Abstract
Reactive oxygen species (ROS) play an important role in the development of complex regional pain syndrome-Type I (CRPS-I), as also demonstrated with the chronic post ischemia pain (CPIP) animal model of CRPS-I. We show that morphine and the antioxidant N-acetylcysteine (NAC) act synergistically to reduce mechanical allodynia in CPIP rats. The tetrapeptide amide [Dmt1]DALDA (H-Dmt-d-Arg-Phe-Lys-NH2) is a potent and selective μ opioid receptor (MOR) agonist with favorable pharmacokinetic properties and with antioxidant activity due to its N-terminal Dmt (2′,6′-dimethyltyrosine) residue. In the CPIP model, [Dmt1]DALDA was 15-fold more potent than morphine in reversing mechanical allodynia and 4.5-fold more potent as analgesic in the heat algesia test. The results indicate that bifunctional compounds with MOR agonist/antioxidant activity have therapeutic potential for the treatment of CRPS-I.
Keywords: complex regional pain syndrome-type I (CRPS-I), chronic post ischemia pain (CPIP) rat model, μ opioid-antioxidant synergy, bifunctional μ opioid/antioxidant peptide, [Dmt1]DALDA, CRPS-I therapeutics
Complex regional pain syndrome-type I (CRPS-I) is a serious pain disorder that may result from fractures, contusions, crush injuries, sprains, arthroscopic surgery, or edematous soft tissue injuries.1,2 It is difficult to treat, with analgesics (including opioids) and anticonvulsants (gabapentin, pregabalin) having limited effect in CRPS-I patients. An animal model of CRPS-I was created by prolonged ischemia of the hind paw in the rat, resulting in chronic postischemia pain (CPIP).3 These so-called CPIP rats exhibit many of the features seen in CRPS-I, including vascular injury,4 chronic ischemia,5 and small fiber nerve degradation.6,7
There is considerable evidence to indicate that reactive oxygen species (ROS) and reactive nitrogen species (RNS) play a major role in the development of neuropathic pain in general,8,9 and of CRPS-I in particular.10 ROS are produced by the enzymes xanthine oxidase and NADPH oxidase, and by the mitochondrial electron transport chain. They include superoxide, hydroxyl radical, and peroxynitrite. In the CPIP rat model the free radical quenchers N-acetyl-l-cysteine (NAC) and Tempol given i.p. at high doses reversed mechanoallodynia.3 NAC has also been shown to be somewhat effective in treatment of CRPS-1 in human patients.10
It has been suggested that antioxidants, such as peroxynitrite decomposition catalysts, may synergize with opiates,11 raising the possibility that bifunctional compounds with combined opioid agonist and antioxidant activity might be effective for the treatment of chronic pain states. The first known compound with a mixed opioid agonist/antioxidant profile is the dermorphin-derived tetrapeptide [Dmt1]DALDA (H-Dmt-dArg-Phe-Lys-NH2; Dmt = 2′,6′-dimethyltyrosine). [Dmt1]-DALDA is a potent μ opioid agonist with subnanomolar μ opioid receptor (MOR) binding affnity () and high μ receptor binding selectivity (selectivity ratio μ/δ/κ = 1/14 700/156).12 It is also an effective scavenger of oxyradicals due to its N-terminal Dmt residue as antioxidant moiety.13 Dmt has greater antioxidant activity than tyrosine because of the two electron-donating methyl groups on its phenol moiety. It has structural similarity to vitamin E; both have a methylated phenol structure. [Dmt1]DALDA showed high antinociceptive potency in the rat and mouse tail-flick assays. In comparison with morphine, its antinociceptive effect was 3000-fold more potent with intrathecal (i.th.) administration14 and 40–220-fold more potent with subcutaneous (s.c.) administration15,16 in these acute pain models. The latter observation indicated that [Dmt1]DALDA is capable of crossing the blood-brain barrier (BBB) to produce potent, centrally mediated antinociception. The duration of its antinociceptive effect was 4 times longer than that of morphine when both compounds were administered at equipotent doses.14,17 Its demonstrated resistance to enzymatic degradation and slow clearance are indicative of good druglike properties.17
