Abstract
Anxiety is an all-inclusive concept incorporating somatic symptoms (palpitations, dizziness, dyspnea), emotional and cognitive elements (negative affect, fear, worry, rumination) and behavioral components (e.g., avoidance). The aim of this study was to examine the specific neural correlates associated with anxiety phenotypes (worry, rumination, somatic anxiety) and negative affect (neuroticism). Twenty-nine anxious participants and thirty healthy controls were included in the study. We analyzed seed-based intrinsic connectivity and used correlation maps in a multivariable regression model to describe the specific effect of each anxiety phenotype independently of the effects of age and the other measures of anxiety. Worry severity was uniquely correlated with increased intrinsic connectivity between right anterior insula (RAI) and the precuneus. Global and somatic anxiety were associated with the limbic and paralimbic structures (increased connectivity between the amygdala, PVN, and hippocampus), while neuroticism was correlated with increased connectivity between limbic and prefrontal structures. Rumination severity did not correlate significantly with any measures of functional connectivity once we controlled for other clinical measures of anxiety. Measures of worry, global anxiety, somatic anxiety, and neuroticism have distinct ‘neural signatures’. These results advocate for a fine-grain approach when analyzing the neural substrates of clinical samples with various anxiety disorders.
1. Introduction
Anxiety is an all inclusive concept incorporating somatic symptoms (e.g., palpitations, dizziness, dyspnea), emotional and cognitive elements (e.g., negative affect, fear, worry, and rumination) and behavioral components (e.g., avoidance) (Zebb and Beck, 1998). Additionally, personality traits such as neuroticism are highly comorbid with anxiety disorders (Clark et al., 1994; Hettema et al., 2006; Hettema et al., 2004). All of these phenotypes have been the object of extensive research and have been characterized by constructs such as defensive reactivity (Lueken et al., 2013), intolerance of uncertainty (Krain et al., 2008; Simmons et al., 2008), anticipatory apprehension (Nitschke et al., 2009), emotional reactivity (Goldin et al., 2009), emotion regulation (Campbell-Sills et al., 2010), and interoceptive sensitivity (Domschke et al., 2010). Multiple studies have described several neuroimaging features of anxiety (for review see (Etkin and Wager, 2007) and (Hilbert et al., 2014)). Most of these studies have focused on specific disorders, such as specific phobia, panic disorder, post-traumatic stress disorder (PTSD), generalized anxiety disorder (GAD), and social phobia. Some of the neurobiological findings implicate structures involved in heightened fear response, especially hyperactivation in the amygdala and insula in specific phobia, PTSD and social anxiety (Etkin and Wager, 2007). PTSD has been additionally linked to hypoactivity in the thalamus, the dorsal and rostral cingulate as well as the ventro- and dorsomedial prefrontal cortex (Etkin and Wager, 2007). GAD has been associated with a more polymorphic pattern, including heightened amygdala response to anticipatory threat (Nitschke et al., 2009), increased amygdala-dorsolateral prefrontal connectivity (Etkin et al., 2009), and greater insula-orbitofrontal connectivity during induction of worry (Andreescu et al., 2014a).
The neurobiological landscape shaped by these studies offers, however, a limited view on specific anxiety phenotypes. Following the RDoC perspective to isolate core neurobiological processes linked to psychopathology, we propose in this study to identify the neural markers related to specific anxiety phenotypes such as worry, rumination, and somatic anxiety. In order to analyze the neural basis of these psychopathological components, we have used specific psychometric scales or homogenous factors extracted from well-validated psychometric scales.
In this study, we examine the functional connectivity markers correlated with three different anxiety phenotypes: worry, somatic anxiety, and rumination. We also include the total HARS as an omnibus measure of global anxiety. Additionally, we include neuroticism in the model in order to isolate the effects of personality traits. We included the neuroticism subscale from the Five Factor Inventory (FFI-N) given the well-described association between neuroticism and anxiety (Clark et al., 1994), including worry (Hale et al., 2010; Watson et al., 1994). A recent neuroimaging study (Servaas et al., 2014) examined the neural correlates of worry in association with neuroticism and found an association between neuroticism and decreased activation in the retrosplenial and visual cortex during worry induction.
In order to examine the functional connectivity correlates of anxious phenotypes, we focused on two neural networks frequently involved in anxious psychopathology, namely the Default Mode Network (DMN) and the Salience Network (SN). The DMN is an organized functional network of several brain regions: posterior cingulate cortex (PCC), medial prefrontal cortex, inferior parietal lobule, and medial temporal regions (Raichle et al., 2001). This network shows a high level of functional connectivity at rest, and its activity consistently decreases during performance of active tasks such as goal directed cognition and task engagement (Buckner et al., 2008; Raichle et al., 2001). Changes in the DMN intrinsic connectivity have been reported in social phobia and generalized anxiety (Andreescu et al., 2014b; Ding et al., 2011; Gentili et al., 2009; Liao et al., 2010; Zhao et al., 2007). The SN, comprised of the anterior insula, dorsal anterior cingulate cortex (ACC), amygdala, ventral tegmental area, and the ventromedial nucleus of the thalamus, is involved in monitoring the salience of interoceptive and external events (Craig, 2009; Menon and Uddin, 2010). Abnormal SN connectivity has been implicated in anxiety disorders as the neural basis for pathologically enhanced salience detection (Andreescu et al., 2014a; Pannekoek et al., 2012a, b; Paulus and Stein, 2006). We explored the functional connectivity of the SN using two seeds: the right anterior insula (RAI) and the left amygdala.
We also explored the functional connectivity of three additional regions-of-interest (ROI) frequently cited in the neurobiological literature of anxiety: the ventral hippocampus, implicated in emotion generation and regulation (Adhikari, 2014; Bishop, 2007; Chen and Etkin, 2013; Davis and Whalen, 2001), the bed nucleus of stria terminalis (BNST), which is considered a key brain ROI for generalized anxiety (Davis, 1998, 1999; Davis et al., 2010; Walker et al., 2009) and stress regulation (Crane et al., 2003), and the paraventricular nucleus (PVN), which critical for both neuroendocrine and autonomic stress regulation (Flandreau et al., 2012; Pego et al., 2010).
We hypothesize that each anxiety phenotype has a different neural signature, but that all overlap partially with the neural signatures of global anxiety and neuroticism. More specifically, we hypothesized that 1) the worry phenotype will be correlated mainly with RAI and BNST connectivity(Andreescu et al., 2015; Walker et al., 2009), 2) the somatic and global anxiety will be correlated mainly with functional connectivity of the limbic/paralimbic structures (amygdala, PVN, ventral hippocampus(Bishop, 2007; Etkin and Wager, 2007)), 3) the rumination phenotype will correlate with PCC connectivity (Berman et al., 2011), and 4) the neuroticism phenotype will have a more diffuse signature, including correlations with PCC, RAI as well as limbic/paralimbic structures (Feinstein et al., 2006; Stein et al., 2007) (Adelstein et al., 2011; Aghajani et al., 2013).
