Abstract
Autoimmune Inner Ear Disease (AIED) is a poorly understood disease marked by bilateral, rapidly progressive hearing loss triggered by unknown stimuli, which is corticosteroid responsive in 60% of patients. Although the mechanism of the disease is not precisely understood, a complex interaction of cytokines is believed to contribute towards the inflammatory disease process and hearing loss. Previously we showed the role of TNF-α in steroid-sensitive, and IL-1β in steroid-resistant immune mediated hearing loss. N-acetylcysteine (NAC), a broad spectrum antioxidant, has been effective in other autoimmune disorders. Other studies have shown NAC to have a protective adjunct role in human idiopathic sudden hearing loss, where the addition of NAC resulted in better hearing recovery than with steroids alone, although the mechanism of this protection was not elucidated. In the present study, we observed PBMCs from AIED patients exhibited higher baseline TNF-α and MPO levels compared to normal healthy controls. NAC effectively abrogates LPS-mediated TNF-α release from PBMC of both AIED patients and controls. We demonstrated that in AIED patients, the TNF-α down-stream signaling pathway appears aberrantly regulated, influencing both MPO and IL-8 expression. Given that NAC effectively abrogated LPS mediated TNF-α release and exerted minimal effects on the downstream targets of this pathway, we feel NAC may be a rational adjunct therapy for this enigmatic disease, worthy of clinical exploration.
Keywords: N-acetylcysteine (NAC), Tumor Necrosis Factor-alpha (TNF-α), Myloperoxidase (MPO), Autoimmune Inner Ear Disease (AIED)
1. Introduction
Autoimmune inner ear disease (AIED) is an enigmatic disease of periodic episodes of bilateral progressive hearing decline activated by unknown stimuli [1]. Recently, we have identified a role of TNF-α in steroid-sensitive and IL-1β in steroid-resistant immune mediated hearing loss [2, 3]. Furthermore, in a small cohort of patients, local administration of the TNF-α inhibitor etanercept by intratympanic injection was able to maintain hearing when systemic corticosteroid therapy was tapered [4], although use of systemic etanercept met with less promising efficacy [5, 6].
Reactive oxygen species (ROS) act as central mediators in the etiology of many autoimmune diseases. Among numerous ROS-generating molecules, inducible nitric oxide synthase (iNOS) and myloperoxidase (MPO) are hypothesized to play key roles. MPO is a pro-oxidant enzyme released from granules of activated leukocytes, neutrophils, monocytes, and macrophages at inflammatory sites. When released as part of the innate host defense, it generates free radicals and ROS [7, 8]. MPO plays a vital role in the autoimmune disease process by escalating oxidative stress and oxidizing lipoproteins [9]. There are several autoimmune diseases, such as Wegener’s granulomatosis [10], systemic lupus erythematosus (SLE) [11], Goodpasture syndrome [12], Churg-Strauss syndrome [13], and Cogan’s disease, a rare autoimmune vasculitis causing sensorineural hearing loss[14], associated with the development of anti-neutrophil cytoplasm (ANCA) autoantibodies against MPO. Furthermore, the perilymph of the inner ear is replete with apolipoproteins and therefore a potential target of MPO [15].
N-acetylcysteine (NAC), a broad spectrum antioxidant, is a precursor in the synthesis of reduced glutathione (GSH) and acts as a free radical scavenger that protects cells from oxidative damage [16]. NAC exerts anti-inflammatory effects [17]. NAC has been identified to be otoprotective, and serve as a beneficial adjunctive therapy in hearing restoration. NAC has been shown to have a protective adjunct role in idiopathic sudden hearing loss, where the addition of NAC to corticosteroid therapy resulted in better hearing recovery than with corticosteroids alone [18]. Furthermore, NAC ameliorated hearing loss in animal models of noise-induced hearing loss in several studies [19–22]. NAC was found to be effective in abrogating LPS-induced TNF alpha release in cardiomyocytes in dose dependent manner [23] and in neuroblastoma cell lines [24].
Pulmonary studies have shown that NAC can reduce MPO levels in the serum/plasma of a small cohort of healthy smokers the concentrations of MPO were reduced after NAC treatment for 8 weeks [25]. The chronic use of NAC inhibited MPO activity in septic lung injury rat model [26].
Adjunct NAC therapy has also been effective in abating autoimmune disease in both animal models of autoimmune disease and human autoimmune disease, although the mechanism of action described has been varied. NAC in combination with aminoguanidine was found to be effective in reducing oxidative stress in experimental autoimmune encephalomyelitis (EAE) [27]. In animal models of autoimmune thyroiditis, adjunct treatment with NAC reduced oxidative stress and the immune infiltration, thereby leading to a restoration of thyroid morphology [28]. NAC therapy also reduced disease severity in a clinical trial of systemic lupus erythematosus (SLE), although the mechanism was ascribed to blocking the mammalian target of rapamycin in T cells [29]. Based on our prior observations that steroid sensitive AIED patients exhibit higher pre-treatment levels of TNF-α [2], we hypothesized that this elevated TNF-α induces expression of MPO which results in oxidative damage and further hearing loss. We further hypothesized that treatment with NAC can partially abrogate TNF-α mediated induction of MPO and subsequent oxidative damage making it a good candidate to be used for adjunct therapy.
Materials and Methods
Subjects
This study was approved and monitored by the Institutional Review Board (IRB) of the North Shore Long Island Jewish Health system. Patients were identified by several neurotologists. All individuals who were included in study gave signed informed consent. Inclusion criteria were based on Niparko et al. study group guidelines [30]. A total of 64 AIED patients and 31 control subjects were recruited for these studies. The control cohort consisted of subjects of similar age, gender and ethnic background, who denied a history of hearing loss or autoimmune disease.
Human PBMC culture and stimulation
PBMCs from AIED patients and control subjects were collected in an identical fashion and were separated by Ficoll density gradient as previously described [3]. Isolated PBMCs were washed twice with 1 X RPMI (GIBCO) and incubated in RPMI 1640 supplemented with 10% (v/v) FBS (Atlanta Biologicals GA; a single lot of FBS was used for all experiments), 100 units/ml penicillin, 100 μg/ml streptomycin and 4.1 mM glutamine and plated at 1x106 cells/ml in 24-well plate (Costar). Preliminary experiments were done to identify the kinetics of TNF-α release after stimulation with LPS, with maximal release plateauing between 4 and 24 hours (supplemental figure 1). PBMCs (1 x 106 per ml) isolated from control subjects were subjected to treatment with and without 1 μg/ml LPS for different time points (1h to 24 h). For rest of the experiments, PBMCs were treated with either 5 mM N-acetyl-L-cysteine (NAC), 1 μg/ml LPS E. coli (0111:B4) (both from Sigma), 10 ng/ml recombinant TNF-α, recombinant myloperoxidase (1.5μg/ml) (both from R&D systems), alone or in combination with NAC. The optimal concentrations of these reagents were previously identified by culturing PBMC with increasing concentrations of these reagents to identify the maximal concentration that could be used without affecting cell viability. All PBMC samples were cultured overnight (16 h) at 37°C in 5% CO2 and compared with similarly cultured, unstimulated PBMC. Cell viability was measured after 16 h by Trypan blue dye exclusion method using the Cellometer Auto T4 automated cell counter (Nexcelom Biosciences). At the end of all incubations, samples were centrifuged and supernatants were collected and stored at −20 °C.