[Dmt1]DALDA is capable of crossing cellular membranes and is taken up into various types of cells, including neuronal cells.18 Importantly, it was shown to selectively target the inner mitochondrial membrane (IMM), where the electron transport chain is located.13 Its ability to penetrate into cells and to distribute to the IMM is due to the structural motif of its amino acid sequence consisting of alternating aromatic and basic residues, as reported elsewhere.13 Because of its antioxidant properties [Dmt1]DALDA can quench mitochondrial ROS that are excessively generated at the IMM. Mitochondrial ROS have been shown to play an important role in the development and maintenance of neuropathic pain.19,20
The effects of s.c. administered [Dmt1]DALDA and morphine on thermal hyperalgesia were compared in the spinal nerve ligation model of neuropathic pain.21 At doses that were equianalgesic in naive animals, [Dmt1]DALDA was more effective in producing an antinociceptive effect than morphine. The superior effect of [Dmt1]DALDA can be explained by its ROS quenching activity and by an additive or synergistic effect of μ opioid agonist activity and antioxidant activity. Furthermore, the compound's norepinephrine uptake inhibitory activity14 may also contribute to the antinociceptive effect, since norepinephrine uptake inhibitors at high doses have been shown to be somewhat effective in neuropathic pain.22 The potent antinociceptive effect of [Dmt1]DALDA seen in the spinal nerve ligation model of neuropathic pain prompted a study of this compound in the CPIP model of CRPS-I in comparison with morphine and with a combination of morphine and NAC.
RESULTS AND DISCUSSION
Compounds were tested in the CPIP model for mechanical allodynia by determining hind paw mechanical sensitivities using von Frey filaments. Furthermore, heat algesia of the tail was assessed as a measure of pain from uninjured tissue in CPIP animals. [Dmt1]DALDA (0.03 to 0.250 mg/kg, s.c.), morphine (0.5 to 2 mg/kg s.c) and a combination of morphine (0.5 to 2 mg/kg, s.c.) and NAC (10 mg/kg, i.p.) were administered. A NAC dose of 10 mg/kg was used in all morphine-NAC combinations because at this dose NAC by itself does not produce a significant effect in the CPIP model.23 This should permit the detection of possible synergistic effects of morphine and NAC in reversing mechanical allodynia or heat algesia.
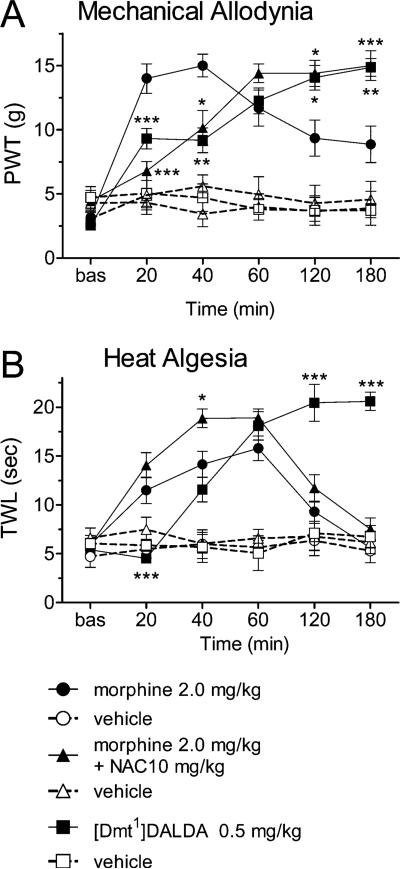
While vehicle administration had no effect on paw withdrawal thresholds (PWTs), the drugs tested produced significant elevations in PWTs that varied over time (drug by time interaction F(10,100) = 5.24, P < 0.00001). Both [Dmt1]DALDA and the morphine + NAC combination produced lower PWTs compared to morphine at 20 and 40 min post treatment, but significantly higher PWTs at the 2 and 3 h post injection time points (Figure 1A). For heat algesia measurements, the time course of drug effects on tail withdrawal latencies (TWLs) also differed among drugs (drug by time interaction F(10,100) = 10.37, P < 0.00001). [Dmt1]DALDA displayed a similar pattern of differences to morphine, with TWLs after [Dmt1]DALDA administration being lower than after morphine administration at 20 min post injection, but higher at 120 and 180 min post injection (Figure 1B). However, the combination of morphine and NAC did not produce a pattern different from that of morphine, except at 40 min post injection, where TWLs were higher after the combination than after morphine alone.