2. Method
2.1. Participants
The data were collected from two studies conducted at the University of Pittsburgh: “Structural and functional neuroanatomy of late-life GAD” and “A pilot fMRI study of emotion modulation in midlife anxiety.” Subjects were recruited from direct advertisement through flyers, local radio and bus ads, as well as from two research registries affiliated with the University of Pittsburgh: The Advanced Center for Intervention and Services Research in Late-Life Mood Disorders (ACISR) registry and the Clinical and Translational Science Institute (CTSI) registry.
This study included participants diagnosed with GAD, as well as non-anxious participants. The primary inclusion criteria for the anxiety participants was a principal diagnosis of GAD for at least six months according to the Structured Clinical Interview for DSM-IV (SCID)(First M, 1995) and a score of 17 or higher on the HARS(Hamilton, 1959) at the time of scanning. Patients with other anxiety disorders were included if GAD was the principal diagnosis (based on severity and duration), as were patients with a past history of alcohol or substance abuse that was in full remission for at least three months. Lifetime comorbid unipolar depression was allowed if GAD was the primary diagnosis (based on duration), but subjects with current Major Depressive Disorder at the time of scanning were excluded.
Other exclusion criteria were lifetime psychosis or bipolar disorder, a diagnosis of dementia, a Mini Mental State Examination score less than 24, increased suicide risk (e.g., current ideation), medical instability according to reviews of medical chart data, ongoing psychotherapy, and current antidepressant or anxiolytic use. All subjects were psychotropic-free at the time of scanning, and they underwent a wash out period of two weeks if previously on an antidepressant (six weeks if on fluoxetine). Participants were allowed to receive non-psychotropic medications. Non-anxious participants had no history of psychiatric disorders. Both studies were approved by the University of Pittsburgh Institutional Review Board.
2.2. Clinical Measures
Participants were assessed using the Hamilton Anxiety Rating Scale (HARS), the self-report Penn State Worry Questionnaire (PSWQ)(Meyer et al., 1990), the Response Style Questionnaire – Rumination Subscale RSQ-RS(Treynor, 2003) and the Five Factor Inventory (FFI) – Neuroticism subscale (FFI-N)(Costa PT, 1992).
The Hamilton Anxiety Rating Scale (HARS) (Hamilton, 1959) is one of the most popular scales to measure the severity of anxiety symptoms. The 14-item assessment measures both psychic anxiety (mental agitation and psychological distress) and somatic anxiety - physical complaints related to anxiety, such as cardiovascular symptoms (e.g., palpitations, chest pain), respiratory symptoms (e.g., choking feelings, sighing, dyspnea) gastrointestinal symptoms (e.g., swallowing difficulties, burning sensations, nausea), genitourinary symptoms (e.g., urgency, premature ejaculation, and etc.). While largely used as a measure of global anxiety (Clark and Donovan, 1994), the HARS has been criticized for its lack of specificity especially with regard to the somatic symptoms (Maier et al., 1988). HARS factor analysis (Serretti et al., 1999) identified two factors: somatic (somatic-sensory, cardiovascular, respiratory, somatic-muscular, gastrointestinal, genitourinary, and autonomic) and ‘psychic’ anxiety (anxious mood, tension, behavior, fears, and insomnia). For this study, we used the total HARS as a measure of global anxiety and the HARS-somatic subscale as a measure of somatic anxiety.
The Penn State Worry Questionnaire (PSWQ) (Meyer et al., 1990) has been developed specifically to measure worry. The questionnaire correlates predictably with several psychological measures related to worry and does not correlate with anxiety measures more remotely related to the worry construct, such as the HARS or State-Trait Anxiety Inventory (Meyer et al., 1990). Correlations between the PSWQ and other measures of anxiety and depression support its convergent and discriminant validity (Brown et al., 1992).
The Response Style Questionnaire – Rumination Subscale (RSQ-RS) (Treynor, 2003) has been developed as a self-report measure of rumination, distinct from depression content. Traditionally associated with depression (Nolen-Hoeksema, 2000), rumination has also been analyzed in relationship to worry (Hong, 2007). Worry is typically associated with anxiety about future events, while rumination is associated with depression over past events (Hong, 2007). Several studies have shown that worry and rumination are distinct factors in joint factor analyses (Hong, 2007), but they share a common higher order factor in the form of perseverative thought (Segerstrom et al., 2010).
2.3. MR Data Acquisition
Data acquisition was identical in both studies. Resting state MRI data were acquired during a five-minute interval while participants looked at a fixation point on the screen. Participants were instructed to think of nothing in particular during this interval. Resting state data were collected prior to the collection of other task-related fMRI data.
Imaging data were collected with a 3-Tesla Siemens Trio TIM scanner, with a 32-channel head coil, located in the MR Research Center at the University of Pittsburgh. T2*-weighted BOLD images were acquired using a gradient-echo echoplanar imaging (EPI) sequence in axial orientation (parallel to AC-PC): TR/TE=2000/34 ms, matrix size=128×128×28, voxel size=2×2×3mm (no gap), flip angle=90°. A total of 150 temporal images were acquired for each subject. The most inferior slice was located below the most inferior aspect of the temporal lobes. High-resolution anatomical images (T1-weighted magnetization-prepared rapid gradient echo MPRAGE) were collected over four minutes and 43 seconds using the following parameters: FOV=256×254mm, voxel size 1×1×1mm, TI=900 ms, TR/TE=2/3.43 ms, flip angle=9°.
2.4. Image Preprocessing
Functional imaging data were processed and analyzed with Statistical Parametric Mapping 8 (SPM8; Wellcome Trust Center for Neuroimaging, London, UK. http://www.fil.ion.ucl.ac.uk/spm/software/spm8) implemented in Matlab (Mathworks, Natick, MA). The temporal functional images for each subject were realigned to the first image using rigid-body transformation. The structural grey matter images were segmented from the MPRAGE image and used to normalize the functional images to the standard MNI space. This was done by first registering the grey matter image to the mean realigned functional images using a linear affine transformation; the grey matter image was then normalized to the SPM Montreal Neurologic Institute grey matter template using a non-linear transformation with basis functions (Ashburner and Friston, 1999). Finally, the same transformation was applied to all the functional images. Normalized functional images were then smoothed using a 10mm Gaussian smoothing kernel to account for the greater morphologic variability in the elderly sample (Reuter-Lorenz and Lustig, 2005).