Monocyte and Neutrophil isolation
Monocytes and Neutrophils were isolated from same patient from same lot of blood by MACS monocyte isolation kit II™ and MACSxpress™ Neutrophil Isolation Kit both from (Miltenyi Biotec, Auburn,CA). The purity of negatively selected monocytes and neutrophils was determined by flow cytometry (FACSCanto II; BD Biosciences) using anti-CD14PE Abs (BD Immunocytometry Systems, San Jose, CA) and anti-CD 15 FITC along with Pacific Blue™ Mouse Anti-Human CD16 Clone 3G8(both from BD Biosciences) respectively. Purity for these experiments exceeded 80% and 90% respectively.
ELISA Methodology
TNF-α and IL-8 ELISA
Supernatant TNF-α (GE Healthcare) and IL-8 (R&D Systems) levels were quantified using a sandwich ELISA as per the manufacturer’s instructions. An 8-point standard curve was constructed for each assay using a quadratic fit, and data were interpolated using BioLinx 2.2 software (Synatech Laboratories Inc). Each sample was run in duplicate. Greater than 70% of all samples were run on replicate plates: the variance between replicate sets was 0.08 and 0.001 for TNF-α and IL-8.
MPO ELISA
Plasma and supernatant MPO levels were quantified using Human MPO Instant ELISA (eBioscience) as per the manufacturer’s instructions. The sensitivity of the assay was 0.026 ng/ml. An 8-point standard curve was constructed for each assay using a quadratic fit, and data were interpolated using BioLinx 2.2 software (Synatech Laboratories Inc). Each sample was run in duplicate. A least 19 samples from AIED patients and 4 control subjects were replicated on different dates. The variance between replicate sets was 0.03.
Thyroid peroxidase (TPO) ELISA
Plasma TPO levels were measured according to the manufacturer’s instructions (Abnova). Concentrations were calculated using a 4-parameter logistic curve. All samples were run in duplicate. The variance between replicate sets was 0.005.
IgG ELISAs for antibodies to MPO and TPO
Plasma anti-MPO IgG and anti-TPO IgG levels were measured by ELISA according to the manufacturer’s instructions (Abnova). Anti-MPO IgG concentrations were calculated using a 4-parameter logistic curve. For the plasma anti-TPO IgG concentration, a standard curve was constructed using a quadratic fit. All samples were run in duplicate. The variance between replicate sets was 0.001, and 0.01 respectively.
Western blotting
Isolated proteins from monocytes, neutrophils and PBMCs were separated on 12 % Mini-PROTEAN® TGX™ Precast Gel (BIO-RAD) and then transferred electrophoretically to PVDF membrane (BIO-RAD). The blot was then blocked with 5% Nonfat dry milk (NFDM) in Tris-buffered saline (TBS) with Tween 20 (0.5%, v/v) for 1 h at room temperature and subsequently incubated overnight at 4 degree with Anti MPO at dilution of 1:750 (DAKO) in TBS-Tween 20 (0.5%, v/v) [TBST] with 5% (w/v) NFDM. Following 3 washes of 5 min each with TBST, the blots were incubated with HRP-conjugated goat anti-rabbit IgG (R &D systems) (1:5000) in blocking buffer for 45 minutes at room temperature. After three washes with TBST, the blot was developed with Clarity ECL Western Blot Substrate kit (BIO-RAD) and exposed to Kodak Scientific Imaging X-OMAT LS Film from Carestream Health (Rochester, NY). Anti-actin antibody (Sigma) was used as loading control.
Statistical analysis
The data are expressed as mean ± standard error of the mean (SEM) and analyzed using GraphPad Prism 5 (GraphPad Software, San Diego, CA). The differences between groups were evaluated using the Mann–Whitney U test or by one-way analysis of variance (ANOVA). Post hoc testing was performed using a Bonferroni’s comparison on selected columns. P values less than or equal to 0.05 were considered significant.
Results
NAC effectively abrogates LPS-mediated TNF-α release from PBMC of both AIED patients and controls
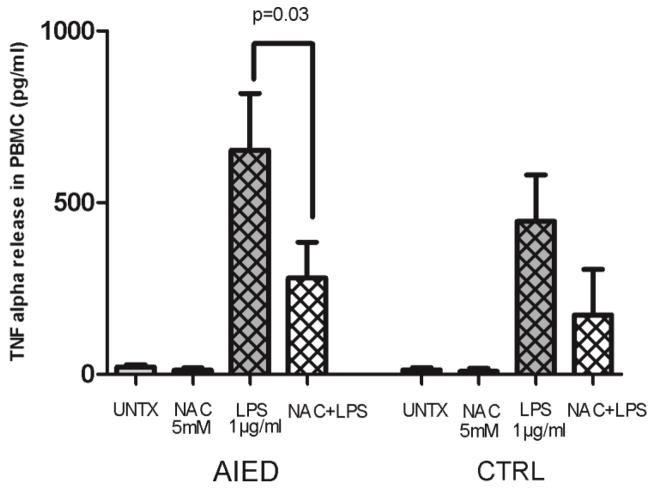
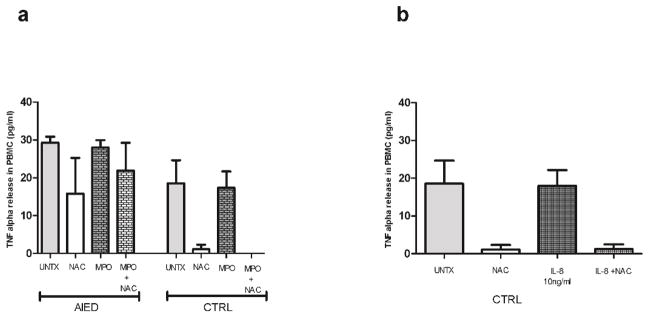
PBMCs isolated from AIED (N=12) patients and healthy control subjects (N=5) were either treated with 1 μg/ml LPS, 5 mM NAC, co-cultured with the combination of 1 μg/ml LPS plus 5 mM NAC, or left untreated for 16 hours. NAC, in combination with LPS, reduced TNF-α release when compared with LPS alone in AIED patients and control subjects: in LPS stimulated PMBC from AIED subjects, NAC reduced TNF-α release from 653 pg/ml to 280 pg/ml (a 57% reduction), and in PBMC from control subjects, NAC reduced TNF-α release from 445 to 173 pg/ml (a 61% reduction) (Fig. 1, p=0.03, Mann–Whitney U test). NAC had a marginal effect on unstimulated PBMC, largely because minimal expression of TNF-α secretion was detected (in PMBC from AIED subjects, NAC reduced TNF-α release from 21 to 12 pg/ml, and in PBMC from control subjects, NAC reduced TNF-α release from 13 to 8 pg/ml). Based on our prior observations of elevated TNF-α secreted protein levels in a subset of AIED patients, we queried whether myeloperoxidase (MPO) was elevated in these AIED patients as well.