Figure 1.
Effects of morphine, morphine and NAC, and [Dmt1]-DALDA (and their vehicles) on mean (±SEM) hind paw withdrawal threshold assayed by von Frey stimulation test for post ischemic mechanical allodynia (A) and mean (±SEM) tail withdrawal latency assayed by the Hargreaves test for tail heat algesia (B). Each curve illustrates the effect of the highest dose of each agent used over 3 h post drug administration. Dmt1]DALDA and the combination of NAC and morphine produced analgesia in the von Frey test with longer onset time but significantly increased duration. Only Dmt1]DALDA had a comparable effect distinct from morphine time course on the Hargreaves test. Asterisks denote the presence of a significant difference from morphine at the same time point: *P < 0.5; **P < 0.01; ***P < 0.001 by Tukey's post hoc test.
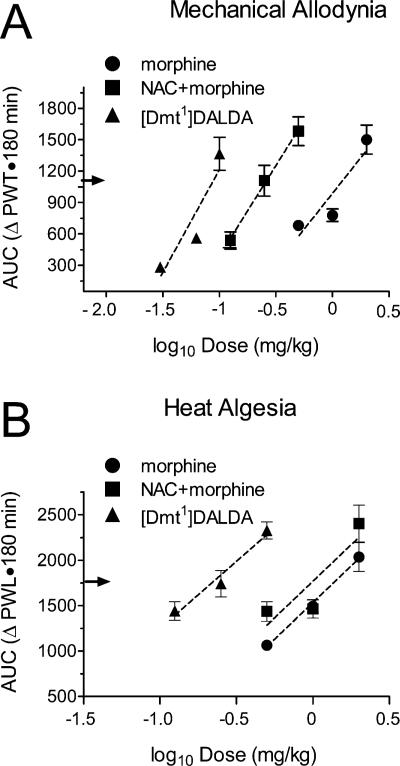
The log–dose AUC plots determined for [Dmt1]DALDA, morphine, and the morphine + NAC combination determined with the von Frey test (mechanical allodynia) are presented in Figure 2A, with calculated ED50 values listed in Table 1. ED50 values varied significantly among drugs (F(2,20) = 65.18, P < 0.00001). [Dmt1]DALDA was 15-fold more potent than morphine in producing an antinociceptive effect (P = 0.00016). In comparison with morphine alone, the morphine + NAC combination also showed statistically significant 4-fold higher potency (P = 0.00034).
Figure 2.
Plots of log–dose AUC for morphine, morphine and NAC and [Dmt1]DALDA on hind paw post ischemic mechanical allodynia assayed by the von Frey test (A) and tail heat algesia assayed by the Hargreaves test (B). ED50 analgesia was defined as 50% of maximum possible effect [(maximal AUC – vehicle AUC) + vehicle AUC)] indicated by an arrow on the y axis. Dotted lines correspond to an unweighted linear regression of AUC on dose. For mechanical allodynia and heat algesia, regressions differed by x-intercept (both P < 0.0001) but not by slope (P = 0.337 and P = 0.928, respectively).
Table 1.
Mean Half-Maximal Analgesic Doses of Morphine, Morphine and N-Acetylcysteine, and [Dmt1]DALDA Measured for Postischemic Hind Paw Mechanical Allodynia and Tail Heat Algesiaa
| CPIP mechanical allodynia |
heat algesia |
|||
|---|---|---|---|---|
| treatment group | ED50 (mg·kg−1) ± SEM | potency ratio | ED50 (mg·kg−1 ± SEM) | potency ratio |
| morphine | 1.546 ± 0.664 | 1.0 | 1.270 ± 0.858 | 1.0 |
| NAC (10 mg·kg−1) + morphine | 0.386 ± 0.291b | 4.05 | 1.057 ± 0.99 | 1.20 |
| [Dmt1]DALDA | 0.103 ± 0.046bc | 15.01 | 0.287 ± 0.03b | 4.42 |
ED50 analgesia was defined as 50% of maximum possible effect [(maximal AUC – vehicle AUC) + vehicle AUC)]. Analgesic ED50 values were individually determined using linear regression analysis (GraphPad Prism, San Diego, CA), averaged for each drug group and compared by ANOVA using post hoc Tukey tests (Statistica, StatSoft, Tulsa, OK). Drugs were given subcutaneously (morphine, [Dmt1]DALDA) or intraperitoneally (NAC).
