Abstract
Reporting the second of two targets within a stream of distracting words during rapid serial visual presentation (RSVP) is impaired when the targets are separated by a single distractor word, a deficit in temporal attention that has been referred to as the attentional blink (AB). Recent conceptual and empirical work has pointed to pre-target brain states as potential mediators of the AB effect. The current study examined differences in pre-target electrophysiology between correctly and incorrectly reported trials, considering amplitude and phase measures of alpha oscillations as well as the steady-state visual evoked potential (ssVEP) evoked by the RSVP stream. For incorrectly reported trials, relatively lower alpha-band power and greater ssVEP inter-trial phase locking were observed during extended time periods preceding presentation of the first target. These results suggest that facilitated processing of the pre-target distracter stream indexed by reduced alpha and heightened phase locking characterizes a dynamic brain state that predicts lower accuracy in terms of reporting the second target under strict temporal constraints. Findings align with hypotheses in which the AB effect is attributed to neurocognitive factors such as fluctuations in pre-target attention or to cognitive strategies applied at the trial level.
Keywords: Visual rapid serial processing, Electroencephalography (EEG), Steady-state visual evoked potential (ssVEP), Attention control, Oscillatory activity
Introduction
Human observers readily extract relevant information from the dense and complex spatiotemporal information stream provided by sensory systems. However, this ability is constrained by limits regarding the temporal and spatial density of the stimulus array. The temporal aspects of these limitations in the human visual system have been extensively examined using rapidly presented stimulus sequences. For instance, rapid serial visual presentation (RSVP) is a widely used paradigm that involves presentation of a series of letters or words. The so-called attentional blink (AB) effect is observed during RSVP when two attended target items are sequentially presented, with at least one intervening distractor item and in close temporal proximity to each other (i.e., within 200–500 ms of time, Raymond et al. 1992). During this time period (referred to as the AB interval), an observer's ability to identify the second of the two targets (T2) is dramatically reduced, compared to conditions in which the duration between the two targets exceeds 500 ms, or when participants are instructed to ignore the first target (T1).
As a striking demonstration of limitations in selective visual processing, the AB phenomenon has attracted attention from experimental psychologists as well as cognitive neuroscientists. A substantial number of theoretical models regarding its origin have been proposed, and recent review papers provide an excellent overview of the main mechanistic views of the origin of the AB (Martens and Wyble 2010; Dell'Acqua et al. 2012). Theoretical perspectives of the AB have highlighted different aspects of sequence processing, attentional selection, and working memory. For instance, work in the context of earlier theoretical models (e.g., Chun and Potter 1995) has emphasized trade-off and interference effects between T1 and T2 processing, using the AB to examine global resource limitations in higher-order stages of stimulus processing such as attention and working memory (Potter et al. 2002), or “access to consciousness” (Marti et al. 2012; Asplund et al. 2014). Other models have capitalized on evidence demonstrating that the impairment in identifying T2 depends to a large extent on the presence of a distractor item between T1 and T2 (Olivers et al. 2007). For instance, the model proposed by Di Lollo et al. (2005) assumes a perceptual/attentional set that is tuned to T1 features and enables rapid processing of targets that match this set (e.g., because of their color or semantic category). Once engaged by a first target, the subsequent disruption of the attentional set by a non-matching distractor is thought to prompt diminished performance for the following targets. In line with the predictions from this model, stringing together multiple targets without intervening distractors is associated with better performance compared to conditions with intervening distractors (Olivers et al. 2007). Examining the limits of this so-called spreading-the-sparing effect (Di Lollo et al. 2005; Olivers et al. 2007) has been a productive research strategy as well, aiding in quantitatively testing competing theoretical models of the AB (Dell'Acqua et al. 2012). Such empirical work has recently led to a renewed interest in the role of temporal attention selection in the AB (Nieuwenstein et al. 2009; Vul et al. 2008). For instance, the AB effect is attenuated when observers are prevented from over-attending to T1, e.g., by listening to music or working on a competing task (Olivers and Nieuwenhuis 2005). Thus, “overzealously applied” selection of T1 has been proposed as a factor contributing to the AB effect, such that the inappropriate selection of T1 + 1 replaces the task-appropriate selection of T2 (Olivers et al. 2007). Finally, recent accounts based on behavioral and neurophysiological data have examined the impact of pre-target cortical states on T2 performance (Keil and Heim 2009; Kranczioch et al. 2007), specifically, the amount of attention paid to T1 as well as the preceding distractor stream (Shapiro et al. 2006).
Taken together, behavioral, computational modeling, and electrophysiological studies have highlighted the role of multiple neurocognitive systems acting over time to protect target items from distraction and potentially suppress distractor items (Dell'Acqua et al. 2012; Kranczioch et al. 2007; Martens and Wyble 2010). Electrophysiological approaches with their exquisite temporal resolution are particularly well suited to investigate potential mechanisms contributing to the AB effect (Hommel et al. 2006). Existing evidence from event-related potentials (ERPs) and steady-state visual evoked potentials (ssVEPs) suggests that larger T1-related neural responses are associated with missed T2 responses, supporting ideas emphasizing the role of T1 processing for the AB (Keil and Heim 2009). In line with such findings, numerous recent theoretical accounts predict that the regulation of attention deployment prior to and around T1 is crucial for T2 performance (for reviews, see Martens and Wyble 2010; Dell'Acqua et al. 2012). Specifically, three of the most widely studied computational models of the AB render clear predictions regarding pre-T1 brain states that affect T2 report: The boost and bounce model proposed by Olivers and Meeter (2008) describes the AB as a result of an initial attention signal to the T1 (the “boost”), followed by a suppression signal (the “bounce”) that prevents facilitated processing of non-targets following T1. If a T2 stimulus occurs during this bounce signal, it is thought to be prevented from entering subsequent working memory stages and thus not reported at the end of the trial. In a similar fashion, the episodic simultaneous type/serial token model by Wyble et al. (2011) argues that the AB results from the dynamic parsing of visual input into temporal packets (attentional episodes) that can be encoded into memory, with attention episodes (e.g., detected targets) separated by periods of suppressed attention. Under both models, a brain state that allows the temporally precise allocation of subsequent facilitation and suppression to targets and non-targets in rapid succession would result in relatively improved performance. Finally, the threaded cognition model (Taatgen et al. 2009) posits that the AB is the consequence of overexertion of cognitive control and suppression of sensory processing while processing the T1. Here, a pre-T1 brain state consistent with low deployment of attention to the RSVP stream would predict correct performance for the T2 stimulus.
Time–frequency analyses of oscillatory activity are particularly well suited to test these predictions as they yield continuous measures of electrocortical activity across the time course of an experimental trial (Keil et al. 2001). At the same time, numerous studies with detection, identification, and selective attention tasks have pointed to a predictive role of pre-task brain states indexed by oscillatory activity (Weisz et al. 2014). Here, we examine the predictive value of pre-target oscillatory brain activity assessed with EEG, focusing on two phenomena readily measured during RSVP: (1) time-varying alpha power (8–13 Hz) and (2) the ssVEP, evoked by the regular stimulus stream.
Alpha power has traditionally been regarded as a signature of cortical tissue that neither receives nor processes sensory input (Adrian and Matthews 1934). In line with this notion, decreases in spectral power in the alpha band reliably occur with sensory processing of external stimuli (Bollimunta et al. 2011; Keil et al. 2006; Ray and Cole 1985). Challenging the traditional view however, power increase in alpha-range oscillations has also been demonstrated in a variety of experimental designs, including working memory load (Jensen et al. 2002; Klimesch et al. 2006) and motor process imagery (Neuper et al. 2005; Pfurtscheller and Berghold 1989). Reviewing the literature, Klimesch et al. (2006) concluded that alpha increase is most often seen in tasks that involve top-down processing in the absence of external stimulation, e.g., when participants maintain an internal representation. In a similar vein, other authors have suggested that alpha-power increase reflects cortical inhibition mechanisms mediating the suppression or “gating” of distracting sensory input (Foxe and Snyder 2011; Jensen and Mazaheri 2010; Mathewson et al. 2011). Thus, the magnitude of alpha oscillations may index processes linked to the management of limited capacity systems across different types of information processing (Romei et al. 2010).