Fig. 1. NAC abrogates LPS-mediated TNF-α release from PBMC of both AIED patients and controls.
PBMCs isolated from AIED patients (N=12) and healthy control subjects (N=5) were treated with 1 μg/ml LPS, 5 mM NAC, 5 mM NAC + 1 μg/ml LPS or left untreated for 16 h. LPS-mediated TNF-α release was significantly reduced by NAC in AIED patients (p = 0.03, Mann–Whitney U test) compared to LPS alone. In case of control subjects, there was trend towards reduction of TNF release, however significance was not achieved (p=0.09). Error bars show ± SEM.
AIED patients have elevated plasma levels of MPO
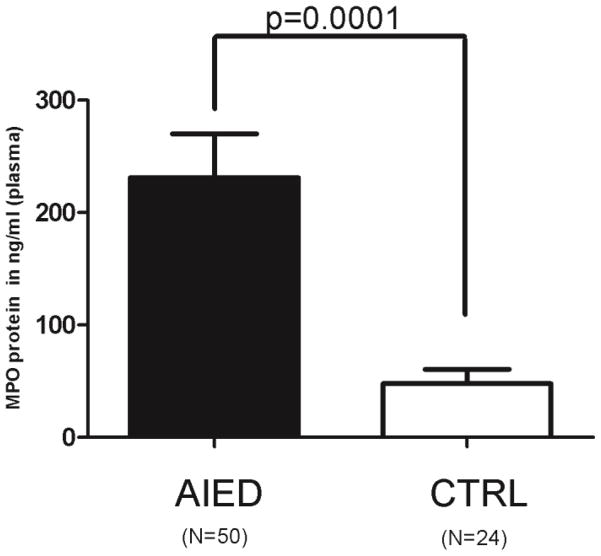
Plasma samples from 50 AIED patients and from 24 normal healthy control subjects were tested by sandwich ELISA to compare their MPO plasma levels. MPO levels were significantly increased in the plasma of AIED patients compared with controls (231 ng/ml of AIED patients plasma versus 47.76 ng/ml of plasma from control subjects (p<0.0001 by the Mann–Whitney U test)) (Fig. 2). The data suggests that increase in MPO levels is associated with AIED disease.
Fig. 2. AIED patients have higher plasma levels of MPO compared to control subjects.
Plasma samples from AIED patients (N=50) and normal healthy (N=24) control subjects were tested by sandwich ELISA to compare their MPO levels. MPO plasma levels were significantly increased in AIED patients (p<0.0001) compared with controls by the Mann–Whitney U test with 95% CI. Error bars show ± SEM
MPO can be marginally induced by TNF-α, and blocked by NAC
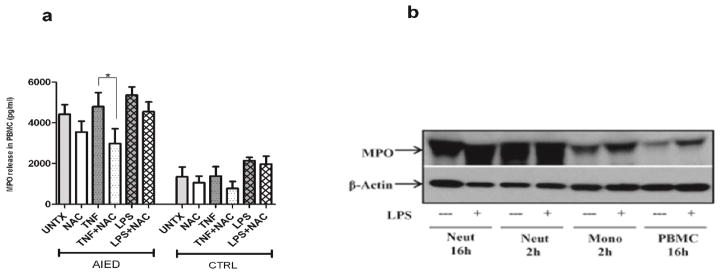
To determine whether MPO induction was TNF-α mediated, PBMCs isolated from AIED (N=8) patients and healthy control subjects (N=6) were either treated with 10 ng/ml TNF-α or 5 mM NAC, co-cultured with 10 ng/ml TNF-α plus 5 mM NAC, or left untreated for 16 h. NAC had a marginal effect on unstimulated PBMC. NAC reduced MPO in unstimulated PMBC from AIED patients and control subjects by 20% and 21%, respectively (Fig. 3a). Similarly, basal MPO release from untreated PBMC of AIED patients (N=12) was 3.3 times greater as compared with release from PBMC of control subjects. NAC reduced the TNF-α-mediated marginal increase in MPO release in AIED patients p<0.05 (one way ANOVA followed by post hoc analysis using a Bonferroni’s comparison on selected columns) (Fig. 3a). LPS stimulated PBMC cultures from AIED patients were largely refractory to NAC treatment, as NAC treatment resulted in only a minimal decrease in MPO levels from LPS stimulated PBMC (Fig. 3a). We suspect this failure of NAC to mitigate the LPS effect may be a result of LPS induction of other pro-inflammatory cytokines other than TNF-α, and that the NAC effect may be limited to TNF-α (Fig. 3a).
Fig. 3. MPO is generated by both neutrophils and monocytes fractions of PBMC and NAC reduces TNF-α-mediated MPO release from PBMC of both AIED patients and controls.
a. A sandwich ELISA was done on stimulated PBMCs isolated from AIED patients and healthy control subjects. PBMC from AIED patients were either treated with 10 ng/ml TNF-α (8 AIED patients) or 1 μg/ml LPS (16 AIED patients) +/− 5 mM NAC, and compared to untreated PBMC, or PBMC from control subjects (N=6) cultured under the same conditions for 16 h. NAC in combination with TNF-α was able to reduce MPO release when compared with TNF-α alone in PBMC from AIED patients and control subjects. Comparisons were made by one-way analysis of variance (ANOVA) p < 0.0001, followed by post hoc testing using a Bonferroni’s comparison test on selected columns. TNF-α induced MPO release compared with TNF-α + NAC achieved statistical significance p < 0.05 in AIED patients. b. Neutrophils (Neut), monocytes (Mono) and total PBMCs were isolated from an AIED patient and treated with LPS 1 μg/ml or (N=4) and healthy control subjects (N=3) were either treated with 10 ng/ml recombinant TNF-α or 5 mM NAC, co-cultured with 10 ng/ml recombinant TNF-α + 5 mM NAC, 1 μg/ml LPS, co-cultured with 1 μg/ml LPS + 5 mM NAC, or left untreated for 16 h. TNF-α and LPS were able to induce IL-8 release from PBMCs of AIED patients. Addition of NAC to either TNF-α or LPS cultures resulted in only a minimal reduction of IL-8 release.