Different from morphine group P < 0.001 (Tukey's test).
Different from NAC + morphine group, P < 0.001 (Tukey's test).
In the heat algesia test, ED50 values also varied significantly among drugs (F(2,20) = 24.48, P = 0.0001). [Dmt1]DALDA again showed enhanced antinociceptive potency as compared to morphine (P = 0.0017, Figure 2B), even though the potency ratio (4.42) was smaller than the one determined in the mechanical allodynia test (Table 1). Unlike in the von Frey test, however, the effect of the morphine + NAC combination was not significantly different from that of morphine alone in the heat algesia test. Nonetheless, like that of morphine, the ED50 value for the morphine + NAC combination was significantly greater than that of [Dmt1]DALDA (P = 0.0004).
The observation that the morphine + NAC combination is more potent than morphine alone in the von Frey test confirms that ROS play an important role in the development of mechanical allodynia in the CPIP rat model of CRPS-I. Since NAC alone reversed mechano-allodynia in CPIP rats only at doses greater than 50 mg/kg,23 it is obvious that morphine and NAC act synergistically in this model. This is in agreement with an earlier suggestion that antioxidants may synergize with opiates to reverse the development of chronic pain states.11 Since NAC is not mitochondria-targeted, ROS generated from sources other than the mitochondrial electron transport chain, such as xanthine oxidase or NADPH oxidase, may be implicated in the development of mechanical allodynia in CPIP rats. Indeed, these ROS sources have been implicated as critical in ischemia-reperfusion injury.24 In contrast to mechanical allodynia, heat algesia is not reversed more effectively by the morphine + NAC combination than by morphine alone in the CPIP rat model. This is likely since the heat algesia testing was performed in the otherwise uninjured tail, so ROS are not expected to play a significant role in this test.
In conclusion, this study confirms the important role of ROS in the development of mechano-allodynia in the CPIP model of CRPS-I and provides the first evidence that an antioxidant (NAC) and a μ opioid agonist (morphine) act synergistically to reverse mechanical allodynia in this model. In a previous evaluation of various analgesics using the CPIP model, anti-inflammatory and antineuropathic drugs were quite ineffective.25 [Dmt1]DALDA is by far the most effective analgesic identified so far for reversal of mechanical allodynia in the CPIP rat model. The use of a single bifunctional compound like [Dmt1]DALDA with combined μ opioid agonist and antioxidant activity is preferable to a two-component cocktail of a μ opioid and an antioxidant because of the more predictable pharmacokinetic and pharmacodynamic relationship resulting from the administration of a single compound.26 The development of [Dmt1]DALDA analogues with increased antioxidant effciency as further improved drug candidates for treatment of CRPS-I is under way in our laboratory.
METHODS
Compounds
[Dmt1]DALDA was synthesized by the solid-phase method and purified by preparative HPLC as described.12 N-Acetyl-l-cysteine was purchased from Sigma-Aldrich, Oakville, ON, and morphine sulfate was obtained from Sabex, Montreal, QC.
Testing of Compounds in CPIP Rats
Animals
This study used Long Evans male rats (250–450 g). The rats were housed in groups of 2–3 and were given food and water ad libitum according to a 12:12 h light/dark cycle. The procedures and treatments used in this experiment were approved by the Animal Care Committee at McGill University and conformed to the ethical guidelines of the Canadian Council on Animal Care and the International Association for the Study of Pain.
Induction of Chronic Postischemia Pain (CPIP)
CPIP was generated by a period of hind paw ischemia maintained for 3 h under general anesthesia, followed by reperfusion, as previously described.3 Briefly, rats were initially anesthetized with a bolus (55 mg/kg, i.p.) followed by i.p. infusion (0.15 mL/h) of sodium pentobarbital (Ceva Santé Animale, Libourne, France) for 2 h. After the induction of anesthesia, a Nitrile 70 durometer O-ring (O-rings West, Seattle, WA) with 5.5 mm internal diameter was placed around the rat's left hind limb proximal to the ankle joint. The ring was left in place for 3 h, and the rats recovered from anesthesia 30–60 min following reperfusion. Sham rats were anesthetized for the same period but without being subjected to the ischemia procedure.