2.5. Functional Connectivity Analysis
The Robust Weighted Least Square (WLS) toolbox (version 3.1, SPM8) was used to detect and adjust for artifacts in the fMRI time series data. This method uses the residual variance to weight the images, resulting in an optimal model estimation of noisy data due to motion artifacts (Diedrichsen and Shadmehr, 2005) (www.icn.ucl.ac.uk/motorcontrol/imaging/robustWLS.html).
2.5.1. Seed Regions (see Fig 1)
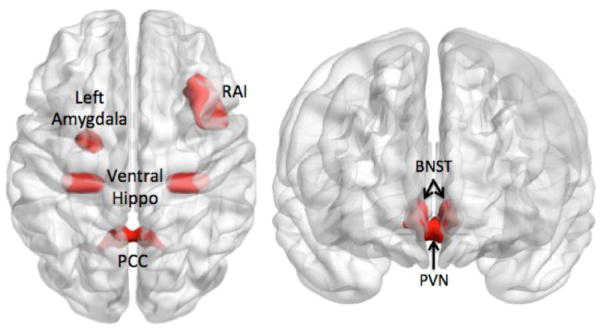
Fig 1.
Seed-based connectivity correlates of anxiety – Seeds used in the analysis
RAI=right anterior insula; PCC=posterior cingulate cortex; BNST=bed nucleus of stria terminalis; PVN=paraventricular nucleus.; Ventral Hippo=ventral hippocampus
The PCC was used as the seed region to examine the connectivity within the DMN (Raichle et al., 2001). The left and right posterior cingulate from the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) (1×1×1mm) in Colin27 space was down-sampled to a voxel resolution of 3.75 × 3.75 × 3.75mm (left and right PCC combined, 200 voxels). A smaller ROI of 39 voxels, centered on the posterior cingulate, was created as seed region performing erosions (2 iterations, 6 connected, 2.5-dimensional) with a 3×3×3mm voxel structuring element. Based on extensive prior literature, the right anterior insula (RAI) was used as the seed region to examine the connectivity within the SN (Cauda et al., 2011; Uddin, 2015). The RAI seed was extracted from the right insula region defined in the AAL atlas in the WFU Pick-Atlas (Maldjian et al., 2003). From the insular cortex, we extracted the anterior insular cortex (landmarked anterior of the central insular sulcus) using ITK(Yoo, 2004). The BNST and the PVN were hand-drawn using MRIcron (version 6/2013) on a built in MNI template (ch2better). These non-overlapping seeds were based on the structures described in the Atlas of the Human Brain (Banihashemi, 2012; Mai, 2008). The BNST was based on plates 18 (using Talairach reference systems, y=−2.7mm) through 24 (y=+2.7mm) and encompassed the central, medial, lateral, and ventral divisions (Mai, 2008). The PVN was based on plates 20 (y=−1.3mm) through 28 (y=8.0mm) and included parvocellular, magnocelluar, dorsal, and posterior subnuclei (Mai, 2008). Given the lateralization effects often described in the literature with regard to the right and left amygdala involvement in emotion regulation(Baas et al., 2004), we have chosen to use the left amygdala as seed, as left amygdala appears to be involved in sustained anxious stimuli evaluation(Glascher and Adolphs, 2003). The left amygdala seed is based on the left amygdala region defined in the AAL atlas. The ventral (bilateral) hippocampus seed has been created from the right and left hippocampus region from the AAL atlas using ITK(Yoo, 2004) and the coordinates described by Chen & Etkin (Chen and Etkin, 2013) [y=−21 to −32].
For each subject, a reference resting state time-series was extracted by averaging the time-series for all voxels (resulted from the Robust WLS) within each seed using the Marsbar plug-in in SPM(Brett M, 2002). The resulting time-series were used as regressors in the general linear model of the first-level analysis in SPM8 to generate connectivity maps for each participant. Additionally, to further control for physiological and movement-related variance, we included in the model, as nuisance covariates, the adjusted cerebrospinal fluid (CSF) time-series extracted from a sphere centered in the fourth ventricle (radius=2mm, center MNI x=0, y=−43, z=−26) and the motion correction parameters. Individual connectivity maps were estimated and submitted for a second-level group analysis.
2.5.2. Voxelwise Regression Analysis
The first-level functional connectivity maps for all participants were included in four separate multiple regression analyses. For every multiple regression model, one of the anxiety measures was used as our predictor of interest while we controlled for age and the other three anxiety measures. Pearson’s correlation coefficient was used to compute the correlation of the anxiety measures. To assess collinearity due to the overlapping of the anxiety measures, the variance inflation factor (VIF) was also computed. The VIF calculates how much the variance is inflated due to collinearity (indicating that one of the predictors is an exact combination of the others). A rule of thumb is that a VIF value larger than 10 signals inflated variance due to collinearity(Kelinbaum DG, 1998). To investigate the presence of collinearity among the set of our predictors we computed the VIF using the associated R2-values resulted from fitting four models using the predictor variables only (each of the four predictor anxiety measures was considered as the dependent variable and the other three anxiety measures were introduced as independent). The resulted R2 for each of these models was then used to compute the VIF using the formula: VIF=1/(1−R2)(Kelinbaum DG, 1998).
The voxelwise analyses were corrected for multiple comparisons by using Monte Carlo simulations implemented in AlphaSim (version 2.0/2002; http://afni.nimh.nih.gov/pub/dist/doc/program_help/AlphaSim.html)(Ward, 2000). A corrected p < 0.05 was deemed significant.
3. Results
A total of 59 participants were included in this study (see Table 1 for clinical and demographic data on the sample). Participants had a mean age of 56.05 [SD 18.3]. Sixty-three percent were women. Twenty-nine participants had a diagnosis of GAD. Of those, two participants also had a diagnosis of social phobia, one of post-traumatic stress disorder, four of panic disorder without agoraphobia, one with binge eating disorder, two of dysthymic disorder, and one of alcohol dependence in full remission. Thirty participants were healthy controls. The Pearson correlation coefficient between any two of the anxiety measures were the following: ρ(HARS & RSQ)=0.698, ρ(HARS& PSWQ)=0.788, ρ(HARS&FFI.N) =0.740, ρ(RSQ&PSWQ)=0.759, ρ(RSQ,FFI-N) =0.796, and ρ(PSWQ&FFI-N)=0.816. The associated R2 and VIF values from fitting the four models were the following: R2(HARS|PSWQ,RSQ,FFI)=0.656, VIF=2.915; R2(PSWQ| HARS, RSQ,FFI)=0.753, VIF=4.057; R2(RSQ|HARS,PSWQ,FFI)=0.677, VIF=3.098; and R2(FFI|HARS,PSWQ,RSQ)= 0.748, VIF=3.976. None of the computed VIF values were greater than 10.