Neutrophils and monocytes produce MPO
To see if increased MPO levels could be ascribed to a specific cell type, we isolated monocytes and neutrophils from an AIED patient and by western blot compared their intracellular MPO contents with total PBMC isolated from same patient from same lot of blood. Western blotting indicated that MPO is expressed by both neutrophils and monocytes (Fig. 3b). However, the majority of MPO is produced by the neutrophil fraction (Fig. 3b).
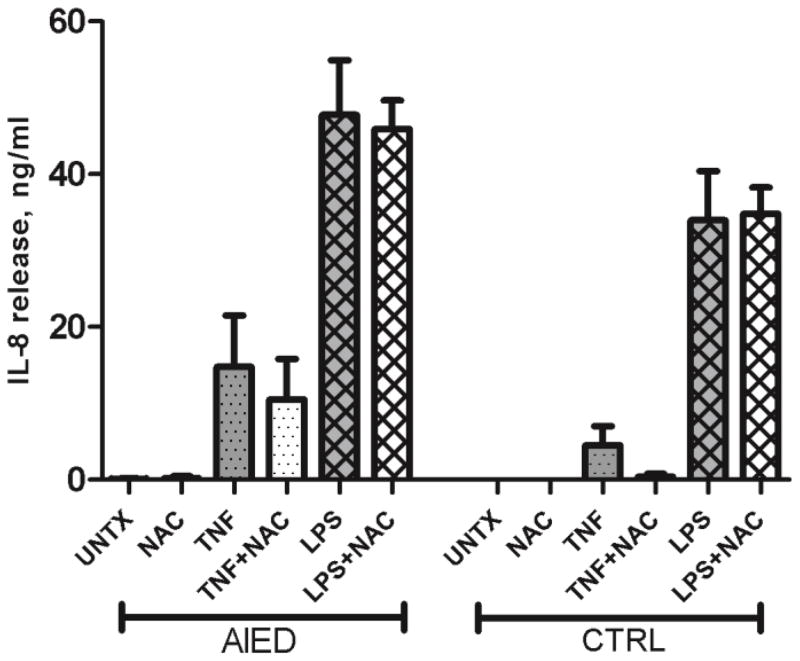
TNF-α induces IL-8 release, which is marginally reduced with NAC co-culture
PBMCs isolated from AIED patients (N=4) and healthy control subjects (N=3) were either treated with 10 ng/ml recombinant TNF-α, LPS, 5 mM NAC, co-cultured with 10 ng/ml recombinant TNF-α plus 5 mM NAC or 1 μg/ml LPS or co-cultured with 1 μg/ml LPS plus 5 mM NAC and compared to untreated PBMC. NAC’s effect on unstimulated PBMC in AIED subjects and control subjects could not be judged as practically there was no basal secretion in both cases. Both TNF-α, and LPS were able to induce IL-8 release from PBMCs of AIED patients, although, again, the LPS effect was substantially greater. The addition of NAC to either TNF-α or LPS stimulated cultures failed to substantially reduce IL-8 levels suggesting NAC exerts a greater effect on TNF-α expression rather than IL-8 (Fig. 4).
Fig. 4.
TNF-α mediated MPO and IL-8 induction is unidirectional
Given that TNF-α is capable of inducing MPO, we queried whether MPO could reciprocally induce TNF-α. PBMCs isolated from AIED patients (N=4) and healthy control subjects (N=5) were either treated with 1.5 μg/ml recombinant MPO, 5 mM NAC, co-cultured with 1.5 μg/ml MPO plus 5 mM NAC or left untreated for 16 h. MPO treatment does not induce TNF-α (Fig. 5a). Interestingly, co-culture with MPO negated the ability of NAC to inhibit TNF-α release in AIED PBMC, suggesting polarization of AIED PBMC to a refractory phenotype. Similarly, we also investigated whether IL-8 can induce TNF-α. PBMCs isolated from healthy control subjects (N=5) were either treated with 10 ng/ml recombinant IL-8, 5 mM NAC, co-cultured with 10 ng/ml IL-8 plus 5 mM NAC, or left untreated for 16 h. IL-8 was similarly unable to induce TNF-α release from PBMCs of control subjects (Fig. 5b). A similar pattern was observed in a small cohort of AIED patients (data not shown). These observations led us to conclude that TNF-α induction of MPO and IL-8 is unidirectional, and a feedback loop does not appear to be present.
Fig. 5. IL-8 or MPO do not augment TNF-α release.
a. PBMCs isolated from AIED patients (N=4) and healthy control subjects (N=5) were either treated with 1.5 μg/ml recombinant MPO, 5 mM NAC, co-cultured with 1.5 μg/ml MPO + 5 mM NAC or left untreated for 16 h. MPO treatment does not induce TNF-α. b. PBMCs isolated from healthy control subjects (N=5) were either treated with 10 ng/ml recombinant IL-8, 5 mM NAC, co-cultured with 10 ng/ml IL-8 + 5 mM NAC or left untreated for 16 h. IL-8 was unable to induce TNF-α release from PBMCs of control subjects.
Anti-MPO IgG antibodies were not detected in AIED patients
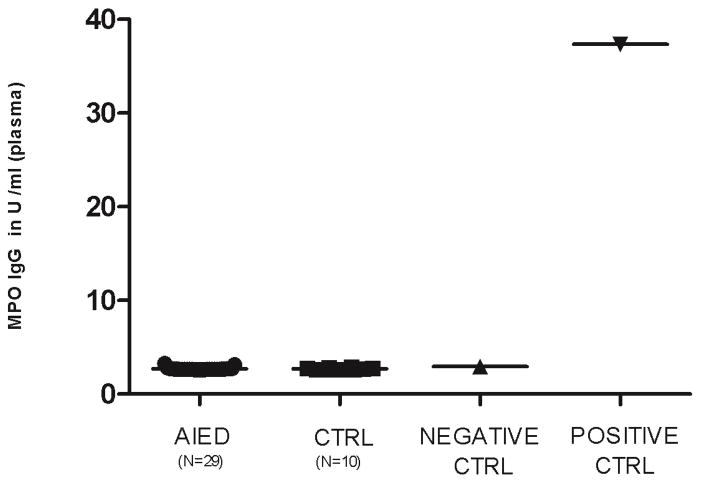
Antibodies to MPO have been detected in many autoimmune diseases. Since plasma MPO levels are elevated in our patient cohort, we hypothesized that our patients may have anti-MPO antibodies. Plasma from 29 AIED patients and 10 normal healthy control subjects was tested by ELISA to determine if AIED patients exhibited elevated anti-MPO plasma IgG levels compared with controls. No significant anti-MPO IgG antibodies were detected in either AIED patients or control subjects (Fig. 6). These results suggest that although MPO is elevated, it is not the target of an aberrant antibody-mediated immune response.
Fig. 6. MPO IgG plasma levels were absent in AIED patients and control subjects.
