Abstract
Background
Primary aldosteronism (PA) is a common and under-diagnosed disease with significant morbidity potentially cured by surgery. We aim to assess if the long-term cardiovascular benefits of identifying and treating surgically correctable PA outweigh the upfront increased costs in patients at the time patients are diagnosed with resistant hypertension (RH).
Methods and Results
A decision-analytic model compares aggregate costs and systolic blood pressure (SBP) changes of six recommended or implemented diagnostic strategies for PA in a simulated population of at-risk RH patients. We also evaluate a seventh “treat all” strategy wherein all patients with RH are treated with a mineralocorticoid-receptor antagonist without further testing at RH diagnosis. Changes in SBP are subsequently converted into gains in quality-adjusted life years (QALYs) by applying National Health and Nutrition Examination Survey data on concomitant risk factors to an existing cardiovascular disease simulation model. QALYs and lifetime costs were then used to calculate incremental cost-effectiveness ratios (ICERs) for the competing strategies. The ICER for the strategy of computerized tomography (CT) followed by adrenal venous sampling (AVS) was $82,000/QALY compared to “treat all”. ICERs for CT alone and AVS alone were $200,000/QALY and $492,000/QALY; the other strategies were more costly and less effective. Integrating differential patient-reported health-related quality of life adjustments for patients with PA, ICERs for screening patients with CT followed by AVS, CT alone, and AVS alone were $52,000/QALY, $114,000/QALY, and $269,000/QALY gained.
Conclusions
CT scanning followed by AVS was a cost-effective strategy to screen for PA among patients with resistant hypertension.
Keywords: hyperaldosteronism, hypertension, cost-effectiveness
Hypertension (HTN, Supplemental Table 1) affects 76 million Americans and is the leading cause of heart disease, stroke and death.1 Prevalence of resistant hypertension (RH) is estimated between 12–30% of the hypertensive population.2–4 The Joint National Committee (JNC) suggests referral to a hypertension specialist in this subset of patients, although practice recommendations for the screening and diagnosis of secondary causes of hypertension vary.2, 5, 6
Primary aldosteronism (PA) is the most common cause of secondary HTN and is characterized by autonomous, inappropriately elevated plasma aldosterone, stemming from an aldosterone producing adenoma (APA) or bilateral adrenal hyperplasia (BAH). Because HTN – frequently the only sign of PA – is so common, the diagnosis of PA is often overlooked.5 The prevalence of PA in the hypertensive population is estimated to be 10% in recent studies, with nearly half being unilateral (i.e. surgically-correctable) disease.5, 7, 8 Patients with PA make up 17–23% of RH patients and have worse outcomes.9–12 In comparison to primary hypertensive patients matched for BP, patients with both subtypes of PA have four times the risk of stroke, seven times the risk of non-fatal heart attack, and 7–12 times the risk of atrial fibrillation.11, 13 Moreover, patients with PA have worse psychosocial and quality of life scores when compared to matched patients with primary hypertension.14–16
Adrenalectomy for APA (i.e., unilateral PA) is effective and is shown to reverse cardiovascular and renal complications.13, 17–19 However, there are costs and different levels of efficiency associated with the various screening strategies used to determine who is most likely to benefit from surgical intervention. Furthermore, even small changes in blood pressure have been shown to have significant downstream effects on cardiovascular events.20, 21 Treatment with mineralocorticoid receptor antagonists also yields significant improvements in blood pressure and regression of left ventricular hypertrophy in all PA subtypes.13, 17, 19, 22
We hypothesize that it is cost-effective to screen the resistant hypertensive population for PA, which is known to have a high proportion of PA patients. Specifically, we postulate that the improvements in blood pressure and consequent reductions in downstream cardiovascular events, in addition to improvements in quality of life resulting from appropriate treatment of PA patients, outweigh the upfront cost to establishing a PA diagnosis and initiating disease-specific medical treatment or surgery when appropriate. In this analysis, we use a decision-analytic approach to compare recommended strategies and strategies commonly used in practice for PA screening and identification of patients with surgically correctable disease in the RH population versus treating all patients with mineralocorticoid receptor antagonists.
Methods
Model structure
A decision-analytic model (Figure 1) was used to compare the aggregate intervention costs and effectiveness associated with six screening strategies that are both diagnostic (i.e. to distinguish primary hyperaldosteronism from primary hypertension) and help with lateralization (i.e. to distinguish surgically-correctable APA from bilateral adrenal hyperplasia in those with positive blood tests) in a simulated cohort of RH patients. As a clearly superior strategy has not been proven, we chose to perform a comprehensive analysis including a number of recommended strategies as well as commonly used algorithms in practice.
Figure 1.
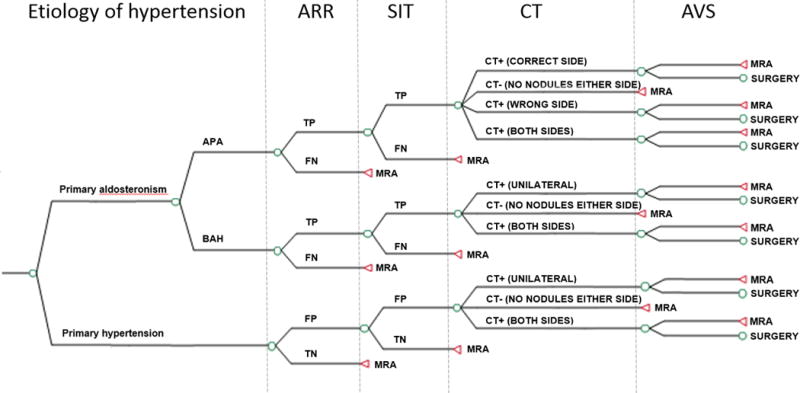
Model schematic for intervention strategies (i.e. SIT/CT/AVS, CT/AVS, SIT/AVS, AVS only, SIT/CT, and CT only). True underlying cause of hypertension (primary hypertension versus PA) and subtype of PA (“unilateral” = APA; “bilateral” = BAH) are based on prevalence in the population of RH patients. All patients in the intervention strategies undergo screening with ARR. Strategies differ on use of SIT, CT, and AVS (Table 1).
APA: Aldosterone-producing adenoma; ARR: Aldosterone-renin ratio; AVS: Adrenal venous sampling; BAH: Bilateral adrenal hyperplasia; SIT: Saline-infusion testing; TP: true-positive given etiology of hypertension; FN: false-negative; MRA: Mineralocorticoid-receptor antagonist.