Table 1.
Clinical and demographic data of the sample (N=59)
| Variable | Group | Mean/Standard Deviation | Group difference (t, df, p) |
|---|---|---|---|
| Age | Total Sample | 56.05, SD=18.35 | T(56.94)=0.147, p=0.88 |
| GAD participants (N=29) | 55.68 (18.53) | ||
| Non-GAD Participants (N=30) | 56.40 (18.48) | ||
| Gender (Female) | Total Sample | 63% (37)* | χ(1)=1.55, p=0.212 # |
| GAD participants | 72% (21) | ||
| Non-GAD participants | 46% (14) | ||
| Education | Total Sample | 16 [IQR=5] ** | W=426.5, p=0.90## |
| GAD participants | 16 (IQR=4) | ||
| Non-GAD participants | 16 (IQR=5.75) | ||
| Hamilton Anxiety Rating Scale | Total Sample | 11.69 (9.54) | T(43.61)= −24.41, p<0.001 |
| GAD participants | 20.89 (3.50) | ||
| Non-GAD participants | 2.80 (1.95) | ||
| Penn State Worry Questionnaire | Total Sample | 46.15 (17.51) | T(52.77)= −10.02, p<0.001 |
| GAD participants | 60.28 (11.87) | ||
| Non-GAD participants | 32.50 (9.20) | ||
| Response Style Questionnaire – Rumination Subscale | Total Sample | 33.42 (10.70) | T(34.84)= −6.28, p<0.001 |
| GAD participants | 40.38 (11.06) | ||
| Non-GAD participants | 26.7 (3.95) | ||
| Five Factor Inventory – Neuroticism | Total Sample | 17.83 (11.41) | T(46.37)= −8.31, p<0.001 |
| GAD participants | 26.38 (9.31) | ||
| Non-GAD participants | 9.57(5.75) | ||
| Mini Mental State Examination | Total Sample | 29.50 [IQR=1.25]*** | W=212.5, p=0.087 ## |
| GAD participants | 29 (IQR=2) | ||
| Non-GAD participants | 30 (IQR=1) |
Proportion;
Median [Interquartile Range];
Available for 36 participants
Chi-square test;
Wilcoxon’s rank sum
Multiple Regression Results (see Table 2)
Table 2.
Intrinsic connectivity correlates for different types of anxiety
| Anxiety Phenotype/Clinical Measure | Seed | Region of interest (x/y/z) | Cluster size (ke), t statistic, degrees of freedom |
|---|---|---|---|
|
| |||
| Global Anxiety/HARS | Bed Nucleus of Stria Terminalis | Left Frontal (BA 6) (−32/1/55) | (510, 3.570, 52) |
| Left Middle Temporal (−50/−52/4) | (181, 3.694, 52) | ||
|
| |||
| Left Amygdala | Left Hippocampus (−18/−40/8) | (607, 3.870, 52) | |
| Left Middle Frontal Gyrus (−38/4/58) | (36, 3.640, 52) | ||
|
| |||
| Paraventricular Nucleus | Left Caudate (tail) (−28/−38/8) | (4378, 4.521, 52) | |
| Left Hippocampus (−26/−38/3) | (4378, 4.427, 52) | ||
| Left Parietal (BA2) (−37/−30/32) | (4378, 5.103, 52) | ||
|
| |||
| Posterior Cingulate Cortex | Right Middle Temporal (BA22) (54/−42/4) | (1413, 4.890, 52) | |
|
| |||
| Ventral Hippocampus | Left Middle Temporal Gyrus (BA22) (−60/−48/2) | (76, 3.633, 52) | |
| Left Dorsal Hippocampus (−23/−40/0) | (144, 3.724, 52) | ||
|
| |||
| Somatic anxiety/HARS-S | Bed Nucleus of Stria Terminalis | Left Middle Temporal (BA21) (−57/−24/−7) | (509, 4.378, 52) |
| Left Inferior Temporal (−50/−54/−8) | (509, 4.217, 52) | ||
|
| |||
| Left Amygdala | Right Dorsal Hippocampus (−12/−38/10) | (288, 4.068, 52) | |
| Left Dorsal Hippocampus (−13/−36/5.6) | (323, 3.811, 52) | ||
|
| |||
| Paraventricular Nucleus | Left Hippocampus (−23/−40/0) | (1814, 4.681, 52) | |
| Left Caudate (tail) (−28/−38/8) | (1814, 4.681, 52) | ||
| Left Cingulate (BA31) (−18.7/−34/39) | (421, 4.39, 52) | ||
|
| |||
| Posterior Cingulate Cortex | Right Insula (BA 13) (44/−14/−2) | (2180, 4.901, 52) | |
| Right Amygdala (24/0/−16) | (77, 4.391, 52) | ||
| Right Superior Temporal Gyrus (BA 22) (55/−32/2) | (2180, 5.260, 52) | ||
|
| |||
| Ventral Hippocampus | Right Temporal Inferior (42/−64/−6) | (99, 3.577, 52) | |
|
| |||
| Worry/PSWQ | Right Anterior Insula | Left cuneus (0/−84/38) | (848, 4.011, 52) |
| Right precuneus (BA 31) (12/−56/32) | (848, 3.836, 52) | ||
| Right precuneus (BA19) (34/−78/37) | (69, 3.94, 52) | ||
|
| |||
| Neuroticism/FFI-N | Left Amygdala | Right Caudate (16/6/21) | (1772, 4.322, 52) |
| Dorsal ACC (BA 32) (12/24/26) | (1772, 3.916, 52) | ||
| Left dlPFC (BA 46) (−46/32/23) | (458, 3.654, 52) | ||
| Right Insula/Frontal Operculum (34/25/8) | (944, 5.25, 52) | ||
| Right Fusiform Gyrus (BA 19) (30/−50/−7) | (268, 3.479, 52) | ||
|
| |||
| Paraventricular Nucleus | Left Fusiform Gyrus (BA 37) (−34/−40/−15) | (148, 4.271, 52) | |
|
| |||
| Ventral Hippocampus | Right Caudate Head (15/10/17) | (1507, 4.128, 52) | |
| Middle Frontal Gyrus (36/4/58) | (26, 3.623, 52) | ||
| Inferior Frontal Gyrus (42/32/10) | (230, 3.595, 52) | ||
Clinical measures: Penn State Worry Questionnaire (PSWQ), Hamilton Anxiety Rating Scale (HARS), Hamilton Anxiety Rating Scale - Somatic (HARS-S), Five Factor Inventory – Neuroticism (FFI-N)
In order to isolate the effect of each phenotype, we included all participants in five separate multiple regression analyses. For every multiple regression model, one of the anxiety measures was used as our predictor of interest while we controlled for age, gender and the other anxiety measures.