Another continuous measure of electrocortical facilitation during RSVP is the ssVEP (Regan 1989). This signal is evoked by a visual stimulus that is rapidly and regularly modulated in luminance or contrast. It is defined in the frequency domain by a very narrow peak at the stimulus modulation frequency (Muller and Hillyard 2000). The amplitude of the ssVEP is increased for attended stimuli (Müller et al. 2003). Similarly, the consistency of the ssVEP's oscillatory phase across trials as measured by inter-trial phase locking (ITPL; Lachaux et al. 1999) is heightened for attended, compared to unattended stimuli.
Previous work has examined alpha power and steady-state potential measures in the AB task, but has yielded somewhat mixed results. In terms of alpha fluctuations in the AB paradigm, Kranczioch et al. (2007) found pre-T1 alpha-power reduction and lower inter-trial phase coherence in trials where T2 was correctly reported. These authors also noted that alpha power (and phase-locking) estimates may have been affected by the RSVP stream, presented at a rate of 10 Hz in their study. By contrast, MacLean and Arnell (2011) reported relatively decreased alpha power during the RSVP stream prior to missed T2s while using a slower RSVP presentation rate (8.56 Hz). Studies employing the ssVEP during the AB have focused on changes in time-varying amplitude and have repeatedly observed that ssVEP amplitude following T1 was heightened in T2-missed trials (Keil and Heim 2009; Keil et al. 2006).
In addition to its amplitude, the phase at which near-threshold visual stimuli are presented within the alpha cycle may impact behavioral outcomes such as hits and misses (Busch et al. 2009; Mathewson et al. 2009). Thus, whereas heightened alpha power has been taken to reflect decreased excitability of sensory cortical areas (Foxe and Snyder 2011), or an active gating mechanism facilitating internal cognitive processing (Klimesch et al. 2006), the ongoing phase may be related to fluctuations in cortical excitability (Bollimunta et al. 2011). A similar effect has been reported for the ssVEP, in which targets are better detected when presented in phase with a preceding RSVP stimulus train (Mathewson et al. 2009, 2011). In line with these results, a recent study reported significant non-uniformity in the ssVEP phase at the time of T1 presentation, when T2 was incorrectly reported (Zauner et al. 2012). Again, this suggests that greater levels of visual-cortical entrainment to the RSVP stimulus train, taken to indicate over-attending to the RSVP stream, may decrease T2 report accuracy.
What is not known is the extent to which pre-target oscillatory markers of attentive states possess predictive value vis-a-vis performance in the AB task. Robust relations between pre-target states and behavior would have implications for theoretical accounts of the AB, but also possess potential for heightening our understanding of the brain mechanisms managing capacity limitations over time. The present study addressed these questions, examining the predictive value of pre-T1 alpha and ssVEP oscillations vis-a-vis performance in the AB task. To the extent that states of heightened attentional engagement prior to and during presentation of the first target predict poor identification of the second target, it was expected that trials with incorrect T2 report would be associated with greater pre-T1 alpha decrease and higher ssVEP phase locking. To explore the extent to which performance-related differences in pre-target oscillatory characteristics are associated with altered communication between cortical sites, we also measured phase-locking values between occipital sensor Oz and the remainder of the sensor array and compared these values between correct and incorrect trials.
Methods
Participants
Participants were 17 (10 female; mean age = 19.15 years) undergraduate students at the University of Florida, who participated in the study for course credit after giving informed consent. Data from two additional participants were discarded because of low trial counts for either correct or incorrect T2 responses in the Lag-2 condition. All participants reported no family history of seizures. All procedures were approved by the Institutional Review Board of the University of Florida.
Stimuli
All words were selected from the Affective Norms of English Words (ANEW) list (Bradley and Lang 1999). The target words were drawn from a list of 182 neutral words (arousal: mean = 3.97, SD = 2.11; valence: mean = 5.25, SD = 1.43, all on a 9-point scale, with a mean of 5.0), which were divided in two 90-word groups to serve as either the first or second target. Each target word was presented once randomly during the first 90 trials and then again, randomly, during the last 90 trials. For all other neutral distractors, 30 additional neutral words were chosen. In addition, this experiment included trials with a manipulation of the affective content of the distracter immediately following T1, which is not the focus of the present report. In brief, 52 unpleasant words (arousal: mean = 6.54, SD = 2.45; valence: mean = 2.17, SD = 1.52), 52 pleasant words (arousal: mean = 6.59, SD = 2.40; valence: mean = 7.90, SD = 1.38) served as emotional distractors, and 52 additional neutral words (arousal: mean = 3.95, SD = 2.17; valence: mean = 5.32, SD = 1.33) served as control distractors immediately following T1 during the inter-target interval. This manipulation did not result in changes of accuracy and will therefore only briefly be reported on below. All distractors were presented in a white font (226.6 cd/m2), and target words were presented in a green font (171.4 cd/m2). All words were presented in a 42-point Helvetica font on a black background (0.31 cd/m2).
Procedure
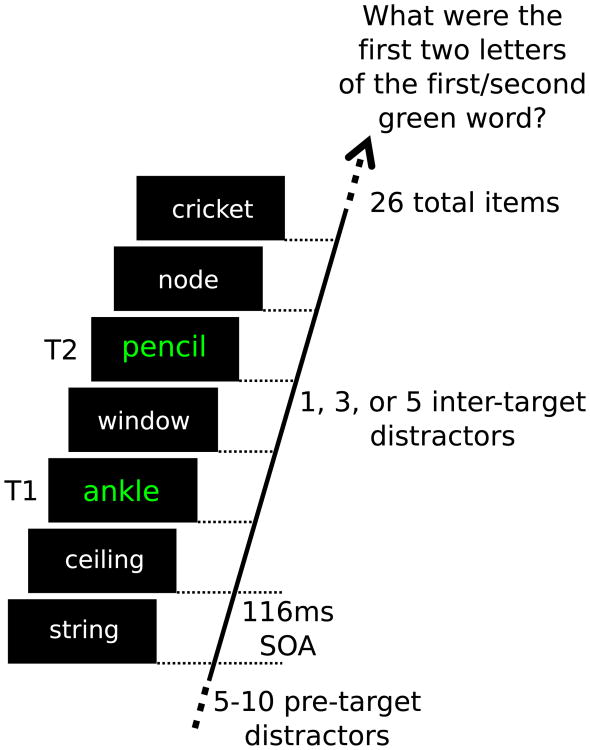
The AB task was implemented using the PsychToolbox suite for MATLAB (Brainard 1997) and presented on a 23-inch LED (Samsung LS23A950) monitor with a refresh rate of 120 Hz and a gray-to-gray response time of 2 ms. Following the application of the EEG sensors (see below), participants entered a sound-attenuated, dimly lit chamber. The participants were centered with and seated 1 m from the screen. Participants then received verbal and on-screen instruction to report two green target words embedded in a RSVP stream of words, by typing the first two letters of each word when prompted at the end of each trial. In addition, participants were instructed to say each word out loud when typing. One practice trial was conducted to ensure the participant understood the instructions and pace of the experimental trials. Each trial started with an initial blank screen for a random interval between 3 and 5 s. The subsequent RSVP stream (Fig. 1) began with a baseline of 5–10 words (i.e., between 580 and 1160 ms in duration) preceding T1. The inter-target interval consisted of 1, 3, or 5 distracter words (Lag 2, Lag 4, Lag 6), and SOAs for each lag condition were 232, 464, and 696 ms, respectively. Following the second target, between 8 and 18 distracter words (1044–2088 ms) completed the RSVP string of 26 words in total. Thus, each trial was 3016 ms in duration for all experimental conditions.