Plasma samples from 29 AIED patients and 10 normal healthy control subjects were tested by ELISA were compared with a positive control (MPO antibodies in serum/buffer) and a negative control (PBS, BSA detergent sodium azide 0.09%) to identify whether AIED patients made anti-MPO IgG antibodies. Anti-MPO IgG plasma levels were in the same range as of negative control for both AIED patients as well as control subjects. Error bars show ± SEM.
AIED patients have mildly elevated TPO levels and autoantibodies to TPO
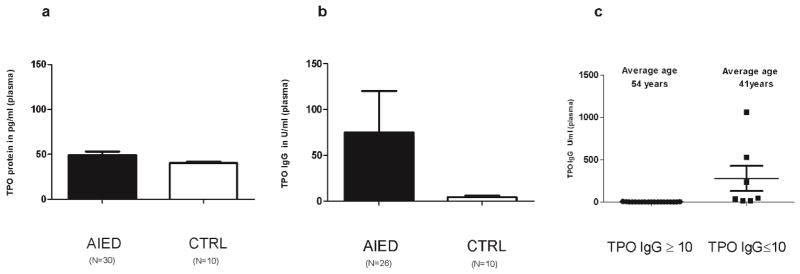
Since MPO shares great extent of homology with thyroperoxidase (TPO) and anti-TPO antibodies are readily detected in patients with various autoimmune diseases, we measured TPO protein levels in plasma of AIED patients (N= 30) and normal healthy controls (N=10) by a sandwich ELISA. TPO plasma levels were slightly greater in AIED patients and controls (49.02 pg/ml of serum for AIED patients versus 40.37 pg/ml of serum for control subjects), although this difference is marginal and would require a substantially larger cohort to draw any conclusions (Fig. 7a). Similar to the rationale for investigating the presence of anti-MPO antibodies, we also questioned whether our AIED cohort had anti-TPO antibodies. Plasma IgG levels of anti-TPO auto-antibodies in the same 26 AIED patients were 17 fold higher than in the 10 normal controls (Fig. 7b). Anti-TPO antibodies are prevalent in older adults[31], to rule out that possibility we segregated our TPO IgG ELISA data (Fig. 7c) on the basis of a threshold of 10 U/ml. We observed that the group with TPO IgG < 10 U/ml (mean= 0.67) had an age mean of 54 years old vs. the group of >10 U/ml (mean value= 277.94) with an age mean of 41 years of age. This discounts the possibility that increased TPO IgG levels in AIED patients are due to more aged AIED population.
Fig. 7. AIED patients have marginally elevated TPO levels; however exhibit enhanced anti-TPO antibodies compared with age matched controls.
a. Plasma samples from 30 AIED patients and 10 normal healthy control subjects were tested by sandwich ELISA to compare their TPO levels. TPO plasma levels were almost same in AIED patients compared with controls (49.02 pg/ml for AIED patients versus 40.37 pg/ml for control subjects). Error bars show ± SEM. b. Plasma samples from AIED patients (N=26) and normal healthy (N=10) control subjects were tested by ELISA to compare their TPO IgG levels. TPO IgG plasma levels were very high (17 fold) in AIED patients’ plasma when compared with control subjects. Error bars show ± SEM. c. Analysis of TPO IgG threshold relative to patient age. We segregated our AIED patient (N=26) TPO IgG ELISA data into two groups on the basis of a threshold of 10 U/ml of TPO IgG. The lower threshold group of <10U/ml (N=19 patients) had a mean value of TPO IgG of 0.67 with a mean age of 54 years old vs. the higher threshold group of >10U/ml (N=7 patients) with a mean value of TPO IgG of 277.94 with a mean age of 41 years old.
Discussion
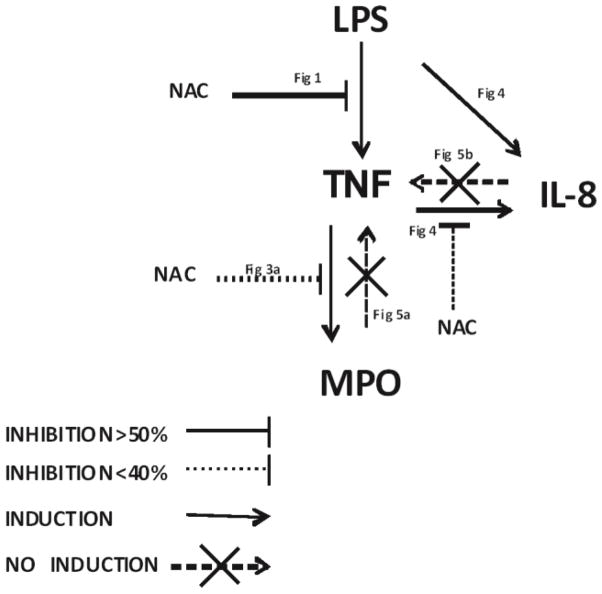
Previously we observed that PBMCs from AIED patients exhibited greater TNF-α release compared to normal healthy controls [2]. Here we show that PBMC treatment with NAC abrogates approximately 60% of LPS-mediated TNF-α release from PBMCs of both AIED patients and controls. Other studies have also noticed similar inhibition with NAC when induced by LPS [23]. We noticed that this effect is dose dependent (data not shown), with the 5 mM NAC concentration being optimal. Given that TNF-α is able to influence MPO [32] and IL-8 [33] expression in other systems, we investigated and ascertained that in AIED patients, the TNF-α down-stream signaling pathway appears aberrantly regulated (Fig 8). This TNF-α mediated IL-8 and MPO induction appears to be unidirectional because MPO and IL-8 treatment does not induce TNF-α, but interestingly, the addition of MPO may cause refractoriness in NAC-mediated TNF-α inhibition in AIED patients (Fig 5). Clinically, NAC treatment has been observed to enhance the effect of corticosteroids in hearing recovery [18]. We have previously demonstrated that, specifically, corticosteroid sensitive AIED correlated with high TNF-α levels [2]. NAC has been effective in inhibiting TNF-α [23] and IL-8 [34, 35] in other studies. Notably, TNF-α appears to be a potential target of NAC, as NAC does not exert a strong effect on the further downstream targets MPO and IL-8. Furthermore, NAC was ineffective in reducing IL-1β levels (data not shown). Others have shown that NAC’s effect may be via suppression of TNF mediated NFkB activation [36], [37], [38] Whether this effect is specific to a particular cell type is unknown, however, neutrophils have been observed to have increased NFkB activation and IL-8 expression [39], although PPARs act to inhibit inflammation through NFkB occurs in monocytes, B and T cells [40] suggesting it may not be limited to one cellular subset. Plasma levels of MPO in AIED patients were almost 5 fold higher when compared with normal healthy subjects (Fig 2), indicating that high MPO levels in AIED could be a result of increased TNF-α expression, although elevation of MPO is likely the result of a number of factors. Future experiments will determine the precise effect of anti-TNF neutralizing antibodies on MPO expression in these patients, although TNF inhibition has not been