-
3.1
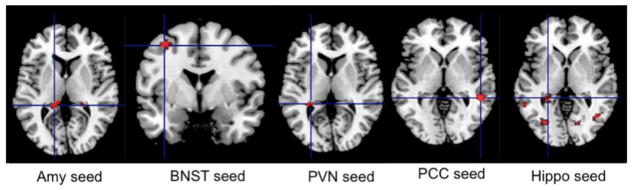
Neural correlates of global anxiety (effects of total HARS). Several regions remained significant after controlling for the effects of age, gender, worry severity, rumination, and neuroticism. Total HARS has been associated with increased connectivity between the left amygdala, left hippocampus, and left middle frontal gyrus, between the BNST and left frontal and left middle temporal gyrus, between the PVN and left caudate, left hippocampus, and left parietal cortex (BA2), between the PCC and right middle temporal cortex, and between the ventral hippocampus and left middle temporal gyrus and left dorsal hippocampus (see Fig 2).
-
3.2
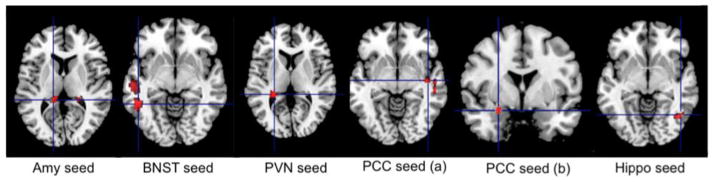
Neural correlates of somatic anxiety (effects of HARS-somatic subscale). Several regions remained significant after controlling for the effects of age, gender, worry severity, rumination, and neuroticism. Thus, somatic anxiety has been associated with increased intrinsic connectivity between the left amygdala and dorsal hippocampus, between the PVN and left hippocampus, left caudate and middle cingulate (BA31), between the BNST and left temporal gyrus (middle and inferior) and middle cingulate gyrus, between the PCC and right insula, right amygdala, and temporal gyrus (superior and middle), and between the ventral hippocampus and right inferior temporal gyrus (see Fig 3).
-
3.3
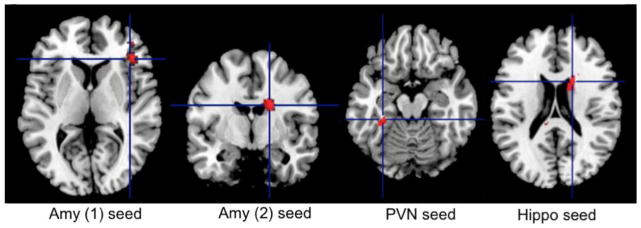
Neural correlates of worry severity (effects of PSWQ). The only network that remained significant after controlling for the effect of age, gender, global anxiety, rumination, and neuroticism was the Salience Network centered on the RAI. Thus, worry severity correlated with greater connectivity between the RAI and left cuneus and right precuneus (see Fig 4).
-
3.4
Neural correlates of neuroticism (effects of FFI-Neuroticism subscale). After controlling for age, gender, severity of worry, rumination, and global anxiety, neuroticism correlated with greater connectivity between the left amygdala and left dorsolateral prefrontal cortex (dlPFC), right caudate, dorsal ACC, right insula/right frontal operculum and right fusiform gyrus, between the PVN and left fusiform gyrus, and between the ventral hippocampus and right caudate, middle, and inferior frontal gyrus (see Fig 5).
-
3.5
Neural effects of rumination (effects of RSQ-RS). In this sample, rumination severity did not correlate significantly with any measures of functional connectivity once we controlled for age, gender and the other clinical measures of anxiety.
Fig 2.
Intrinsic connectivity correlates of global anxiety severity as measured by HARS
Global anxiety correlated positively with increased intrinsic connectivity between the left amygdala, left hippocampus, and left middle frontal gyrus, between the BNST and left frontal and left middle temporal gyrus, between the PVN and left caudate, left hippocampus, and left parietal cortex (BA2), between the PCC and right middle temporal cortex, between the ventral hippocampus and left middle temporal gyrus and left dorsal hippocampus. For coordinates, cluster size, t-statistic and degrees of freedom – see Table 2
HARS=Hamilton Anxiety Rating Scale, Amy=amygdala; BNST= bed nucleus of stria terminalis; PVN=paraventricular nucleus; PCC= posterior cingulate cortex; Hippo=hippocampus
Fig 3.
Intrinsic connectivity correlates of somatic anxiety severity as measured by the HARS somatic subscale
Somatic anxiety positively correlated with increased intrinsic connectivity between the left amygdala and dorsal hippocampus, between the PVN and left hippocampus, and left caudate, between the BNST and left temporal gyrus (middle and inferior) and middle cingulate gyrus, between the PCC and right insula, right amygdala, and the temporal gyrus (superior and middle), and between the ventral hippocampus and right inferior temporal gyrus. For coordinates, cluster size, t-statistic and degrees of freedom – see Table 2
HARS = Hamilton Anxiety Rating Scale, Amy=amygdala; BNST= bed nucleus of stria terminalis; PVN=paraventricular nucleus; PCC= posterior cingulate cortex; Hippo=hippocampus
Fig 4.
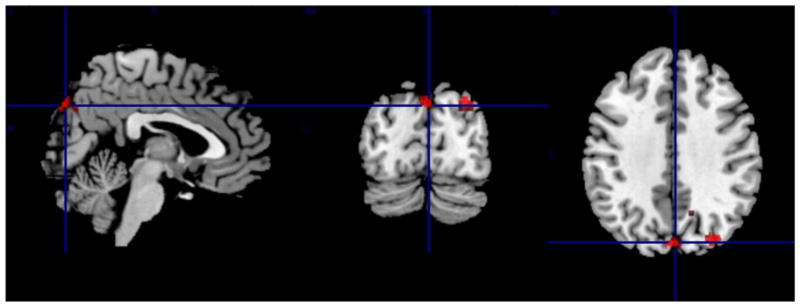
Intrinsic connectivity correlates of worry severity as measured by the PSWQ
Worry severity correlated positively with increased intrinsic connectivity between the RAI and right precuneus and left cuneus. For coordinates, cluster size, t-statistic and degrees of freedom – see Table 2
PSWQ= Penn State Worry Questionnaire; RAI= Right Anterior Insula
Fig 5.