Fig. 1.
The sequence of one trial of the RSVP paradigm. Items were presented for 116 ms each resulting in a presentation rate of 8.56 Hz. The present illustration depicts a trial in which the target words are separated by a single distractor word. Target words were neutrally valenced and displayed in a green font, whereas all distractor words were presented in a white font. All words were presented in the center of a computer screen
In each lag condition, the affective content of the first inter-target distracter (immediately following T1) was pleasant, neutral, or unpleasant. The entire experiment consisted of 180 trials, with 20 trials in each of the 9 (3 Lags × 3 distractor valence categories) experimental conditions.
Electrophysiological recordings and data reduction
EEG data were collected using a 257-electrode system (Electrical Geodesics Incorporated; Eugene, Oregon, USA). Following application of the sensors, impedances were reduced to below 70 kΩ as recommended for this high-input impedance amplifier (200 MΩ input impedance) or excluded from analysis if this was not possible. Data were collected continuously at 250 Hz, constrained by two online elliptic filters at 0.1 Hz (high-pass) and 100 Hz (low-pass). Additional filtering was performed offline on the continuous data, which were convolved with Butterworth filters having their 3 dB points at 1 Hz (19th order high-pass) and 40 Hz (6th order low-pass). Data were then segmented into 3304 ms epochs relative to the onset of T1 (1100 ms pre-T1 onset; 2204 ms post-T1 onset).
The focus of the present analysis was to compare electrocortical oscillatory activity prior to the onset of T1 during correct and incorrect trials. Because error rate was low for Lag 4 and Lag 6, only trials in the Lag 2 condition were used for the analyses. In addition, only activity prior to T1 was considered in our analyses. Across participants, out of a total of 1139 trials, T2 was incorrectly reported in 452 trials and correctly reported in 687 trials in this condition. Following automatic rejection, EEG segments were available for 358 incorrect (mean = 18.84; SD = 10.15) and 510 correct (mean = 26.84; SD = 7.36) trials. To ensure identical signal-to-noise ratio, the number of correct and incorrect trials was made equal by randomly discarding trials for each participant. Data from two participants were discarded for retaining <8 trials in one condition. Thus, subsequent analyses are based on 275 (SD = 5.60) segments that were selected at random from the pool of correct trials at Lag 2.
Wavelet analysis
To examine the temporal dynamics of oscillatory brain activity, the artifact-free EEG epochs were convolved with a family of Morlet wavelets (see, e.g., Tallon-Baudry and Bertrand 1999; Tallon-Baudry et al. 1998). In this analysis, the final 132 ms of each EEG epoch was excluded to achieve a frequency resolution allowing for a frequency bin (f0, see below) centered at the RSVP frequency of 8.56 Hz. To obtain time–frequency representations of the data across a range of frequencies, one wavelet is typically convolved with the data for each frequency of interest (f0). With increasing values of f0, these wavelets systematically decrease their resolution (σf) in the frequency domain, while achieving higher temporal specificity. Here, the relation between analytic frequency f0 and spectral resolution (the standard deviation σf of the Gaussian representation of the wavelet in the frequency domain) was set to be a constant f0/σf = 7. Complex wavelets were calculated for f0 ranging from 3.06 to 27.54 Hz, in steps of 0.306 Hz and normalized to have equal amounts of energy. The alpha band was defined as the frequency range between 9.49 and 10.40 in the present study, and the resulting frequency resolution was 1.35 Hz (full width at half maximum) and a time resolution of 116 ms (full width at half maximum). The ssVEP frequency was defined as the frequency band centered at 8.56, with a frequency resolution of 1.22 Hz (full width at half maximum) and a time resolution of 138 ms (full width at half maximum). EEG segments were multiplied channel-wise with cosine-square taper window of 200 ms length (zero to unity), applied at the beginning and end of each segment, and then convolved with the complex wavelets. For each frequency of interest, two evolutionary spectra were then calculated: (1) the time-varying total amplitude and (2) the time-varying inter-trial phase locking. Time-varying amplitude for each trial was computed as the square absolute value of the convolution of the cosine-square-tapered EEG signal with the wavelet. Time-by-frequency matrices were then averaged separately across trials with correct and incorrect responses, for each participant and electrode site. In addition to total amplitude, we calculated the evoked amplitude spectrum by applying the same wavelet family to the time-domain averaged data, rather than the individual trials.
Time-varying inter-trial phase locking was obtained in a similar fashion, by averaging the normalized (divided by the modulus), complex coefficients (complex instantaneous phase) resulting from convolution of the wavelets and the data across trials, as described in Lachaux et al. (1999). The same method (Lachaux et al. 1999) was extended to measuring inter-site phase locking (ISPL) between Oz and all remaining sensors. Here, complex phase difference scores were first calculated by subtracting the complex coefficients at Oz from the coefficients at each other sensors, for each trial, time point, and frequency. These differences were again normalized and averaged across all epochs, resulting in a time-varying estimate of inter-site phase locking between all sensor locations and Oz, at each frequency.
Statistical analysis
Analysis of behavioral data
Only trials in which T1 was correctly reported were included in the analyses. Percentage of correctly reported T2 trials was calculated for each lag (3; Lag 2, Lag 4, Lag 6) and valence (3; unpleasant, neutral, pleasant) condition for a total of nine accuracy ratings per participant. A repeated measures ANOVA with factors of Lag (2, 4, 6) and distracter content (pleasant, neutral, unpleasant) was conducted to determine whether accuracy was affected by the temporal position of the second target relative to the first and whether accuracy during any of the lag conditions was affected by the valence of the first intervening distractor.
Analysis of electrophysiological data
Time-varying t tests
To examine differences in the time-varying spectral power, inter-trial phase locking, and evoked spectral power, between T2-incorrect and T2-correct trials, a series of t tests were performed at each sensor between incorrect and correct trials across all time points for the frequency bands corresponding to the ssVEP and alpha, separately. To determine the point at which t values reached significance, a permutation-based control for multiple comparisons was performed. Here, for each comparison separately, values at each time point were randomly assigned to either the correct or incorrect condition, and then a t value was calculated. This process was repeated 5000 times, and the resulting t values entered a permutation distribution, used for determining the critical t values. Critical t values were defined as the t values that marked the 2.5 and 97.5 percentile of the permutation distribution, respectively, thus indicating a corrected significance level of 0.05. Following this computation, the cutoff t scores were −3.10 and 3.10 for ssVEP ITPL, −3.10 and 3.11 for evoked ssVEP power, −3.03 and 3.07 for evoked alpha power. Because maximum activation for ssVEP and alpha occurred over occipital and parietal areas, only sensors over these regions were considered for analysis. As an additional precaution, sensors located on the edges of the sensor array were excluded due to their high susceptibility to noise. To avoid wavelet artifacts related to the discontinuous onset and offset of each data segment, the initial 200 ms treated with the cosine taper window was not included. Because T1-evoked activity may appear in pre-T1 time periods due to the temporal smearing of the wavelets, the 138 ms leading up to T1 presentation was not considered in this analysis. Significant t values for specific electrodes and time points are reported when t values reached significance in at least two contiguous sensors simultaneously.