We considered six screening strategies (Table 1) to identify those patients with unilateral, surgically correctable hyperaldosteronism (i.e. APA). All of these strategies begin with an aldosterone to renin ratio (ARR); patients with a negative ARR are started on a mineralocorticoid-receptor antagonist (MRA). Following a positive ARR, patients received one of the following testing options: 1) Confirmatory saline-infusion test (SIT), abdominal computerized tomography (CT), and adrenal venous sampling (AVS) (strategy SIT/CT/AVS), 2) CT and AVS (CT/AVS), 3) SIT and AVS (SIT/AVS), 4) AVS only, 5) SIT and CT (SIT/CT), or 6) CT only. A seventh strategy included upfront treatment with an MRA in all RH patients without further testing. Spironolactone was chosen as the base-case MRA as it is the least costly yet still effective medication against which to compare potential “surgical” strategies. We aimed to assess the consequences of immediate action at RH diagnosis as it has been shown that length of time with PA is correlated with failure of cure. Therefore, a strategy of medical trial followed by surgery was not considered.
Table 1.
Diagnostic and lateralization strategies for intervention model.
| Strategy | Screen (ARR) | Confirmatory (SIT) | Imaging (CT) | AVS | Surgery |
|---|---|---|---|---|---|
| 1 – SIT/CT/AVS | + | + | + | + | + |
| 2 – CT/AVS | + | − | + | + | + |
| 3 – SIT/AVS | + | + | − | + | + |
| 4 – AVS only | + | − | − | + | + |
| 5 – SIT/CT | + | + | + | − | + |
| 6 – CT only | + | − | + | − | + |
| 7 – MRA only | − | − | − | − | − |
ARR: aldosterone to renin ratio; SIT: saline-infusion testing; AVS: adrenal venous sampling; MRA: Mineralocorticoid-receptor antagonist.
Based on the best available published evidence (Table 2 and Supplemental Table 2), we assumed that patients with surgically treated APA would obtain an additional 10 mmHg reduction in systolic blood pressure (SBP) compared to PA patients treated with MRA. In strategy MRA only, all RH patients were treated upfront with MRA without ARR screening as it has been shown that primary hypertensive patients also respond, albeit to a lesser extent, to MRA therapy.22, 23 All patients in all other strategies who had a negative ARR, negative CT (i.e. no nodules found in either adrenal) or who underwent unsuccessful surgery (i.e. those with BAH or primary hypertension undergoing surgery) were given MRA therapy with resultant decreases in blood pressure at varying levels predicted by the true underlying RH etiology (i.e. patients with primary hypertension would have a reduction in SBP of 10 mmHg and those with PA would have a reduction of 20 mmHg).
Table 2.
Intervention model inputs for base-case analysis and sensitivity analysis (see Supplemental Table 2 for the respective references).
| Parameter | Value | Sensitivity analysis range |
|---|---|---|
| Epidemiology | ||
| Prevalence of PA in resistant HTN | 0.20 | 0.11–0.23 |
| Proportion of unilateral PA | 0.43 | 0.35–0.60 |
| Prevalence of incidental adrenal nodules | 0.05 | 0.01–0.09 |
| Test characteristics | ||
| Sensitivity of screening testing (ARR) | 0.78 | 0.66–0.98 |
| Specificity of screening testing (ARR) | 0.83 | 0.63–0.99 |
| Sensitivity of confirmatory testing (SIT) | 0.83 | 0.55–0.90 |
| Specificity of confirmatory testing (SIT) | 0.75 | 0.75–1.00 |
| Probability of contralateral nodule CT in APA | 0.12 | 0.06–0.13 |
| Probability of true positive CT in APA (sensitivity given APA) | 0.59 | 0.49–0.62 |
| Probability of bilateral CT abnormalities in APA | 0.15 | 0.13–0.36 |
| Probability of normal CT in APA | 0.14 | 0.07–0.25 |
| Probability of bilateral CT abnormalities in BAH (sensitivity given BAH) | 0.41 | 0.19–0.46 |
| Probability of normal CT in BAH | 0.22 | 0.22–0.43 |
| Probability of unilateral CT in BAH | 0.36 | 0.33–0.38 |
| Lateralizing AVS with BAH (false-positive given true bilateral disease) | 0.02 | 0.02–0.20 |
| Sensitivity of AVS for unilateral disease (true-positive given true unilateral disease) | 0.93 | 0.80–0.93 |
| Proportion of unsuccessful adrenal vein cannulation | 0.18 | 0.04–0.37 |
| Procedural morbidity | ||
| Morbidity from AVS (bleeding) | 0.01 | 0.006–0.07 |
| Morbidity from surgery | 0.07 | 0.06–0.08 |
| Mortality from adrenalectomy | 0.01 | 0.00–0.01 |
| Treatment effects (ΔmmHg) | ||
| SBP change with death/no treatment | 0.00 | – |
| SBP change treatment of primary hypertensive patients with MRA | 10.00 | 4–22 |
| SBP change treatment PA with MRA | 20.00 | 11–33 |
| Incremental SBP change with PA adrenalectomy (over MRA) | 10.00 | 0–20 |
| Costs | ||
| Screening ARR (CPT 82088, 84244,84132) | $93 | (0.5–1.5) BCE |
| Confirmatory saline infusion testing (CPT 96365, 93666) | $141 | (0.5–1.5) × BCE |
| Abdominal CT (CPT 74170) | $329 | (0.5–1.5) BCE |
| Adrenal venous sampling (CPT 75893, 36500) | $2,645 | (0.5–1.5) × BCE |
| Adrenalectomy (surgery + anesthesia)a (CPT 60650, 00866) | $3,054 | (0.5–1.5) × BCE |
| Hospitalization (DRGb 615) | $7,867 | (0.5–1.5) × BCE |
| Hospitalization w/MCC (DRG 614) | $16,833 | (0.5–1.5) BCE |
| One year cost of spironolactone | $158 | (0.5–1.5) × BCE |
ARR – aldosterone to renin ratio; SIT – saline-infusion testing; APA – aldosterone producing adenoma; BAH – bilateral adrenal hyperplasia; MCC – major comorbidities or complications; SBP – systolic blood pressure; MRA: Mineralocorticoid-receptor antagonist; BCE – base-case estimate.
Cost of anesthesia was based on the product of average anesthesia time (15 minute increments), 2013 HCPCS Anesthesia Base Units, and the national anesthesia conversion factor.