Intrinsic connectivity correlates of neuroticism as measured by the FFI-N
Neuroticism correlated with greater connectivity between the left amygdala and left dlPFC, right caudate, dorsal ACC, and right fusiform gyrus, between the PVN and left fusiform gyrus, and between the ventral hippocampus and right caudate, middle, and inferior frontal gyrus. For coordinates, cluster size, t-statistic and degrees of freedom – see Table 2
FFI-N= Five Factor Inventory, Neuroticism. Amy=amygdala; PVN=paraventricular nucleus; Hippo=hippocampus
4. Discussion
In conclusion, our analysis identifies unique neural correlates for specific anxiety phenotypes. Thus, we can envision an anxiety matrix with multiple dimensions (e.g., somatic anxiety, worry, rumination, neuroticism), each with different neural correlates. Our results support decades of phenomenological research advocating for different substrates for worry and anxiety (Borkovec, 1991; Gana et al., 2001; Heller et al., 1997; Zebb and Beck, 1998). Thus, worry seems to have a completely unique profile compared all the other types of anxiety explored in this analysis. It is the only form of anxiety that correlates specifically with the intrinsic connectivity of the SN (centered on the RAI seed). Abnormal SN connectivity at rest and during worry induction has been implicated in anxiety disorders (Andreescu et al., 2014a; Pannekoek et al., 2012a, b; Paulus and Stein, 2006), as the basis for pathologically enhanced salience detection (misattributing emotional salience to mundane events)(Menon and Uddin, 2010; Uddin, 2015). The increased insula-precuneus/cuneus connectivity may indicate the importance of thought-action fusion in the worry process (Jones and Bhattacharya, 2013). This phenomenon has been described especially in connection to obsessive-compulsive disorder (cognitive bias in which individuals believe that the mere thought of an event increases its likelihood of its occurring in reality), but it has been recently advocated that is represents a transdiagnostic feature strongly predictive of GAD (Thompson-Hollands et al., 2013) and correlated with activation of the precuneus.
Given the heterogeneous nature of the HARS (Maier et al., 1988), it is not surprising that global anxiety seems to have a more diffuse signature, connecting multiple limbic and cortical structures. This emphasizes the importance of selecting specific anxiety phenotypes when exploring clinical-neural correlations. Once we isolated the effects of somatic anxiety we noticed a more vigorous association of the HARS-S with intrinsic connectivity in subcortical regions implicated in pathologic anxiety (e.g., amygdala – dorsal hippocampus, PVN – dorsal hippocampus, PVN - caudate, PCC – amygdala, and PCC - insula). Our results indicate that the somatic components of anxiety rest on a subcortical network anchored predominantly in the dorsal hippocampus and the amygdala, both regions involved in emotion processing (Adhikari, 2014; Bishop, 2007).
Resting state connectivity markers of neuroticism described in the literature include increased amygdala – precuneus connectivity (Aghajani et al., 2013), PCC – precuneus (Adelstein et al., 2011), precuneus – dmPFC, and precuneus – middle temporal gyrus and temporal pole (Adelstein et al., 2011). Overall, these markers indicate a consistent involvement of regions implicated in self-referential thought processing and emotion regulation. Our results emphasize the increased connectivity between regions implicated in emotion generation (e.g., amygdala and ventral hippocampus) and stress response (BNST and PVN) together with regions implicated in higher-order emotion regulation and attention modulation (e.g., insular cortex (Goldin et al., 2008), frontal cortex(Ochsner and Gross, 2005), caudate(Caligiuri et al., 2006), and fusiform gyrus(Fonville et al., 2014; Lepsien and Nobre, 2006)). Additionally, the results indicate a single common neural pattern shared by neuroticism and the different anxiety subtypes: both neuroticism and global anxiety have increased intrinsic connectivity between the amygdala and the frontal cortex, suggesting probably a more active regulatory role of the frontal cortex(Ochsner et al., 2004) due to the heightened negative emotional experiences related by both neuroticism and global anxiety (Canli, 2004; Kumari et al., 2007).
In regards to the construct of rumination, once we controlled for various other types of anxiety, rumination did not correlate with any specific neural marker. This may be due to high correlation between the RSQ and the other scales (mainly the PSWQ and FFI-N), but may also be a reflection of the subjects’ clinical characteristics (anxious but not depressed) and our choice of seeds, a choice weighted toward anxiety-relevant subcortical regions.
Two seeds – the PVN and the left amygdala - had increased connectivity with the hippocampus. This is noticeable when analyzing both global and somatic anxiety and supports the role of the hippocampus, especially ventral hippocampus, in a distributed network that moderates anxiety(Adhikari, 2014). Our results indicate a specific interplay between amygdala, ventral hippocampus, and PVN related uniquely to somatic anxiety, suggesting the dysfunction of autonomic and neuroendocrine response to emotionally charged stimuli (Kheirbek et al., 2012).
Several limitations are worth noting. First, our study did not explore other anxiety phenotypes such as panic or avoidance. While our sample contained both participants with anxiety disorders and non-anxious participants, GAD is overrepresented at the expense of other anxiety disorders, thus the generalizability of our findings would require future replication in wider samples. We did not differentiate between the BNST subnuclei, cited as having antagonistic effects in generating anxious apprehension (Ventura-Silva et al., 2012) or between amygdalar subregions, which may have distinct connectivity patterns (Etkin et al., 2009).
In conclusion, we present novel data suggesting that different anxious phenotypes are associated with distinct connectivity patterns in two canonical functional networks. These results advocate for a fine-grain approach when analyzing the neural substrates of clinical sample with various anxiety disorders.
Highlights.
The aim of this study was to examine the specific neural correlates associated with anxiety phenotypes (worry, rumination, somatic anxiety) and negative affect (neuroticism).
Our results show that measures of worry, global anxiety, somatic anxiety, and neuroticism have distinct ‘neural signatures’.
Worry severity was uniquely correlated with increased intrinsic connectivity between right anterior insula and the precuneus.
Somatic anxiety was associated with increased connectivity in the limbic and paralimbic structures.
Neuroticism was correlated with increased connectivity between limbic and prefrontal structures.
Acknowledgments
Supported by NIMH MH 086686, MH 071944, the Brain and Behavior Research Foundation (NARSAD) Young Investigator Award (Dr. Andreescu).
The authors would like to thank the Geriatric Psychiatry Neuroimaging Lab staff for their support.