Cross-correlation function
Because ssVEP ITPL and alpha power reflect complementary, opposite processes, the relationship between ssVEP ITPL and alpha power was investigated using a cross-correlation function between the two time series, at each sensor. First, the average epoched ssVEP ITPL and alpha-power time series were calculated for each participant. Then, the first 816 ms of the ITPL time course was correlated with the first 816 ms of alpha-power time course for each participant separately. The ITPL segment was then shifted in 4-ms steps across the entire alpha-power time series, and a Spearman's correlation coefficient was calculated for each shift. Thus, 612 correlation coefficients were produced for each sensor, representing 2448 ms of data. These time series were averaged across participants after performing a Fisher's Z transformation.
Inter-site phase locking
Differences in ssVEP and alpha oscillatory activity may be influenced by communication between cortical sites. To investigate whether any potential differences observed in the preceding analysis are related to such inter-cortical communication, ISPL values between correct and incorrect trials were compared using a series of t tests as described in the previous analysis. Cutoff t scores for this comparison were −3.16 and 3.38 for both ssVEP and alpha ISPL. This follow-up analysis was limited to the time range (time segments within 100 ms) of significant differences in the analyses of power and inter-trial phase locking, but was not limited to only parietal and occipital sensors.
Comparisons of oscillatory phase
To examine the potential role of oscillatory phase at the alpha and ssVEP frequency for identification performance, we conducted an exploratory comparison of phase differences between correct-T2 and incorrect-T2 trials at each sensor and time point. To this end, phase angles were extracted from the complex matrix resulting from the convolution of the wavelet family and the single-trial data, for the ssVEP and alpha frequency bands separately. Phase values (obtained for each time point and electrode location) were then pooled across participants and phase angles of correct and incorrect trials compared with Fisher's nonparametric test for equality of circular means (Fisher 1995), resulting in a sensor by time matrix of p values for each frequency. Analogous to the power and phase-locking analyses described above, these p values were controlled for multiple comparisons by the permutation technique. A distribution of p values was generated by repeating the comparisons 8000 times, with assignments of phase angles to correct and incorrect trial categories shuffled randomly. Phase differences were considered significant at the p < 0.05 level, if below the permutation corrected threshold of 0.011. As with the previous time-varying t tests, only when the significance threshold was exceeded in at least two spatially contiguous sensors with temporal simultaneity are results reported. Unlike the previous time-varying t tests, no points were excluded from analysis.
Rank correlations between oscillatory activity and behavior
In a final analysis aimed to parametrically relate ssVEP ITPL and alpha power, for each participant the difference between correct and incorrect trials was calculated at each sensor during a 120-ms segment corresponding to the temporal window of the effects observed for the running t test for ssVEP ITPL and alpha power separately. A Spearman's rank correlation between each participant's ssVEP ITPL and alpha-power difference score was then calculated at each sensor. To investigate the relationship of these two measures with inter-individual performance on the AB task, the difference in performance between Lag 4 and Lag 2 was calculated and submitted to a Spearman's rank correlation with both the ssVEP ITPL and alpha-power condition difference.
Results
Behavioral results
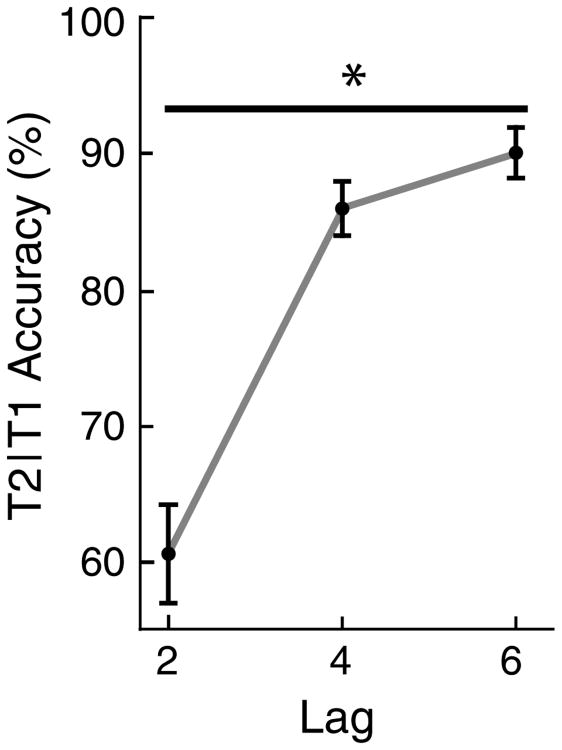
As illustrated in Fig. 2, a main effect of lag was observed (F2,32 = 138.73, p < 0.001). This main effect was characterized by a significant linear trend (F1,16 = 184.27, p < 0.001), such that T2 report accuracy improved systematically with increasing SOA. Neither the main effect of valence nor the lag by valence interaction reached significance, indicating that the valence of the distractor immediately following T1 had no effect on T2 report. As a consequence, this factor is not considered in the remainder of this report.
Fig. 2.
Mean (n = 17) report accuracy for T2 on trials in which T1 was reported correctly for the Lag 2 (M = 60.72 %, SE = 3.69 %), Lag 4 (M = 86.06 %, SE = 2.30 %), and Lag 6 (M = 89.88 %, SE = 2.20 %) conditions. Standard errors are indicated by the vertical bars. A significant linear trend was observed across all three lag conditions
EEG data
ssVEP inter-trial phase locking
As shown in Fig. 3 (top), ssVEP inter-trial phase locking values were higher for incorrect compared to correct trials at occipital midline sensors, in a time segment extending between −268 and −138 ms pre-T1. During this time interval, two adjacent occipital electrodes near Oz exceeded the permutation-controlled significance threshold of 3.10. Their peak t values were 3.18 (p < 0.05) and 3.46 (p < 0.05).
Fig. 3.
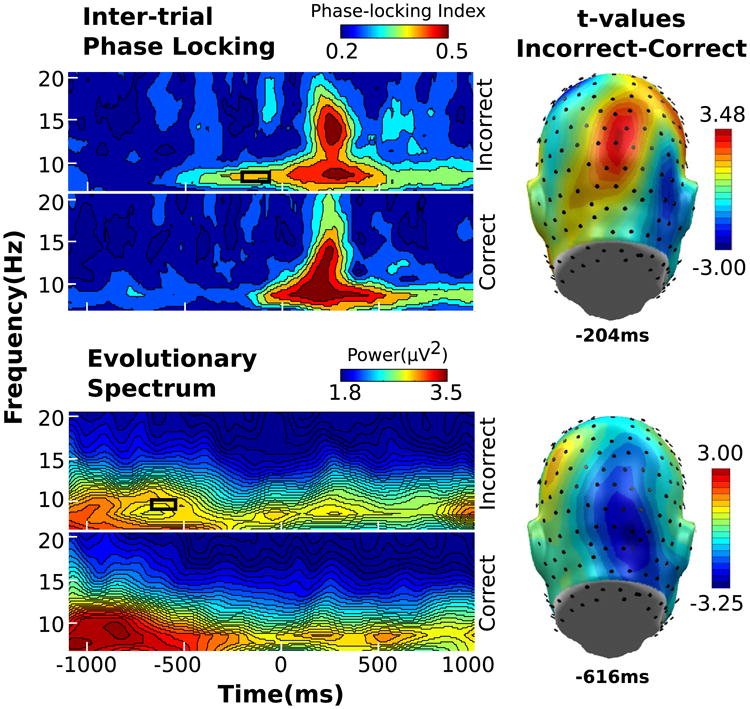
Wavelet illustrations for ITPL (left, top), power (left, bottom), for frequencies 6.73–20.50 Hz from −1100 to 1104 ms relative to T1 onset from one representative sensor, with time periods of significant incorrect versus correct differences indicated by the black box. Topographical distribution of t values (right) taken at the time point containing the maximum t value
Induced alpha power
Alpha power was also sensitive to performance
Two contiguous right occipital sensors showed significant power increase for correct compared to incorrect trials in the time range between −648 and −548 ms (see Fig. 3, bottom). The peak t values for within this sensor cluster were −3.15 (p < 0.05) and −3.25 (p < 0.05).