DRG – Diagnosis-related group for adrenal procedures with and without major comorbidities or complications.
For the intervention decision tree, the clinical starting point was a patient with RH. For the primary analysis, we made a number of important assumptions: 1) patients were all considered surgical candidates; 2) patients diagnosed with unilateral PA (appropriately or inappropriately) all underwent laparoscopic adrenalectomy; 3) false-positive rates (i.e. falsely determined to be PA) of CT results for RH patients with primary hypertension were reflective of prevalence of incidental adrenal nodules in the population24, 25; 4) if abdominal CT indicated an abnormality on both sides, patients either proceeded to AVS and surgery if AVS lateralized to one adrenal gland (strategies SIT/CT/AVS and CT/AVS), or in CT-only strategies (strategies SIT/CT and CT only) patients were treated with MRA; and 5) if CT did not show abnormalities in either adrenal gland, in CT only strategies (strategies SIT/CT and CT only) patients were treated with MRA.
TreeAge Pro 2014 (TreeAge Software, Williamstown, MA) was used to construct and analyze the intervention model. The change in SBP, change in the number of antihypertensive medications following treatment (estimates obtained from the literature), and differential projected annual costs of anti-hypertensive regimens were calculated for each strategy in the immediate intervention model.
Costs
The analysis was performed from a health care system perspective. Best available cost and probability estimates were extracted from the literature (Table 2 and Supplemental Table 2). The costs of screening, surgery, complications, and medications were included in the analysis, but non-health care related costs to the patient were not.
Quality-adjusted life years and lifetime costs
Changes in SBP and cost of antihypertensive medications per strategy from the decision tree model were subsequently converted into gains in quality-adjusted life years (QALYs) and changes in lifetime cardiovascular disease (CVD) costs using a previously developed and validated CVD model which is able to evaluate the long term cardiovascular, costs, events and mortality for each change in blood pressure reduction.26 The reductions in blood pressure and costs associated with each intervention strategy were applied to a National Health and Nutrition Examination Survey (NHANES) population with additional risk factor data to project CVD events.
From 40,790 patients available in the continuous NHANES database from 2005–2012, a cohort of 836 patients was selected according to the following criteria: 1) patients with SBP ≥ 160 mmHg (presumed RH) and 2) patients with available data on cardiovascular risk factors required to assess 10-year Framingham risk score.20 These patients were sampled with replacement to create the simulation cohort (1,000,000 patients) and entered into the CVD Markov model (Supplemental Figure 1) with microsimulation to assess comparative discounted lifetime costs and QALYs (i.e. incremental cost-effectiveness ratios, ICERs).26 Briefly, each year, patients had a probability of developing CVD (i.e. coronary heart disease, stroke, or CVD-related death) based on Framingham risk function, and a probability of death from other causes based on age and sex-based life tables (Supplemental Table 3). Differential annual costs of anti-hypertensive regimens for the years following the initial intervention were calculated for each strategy and factored into the CVD model (detailed in Supplemental Methods & Supplemental Table 2).
In the base case analysis we consider only CVD effects on health-related quality-of-life (HRQoL). Utility weights for baseline primary hypertensive patients and downstream health states within the CVD model were based on a broad national sample of community-based, patient-reported EQ-5D utility scores associated with chronic diseases.27 We also performed an alternative analysis that incorporated a possible utility reduction owing to untreated PA. Prior data suggest that patients with PA have worse quality of life scores when compared to patients with primary hypertension.14–16 We derived estimates of utility weights (i.e. measure of HRQoL) from longitudinal survey data using the Short Form-12v1® in a cohort of patients before and after treatment with adrenalectomy or MRA. Data were catalogued and translated into interval scale utilities (0 = dead to 1 = perfect health) using the QualityMetric health state score system, SF6D®, for integration of a change of utility into the CVD model. The study was approved by the institutional review board and subjects gave informed consent. For the purposes of this study, median differences post-treatment between surgical versus medically treated PA patients were used to adjust the base utility for each intervention strategy for the CVD model.
QALYs and lifetime CVD costs from the simulation model and per-patient diagnostic and treatment costs from the decision tree model were then used to calculate ICERs (cost per QALY) for the seven competing strategies. Future costs and QALYs were discounted at 3% per annum.28 A willingness-to-pay (WTP) threshold of $US 150,000/QALY gained was used as a benchmark for cost-effectiveness, following the American College of Cardiology/American Heart Association paper position paper on integration of cost-effective data into clinical practice.29
Sensitivity analysis
We performed univariate sensitivity analyses on all variables to assess effects of varying key model parameters upon our results (Table 2 and Supplemental Figures 2 to 6). Given recommendations for utilization of CT for surgical planning and to assess for potential malignancy6, 30, we also tested the additional cost of the CT (with all patients still undergoing AVS regardless of the CT results) in the AVS-only strategies (SIT/AVS and AVS only). Probabilistic sensitivity analysis (PSA) was performed to assess the effects of parameter estimate uncertainty.
Results
Costs and outcomes by strategy are shown in Table 3. Of the strategies evaluated, proceeding directly to adrenal venous sampling (AVS only) yielded the greatest SBP reduction (12.49 mmHg) and cost. Treating all patients with MRA was the least costly – given no further testing or surgery – but with the lowest SBP reduction. Using preliminary patient-reported survey data to measure the effects of treatment on HRQoL in patients with PA, we found a greater improvement with surgery as compared to MRA. A median utility decrement of 0.054 [IQR 0, 0.079] was found for those with PA treated medically versus surgically. Moreover, the strategies that resulted in more patients with true surgically correctable PA being treated with surgery also had a greater increase in quality of life (i.e. AVS only strategy).
Table 3.