clinically effective in AIED patients [5], [6], despite possible benefit in patients with Cogan’s disease [14]. As anticipated, MPO expression is predominantly observed in the neutrophil fraction and to a lesser degree in the monocytes fraction which is consistent with prior observations [41] Future studies will characterize the cell frequency in AIED patients, as neither we, or other investigators have determined if any irregularities exist. Others have shown that MPO is important in LDL oxidation and resultant monocyte differentiation into classical or alteratively activated macrophages based on the amount of oxidized LDL [42], suggesting that MPO activity may dictate monocyte differentiation and disease progression or remission in other systems based on the local microenvironment. It is certainly possible that prolonged treatment with NAC may improve MPO repression. Given that we detected elevated MPO in our patient cohort, and MPO autoantibodies have been widely observed in different autoimmune diseases, we decided to investigate MPO antibody levels in our patient cohort. We were unable to detect MPO autoantibodies in these patients as measured by plasma anti-MPO IgG ELISA (Fig. 6), which highlights the difference between this patient cohort and Cogan’s patients as they have anti-MPO antibodies [14]. MPO and TPO share up to 42 % sequence homology [43–45]. The relationship between TPO and MPO is also manifested by potentially shared epitopes in autoimmune thyroid disease patients between these two antigens [46]. Although TPO levels in the plasma were marginally higher in AIED patients compared with controls, AIED patients had significantly higher anti-TPO IgG levels when compared with normal healthy controls (Fig. 7). Although anti-TPO antibodies are prevalent in older adults[31], when we segregated our TPO IgG ELISA data on the basis age, we observed that the younger patients had substantially higher anti-TPO titers. The group with low anti-TPO IgG < 10 U/ml (mean= 0.67 U/ml) had a mean age of 54 years old versus the group of >10 U/ml (mean = 277.94 U/ml) with a mean age of 41 years old. These results suggest that the high anti-TPO IgG values are not due to a more aged AIED population, but rather may be associated with development of an autoimmune disease (Fig. 8), as many AIED patients have a wide variety of autoantibodies detected in their sera [47]. It is unlikely, however, that these autoantibodies have a pathogenic role in the disease process. Presence of these and other auto-antibodies in AIED is variable, and neither assists in clarifying the diagnosis of AIED, nor do they dictate treatment[47].
Fig. 8.
Schematic diagram illustrating the proposed signaling pathway.
Our studies demonstrated that TNF-α is capable of inducing IL-8 release (Fig. 4). IL-8 instilled into the round window membrane of the inner ear of rats resulted in an inflammatory infiltrate, however, interestingly, at the time of resultant sensorineural hearing loss, the neutrophil infiltrate had resolved, leaving only resident macrophages, suggesting their potential role in the development of immune mediated hearing loss [48]. IL-8 expression is enhanced in Th17 polarizing conditions, consistent with development of an autoimmune phenotype [49], especially in the background of TNF-α expression [50]. Interestingly, reduced IL-8 levels have been observed in patients with thyroid disease [51]. Given that AIED patients that appear to have anti-TPO antibodies, and elevated IL8 in response to TNF, future studies will further investigate the role of IL-8 in these patients.
Taken together, our data demonstrates the ability of NAC to inhibit TNF-α expression in these AIED patients, and may elucidate why adjuvant therapy with NAC has shown to be clinically beneficial in hearing recovery. Given that we have observed that TNF-α influences the expression of MPO and IL-8 in these patients and may potentiate autoimmune disease, the equilibrium between the release of oxidants, and their inactivation by antioxidants could therefore be decisive in clinical disease progression or remission. The above data emphasize that use of antioxidants like NAC may be an important adjunct therapy for the treatment of this poorly understood disease, and further understanding of the molecular mechanism of action may elucidate further biologic targets for intervention.
In conclusion, we demonstrate the ability of NAC to inhibit TNF-α expression in AIED patients, which may explain the clinical benefit of adjuvant NAC antioxidant therapy hearing recovery.
Supplementary Material
Acknowledgments
This study was supported by a National Institutes of Health grant R21/R33DC011827 (AV) and Merrill & Phoebe Goodman Otology Research Center
Abbreviations used in this article
- AIED
Autoimmune Inner Ear Disease
- NAC
N-acetyl-L-cysteine
- GSH
L-γ-glutamyl-L-cysteinyl-glycine
- ROS
reactive oxygen species
- MPO
Myeloperoxidase
- TPO
Thyroperoxidase
- EAE
experimental autoimmune encephalomyelitis
Footnotes
Conflict of Interest
Dr. Vambutas and the Feinstein Institute for Medical Research holds a patent application for the use of IL-1β receptor antagonists for the treatment of AIED and related diseases.
Author Contributions Conceived and designed the experiments: SP, AV. Performed the experiments: SP, CS. Analyzed the data: SP, AV. Contributed reagents/materials/analysis tools: AV. Wrote the paper: SP, AV.