Evoked ssVEP and alpha power
When comparing the evoked spectrum for T2-correct and T2-incorrect trials using permutation-controlled t tests, no sensor clusters met the criteria for significance in either frequency band.
Cross-correlation function
Given that ssVEP ITPL and induced alpha power both showed sensitivity to T2 performance, their linear dependency was examined in a control analysis, aiming to establish the extent to which both measures reflect overlapping neural activity. Here, the cross-correlation function between the ssVEP ITPL and alpha-power time series was calculated at each sensor, resulting in a metric of linear dependency between the two variables at all possible temporal lags. This analysis suggested that the time courses of alpha power and ssVEP ITPL were not linearly related at any lag, supporting the statistical independency of these two measures.
Inter-site phase locking
Performance-related differences in ssVEP ITPL and alpha power were also followed up by connectivity analyses, focusing on the pre-T1 temporal regions of interest identified above for ssVEP ITPL and alpha power. Greater ssVEP phase locking between Oz and two spatially and temporally separate sensor clusters was observed for correct compared to incorrect trials. The first sensor cluster consisted of seven contiguous right frontal sensors and exceeded the significance threshold of −3.16 (p < 0.05) during a time period lasting from −676 to −608 ms, relative to T1 onset (Fig. 4, top). Within this cluster, the maximum t values ranged from −3.40 (p < 0.05) to −4.16 (p < 0.05). The second sensor cluster comprised of four contiguous left parietal sensors which exceeded significance from −152 to −138 ms (Fig. 4, bottom), with peak t values ranging from −3.52 (p < 0.05) to −5.26 (p < 0.05). In the alpha frequency band, ISPL values for correct and incorrect T2 trials did not differ.
Fig. 4.
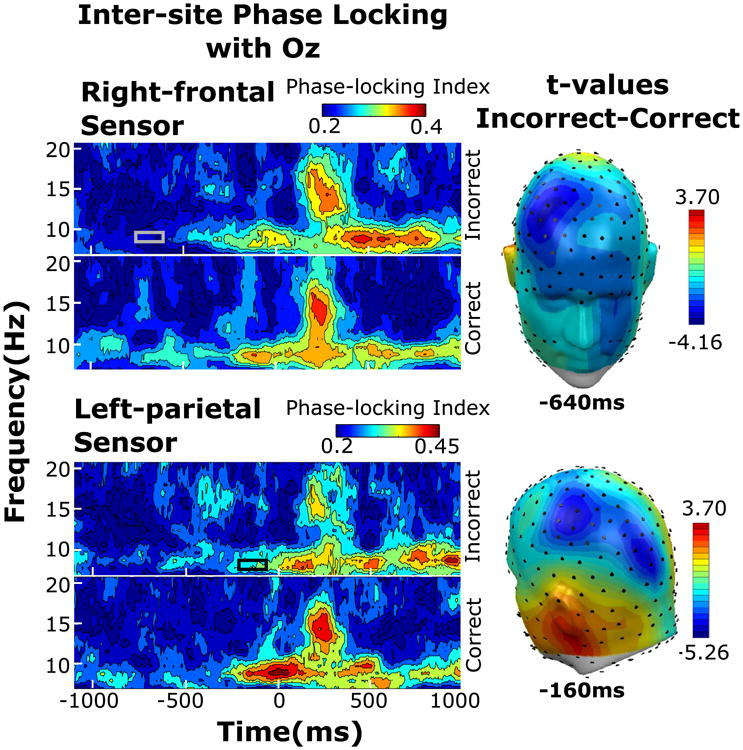
Wavelet illustrations for ISPL taken from one representative sensor within the right frontal (left, top) and right parietal (left, bottom) for frequencies 6.73–20.50 Hz from −1100 to 1104 ms relative to T1 onset from one representative sensor, with time periods of significant incorrect versus correct differences indicated by the gray or black box. Topographical distribution of t values (right) taken at the time point containing the maximum t value for either sensor cluster
Phase differences between T2-correct and T2-incorrect trials
Permutation-controlled comparisons of phase angles at the ssVEP frequency did not exceed the significance threshold. By contrast, alpha phase was related to performance: Centro-parietal midline sensors and a cluster of left superior temporal sensors (see Fig. 5b) showed sustained differences in the distribution of phase angles in a time segment between −294 and −188 ms relative to T1 onset (see Fig. 5a). No differences were observed during the early part of the epoch or after presentation of the targets. Follow-up tests examining the uniformity of the distribution of phase angles (see Fig. 5b) showed that, throughout the duration of the segment from −294 and −188 ms, phase angles during correct trials were non-uniform, showing significant (Rayleigh's R > 0.21, p < 0.05) preference for a specific direction. By contrast, phase angles during incorrect trials were uniformly distributed and did not evince any preference during the time segment of interest.
Fig. 5.
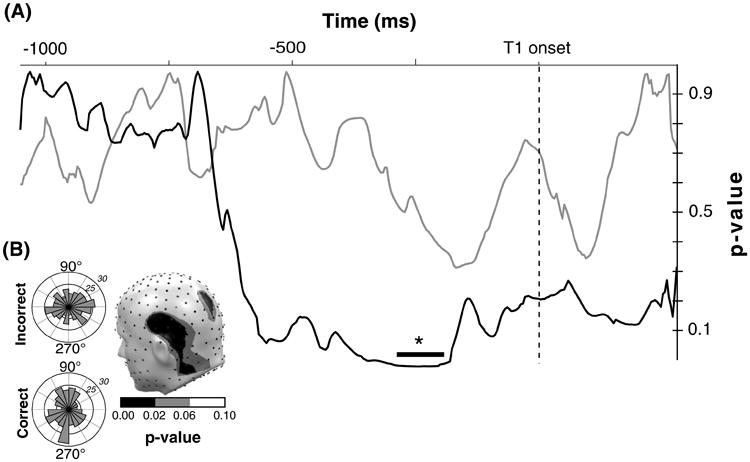
a p values across time for incorrect versus correct comparison of ongoing phase for ssVEP (gray) and alpha (black) at one representative sensor. The black bar indicates the time window in which the p values exceeded significance. b Rose plots of the phase angle distribution for all correct (left, bottom) and incorrect trials (left, top) at one representative time point within the time window of significant alpha p values. The topographical distribution of p values of correct versus incorrect alpha phase angles at this time point is illustrated (right)
Correlation between performance and oscillatory brain activity
Inter-individual AB performance was related to alpha-power differences between correct and incorrect trials at eight contiguous left occipito–parietal sensors near Oz (Fig. 6). In this cluster, Spearman rank correlation coefficients ranged from −0.48 (p < 0.05) to −0.78 (p < 0.05). Here, participants with greater pre-T1 alpha power during correct, compared to incorrect trials, displayed better performance during Lag 2, relative to Lag 4. By contrast, no relationship between ssVEP ITPL and AB performance was observed.
Fig. 6.