Aggregate intervention costs, average change from baseline in: systolic blood pressure (Δ SBP), average number of antihypertensive medications (Δ MED), and health-related quality of life (Δ QALY) measurement per strategy*. Δ SBP and Δ MED per strategy from the decision tree model were converted into aggregate lifetime discounted costs and QALYs using the CVD model.
| Strategy | Etiology | Intervention cost | Δ SBP | Δ MED | Δ QALYs* | Discounted cost ($)** | Discounted QALYs** |
|---|---|---|---|---|---|---|---|
| 1 SIT/CT/AVS | All | $1,064 | −12.35 | −0.61 | 0.0018 | 35,563 | 11.568 |
| PH | $295 | −10.00 | −0.50 | ||||
| PA | $4,139 | −21.75 | −1.04 | ||||
| 2 CT/AVS | All | $1,226 | −12.42 | −0.62 | 0.0022 | 35,654 | 11.5694 |
| PH | $332 | −10.00 | −0.50 | ||||
| PA | $4,802 | −22.11 | −1.11 | ||||
| 3 SIT/AVS | All | $1,240 | −12.41 | −0.62 | 0.0021 | 35,682 | 11.5692 |
| PH | $399 | −10.00 | −0.50 | ||||
| PA | $4,606 | −22.04 | −1.09 | ||||
| 4 AVS only | All | $1,670 | −12.49 | −0.63 | 0.0026 | 36,030 | 11.5704 |
| PH | $747 | −10.00 | −0.50 | ||||
| PA | $5,365 | −22.45 | −1.17 | ||||
| 5 SIT/CT | All | $1,132 | −12.38 | −0.61 | 0.0019 | 35,600 | 11.5686 |
| PH | $310 | −10.00 | −0.50 | ||||
| PA | $4,417 | −21.88 | −1.07 | ||||
| 6 CT only | All | $1,341 | −12.45 | −0.63 | 0.0024 | 35,734 | 11.5698 |
| PH | $391 | −10.00 | −0.50 | ||||
| PA | $5,138 | −22.26 | −1.14 | ||||
| 7 MRA only | All | $158 | −12.00 | −0.54 | 0 | 35,003 | 11.5615 |
| PH | $158 | −10.00 | −0.50 | ||||
| PA | $158 | −20.00 | −0.70 |
AVS: adrenal venous sampling; PH: primary hypertension; PA: primary aldosteronism; MRA: mineralocorticoid-receptor antagonist.
The utility gain attributable to surgical correction of PA was used only in an alternative analysis, not in the base case.
Future costs and QALYs were discounted at 3% per annum.
The 836 patients from the NHANES database had a mean age of 67.6 years, were 58.0% female, and had an initial mean SBP of 175 mmHg. After entering intervention costs and changes in SBP into the CVD model, we found that none of the strategies with confirmatory saline-infusion testing (SIT) were cost-effective (i.e., all were dominated). CT/AVS, CT only, and AVS only strategies all resulted in gains in life expectancy compared to treating all patients with MRA (i.e. on the efficiency frontier) at an increased cost. At a U.S. WTP threshold of $150,000/QALY and without adjustments in HRQoL for patients with surgically untreated PA, CT/AVS strategy is the cost-effective choice with an ICER of $82,000/QALY (Figure 2). After integrating conservative HRQoL reductions for PA patients treated with MRAs alone, CT only strategy (with an ICER of $114,000/QALY) would be the cost-effective choice at this WTP threshold (Figure 3).
Figure 2.
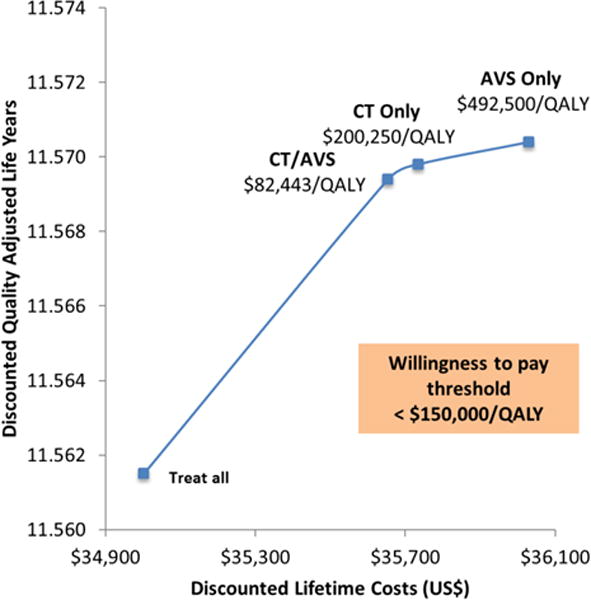
Efficiency frontier for base-case analysis unadjusted for HRQoL adjustment for PA patients not undergoing surgery. Only non-dominated strategies shown.
Figure 3.
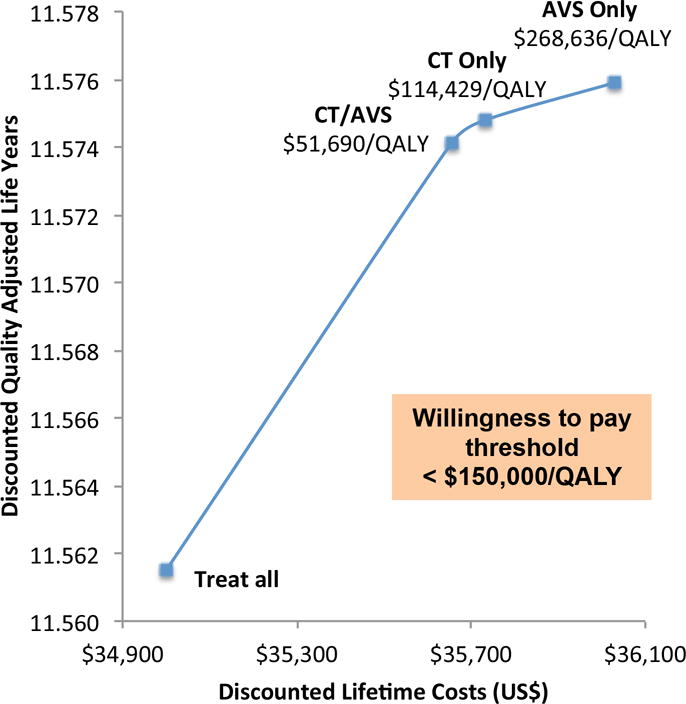
Efficiency frontier for secondary analysis with HRQoL adjustment for PA patients treated with mineralocorticoid-receptor antagonist alone. Only non-dominated strategies shown.
The proportion of patients undergoing surgery by underlying disease is shown in Supplemental Table 4. Using the CT/AVS strategy, the preferred strategy at the WTP threshold of $150,000/QALY, 50% of patients with true unilateral PA are treated appropriately with adrenalectomy with a surgical mortality of less than 0.1%. At the same time, potentially ineffective surgery would occur in 0.1% of patients (i.e. patients with BAH or primary hypertension). Surgical mortality in BAH and PH patients undergoing surgery nears zero.