References
- 1.McCabe BF. Autoimmune sensorineural hearing loss. The Annals of otology, rhinology, and laryngology. 1979;88(5 Pt 1):585–9. doi: 10.1177/000348947908800501. [DOI] [PubMed] [Google Scholar]
- 2.Svrakic M, Pathak S, Goldofsky E, Hoffman R, Chandrasekhar SS, Sperling N, et al. Diagnostic and prognostic utility of measuring tumor necrosis factor in the peripheral circulation of patients with immune-mediated sensorineural hearing loss. Archives of otolaryngology--head & neck surgery. 2012;138(11):1052–8. doi: 10.1001/2013.jamaoto.76. [DOI] [PubMed] [Google Scholar]
- 3.Pathak S, Goldofsky E, Vivas EX, Bonagura VR, Vambutas A. IL-1beta is overexpressed and aberrantly regulated in corticosteroid nonresponders with autoimmune inner ear disease. Journal of immunology (Baltimore, Md : 1950) 2011;186(3):1870–9. doi: 10.4049/jimmunol.1002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Wijk F, Staecker H, Keithley E, Lefebvre PP. Local perfusion of the tumor necrosis factor alpha blocker infliximab to the inner ear improves autoimmune neurosensory hearing loss. Audiology & neuro-otology. 2006;11(6):357–65. doi: 10.1159/000095897. [DOI] [PubMed] [Google Scholar]
- 5.Matteson EL, Choi HK, Poe DS, Wise C, Lowe VJ, McDonald TJ, et al. Etanercept therapy for immune-mediated cochleovestibular disorders: a multi-center, open-label, pilot study. Arthritis and rheumatism. 2005;53(3):337–42. doi: 10.1002/art.21179. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S, Shoup A, Weisman MH, Harris J. Etanercept treatment for autoimmune inner ear disease: results of a pilot placebo-controlled study. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2005;26(5):903–7. doi: 10.1097/01.mao.0000185082.28598.87. [DOI] [PubMed] [Google Scholar]
- 7.Klebanoff SJ. Myeloperoxidase. Proceedings of the Association of American Physicians. 1999;111(5):383–9. doi: 10.1111/paa.1999.111.5.383. [DOI] [PubMed] [Google Scholar]
- 8.Klebanoff SJ. Myeloperoxidase: friend and foe. Journal of leukocyte biology. 2005;77(5):598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 9.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. The Journal of clinical investigation. 1994;94(1):437–44. doi: 10.1172/jci117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seo P, Stone JH. The antineutrophil cytoplasmic antibody-associated vasculitides. The American journal of medicine. 2004;117(1):39–50. doi: 10.1016/j.amjmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 11.Nassberger L, Sjoholm AG, Jonsson H, Sturfelt G, Akesson A. Autoantibodies against neutrophil cytoplasm components in systemic lupus erythematosus and in hydralazine-induced lupus. Clinical and experimental immunology. 1990;81(3):380–3. doi: 10.1111/j.1365-2249.1990.tb05342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arimura Y, Minoshima S, Kamiya Y, Nakabayashi K, Kitamoto K, Nagasawa T. A case of Goodpasture’s syndrome associated with anti-myeloperoxidase antibodies. Internal medicine (Tokyo, Japan) 1992;31(2):239–43. doi: 10.2169/internalmedicine.31.239. [DOI] [PubMed] [Google Scholar]
- 13.Tervaert JW, Goldschmeding R, Elema JD, von dem Borne AE, Kallenberg CG. Antimyeloperoxidase antibodies in the Churg-Strauss syndrome. Thorax. 1991;46(1):70–1. doi: 10.1136/thx.46.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greco AGA, Fusconi M, Magliulo G, Turchetta R, Marinelli C, Macri GF, De Virgilio A, de Vincentiis M. Cogan’s syndrome: an autoimmune inner ear disease. Autoimmun Rev. 2013;12(3):396–400. doi: 10.1016/j.autrev.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Thalmann I, Kohut RI, Ryu J, Comegys TH, Senarita M, Thalmann R. Protein profile of human perilymph: in search of markers for the diagnosis of perilymph fistula and other inner ear disease. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1994;111(3 Pt 1):273–80. doi: 10.1177/01945998941113P117. [DOI] [PubMed] [Google Scholar]
- 16.Cotgreave IA. N-acetylcysteine: pharmacological considerations and experimental and clinical applications. Advances in pharmacology (San Diego, Calif) 1997;38:205–27. [PubMed] [Google Scholar]
- 17.Blesa S, Cortijo J, Mata M, Serrano A, Closa D, Santangelo F, et al. Oral N-acetylcysteine attenuates the rat pulmonary inflammatory response to antigen. The European respiratory journal. 2003;21(3):394–400. doi: 10.1183/09031936.03.00039602. [DOI] [PubMed] [Google Scholar]
- 18.Angeli SI, Abi-Hachem RN, Vivero RJ, Telischi FT, Machado JJ. L-N-Acetylcysteine treatment is associated with improved hearing outcome in sudden idiopathic sensorineural hearing loss. Acta oto-laryngologica. 2012;132(4):369–76. doi: 10.3109/00016489.2011.647359. [DOI] [PubMed] [Google Scholar]
- 19.Kopke RD, Weisskopf PA, Boone JL, Jackson RL, Wester DC, Hoffer ME, et al. Reduction of noise-induced hearing loss using L-NAC and salicylate in the chinchilla. Hearing research. 2000;149(1–2):138–46. doi: 10.1016/s0378-5955(00)00176-3. [DOI] [PubMed] [Google Scholar]
- 20.Ohinata Y, Miller JM, Schacht J. Protection from noise-induced lipid peroxidation and hair cell loss in the cochlea. Brain research. 2003;966(2):265–73. doi: 10.1016/s0006-8993(02)04205-1. [DOI] [PubMed] [Google Scholar]
- 21.Duan M, Qiu J, Laurell G, Olofsson A, Counter SA, Borg E. Dose and time-dependent protection of the antioxidant N-L-acetylcysteine against impulse noise trauma. Hearing research. 2004;192(1–2):1–9. doi: 10.1016/j.heares.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Kopke R, Bielefeld E, Liu J, Zheng J, Jackson R, Henderson D, et al. Prevention of impulse noise-induced hearing loss with antioxidants. Acta oto-laryngologica. 2005;125(3):235–43. doi: 10.1080/00016480410023038. [DOI] [PubMed] [Google Scholar]
- 23.Peng TLX, Feng Q. Pivotal role of gp91phox-containing NADH oxidase in lipopolysaccharide-induced tumor necrosis factor-alpha expression and myocardial depression. Circulation. 2005;111(13):1637–44. doi: 10.1161/01.CIR.0000160366.50210.E9. [DOI] [PubMed] [Google Scholar]
- 24.Álvarez SM-FM. TNF-A may mediate inflammasome activation in the absence of bacterial infection in more than one way. PLoS One. 2013 doi: 10.1371/journal.pone.0071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eklund A, Eriksson O, Hakansson L, Larsson K, Ohlsson K, Venge P, et al. Oral N-acetylcysteine reduces selected humoral markers of inflammatory cell activity in BAL fluid from healthy smokers: correlation to effects on cellular variables. The European respiratory journal. 1988;1(9):832–8. [PubMed] [Google Scholar]