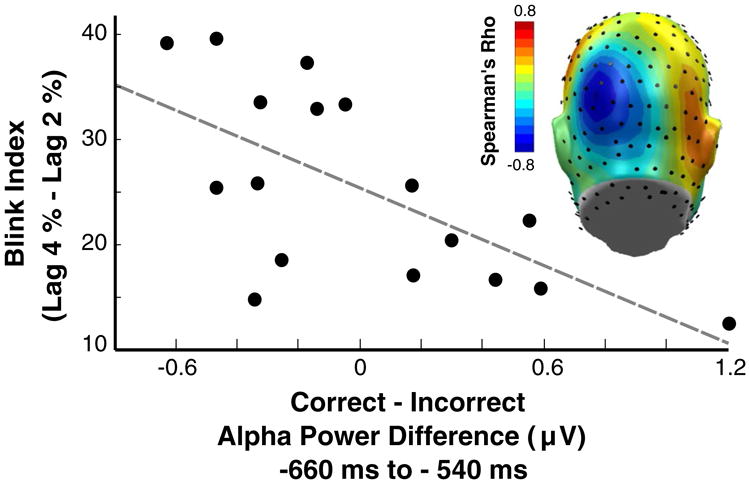
Inter-individual relationship between alpha-power differences (correct–incorrect) and AB performance (Lag 4 % correct = Lag 2 % correct) from one representative sensor with a Spearman's Rho of −0.60 (p < 0.05). Spearman's Rho values across sensors illustrated in the topographical distribution
Discussion
The purpose of this study was to investigate the extent to which pre-T1 EEG characteristics predict T2 report accuracy, focusing on differences in induced alpha power and ssVEP phase locking. Several hundreds of milliseconds prior to T1 onset, correct trials showed relatively heightened induced alpha power and increased ssVEP ISPL between Oz and right frontal electrodes. In a temporal window more proximate to T1 onset, correct trials were associated with relatively lower ssVEP ITPL and higher connectivity between Oz and a left parietal sensor cluster. In addition, a significant difference in the ongoing alpha phase was observed between correct and incorrect trials during this second temporal window, immediately preceding T1. Importantly, alpha power also predicted behavioral performance across participants, with individuals showing heightened alpha power prior to T1 in correct trials overall outperforming those with no or opposite alpha-power modulations.
To the extent that alpha power and ssVEP phase locking showed complementary, opposite, patterns regarding their prediction of behavioral accuracy, one may ask whether the two measures were negatively related and possibly reflect the same underlying process. An analysis of the cross-correlation function between the two metrics demonstrated that they were statistically independent. While a linear relationship between the markers of oscillatory activity considered here was not supported, their temporal dynamics vis-a-vis performance suggested significant functional overlap: During an early time period beginning around −650 ms relative to T1, increased alpha power at occipital electrodes and increased ssVEP phase locking between Oz and frontal sensors were associated with correct T2 report. The former effect (heightened pre-T1 alpha power in correct trials) also predicted inter-individual differences in T2 accuracy at Lag 2, relative to Lag 4: More alpha enhancement in correct trials was related to better performance during the AB interval. More proximate to T1 onset, a combination of (a) decreased ssVEP inter-trial phase locking, (b) heightened ssVEP ISPL with left occipito–parietal sensors, and (c) alignment of the alpha phase at posterior sensors was predictive of T2 being correctly reported. Together, these differences are consistent with a mechanism of active suppression of sensory cortical processing during the distractor stream, prior to T1. Such an inhibitory role has been proposed in traditional and contemporary neurophysiological models of alpha oscillations (Steriade and Llinás 1988; Klimesch et al. 2006), in which alpha is seen as an index of cortical excitability, potentially reflecting the thalamocortical information flow (Steriade and Llinás 1988). The interactions between evoked and induced oscillations during AB at the level of power, phase, and connectivity may be a productive area of future research.
Relatively heightened alpha power has traditionally been considered an index of a more controlled and restricted flow of information from the thalamus to the cortex (Adrian and Matthews 1934) and has recently been linked to a range of processes involving top-down, or “controlled” processing (Klimesch et al. 2006). In a similar vein, heightened alpha activity may reflect an attentional gating mechanism in which the cortical processing of external, sensory information is reduced (Klimesch et al. 2006), potentially through changes in excitability (Romei et al. 2010). In line with this notion, visual stimuli presented at near-threshold levels are more likely to be detected at lower levels of alpha power (Ergenoglu et al. 2004), with some researchers suggesting that intermediate levels of alpha power index near-optimal states for stimulus processing (Rajagovindan and Ding 2011). Together, these studies suggest that high alpha power may characterize a state of less sensory cortical excitability, potentially related to lower levels of external attention control. The present finding of heightened alpha activity prior to T1 selectively during T2-correct trials is therefore consistent with contemporary models that emphasize the role of pre-T1 neurocognitive processes for AB performance as reviewed in Dell'Acqua et al. (2012), or Martens and Wyble (2010). Experimental and computational evidence has converged to demonstrate that relatively heightened activation of the internal representation of the targets, in combination with relatively less attention directed to pre-target distractors and the T1, predicts correct T2 report during the AB (Olivers and Nieuwenhuis 2005). Specifically, the present result aligns with overinvestment theories of the AB in which excessively applied attention control in the time region around T1 negatively affects T2 report accuracy (Olivers and Meeter 2008; Taatgen et al. 2009). Whether the AB effect holds a systematic relationship with alpha power and whether such an effect holds only for intermediate levels is outside the scope of the current experiment.
Consistent with these considerations, inter-trial ssVEP phase locking was heightened immediately prior to T1 onset, in trials with incorrect T2 report. Paralleling the findings with alpha power, this supports the notion that facilitated processing of the pre-target distracters is detrimental for correctly reporting T2: When evoked by an external stimulus, a more consistent ssVEP phase across trials reflects more synchronous and temporally stable engagement of the neural tissue, which has been linked to increased selective attention (Ding et al. 2006; Porcu et al. 2013). Thus, in addition to differences in alpha power, ssVEP ITPL differences are pertinent for hypotheses of the AB that highlight “overzealously applied” attentional selection of RSVP items (Olivers and Meeter 2008; Taatgen et al. 2009). Visuocortical over-engagement early in a trial is predictive of relatively poorer performance later in the same trial in sustained attention tasks, assessed through behavioral experiments (Ling and Carrasco 2006) as well as ssVEPs (Wieser and Keil 2011). Consistent with the present findings, detecting multiple targets may benefit from directing less attention toward the pre-target distractor stream. One may speculate, then, that an initially less attentive state is beneficial for rapid and accurate processing of alternating distractors and targets.
The present results replicate the report by MacLean and Arnell (2011) who observed greater alpha reduction on T2 missed trials. By contrast, Kranczioch et al. (2007) reported heightened pre-T1 alpha power for incorrect trials. As noted by these same authors, this discrepant finding may be related to the fact that the RSVP frequency (10 Hz) in Kranczioch et al. (2007) was centered in the alpha range, preventing a spectral separation of alpha and ssVEP responses. In line with the present results however, Kranczioch et al. (2007) found pre-T1 inter-trial phase coherency to be higher on T2 incorrect trials.
Given the close spectral proximity of the alpha band and the ssVEP frequency in the present study, the question arises regarding the independence of the oscillatory activity at the RSVP frequency and in the alpha frequency range. In the current results, induced power at the ssVEP frequency followed a similar pattern as the alpha band, showing greater power for correct compared to incorrect trials in time ranges preceding T1. However, a difference in ssVEP ITPL values was not observed during this time, which provides evidence that the difference in power during this time period is likely not due to oscillatory activity evoked by the RSVP train. In addition, performance-related differences in induced power at the ssVEP or alpha band did not emerge during the difference in ITPL beginning at −250 ms. Together, these results suggest that the temporal dynamics of the ssVEP ITPL are unaffected by induced power, suggesting that oscillatory activity evoked by the RSVP stimulus stream may be separable from alpha using ITPL measurements.