Sensitivity analysis
The same four strategies remain on the efficiency frontier over a wide-range of sensitivity analyses (Table 2), specifically through a wide range of prevalence of PA in the RH population (Supplemental Figure 2), prevalence of adrenal incidentalomas, and testing characteristics of screening ARR (Supplemental Figures 3 and 4). When the prevalence of unilateral PA (APA) increased above 50%, strategy CT/AVS fell off the efficiency frontier. There was no effect on results for sensitivity of screening ARR and confirmatory testing. When the sensitivity and specificity of CT or AVS were diminished or costs of the tests changed significantly, competing strategies became more desirable. However within reported ranges, our results were consistent. The efficiency frontier remained the same with the additional cost of the CT for surgical planning. Some patients require higher doses of MRA to attain improvement in blood pressure. No significant changes in the efficiency frontier were seen with increasing the cost of this medication (Supplemental Figure 5).
Efficient strategies are dependent on treatment effect, with the key parameters being the SBP change with MRA in PA patients and the incremental effect of surgery versus MRA in patients with PA, illustrated in Figure 4. However, even with a very small incremental SBP effect between PA patients treated with surgery versus medication, screening strategies to identify appropriate patients for surgery remained on the efficiency frontier (Figure 5). In the base case analysis, with a 6 mmHg change in SBP with surgery over MRA, CT/AVS is cost-effective. With including utility adjustments for untreated PA, at a 2 mmHg improvement CT/AVS is cost-effective.
Figure 4.
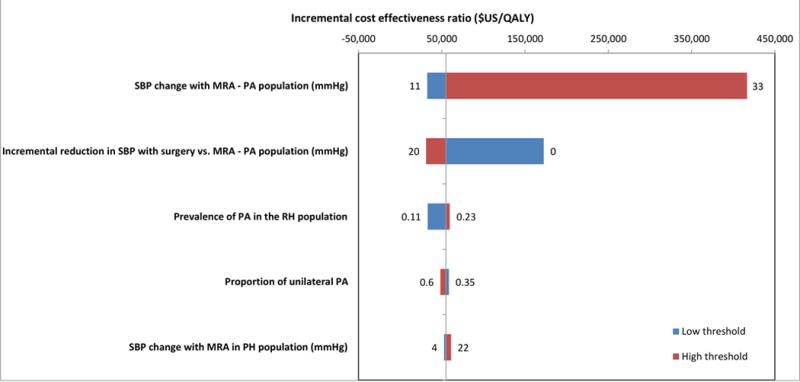
Tornado plot illustrating univariate sensitivity analyses of key parameters for the strategy for the preferred strategy (CT only) versus MRA only. The width of the horizontal bars illustrate the effects of each parameter on the ICER ($/QALY). The vertical line represents the base case result.
Figure 5.
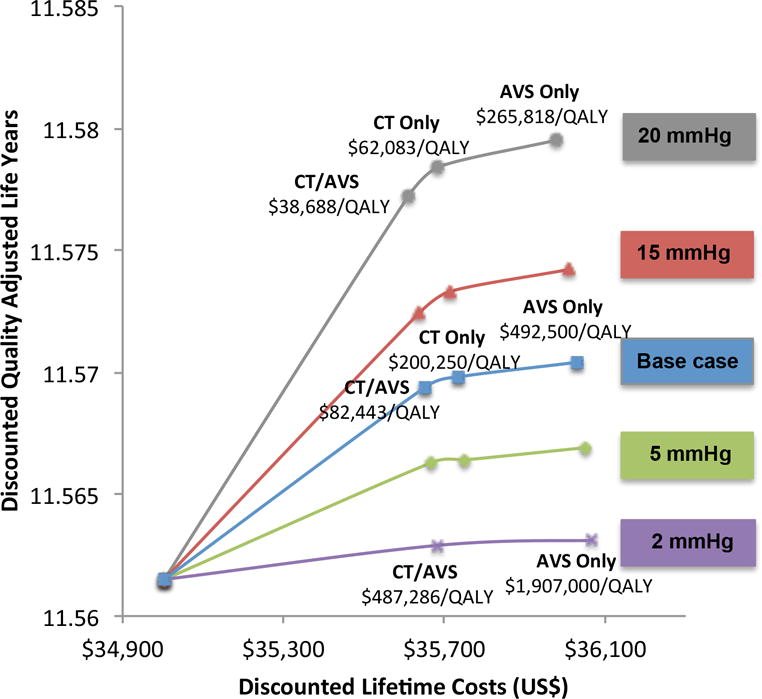
Sensitivity analysis of the incremental blood pressure changes of surgery over MRA for PA patients without utility adjustments for PA patients treated with MRA alone. Only non-dominated strategies shown.
In most reports, the average age at diagnosis is younger in patients with PA than with primary hypertension. While patients entering the model did not have a diagnosis of PA as the cause of RH (by design), we tested our hypothesis that screening for PA at a younger age may increase the benefit of screening by performing a subgroup analysis in patients <50 years old from our NHANES cohort. We found the same strategies were cost-effective with substantial improvements in the ICERs (Figure 6). Very few patients with RH are younger than 40 years old (average age in our cohort was 67), therefore, we chose not to test stratifying patients for surgery following a unilateral CT findings by age as recommended by the Endocrine Practice guidelines.6
Figure 6.
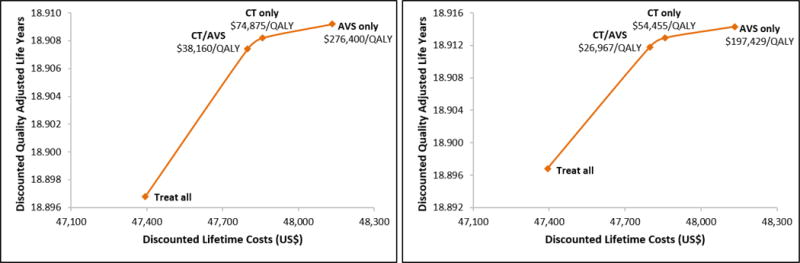
Efficiency frontiers in a cohort of patients <50 years old: A) base-case analysis considering only effects of CVD on HRQoL, and B) base-case analysis with differential patient-reported HRQoL associated with untreated PA. Only non-dominated strategies shown. In strategy CT/AVS, all patients proceed to AVS unless no abnormality is found on CT scan.’
Finally, in order to test our assumption that NHANES patients in our sample had RH (versus inadequate treatment or poor patient adherence to antihypertensive regimen), we performed a sub-group analysis on patients who confirmed having a current prescription of antihypertensive medications on the NHANES questionnaire (Supplemental Figure 6). We found that the same strategies were cost-effective in this subgroup analysis.