- 26.Ozdulger A, Cinel I, Koksel O, Cinel L, Avlan D, Unlu A, et al. The protective effect of N-acetylcysteine on apoptotic lung injury in cecal ligation and puncture-induced sepsis model. Shock (Augusta, Ga) 2003;19(4):366–72. doi: 10.1097/00024382-200304000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Ljubisavljevic S, Stojanovic I, Pavlovic D, Sokolovic D, Stevanovic I. Aminoguanidine and N-acetyl-cysteine supress oxidative and nitrosative stress in EAE rat brains. Redox report : communications in free radical research. 2011;16(4):166–72. doi: 10.1179/1351000211y.0000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poncin S, Colin IM, Decallonne B, Clinckspooor I, Many MC, Denef JF, et al. N-acetylcysteine and 15 deoxy-{delta}12,14-prostaglandin J2 exert a protective effect against autoimmune thyroid destruction in vivo but not against interleukin-1{alpha}/interferon {gamma}-induced inhibitory effects in thyrocytes in vitro. The American journal of pathology. 2010;177(1):219–28. doi: 10.2353/ajpath.2010.091253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai ZW, Hanczko R, Bonilla E, Caza TN, Clair B, Bartos A, et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis and rheumatism. 2012;64(9):2937–46. doi: 10.1002/art.34502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niparko JK, Wang NY, Rauch SD, Russell GB, Espeland MA, Pierce JJ, et al. Serial audiometry in a clinical trial of AIED treatment. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2005;26(5):908–17. doi: 10.1097/01.mao.0000185081.28598.5c. [DOI] [PubMed] [Google Scholar]
- 31.Marcocci C, Chiovato L. Werner and Ingbar’s the thyroid: a fundamental and clinical text. 8. Philadelphia: Lippincott Williams & Wilkins; 2000. Thyroid-directed antibodies; pp. 414–31. [Google Scholar]
- 32.Yang MCJ, Zhao J, Meng M. Etanercept attenuates myocardial ischemia/reperfusion injury by decreasing inflammation and oxidative stress. PLoS One. 2014 doi: 10.1371/journal.pone.0108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osawa YNM, Banno Y, Brenner DA, Asano T, Nozawa Y, Moriwaki H, Nakashima S. Tumor necrosis factor alpha-induced interleukin-8 production via NF-kappaB and phosphatidylinositol 3-kinase/Akt pathways inhibits cell apoptosis in human hepatocytes. Infect Immun. 2002;70(11):6294–301. doi: 10.1128/IAI.70.11.6294-6301.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarir HME, Karimi K, Kraneveld AD, Rahman I, Caldenhoven E, et al. Cigarette smoke regulates the expression of TLR4 and IL-8 production by human macrophages. Journal of inflammation. 2009;6(12) doi: 10.1186/1476-9255-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumoto KHS, Gon Y, Nakayama T, Takizawa H, Horie T. N-acetylcysteine inhibits IL-1 alpha-induced IL-8 secretion by bronchial epithelial cells. Respir Med. 1998;92(3):512–5. doi: 10.1016/S0954-6111(98)90300-6. [DOI] [PubMed] [Google Scholar]
- 36.Oka SKH, Kamata K, Yagisawa H, Hirata H. N-acetylcysteine suppresses TNF-induced NF-kappaB activation through inhibition of IkappaB kinases. FEBS letters. 2000;472(2–3):196–202. doi: 10.1016/s0014-5793(00)01464-2. [DOI] [PubMed] [Google Scholar]
- 37.Park JHKS, Kim JY, Tchah H. The Antioxidant N-Acetylcysteine Inhibits Inflammatory and Apoptotic Processes in Human Conjunctival Epithelial Cells in a High-Glucose Environment. Invest Ophthalmol Vis Sci. 2015;56(9):5614–21. doi: 10.1167/iovs.15-16909. [DOI] [PubMed] [Google Scholar]
- 38.Carroll JEHE, Hess DC, Wakade CG, Chen Q, Cheng C. Nuclear factor-kappa B activation during cerebral reperfusion: effect of attenuation with N-acetylcysteine treatment. Brain Res Mol Brain Res. 1998;56(1–2):186–91. doi: 10.1016/s0169-328x(98)00045-x. [DOI] [PubMed] [Google Scholar]
- 39.Stegmaier JCKC, Bogner V, Matz M, Kanz KG, Mutschler W, Biberthaler P. Dynamics of neutrophilic NF-kB translocation in relation to IL-8 mRNA expression after major trauma. Inflammation research : official journal of the European Histamine Research Society [et al] 2008;57(11):547–54. doi: 10.1007/s00011-008-7207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Genolet RWW, Michalik L. PPARs as drug targets to modulate inflammatory responses? Curr Drug Targets Inflamm Allergy. 2004;3(4):361–75. doi: 10.2174/1568010042634578. [DOI] [PubMed] [Google Scholar]
- 41.Hansson MOI, Nauseef WM. Biosynthesis, processing, and sorting of human myeloperoxidase. Arch Biochem Biophys. 2006;445(2):214–24. doi: 10.1016/j.abb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Seo JWYE, Yoo KH, Choi IH. Macrophage Differentiation from Monocytes Is Influenced by the Lipid Oxidation Degree of Low Density Lipoprotein. Mediators of inflammation. 2015 doi: 10.1155/2015/235797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Libert F, Ruel J, Ludgate M, Swillens S, Alexander N, Vassart G, et al. Thyroperoxidase, an auto-antigen with a mosaic structure made of nuclear and mitochondrial gene modules. The EMBO journal. 1987;6(13):4193–6. doi: 10.1002/j.1460-2075.1987.tb02766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimura S, Ikeda-Saito M. Human myeloperoxidase and thyroid peroxidase, two enzymes with separate and distinct physiological functions, are evolutionarily related members of the same gene family. Proteins. 1988;3(2):113–20. doi: 10.1002/prot.340030206. [DOI] [PubMed] [Google Scholar]
- 45.Banga JP, Mahadevan D, Barton GJ, Sutton BJ, Saldanha JW, Odell E, et al. Prediction of domain organisation and secondary structure of thyroid peroxidase, a human autoantigen involved in destructive thyroiditis. FEBS letters. 1990;266(1–2):133–41. doi: 10.1016/0014-5793(90)81524-r. [DOI] [PubMed] [Google Scholar]
- 46.Banga JP, Tomlinson RW, Doble N, Odell E, McGregor AM. Thyroid microsomal/thyroid peroxidase autoantibodies show discrete patterns of cross-reactivity to myeloperoxidase, lactoperoxidase and horseradish peroxidase. Immunology. 1989;67(2):197–204. [PMC free article] [PubMed] [Google Scholar]
- 47.Agrup C, Luxon LM. Immune-mediated inner-ear disorders in neuro-otology. Current opinion in neurology. 2006;19(1):26–32. doi: 10.1097/01.wco.0000194143.02171.46. [DOI] [PubMed] [Google Scholar]
- 48.Iguchi HAM. Interleukin 8 can affect inner ear function. ORL J Otorhinolaryngol Relat Spec. 1998;60(4):181–9. doi: 10.1159/000027591. [DOI] [PubMed] [Google Scholar]
- 49.Gasch M, Goroll T, Bauer M, Hinz D, Schutze N, Polte T, et al. Generation of IL-8 and IL-9 producing CD4(+) T cells is affected by Th17 polarizing conditions and AHR ligands. Mediators of inflammation. 2014;2014:182549. doi: 10.1155/2014/182549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bogiatzi SI, Guillot-Delost M, Cappuccio A, Bichet JC, Chouchane-Mlik O, Donnadieu MH, et al. Multiple-checkpoint inhibition of thymic stromal lymphopoietin-induced TH2 response by TH17-related cytokines. The Journal of allergy and clinical immunology. 2012;130(1):233–40e5. doi: 10.1016/j.jaci.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 51.Provatopoulou X, Georgiadou D, Sergentanis TN, Kalogera E, Spyridakis J, Gounaris A, et al. Interleukins as markers of inflammation in malignant and benign thyroid disease. Inflammation research : official journal of the European Histamine Research Society [et al] 2014;63(8):667–74. doi: 10.1007/s00011-014-0739-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.