Footnotes
Financial disclosures: Carmen Andreescu, Douglas Mennin, Dana Tudorascu, Sarah Walker, Lei K Sheu and Layla Banihashemi do not have any potential conflict of interest to acknowledge. Howard Aizenstein has received research support from Novartis Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adelstein JS, Shehzad Z, Mennes M, Deyoung CG, Zuo XN, Kelly C, Margulies DS, Bloomfield A, Gray JR, Castellanos FX, Milham MP. Personality is reflected in the brain’s intrinsic functional architecture. PLoS One. 2011;6:e27633. doi: 10.1371/journal.pone.0027633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari A. Distributed circuits underlying anxiety. Frontiers in behavioral neuroscience. 2014;8:112. doi: 10.3389/fnbeh.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajani M, Veer IM, van Tol MJ, Aleman A, van Buchem MA, Veltman DJ, Rombouts SA, van der Wee NJ. Neuroticism and extraversion are associated with amygdala resting-state functional connectivity. Cogn Affect Behav Neurosci. 2013 doi: 10.3758/s13415-013-0224-0. [DOI] [PubMed] [Google Scholar]
- Andreescu C, Sheu LK, Tudorascu D, Gross JJ, Walker S, Banihashemi L, Aizenstein H. Emotion Reactivity and Regulation in Late-Life Generalized Anxiety Disorder: Functional Connectivity at Baseline and Post-Treatment. Am J Geriatr Psychiatry. 2014a doi: 10.1016/j.jagp.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C, Sheu LK, Tudorascu D, Gross JJ, Walker S, Banihashemi L, Aizenstein H. Emotion reactivity and regulation in late-life generalized anxiety disorder: functional connectivity at baseline and post-treatment. Am J Geriatr Psychiatry. 2015;23:200–214. doi: 10.1016/j.jagp.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C, Sheu LK, Tudorascu D, Walker S, Aizenstein H. The ages of anxiety--differences across the lifespan in the default mode network functional connectivity in generalized anxiety disorder. Int J Geriatr Psychiatry. 2014b;29:704–712. doi: 10.1002/gps.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Brain Res Rev. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Banihashemi L, Sheu LK, Gianaros PJ. Childhood physical abuse predicts adulthood stressor-evoked activity in the limbic forebrain and hypothalamic regions. Society of Biological Psychiatry Annual Meeting; Philadelphia. 2012. [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6:548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Shadick RN, Hopkins M. The nature of normal and pathological worry. In: Rappe RM, Barlow DH, editors. Chronic anxiety: Generalized anxiety disorder and mixed anxiety-depression. 1991. pp. 29–51. [Google Scholar]
- Brett MAJ-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- Brown TA, Antony MM, Barlow DH. Psychometric properties of the Penn State Worry Questionnaire in a clinical anxiety disorders sample. Behav Res Ther. 1992;30:33–37. doi: 10.1016/0005-7967(92)90093-v. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Brown GG, Meloy MJ, Eberson S, Niculescu AB, Lohr JB. Striatopallidal regulation of affect in bipolar disorder. J Affect Disord. 2006;91:235–242. doi: 10.1016/j.jad.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Simmons AN, Lovero KL, Rochlin AA, Paulus MP, Stein MB. Functioning of neural systems supporting emotion regulation in anxiety-prone individuals. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T. Functional brain mapping of extraversion and neuroticism: learning from individual differences in emotion processing. Journal of personality. 2004;72:1105–1132. doi: 10.1111/j.1467-6494.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Chen AC, Etkin A. Hippocampal Network Connectivity and Activation Differentiates Post-Traumatic Stress Disorder From Generalized Anxiety Disorder. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Donovan JE. Reliability and validity of the Hamilton Anxiety Rating Scale in an adolescent sample. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:354–360. doi: 10.1097/00004583-199403000-00009. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. J Abnorm Psychol. 1994;103:103–116. [PubMed] [Google Scholar]
- Costa PT, MR . Revised NEO personality inventory and NEO five-factor inventory professional manual. Odessa: 1992. [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Crane JW, Buller KM, Day TA. Evidence that the bed nucleus of the stria terminalis contributes to the modulation of hypophysiotropic corticotropin-releasing factor cell responses to systemic interleukin-1beta. J Comp Neurol. 2003;467:232–242. doi: 10.1002/cne.10918. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi Changjun. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Annals of the New York Academy of Sciences. 1999;877:281–291. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Davis M, Young Lim Lee. Fear and Anxiety: Possible Roles of the Amygdala and Bed Nucleus of Stria Terminalis. Cognition and Emotion. 1998;12:277–305. [Google Scholar]
- Diedrichsen J, Shadmehr R. Detecting and adjusting for artifacts in fMRI time series data. Neuroimage. 2005;27:624–634. doi: 10.1016/j.neuroimage.2005.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Chen H, Qiu C, Liao W, Warwick JM, Duan X, Zhang W, Gong Q. Disrupted functional connectivity in social anxiety disorder: a resting-state fMRI study. Magn Reson Imaging. 2011;29:701–711. doi: 10.1016/j.mri.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Domschke K, Stevens S, Pfleiderer B, Gerlach AL. Interoceptive sensitivity in anxiety and anxiety disorders: an overview and integration of neurobiological findings. Clin Psychol Rev. 2010;30:1–11. doi: 10.1016/j.cpr.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66:1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Stein MB, Paulus MP. Anterior insula reactivity during certain decisions is associated with neuroticism. Soc Cogn Affect Neurosci. 2006;1:136–142. doi: 10.1093/scan/nsl016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, SR, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P), 2.0 ed. 1995. [Google Scholar]
- Flandreau EI, Ressler KJ, Owens MJ, Nemeroff CB. Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology. 2012;37:27–38. doi: 10.1016/j.psyneuen.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonville L, Giampietro V, Surguladze S, Williams S, Tchanturia K. Increased BOLD signal in the fusiform gyrus during implicit emotion processing in anorexia nervosa. NeuroImage Clinical. 2014;4:266–273. doi: 10.1016/j.nicl.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gana K, Martin B, Canouet MD. Worry and anxiety: is there a causal relationship? Psychopathology. 2001;34:221–229. doi: 10.1159/000049314. [DOI] [PubMed] [Google Scholar]