Hind Paw Mechanical Sensitivity Testing
The plantar surface of the injured hind paw was tested for mechanical allodynia by measuring the PWT in CPIP and sham-treated rats. Rats were placed in acrylic enclosures (10.5 cm wide × 180 cm long × 150 cm high) over a wire mesh floor (6 mm × 6 mm openings). Nylon monofilaments were applied to the plantar surface of the injured (CPIP) hind paw along the midline. Starting with a filament producing a force of 2 g, in either ascending (after a negative response) or descending (after positive response) force, each filament was applied for 10 s or until a flexion reflex occurred. The minimum stimulus intensity was 0.25 g, and the maximum (cutoff value) was 15 g. The 50% threshold (grams) was calculated as (10[Xf+kδ])/10 000 where Xf = filament number of the final von Frey hair used, k = value for the pattern of positive/negative responses, and δ = mean difference in log unit between stimuli (for the present experiments, δ = 0.220); for more details, see Chaplan et al.27
Tail Heat Algesia Testing
TWL tests always took place after von Frey testing. Each rat was placed in a closed acrylic enclosure (10.5 cm wide × 180 cm long × 150 cm high), on the glass surface of a Hargreaves apparatus (#390G, IITC Life Science, Woodland Hills, CA). A point 5–10 cm from the tip of the tail was subjected to irradiation by a focused light beam approximately 4 × 6 mm in area. The time to tail withdrawal from the light was recorded and the light switched off. The intensity of the light had been set to produce a tail flick within approximately 6 s of stimulation (as previously determined in other animals, data not shown). In the absence of a response, a cutoff time of 21 s was applied to each stimulation period. Each measurement during the course of any session consisted of the average of three tests, given at intervals of 5 min.
Pharmacological Treatments
A total of 23 animals were used for the drug trials. The compounds and compound combination were tested for their effects on CPIP PWT, each in a separate group of animals: [Dmt1]DALDA (n = 9), morphine (as morphine sulfate) (n = 8), and a combination of morphine and NAC (10 mg) (n = 6). The same compounds and compound combination were tested for their effects on TWL: [Dmt1]DALDA (n = 9), morphine (n = 8), and the combination of morphine and NAC (10 mg) (n = 6). All drugs were dissolved in sterile saline and were administered s.c. in a volume of 0.5 mL/kg. Rats were tested in groups of three in order to allow the measurement of both PWT and PWL in the same animal at time intervals of 20 min and more. After a 20 min period of acclimatization, baseline measurements were obtained for PWT and then TWL before drug injection and 20, 40, 60, 120, and 180 min after administration of each drug or drug combination. All pharmacological testing was performed between 7 and 22 days post ischemia
Data Analysis
For each drug (and drug combination), PWTs and TWLs were averaged by dose and treatment time and subjected to analysis of variance (ANOVA) using repeated measures. Drug effects on CPIP were then compared at each testing time point by pairwise comparisons using Tukey's test when a significant time by drug interaction was observed.. The area under the curve (AUC) was calculated for PWTs and TWLs by the trapezoidal method from the time course data from each subject. For each drug, AUCs were averaged by dose and subjected to ANOVA, followed by post hoc Tukey tests after the observation of a significant dose effect.
For each drug tested, unweighted linear regressions of log AUC versus log dose were calculated for individual subjects. The regression x-intercept giving an AUC equal to 50% of the maximal drug effect above vehicle AUC value was calculated from individual subjects, and averaged by drug group to obtain an estimated ED50 AUC value. These ED50s were subjected to ANOVA followed by post hoc Tukey's tests to compare the drugs tested (Table 1). Statistical analyses were performed using Statistica 6 (StatSoft, Tulsa, OK), and GraphPad Prism (Version5, GraphPad Software, San Diego, CA).
Acknowledgments
Funding
Research was supported, in part, by research grants from the National Institute on Drug Abuse, NIH (DA004443) to P.W.S., the Natural Sciences and Engineering Research Council (RGPIN 194521) to T.J.C., and the Canadian Institutes of Health Research (MOP-89716 to P.W.S. and MOP-119279 to T.J.C.).
