of other studies have reported that AD and additional neurological disorders may be misdiagnosed as prion diseases 6,8,9 ; this is not surprising given the findings of one study that found while most AD cases (90%) met AD clinical classification criteria, a high proportion also fulfilled clinical criteria for CJD (58%). 8 Chitravas et al retrospectively reviewed pathological findings of cases clinically suspected to have prion disease that were negative upon autopsy from the National Prion Disease Pathology Surveillance Center (NPDPSC) and reported that many patients who were suspected to have prion disease actually had potentially treatable neurologic diseases such as immune-mediated disorders, neoplasms, infections, and metabolic or toxic encephalopathies. 6
A limitation of the present study is that the NACC data sets may not be a representative sample of patients diagnosed with AD in the United States. The NACC collects data from various centers around the country, each with its own criteria for the inclusion of cases, and these specialized centers may be better equipped to provide accurate diagnoses compared to other institutions. However, if misdiagnosis of prion disease cases prior to autopsy was occurring at a substantial rate, the data sets could potentially reflect that. Furthermore, because neuropathologic analyses would be expected to be more commonly performed among patients with clinically atypical AD, this study, which only included autopsied cases, likely overestimates the percentage of clinically unsuspected prion disease cases that would be found among the overall AD population. Further limitations of the present study are that descriptions of neuropathological diagnoses for patients with a neuropathology code indicating prion disease were restricted to what was provided on the short form submitted by the ADC, and information on the precise diagnostic techniques applied by the pathologists who conducted the brain autopsies in the different ADCs over the 20+ years of the study was not available for review. Thus, although brain autopsy results are generally recognized as the gold standard for diagnosing prion diseases, it is possible that not all autopsied cases of these diseases were ascertained.
Seven decedents, who were identified in the database as having clinically unsuspected prion disease, were eventually appropriately classified as having prion disease due to positive autopsy findings. The present study therefore underscores the importance of neuropathologic testing for all possible prion disease cases, at least until a definitive antemortem, non brain tissue based, diagnostic test becomes established. In addition to confirming clinically suspected cases, testing is useful to identify unsuspected clinically atypical cases. To augment national prion disease surveillance, the CDC, in collaboration with the American Association of Neuropathologists, established the NPDPSC. 10 Personnel at the center perform diagnostic testing of suspected prion disease cases in the United States. Neurologists treating patients with possible prion disease are encouraged to use the services of the NPDPSC for disease confirmation.
Acknowledgments
The authors thank Woodrow Deitrich and Sarah Monsell for their assistance in providing and interpreting the NACC data and also express gratitude to the ADC staff who shared additional information for cases of interest.
Footnotes
Authors’ Note: Contributions by Blase and Harvey were made while they were affiliated with the National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention. They are now with different organizations. Contributions by Mercaldo were largely made while he was with the National Alzheimer’s Coordinating Center, University of Washington, but continued after he relocated to Vanderbilt University. A portion of this work was presented as an abstract/poster at the Prion 2008 Conference, October 8-10, 2008, Madrid, Spain. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported in part by National Institute on Aging Grant U01 AG016976 to the National Alzheimer’s Coordinating Center.
References
- 1. Belay ED, Holman RC, Schonberger LB. Creutzfeldt-Jakob disease surveillance and diagnosis. Clin Infect Dis. 2005;41(6):834–836. [DOI] [PubMed] [Google Scholar]
- 2. Belay ED, Maddox RA, Gambetti P, Schonberger LB. Monitoring the occurrence of emerging forms of Creutzfeldt-Jakob disease in the United States. Neurology. 2003;60(2):176–181. [DOI] [PubMed] [Google Scholar]
- 3. Josephs KA, Ahlskog JE, Parisi JE, et al. Rapidly progressive neurodegenerative dementias. Arch Neurol. 2009;66(2):201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beekly DL, Ramos EM, van Belle G, et al. The national Alzheimer’s coordinating center (NACC) database—an Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18(4):270–277. [PubMed] [Google Scholar]
- 5. Cochran EJ, Bennett DA, Cervenakova L, et al. Familial Creutzfeldt-Jakob disease with a five-repeat octapeptide insert mutation. Neurology. 1996;47(3):727–733. [DOI] [PubMed] [Google Scholar]
- 6. Chitravas N, Jung RS, Kofskey DM, et al. Treatable neurological disorders misdiagnosed as Creutzfeldt-Jakob disease. Ann Neurol. 2011;70(3):437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Manuelidis E, Manuelidis L. Suggested links between different types of dementias: Creutzfeldt-Jakob disease, Alzheimer’s disease, and retroviral CNS infections. Alzheimer Dis Assoc Disord. 1989;3(1-2):100–109. [DOI] [PubMed] [Google Scholar]
- 8. Tschampa HJ, Neumann M, Zerr I, et al. Patients with Alzheimer’s disease and dementia with Lewy bodies mistaken for Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry. 2001;71(1):33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poser S, Mollenhauer B, Krauss A, et al. How to improve the clinical diagnosis of Creutzfeldt-Jakob disease. Brain. 1999;122 (pt 12):2345–2351. [DOI] [PubMed] [Google Scholar]
- 10. National Prion Disease Pathology Surveillance Center. http://www.cjdsurveillance.com/. Accessed August 14, 2015.