Whereas high inter-trial phase locking indexes temporally stable engagement of neural tissue, greater phase locking between electrode sites as measured by ISPL represents a higher degree of coordinated activation between cortical sites and suggests a degree of cortico-cortical communication (Lachaux et al. 1999). The present study demonstrated greater ISPL at the ssVEP frequency between Oz and a right frontal sensor cluster as well as a left parietal sensor cluster for T2-correct compared to incorrect trials. These differences in connectivity temporally coincided with increased alpha power and decreased ssVEP ITPL, respectively, which may be taken to indicate that changes in neural connectivity at the RSVP frequency were related to local modulations across both frequencies. Relatively increased hemodynamic activity in both parietal (Marois et al. 2004) and frontal cortices (Marois et al. 2000, 2004) has been observed during correct, compared to incorrect trials in RSVP tasks. Electromagnetic indices have converged with these findings, also showing increased phase locking between fronto-parietal regions and the occipital cortex selectively during correct T2 report (Gross et al. 2004). Integrating this literature, Hommel et al. (2006) concluded that heightened communication between the visual cortex and widespread cortical sites represents a potential mechanism mediating temporal attention control. Replicating recent connectivity analyses of the AB (Glennon et al. 2015), the present study extends such notions by demonstrating heightened inter-site connectivity during specific epochs preceding T1 for T2-correct trials, accompanying local modulations of alpha and ssVEP signals, respectively.
Significant differences in the phase angle for alpha, but not ssVEP, were found during a pre-T1 time period coinciding with the temporal window of ssVEP ITPL differences, immediately preceding T1. Mid-occipital alpha phases during this time period were uniform for incorrect but not correct trials, indicating that a specific phase angle was characteristic of correct, but not incorrect trials. Overlapping in time with changes in ITPL and inter-site connectivity, this systematic change in phase alignment may be part of altered connectivity and synchrony also characterized by local power and phase-locking measures, consistent with modulations of attention or attention control. Notably, we did not observe differences in phase angle distribution at the time of T1 and T2 onset, reported previously by Zauner et al. (2012), which may be due to the fact that these authors used a 10-Hz presentation rate for the RSVP, thus evoking oscillations at the center of the alpha frequency range.
No effect of distractor valence on T2 report was observed. Given the growing literature on attention capture effects by emotional distracters, this negative result may well indicate that manipulating stimulus saliency at the serial position just after T1 does not affect the AB. Considering the significance of pre-T1 attention-related EEG in predicting the AB observed in the present study however, one may hypothesize that emotionally salient distracters may affect performance when presented prior to rather than in between the targets. Future work may investigate whether salient distractors presented prior to T1 prompt differential T2 report and/or impact the pre-T1 alpha and ssVEP oscillations.
In conclusion, temporally specific modulations of pre-T1 alpha power, alpha phase, and ssVEP phase locking predicted whether T2 was correctly reported in the critical Lag 2 condition. Furthermore, inter-individual differences in pre-T1 alpha-power modulations predicted variability in AB performance across participants. Together, these results can be taken to highlight the important role of pre-target attentional states for T2 performance, with T2 report being hindered by states of heightened attentional control applied to distracters preceding T1 and likely T1 itself. This interpretation is consistent with over-investment theories of the AB.
Acknowledgments
The authors thank Nadia Cacodcar for her assistance during data collection. This research was funded by a Grant from the NIH (R01 MH097320).
Footnotes
Compliance with ethical standards: Conflict of interest The authors declare that they have no conflicts of interest.
References
- Adrian ED, Matthews BHC. The Berger rhythm: potential changes from the occipital lobes in man. Brain. 1934;57(4):355–385. doi: 10.1093/brain/57.4.355. [DOI] [PubMed] [Google Scholar]
- Asplund CL, Fougnie D, Zughni S, Martin JW, Marois R. The attentional blink reveals the probabilistic nature of discrete conscious perception. Psychol Sci. 2014;25(3):824–831. doi: 10.1177/0956797613513810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimunta A, Mo J, Schroeder CE, Ding M. Neuronal mechanisms and attentional modulation of corticothalamic alpha oscillations. J Neurosci. 2011;31(13):4935–4943. doi: 10.1523/JNEUROSCI.5580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective norms for English words (ANEW): instruction manual and affective ratings. University of Florida, Center for the Study of Emotion and Attention; Gainesville: 1999. [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Busch NA, Dubois J, VanRullen R. The phase of ongoing EEG oscillations predicts visual perception. J Neurosci. 2009;29(24):7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM, Potter MC. A two-stage model for multiple target detection in rapid serial visual presentation. J Exp Psychol Hum Percept Perform. 1995;21(1):109–127. doi: 10.1037//0096-1523.21.1.109. [DOI] [PubMed] [Google Scholar]
- Dell'Acqua R, Dux PE, Wyble B, Jolicoeur P. Sparing from the attentional blink is not spared from structural limitations. Psychon Bull Rev. 2012;19(2):232–238. doi: 10.3758/s13423-011-0209-3. [DOI] [PubMed] [Google Scholar]
- Di Lollo V, Kawahara J, Shahab Ghorashi SM, Enns JT. The attentional blink: resource depletion or temporary loss of control? Psychol Res. 2005;69(3):191–200. doi: 10.1007/s00426-004-0173-x. [DOI] [PubMed] [Google Scholar]
- Ding J, Sperling G, Srinivasan R. Attentional modulation of SSVEP power depends on the network tagged by the flicker frequency. Cereb Cortex. 2006;16(7):1016–1029. doi: 10.1093/cercor/bhj044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergenoglu T, Demiralp T, Bayraktaroglu Z, Ergen M, Beydagi H, Uresin Y. Alpha rhythm of the EEG modulates visual detection performance in humans. Cogn Brain Res. 2004;20(3):376–383. doi: 10.1016/j.cogbrainres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Fisher NI. Statistical analysis of circular data. Cambridge University Press; Cambridge: 1995. [Google Scholar]
- Foxe JJ, Snyder AC. The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front Psychol. 2011;2:154. doi: 10.3389/fpsyg.2011.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon M, Keane MA, Elliott MA, Sauseng P. Distributed cortical phase synchronization in the EEG reveals parallel attention and working memory processes involved in the attentional blink. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv023. [DOI] [PubMed] [Google Scholar]
- Gross J, Schmitz F, Schnitzler I, Kessler K, Shapiro K, Hommel B, Schnitzler A. Long-range neural synchrony predicts temporal limitations of visual attention in humans. Proc Natl Acad Sci USA. 2004;101:13050–13055. doi: 10.1073/pnas.0404944101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel B, Kessler K, Schmitz F, Gross J, Akyurek E, Shapiro K, Schnitzler A. How the brain blinks: towards a neurocognitive model of the attentional blink. Psychol Res. 2006;70(6):425–435. doi: 10.1007/s00426-005-0009-3. [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Human Neurosci. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex. 2002;12(8):877–882. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- Keil A, Heim S. Prolonged reduction of electrocortical activity predicts correct performance during rapid serial visual processing. Psychophysiology. 2009;46:718–725. doi: 10.1111/j.1469-8986.2009.00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Gruber T, Muller MM. Functional correlates of macroscopic high-frequency brain activity in the human visual system. Neurosci Biobehav Rev. 2001;25(6):527–534. doi: 10.1016/s0149-7634(01)00031-8. [DOI] [PubMed] [Google Scholar]