ABBREVIATIONS
- AUC
area under the curve
- CPIP
chronic post ischemia pain
- CRPS-I
complex regional pain syndrome-type I
- Dmt
2′,6′-dimethyltyrosine
- [Dmt1]DALDA
H-Dmt-d-Arg-Phe-Lys-NH2
- IMM
inner mitochondrial membrane
- MOR
μ opioid receptor
- NAC
N-acetyl-l-cysteine
- PWT
paw withdrawal threshold
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- TWL
tail withdrawal latency
Footnotes
Author Contributions
P.W.S. discovered [Dmt1]DALDA, conceived the study, and wrote the manuscript. T.M.-D.N. synthesized and purified [Dmt1]DALDA. A.S. performed behavioral tests with [Dmt1]-DALDA and morphine. A.W.H.P. performed behavioral tests with NAC and morphine. A.L. planned experiments, performed data analysis, prepared figures, and edited the manuscript. T.J.C. was involved in the conception and planning of the study and edited the manuscript.
The authors declare no competing financial interest.
REFERENCES
- 1.Allen G, Galer BS, Schwartz L. Epidemiology of complex regional pain syndrome: a retrospective chart review of 134 patients. Pain. 1999;80:539–44. doi: 10.1016/S0304-3959(98)00246-2. [DOI] [PubMed] [Google Scholar]
- 2.Galer BS, Henderson J, Perander J, Jensen MP. Course of symptoms and quality of life measurement in complex regional pain syndrome: a pilot survey. J. Pain Symptom Manage. 2000;20:286–92. doi: 10.1016/s0885-3924(00)00183-4. [DOI] [PubMed] [Google Scholar]
- 3.Coderre TJ, Xanthos DN, Francis L, Bennett GJ. Chronic post-ischemia pain (CPIP): a novel animal model of complex regional pain syndrome-type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain. 2004;112:94–105. doi: 10.1016/j.pain.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Wasner G, Heckmann K, Maier C, Baron R. Vascular abnormalities in acute reflex sympathetic dystrophy (CRPS-I): complete inhibition of sympathetic nerve activity with recovery. Arch. Neurol. 1999;56:613–20. doi: 10.1001/archneur.56.5.613. [DOI] [PubMed] [Google Scholar]
- 5.Koban M, Leis S, Schultze-Mosgau S, Birklein F. Tissue hypoxia in complex regional pain syndrome. Pain. 2003;104:149–57. doi: 10.1016/s0304-3959(02)00484-0. [DOI] [PubMed] [Google Scholar]
- 6.Albrecht PJ, Hines S, Eisenberg E, Pud D, Finlay DR, Connolly MK, Pare M, Davar G, Rice FL. Pathological alterations of cutaneous innervation and vasculature in affected limbs from patients with complex regional pain syndrome. Pain. 2006;120:244–66. doi: 10.1016/j.pain.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 7.Oaklander AL, Rissmiller JG, Gelman LB, Zheng L, Chang Y, Gott R. Evidence of focal small-fiber axonal degeneration in complex regional pain syndrome. Pain. 2006;120:235–43. doi: 10.1016/j.pain.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 8.Kim HK, Park SK, Zhou J-L, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–24. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Salvemini D, Little JW, Doyle T, Neumann WL. Roles of reactive oxygen and nitrogen species in pain. Free Radical Biol. Med. 2011;51:951–66. doi: 10.1016/j.freeradbiomed.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez RSGM, Zuurmond WWA, Bezemer PD, Kuik D, van Loenen AC, de Lange JJ, Zuidhof AJ. The treatment of complex regional pain syndrome type I with free radical scavengers: a randomized controlled study. Pain. 2003;102:297–307. doi: 10.1016/S0304-3959(02)00414-1. [DOI] [PubMed] [Google Scholar]
- 11.Janes K, Neumann WL, Salvemini D. Anti-superoxide and anti-peroxynitrite strategies in pain suppression. Biochim. Biophys. Acta, Mol. Basis Dis. 2012;1822:815–821. doi: 10.1016/j.bbadis.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiller PW, Nguyen TM-D, Berezowska I, Dupuis S, Weltrowska G, Chung NN, Lemieux C. Synthesis and in vitro opioid activity profiles of DALDA analogues. Eur. J. Med. Chem. 2000;35:895–901. doi: 10.1016/s0223-5234(00)01171-5. [DOI] [PubMed] [Google Scholar]