The results of the probabilistic sensitivity analysis (PSA) are shown as a cost-effectiveness acceptability curve (Figure 7). This graph shows the proportion of the random samplings of parameter distributions resulting in the greatest net health benefit (vertical axis) at increasing WTP thresholds (horizontal axis). At a WTP threshold of $150,000/QALY, a strategy involving screening and subtype diagnosis of PA was cost-effective in 87% of simulations.
Figure 7.
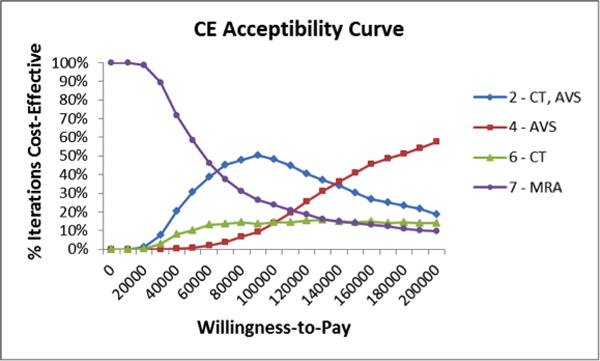
Second order probabilistic sensitivity analysis shown as a cost-effectiveness acceptability curve. Proportion of the random samplings of parameter distributions resulting in the greatest net health benefit on the vertical axis and WTP thresholds on the horizontal axis.
Discussion
Based on this analysis, our results support our hypothesis that screening for PA and surgically treating those with appropriate indications in all patients with RH (versus the MRA only strategy) is cost-effective. This result was consistent in the base case and exhaustive sensitivity analyses. Which of the remaining intervention strategies is preferred depends on the WTP threshold. Prior to integrating the effects of untreated PA on health-related quality of life, the intervention strategy CT/AVS was the only cost-effective management strategy with an ICER of $82,000/life year saved. While it may be counterintuitive that CT/AVS would be more cost-effective than CT alone, this can be explained by the costs incurred from downstream medical and CVD-event related costs from removing the “wrong” adrenal gland (i.e. false-positive CT) and failing to remove a functional adenoma in cases with bilateral CT abnormalities in the CT-only strategy. This highlights the value of AVS in helping to lateralize lesions preoperatively. Finally, our study found that the differential effect of treatment on SBP (i.e. medical versus surgical in PA patients) had the greatest impact on the ICERs. A definitive controlled trial directly comparing the impact of medical management versus surgery would be needed to clarify the magnitude of benefit that would be expected with adrenalectomy relative to treatment with MRA. Despite this variation, we found that with only small incremental differences in SBP in PA patients undergoing surgery versus medical therapy (i.e. 6 mmHg in the base case and 2 mmHg when additional quality of life decrements for those with PA were taken into account), screening for surgically-correctable PA was cost-effective, as small changes in blood pressure have been shown to have significant downstream effects on cardiovascular events.20, 21
When a modest reduction in utility associated with untreated PA was incorporated into the model, there were notable decreases in the ICERs for all screening strategies. At a threshold of $150,000/QALY, CT only becomes the preferred strategy, with an ICER of $114,000/QALY. We postulate that an early improvement in quality of life may be derived from a number of factors including taking fewer medications and associated side-effects, decreased financial burden and fewer office visits. Downstream effects of health-related quality of life may be attributable to lower blood pressure resulting in fewer interventions and cardiovascular events. Psychometric survey data show that patients with PA have increased anxiety, depression and SF36 domains compared to the general population and primary hypertensive controls.14, 16 Studies suggest that patients show greater improvements following adrenalectomy compared to MRA at six months post-treatment.16 In addition, patients can experience debilitating side effects from MRAs including gynecomastia and impotence. While we do not currently have data on the differential effects of spironolactone versus eplerenone on HRQoL, we expect the potential improvement in HRQoL from fewer medication side effects to be offset by the incremental cost of eplerenone.
Our results show that the strategy with AVS alone following positive screening ARR resulted in the greatest reductions in SBP in the intervention model and greatest quality-adjusted life expectancy; however, this came at significant cost, leading to a very high ICER. Use of AVS in all patients screening positive for PA is controversial. Proponents cite the need for AVS given the high false-negative and false-positive rates of CT for small aldosterone-secreting adrenal adenomas, whereas opponents cite technical difficulty, cost, and risk of adverse effects.31–36 The generalizability of published reports on the performance of AVS in tertiary care centers is open to question, given the lower success rates seen in the few published reports from non-academic centers.30, 32 Moreover, strategies with AVS remained on the efficiency frontier over a wide range of reported success rates of AVS. The addition of a CT for pre-surgical planning – commonly practiced – only adds a small incremental cost to this strategy and does not alter the effectiveness. Lastly, it is important to remember that although WTP thresholds are commonplace in Europe, they are contentious in the U.S.; values vary depending on the economy, location, relevant decision maker, values of the population, and the resources available.37 Estimated on current medical care spending in the U.S., a range of $183,000/QALY – $264,000/QALY may be considered more reflective of current willingness to pay.38
Moreover, while guidelines recommend the use of confirmatory testing, we did not find any strategy that included SIT to be cost-effective. This is likely due to the fact the ARR as a screening test is fairly accurate and that the small incremental benefit from confirmatory testing does not outweigh the cost. It should be noted that we did not include the costs of SIT implementation (i.e. two hours of staff observation for infusion) nor potential morbidity associated with changing and/or withholding hypertensive regimens or of salt-loading in this analysis. Inclusion of these additional costs would make strategies that include confirmatory testing even less cost-effective.