- Keil A, Ihssen N, Heim S. Early cortical facilitation for emotionally arousing targets during the attentional blink. BMC Biol. 2006;4:23. doi: 10.1186/1741-7007-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Hanslmayr S. Upper alpha ERD and absolute power: their meaning for memory performance. In: Neuper C, Klimesch W, editors. Progress in brain research. Vol. 159. Elsevier; 2006. pp. 151–165. Retrieved from http://www.sciencedirect.com/science/article/pii/S0079612306590107. [DOI] [PubMed] [Google Scholar]
- Kranczioch C, Debener S, Maye A, Engel AK. Temporal dynamics of access to consciousness in the attentional blink. NeuroImage. 2007;37(3):947–955. doi: 10.1016/j.neuroimage.2007.05.044. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8(4):194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S, Carrasco M. When sustained attention impairs perception. Nat Neurosci. 2006;9(10):1243–1245. doi: 10.1038/nn1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean MH, Arnell KM. Greater attentional blink magnitude is associated with higher levels of anticipatory attention as measured by alpha event-related desynchronization (ERD) Brain Res. 2011;1387:99–107. doi: 10.1016/j.brainres.2011.02.069. [DOI] [PubMed] [Google Scholar]
- Marois R, Chun MM, Gore JC. Neural correlates of the attentional blink. Neuron. 2000;28:299–308. doi: 10.1016/s0896-6273(00)00104-5. [DOI] [PubMed] [Google Scholar]
- Marois R, Yi DJ, Chun MM. The neural fate of consciously perceived and missed events in the attentional blink. Neuron. 2004;41:465–472. doi: 10.1016/s0896-6273(04)00012-1. [DOI] [PubMed] [Google Scholar]
- Martens S, Wyble B. The attentional blink: past, present, and future of a blind spot in perceptual awareness. Neurosci Biobehav Rev. 2010;34(6):947–957. doi: 10.1016/J.Neubiorev.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti S, Sigman M, Dehaene S. A shared cortical bottleneck underlying attentional blink and psychological refractory period. NeuroImage. 2012;59(3):2883–2898. doi: 10.1016/j.neuroimage.2011.09.063. [DOI] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. To see or not to see: prestimulus α phase predicts visual awareness. J Neurosci. 2009;29(9):2725–2732. doi: 10.1523/JNEUROSCI.3963-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson KE, Lleras A, Beck DM, Fabiani M, Ro T, Gratton G. Pulsed out of awareness: EEG alpha oscillations represent a pulsed-inhibition of ongoing cortical processing. Front Psychol. 2011 doi: 10.3389/fpsyg.2011.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MM, Hillyard S. Concurrent recording of steady-state and transient event-related potentials as indices of visual-spatial selective attention. Clin Neurophysiol. 2000;111(9):1544–1552. doi: 10.1016/s1388-2457(00)00371-0. [DOI] [PubMed] [Google Scholar]
- Müller MM, Malinowski P, Gruber T, Hillyard SA. Sustained division of the attentional spotlight. Nature. 2003;424(6946):309–312. doi: 10.1038/nature01812. [DOI] [PubMed] [Google Scholar]
- Neuper C, Scherer R, Reiner M, Pfurtscheller G. Imagery of motor actions: differential effects of kinesthetic and visual-motor mode of imagery in single-trial EEG. Cogn Brain Res. 2005;25(3):668–677. doi: 10.1016/j.cogbrainres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Nieuwenstein MR, Potter MC, Theeuwes J. Unmasking the attentional blink. J Exp Psychol Hum Percept Perform. 2009;35(1):159–169. doi: 10.1037/0096-1523.35.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivers CNL, Meeter M. A boost and bounce theory of temporal attention. Psychol Rev. 2008;115(4):836–863. doi: 10.1037/a0013395. [DOI] [PubMed] [Google Scholar]
- Olivers CNL, Nieuwenhuis S. The beneficial effect of concurrent task-irrelevant mental activity on temporal attention. Psychol Sci. 2005;16(4):265–269. doi: 10.1111/j.0956-7976.2005.01526.x. [DOI] [PubMed] [Google Scholar]
- Olivers CN, van der Stigchel S, Hulleman J. Spreading the sparing: against a limited-capacity account of the attentional blink. Psychol Res. 2007;71:126–139. doi: 10.1007/s00426-005-0029-z. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Berghold A. Patterns of cortical activation during planning of voluntary movement. Electroencephalogr Clin Neurophysiol. 1989;72(3):250–258. doi: 10.1016/0013-4694(89)90250-2. [DOI] [PubMed] [Google Scholar]
- Porcu E, Keitel C, Müller MM. Concurrent visual and tactile steady-state evoked potentials index allocation of inter-modal attention: a frequency-tagging study. Neurosci Lett. 2013;556:113–117. doi: 10.1016/j.neulet.2013.09.068. [DOI] [PubMed] [Google Scholar]
- Potter MC, Staub A, O'Connor DH. The time course of competition for attention: attention is initially labile. J Exp Psychol Hum Percept Perform. 2002;28(5):1149–1162. doi: 10.1037//0096-1523.28.5.1149. [DOI] [PubMed] [Google Scholar]
- Rajagovindan R, Ding M. From prestimulus alpha oscillation to visual-evoked response: an inverted-U function and its attentional modulation. J Cogn Neurosci. 2011;23(6):1379–1394. doi: 10.1162/jocn.2010.21478. [DOI] [PubMed] [Google Scholar]
- Ray WJ, Cole HW. EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science. 1985;228(4700):750–752. doi: 10.1126/science.3992243. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: an attentional blink? J Exp Psychol Hum Percept Perform. 1992;18(3):849–860. doi: 10.1037/0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Regan D. Human brain electrophysiology: evoked potentials and evoked magnetic fields in science and medicine. Elsevier; New York: 1989. [Google Scholar]
- Romei V, Gross J, Thut G. On the role of prestimulus alpha rhythms over occipito-parietal areas in visual input regulation: correlation or causation? J Neurosci. 2010;30(25):8692–8697. doi: 10.1523/JNEUROSCI.0160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro K, Schmitz F, Martens S, Hommel B, Schnitzler A. Resource sharing in the attentional blink. NeuroReport. 2006;17(2):163–166. doi: 10.1097/01.wnr.0000195670.37892.1a. [DOI] [PubMed] [Google Scholar]
- Steriade M, Llinás RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68(3):649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Taatgen NA, Juvina I, Schipper M, Borst JP, Martens S. Too much control can hurt: a threaded cognition model of the attentional blink. Cogn Psychol. 2009;59(1):1–29. doi: 10.1016/j.cogpsych.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3(4):151–162. doi: 10.1016/S1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced γ-band activity during the delay of a visual short-term memory task in humans. J Neurosci. 1998;18(11):4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E, Nieuwenstein M, Kanwisher N. Temporal selection is suppressed, delayed, and diffused during the attentional blink. Psychol Sci. 2008;19(1):55–61. doi: 10.1111/j.1467-9280.2008.02046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz N, Wühle A, Monittola G, Demarchi G, Frey J, Popov T, Braun C. Prestimulus oscillatory power and connectivity patterns predispose conscious somatosensory perception. Proc Natl Acad Sci. 2014;111(4):E417–E425. doi: 10.1073/pnas.1317267111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser MJ, Keil A. Temporal trade-off effects in sustained attention: dynamics in visual cortex predict the target detection performance during distraction. J Neurosci. 2011;31(21):7784–7790. doi: 10.1523/JNEUROSCI.5632-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyble B, Potter MC, Bowman H, Nieuwenstein M. Attentional episodes in visual perception. J Exp Psychol Gen. 2011;140(3):488. doi: 10.1037/a0023612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauner A, Fellinger R, Gross J, Hanslmayr S, Shapiro K, Gruber W, Klimesch W. Alpha entrainment is responsible for the attentional blink phenomenon. NeuroImage. 2012;63(2):674–686. doi: 10.1016/j.neuroimage.2012.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]