- Gentili C, Ricciardi E, Gobbini MI, Santarelli MF, Haxby JV, Pietrini P, Guazzelli M. Beyond amygdala: Default Mode Network activity differs between patients with social phobia and healthy controls. Brain Res Bull. 2009;79:409–413. doi: 10.1016/j.brainresbull.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Glascher J, Adolphs R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J Neurosci. 2003;23:10274–10282. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. 2009;66:170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale WW, 3rd, Klimstra TA, Meeus WH. Is the generalized anxiety disorder symptom of worry just another form of neuroticism? a 5-year longitudinal study of adolescents from the general population. J Clin Psychiatry. 2010;71:942–948. doi: 10.4088/JCP.09m05506blu. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Heller W, Nitschke JB, Etienne MA, Miller GA. Patterns of regional brain activity differentiate types of anxiety. J Abnorm Psychol. 1997;106:376–385. doi: 10.1037//0021-843x.106.3.376. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Myers JM, Prescott CA, Kendler KS. A population-based twin study of the relationship between neuroticism and internalizing disorders. Am J Psychiatry. 2006;163:857–864. doi: 10.1176/ajp.2006.163.5.857. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, Kendler KS. Genetic and environmental sources of covariation between generalized anxiety disorder and neuroticism. Am J Psychiatry. 2004;161:1581–1587. doi: 10.1176/appi.ajp.161.9.1581. [DOI] [PubMed] [Google Scholar]
- Hilbert K, Lueken U, Beesdo-Baum K. Neural structures, functioning and connectivity in Generalized Anxiety Disorder and interaction with neuroendocrine systems: A systematic review. J Affect Disord. 2014;158C:114–126. doi: 10.1016/j.jad.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Hong RY. Worry and rumination: differential associations with anxious and depressive symptoms and coping behavior. Behav Res Ther. 2007;45:277–290. doi: 10.1016/j.brat.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Jones R, Bhattacharya J. A role for the precuneus in thought-action fusion: Evidence from participants with significant obsessive-compulsive symptoms. NeuroImage Clinical. 2013;4:112–121. doi: 10.1016/j.nicl.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelinbaum DG, KL, Muller KE, Nizam A. Applied Regression Analysis and other multivariable methods. 3. Duxbury Press; 1998. [Google Scholar]
- Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012;15:1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krain AL, Gotimer K, Hefton S, Ernst M, Castellanos FX, Pine DS, Milham MP. A functional magnetic resonance imaging investigation of uncertainty in adolescents with anxiety disorders. Biol Psychiatry. 2008;63:563–568. doi: 10.1016/j.biopsych.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Kumari V, ffytche DH, Das M, Wilson GD, Goswami S, Sharma T. Neuroticism and brain responses to anticipatory fear. Behavioral neuroscience. 2007;121:643–652. doi: 10.1037/0735-7044.121.4.643. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Nobre AC. Cognitive control of attention in the human brain: insights from orienting attention to mental representations. Brain Res. 2006;1105:20–31. doi: 10.1016/j.brainres.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Liao W, Chen H, Feng Y, Mantini D, Gentili C, Pan Z, Ding J, Duan X, Qiu C, Lui S, Gong Q, Zhang W. Selective aberrant functional connectivity of resting state networks in social anxiety disorder. Neuroimage. 2010;52:1549–1558. doi: 10.1016/j.neuroimage.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Lueken U, Hilbert K, Stolyar V, Maslowski NI, Beesdo-Baum K, Wittchen HU. Neural substrates of defensive reactivity in two subtypes of specific phobia. Soc Cogn Affect Neurosci. 2013 doi: 10.1093/scan/nst159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Voss T. Atlas of the Human Brain. 3. Academic Press; New York: 2008. [Google Scholar]
- Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord. 1988;14:61–68. doi: 10.1016/0165-0327(88)90072-9. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, Kalin NH. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166:302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol. 2000;109:504–511. [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Pannekoek JN, Veer IM, van Tol MJ, van der Werff SJ, Demenescu LR, Aleman A, Veltman DJ, Zitman FG, Rombouts SA, van der Wee NJ. Aberrant limbic and salience network resting-state functional connectivity in panic disorder without comorbidity. J Affect Disord. 2012a doi: 10.1016/j.jad.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Pannekoek JN, Veer IM, van Tol MJ, van der Werff SJ, Demenescu LR, Aleman A, Veltman DJ, Zitman FG, Rombouts SA, van der Wee NJ. Resting-state functional connectivity abnormalities in limbic and salience networks in social anxiety disorder without comorbidity. Eur Neuropsychopharmacol. 2012b doi: 10.1016/j.euroneuro.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Pego JM, Sousa JC, Almeida OF, Sousa N. Stress and the neuroendocrinology of anxiety disorders. Current topics in behavioral neurosciences. 2010;2:97–117. doi: 10.1007/7854_2009_13. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Roach AR, Evans DR, Schipper LJ, Darville AK. The structure and health correlates of trait repetitive thought in older adults. Psychol Aging. 2010;25:505–515. doi: 10.1037/a0019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serretti A, Jori MC, Casadei G, Ravizza L, Smeraldi E, Akiskal H. Delineating psychopathologic clusters within dysthymia: a study of 512 out-patients without major depression. J Affect Disord. 1999;56:17–25. doi: 10.1016/s0165-0327(99)00056-7. [DOI] [PubMed] [Google Scholar]
- Servaas MN, Riese H, Ormel J, Aleman A. The neural correlates of worry in association with individual differences in neuroticism. Hum Brain Mapp. 2014 doi: 10.1002/hbm.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A, Matthews SC, Paulus MP, Stein MB. Intolerance of uncertainty correlates with insula activation during affective ambiguity. Neurosci Lett. 2008;430:92–97. doi: 10.1016/j.neulet.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Thompson-Hollands J, Farchione TJ, Barlow DH. Thought-action fusion across anxiety disorder diagnoses: specificity and treatment effects. J Nerv Ment Dis. 2013;201:407–413. doi: 10.1097/NMD.0b013e31828e102c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination Reconsidered: A psychometric analysis. Cognitive Therapy and Research. 2003;27:247–259. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Ventura-Silva AP, Pego JM, Sousa JC, Marques AR, Rodrigues AJ, Marques F, Cerqueira JJ, Almeida OF, Sousa N. Stress shifts the response of the bed nucleus of the stria terminalis to an anxiogenic mode. Eur J Neurosci. 2012;36:3396–3406. doi: 10.1111/j.1460-9568.2012.08262.x. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B. AFNI AlphaSim documentation. Biophysics Research Institute, Medical College of Wisconsin; Milwaukee, WI: 2000. Simulatenous inference for fMRI data. [Google Scholar]
- Watson D, Clark LA, Harkness AR. Structures of personality and their relevance to psychopathology. J Abnorm Psychol. 1994;103:18–31. [PubMed] [Google Scholar]
- Yoo TS. Principles and Practice for Segmentation, Registration and Image Analysis. 1. A K Peters/CRC Press; 2004. Insights into Images. [Google Scholar]
- Zebb BJ, Beck JG. Worry versus anxiety. Is there really a difference? Behavior modification. 1998;22:45–61. doi: 10.1177/01454455980221003. [DOI] [PubMed] [Google Scholar]
- Zhao XH, Wang PJ, Li CB, Hu ZH, Xi Q, Wu WY, Tang XW. Altered default mode network activity in patient with anxiety disorders: an fMRI study. Eur J Radiol. 2007;63:373–378. doi: 10.1016/j.ejrad.2007.02.006. [DOI] [PubMed] [Google Scholar]