Our study has limitations, common to decision-analytic methods, which arise when using simplifying assumptions to model complex disease care pathways. First, we made the presumption that patients above a SBP threshold in the NHANES database had RH and not untreated hypertension. If our assumption was incorrect, this would translate into a lower percentage of truly resistant patients and, therefore, a decreased proportion of PA patients. We would expect that in a pure RH population our screening strategies would be even more cost-effective.16, 22, 39 Indeed, our subgroup analysis of NHANES patients self-reporting use of anti-hypertensives resulted in the same cost-effective strategies (Supplemental Figure 6). Secondly, many retrospective studies may have misclassified patients at diagnosis by not properly identifying subtype (i.e. by not performing AVS), or by leaving open to possibility that failure of adrenalectomy was from underlying primary hypertension (i.e. because no post-operative ARR was performed). We expect that the majority of the misclassification is in failed identification of appropriate surgical candidates who would benefit most from diagnosis and treatment. For example, being younger or female is positively associated with hypertension cure after surgery for APA, but these patient characteristics were not taken under account in the base case analysis.40–42 Furthermore, given that patients with BAH do not undergo surgery, it is impossible to confirm the diagnosis of these patients, making specificity of tests challenging to determine. Lastly, while medical and surgical treatment have been shown to have positive treatment effects, there are limited data on the comparative effect of treatment on blood pressure between the following three groups in controlled trials: 1) primary hypertension patients receiving MRAs, 2) PA patients receiving MRAs, and 3) PA patients undergoing adrenalectomy. Rossi et al. showed comparable efficacy of surgery and MRAs in lowering BP in PA patients.13 Other reports cite greater improvement in BP between patients undergoing surgery compared to MRAs in PA patients (Supplemental Table 2). However, more efficacy data are needed to strengthen the conclusions of this study. In a cost analysis of patients with PA (without comparison to MRA alone), Reimel et al. found surgery to be significantly less expensive over a lifetime.43 It is important to note that while the incremental increased in life expectancy between strategies may be interpreted as small, it should be emphasized that this is an average result over a million simulated patients. Therefore, while many patients in this population (i.e. 80% of whom have primary hypertension) have only a minimum benefit with these strategies, there is a smaller but substantial subset of patients (approximately 10% of the RH population with APA) that gain substantial health benefits.
While we did include NHANES patients with a prior CVD event in the current study, we chose not to enhance the relative risk of prior CVD events in the PA sub-population. In a cross-sectional study, Milliez et al. found that compared to matched controls, PA patients were significantly more likely to have had a previous stroke or myocardial infarction.11 Catena and colleagues confirmed the findings of higher pre-treatment risk of CVD events and attenuation of this differential effect with treatment.10 We chose not to incorporate this into the model in order to isolate the benefit of screening and subsequent comparative improvement in SBP on outcomes. Our findings would be strengthened by integrating this differential history of CVD events given the significantly worse long-term outcomes in those with a prior CVD event. In practice, there is frequently a delay in diagnosing PA. Given that time with PA correlates with more CVD events and with failure of cure from surgery, early diagnosis of patients with PA – and in particular those with surgically-correctable disease – is essential. Integration of this a priori differential risk of CVD between PA and primary hypertensive patients is an important area for investigation.
In conclusion, our study addresses an increasingly important public health concern. Primary hyperaldosteronism is a common disease that is currently grossly underdiagnosed and treated. Given a conservative estimate that 12.5% of the hypertensive population has RH, 20% of RH patients have PA, and half of patients with PA have unilateral disease, we estimate that one million hypertensive patients in the U.S. could be cured with surgery.9, 12, 44 At accepted willingness to pay thresholds in the U.S., our results suggest that CT followed by AVS is a cost-effective strategy to screen for PA among patients with resistant hypertension. Given that CVD events are more likely and that HRQoL is significantly worse in untreated PA patients, and given that there is a reversal of this increased risk with treatment, identifying and appropriately treating RH patients could have significant impact.
Supplementary Material
What is Known
Primary hyperaldosteronism is the most common cause of secondary hypertension and makes up approximately 20% of the resistant hypertensive population.
Patients with primary hyperaldosteronism have worse cardiovascular outcomes compared to matched primary hypertensive patients.
Adrenalectomy is an effective treatment for primary hyperaldosteronism in approximately 50% of patients.
What the Study Adds
At an accepted willingness-to-pay threshold, screening for primary hyperaldosteronism in the resistant hypertensive population is cost-effective in comparison to medical treatment alone.
CT followed by confirmatory adrenal venous sampling is the optimal screening strategy for identifying patients with surgically-correctable (i.e. unilateral) adrenal disease.
Acknowledgments
Sources of Funding: Program in Cancer Outcomes Research Training Grant (NCI R25CA092203), National Cancer Institute (K07CA177900), Massachusetts General Hospital Department of Surgery, National Heart, Lung, and Blood Institute (5R01HL104284), and by a grant of the Else Kröner-Fresenius-Stiftung to M.R.
Footnotes
Disclosures
None.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics–2012 update: A report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension: Diagnosis, evaluation, and treatment: A scientific statement from the american heart association professional education committee of the council for high blood pressure research. Circulation. 2008;117:e510–526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 3.Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the united states, 1988 to 2008. Circulation. 2011;124:1046–1058. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Persell SD. Prevalence of resistant hypertension in the united states, 2003–2008. Hypertension. 2011;57:1076–1080. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 5.Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young WF, Jr, Montori VM. Case detection, diagnosis, and treatment of patients with primary aldosteronism: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 6.Zeiger MA, Thompson GB, Duh QY, Hamrahian AH, Angelos P, Elaraj D, Fishman E, Kharlip J. The american association of clinical endocrinologists and american association of endocrine surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(Suppl 1):1–20. doi: 10.4158/EP.15.S1.1. [DOI] [PubMed] [Google Scholar]