- 13.Zhao K, Zhao G-M, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death and reperfusion injury. J. Biol. Chem. 2004;279:34682–90. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 14.Shimoyama M, Shimoyama N, Zhao G-M, Schiller PW, Szeto HH. Antinociceptive and respiratory effects of intrathecal H-Tyr-D-Arg-Phe-Lys-NH2 (DALDA) and [Dmt1]-DALDA. J. Pharmacol. Exp. Ther. 2001;297:364–71. [PubMed] [Google Scholar]
- 15.Neilan CL, Nguyen TM-D, Schiller PW, Pasternak GW. Pharmacological characterization of the dermorphin analog [Dmt1]DALDA, a highly selective μ-opioid peptide. Eur. J. Pharmacol. 2001;419:15–23. doi: 10.1016/s0014-2999(01)00946-3. [DOI] [PubMed] [Google Scholar]
- 16.Zhao G-M, Wu D, Soong Y, Shimoyama M, Berezowska I, Schiller PW, Szeto HH. Profound spinal tolerance after repeated exposure to a highly selective μ-opioid peptide agonist: role of δ-opioid receptors. J. Pharmacol. Exp. Ther. 2002;302:188–96. doi: 10.1124/jpet.302.1.188. [DOI] [PubMed] [Google Scholar]
- 17.Szeto HH, Lovelace JL, Fridland G, Soong Y, Fasolo J, Wu D, Desiderio DM, Schiller PW. In vivo pharmacokinetics of selective μ-opioid peptide agonists. J. Pharmacol. Exp. Ther. 2001;298:57–61. [PubMed] [Google Scholar]
- 18.Zhao K, Luo G, Zhao G-M, Schiller PW, Szeto HH. Transcellular transport of a highly polar 3+ net charge opioid tetrapeptide. J. Pharmacol. Exp. Ther. 2003;304:425–32. doi: 10.1124/jpet.102.040147. [DOI] [PubMed] [Google Scholar]
- 19.Park E-S, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species in rat neuropathic spinal dorsal horn neurons. Neurosci. Lett. 2006;391:108–11. doi: 10.1016/j.neulet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 20.Kim HY, Chung JM, Chung K. Increased production of mitochondrial superoxide in the spinal cord induces pain behaviors in mice: the effect of mitochondrial electron transport inhibitors. Neurosci. Lett. 2008;447:87–91. doi: 10.1016/j.neulet.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimoyama M, Schiller PW, Shimoyama N, Toyama S, Szeto HH. Superior analgesic effect of H-Dmt-D-Arg-Phe-Lys-NH2 ([Dmt1]DALDA), a multifunctional opioid peptide, compared to morphine in a rat model of neuropathic pain. Chem. Biol. Drug Des. 2012;80:771–74. doi: 10.1111/cbdd.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leventhal L, Smith V, Hornby G, Andree TH, Brandt MR, Rogers KE. Differential and synergistic effects of selective norepinephrine and serotonin reuptake inhibitors in rodent models of pain. J. Pharmacol. Exp. Ther. 2007;320:1178–85. doi: 10.1124/jpet.106.109728. [DOI] [PubMed] [Google Scholar]
- 23.Laferrière A, Millecamps M, Xanthos DN, Xiao WH, Siau C, de Mos M, Sachot C, Ragavendran V, Huygen FJPM, Bennett GJ, Coderre TJ. Cutaneous tactile allodynia associated with microvascular dysfuntion in muscle. Mol. Pain. 2008;4:49. doi: 10.1186/1744-8069-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inauen W, Suzuki M, Granger DN. Mechanisms of cellular injury: potential sources of oxygen free radicals in ischemia/reperfusion. Microcirc., Endothelium, Lymphatics. 1989;5:143–55. [PubMed] [Google Scholar]
- 25.Millecamps M, Coderre TJ. Rats with chronic post-ischemia pain exhibit an analgesic sensitivity profile similar to human patients with complex regional pain syndrome-type I. Eur. J. Pharmacol. 2008;583:97–102. doi: 10.1016/j.ejphar.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morphy R, Rankovic Designed multiple ligands. An emerging drug discovery paradigm. J. Med. Chem. 2005;48:6523–43. doi: 10.1021/jm058225d. [DOI] [PubMed] [Google Scholar]
- 27.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]