- 7.Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez CE, Veglio F, Young WF., Jr Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89:1045–1050. doi: 10.1210/jc.2003-031337. [DOI] [PubMed] [Google Scholar]
- 8.Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Mannelli M, Mattarello MJ, Moretti A, Palumbo G, Parenti G, Porteri E, Semplicini A, Rizzoni D, Rossi E, Boscaro M, Pessina AC, Mantero F. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 9.Calhoun DA. Hyperaldosteronism as a common cause of resistant hypertension. Annu Rev Med. 2013;64:233–247. doi: 10.1146/annurev-med-042711-135929. [DOI] [PubMed] [Google Scholar]
- 10.Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, Sechi LA. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168:80–85. doi: 10.1001/archinternmed.2007.33. [DOI] [PubMed] [Google Scholar]
- 11.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Strauch B, Zelinka T, Hampf M, Bernhardt R, Widimsky J., Jr Prevalence of primary hyperaldosteronism in moderate to severe hypertension in the central europe region. J Hum Hypertens. 2003;17:349–352. doi: 10.1038/sj.jhh.1001554. [DOI] [PubMed] [Google Scholar]
- 13.Rossi GP, Cesari M, Cuspidi C, Maiolino G, Cicala MV, Bisogni V, Mantero F, Pessina AC. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension. 2013;62:62–69. doi: 10.1161/HYPERTENSIONAHA.113.01316. [DOI] [PubMed] [Google Scholar]
- 14.Sonino N, Tomba E, Genesia ML, Bertello C, Mulatero P, Veglio F, Fava GA, Fallo F. Psychological assessment of primary aldosteronism: A controlled study. J Clin Endocrinol Metab. 2011;96:E878–883. doi: 10.1210/jc.2010-2723. [DOI] [PubMed] [Google Scholar]
- 15.Kunzel HE, Apostolopoulou K, Pallauf A, Gerum S, Merkle K, Schulz S, Fischer E, Brand V, Bidlingmaier M, Endres S, Beuschlein F, Reincke M. Quality of life in patients with primary aldosteronism: Gender differences in untreated and long-term treated patients and associations with treatment and aldosterone. J Psychiatr Res. 2012;46:1650–1654. doi: 10.1016/j.jpsychires.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed AH, Gordon RD, Sukor N, Pimenta E, Stowasser M. Quality of life in patients with bilateral primary aldosteronism before and during treatment with spironolactone and/or amiloride, including a comparison with our previously published results in those with unilateral disease treated surgically. J Clin Endocrinol Metab. 2011;96:2904–2911. doi: 10.1210/jc.2011-0138. [DOI] [PubMed] [Google Scholar]
- 17.Catena C, Colussi G, Lapenna R, Nadalini E, Chiuch A, Gianfagna P, Sechi LA. Long-term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension. 2007;50:911–918. doi: 10.1161/HYPERTENSIONAHA.107.095448. [DOI] [PubMed] [Google Scholar]
- 18.Letavernier E, Peyrard S, Amar L, Zinzindohoue F, Fiquet B, Plouin PF. Blood pressure outcome of adrenalectomy in patients with primary hyperaldosteronism with or without unilateral adenoma. J Hypertens. 2008;26:1816–1823. doi: 10.1097/HJH.0b013e3283060f0c. [DOI] [PubMed] [Google Scholar]
- 19.Sechi LA, Di Fabio A, Bazzocchi M, Uzzau A, Catena C. Intrarenal hemodynamics in primary aldosteronism before and after treatment. J Clin Endocrinol Metab. 2009;94:1191–1197. doi: 10.1210/jc.2008-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaziano TA, Steyn K, Cohen DJ, Weinstein MC, Opie LH. Cost-effectiveness analysis of hypertension guidelines in south africa: Absolute risk versus blood pressure level. Circulation. 2005;112:3569–3576. doi: 10.1161/CIRCULATIONAHA.105.535922. [DOI] [PubMed] [Google Scholar]
- 21.Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: Results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 22.Vaclavik J, Sedlak R, Plachy M, Navratil K, Plasek J, Jarkovsky J, Vaclavik T, Husar R, Kocianova E, Taborsky M. Addition of spironolactone in patients with resistant arterial hypertension (aspirant): A randomized, double-blind, placebo-controlled trial. Hypertension. 2011;57:1069–1075. doi: 10.1161/HYPERTENSIONAHA.111.169961. [DOI] [PubMed] [Google Scholar]
- 23.Nishizaka MK, Calhoun DA. The role of aldosterone antagonists in the management of resistant hypertension. Curr Hypertens Rep. 2005;7:343–347. doi: 10.1007/s11906-005-0067-3. [DOI] [PubMed] [Google Scholar]
- 24.Boland GW, Blake MA, Hahn PF, Mayo-Smith WW. Incidental adrenal lesions: Principles, techniques, and algorithms for imaging characterization. Radiology. 2008;249:756–775. doi: 10.1148/radiol.2493070976. [DOI] [PubMed] [Google Scholar]
- 25.Young WF., Jr Clinical practice. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:601–610. doi: 10.1056/NEJMcp065470. [DOI] [PubMed] [Google Scholar]
- 26.Pandya A, Sy S, Cho S, Weinstein MC, Gaziano TA. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314:142–150. doi: 10.1001/jama.2015.6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan PW, Ghushchyan V. Preference-based eq-5d index scores for chronic conditions in the united states. Med Decis Making. 2006;26:410–420. doi: 10.1177/0272989X06290495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in health and medicine. Oxford University Press; 1996. [Google Scholar]
- 29.Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, Masoudi FA, Peterson ED, Shaw LJ. Acc/aha statement on cost/value methodology in clinical practice guidelines and performance measures: A report of the american college of cardiology/american heart association task force on performance measures and task force on practice guidelines. Circulation. 2014;129:2329–2345. doi: 10.1161/CIR.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 30.Rossi GP, Auchus RJ, Brown M, Lenders JW, Naruse M, Plouin PF, Satoh F, Young WF., Jr An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014;63:151–160. doi: 10.1161/HYPERTENSIONAHA.113.02097. [DOI] [PubMed] [Google Scholar]
- 31.Stewart PM, Allolio B. Adrenal vein sampling for primary aldosteronism: Time for a reality check. Clin Endocrinol (Oxf) 2009 doi: 10.1111/j.1365-2265.2009.03714.x. [DOI] [PubMed] [Google Scholar]
- 32.Vonend O, Ockenfels N, Gao X, Allolio B, Lang K, Mai K, Quack I, Saleh A, Degenhart C, Seufert J, Seiler L, Beuschlein F, Quinkler M, Podrabsky P, Bidlingmaier M, Lorenz R, Reincke M, Rump LC, German Conn’s R. Adrenal venous sampling: Evaluation of the german conn’s registry. Hypertension. 2011;57:990–995. doi: 10.1161/HYPERTENSIONAHA.110.168484. [DOI] [PubMed] [Google Scholar]
- 33.Magill SB, Raff H, Shaker JL, Brickner RC, Knechtges TE, Kehoe ME, Findling JW. Comparison of adrenal vein sampling and computed tomography in the differentiation of primary aldosteronism. J Clin Endocrinol Metab. 2001;86:1066–1071. doi: 10.1210/jcem.86.3.7282. [DOI] [PubMed] [Google Scholar]
- 34.Nishikawa T, Omura M. Clinical characteristics of primary aldosteronism: Its prevalence and comparative studies on various causes of primary aldosteronism in yokohama rosai hospital. Biomed Pharmacother. 2000;54(Suppl 1):83s–85s. doi: 10.1016/s0753-3322(00)80019-0. [DOI] [PubMed] [Google Scholar]
- 35.Rossi GP, Sacchetto A, Chiesura-Corona M, De Toni R, Gallina M, Feltrin GP, Pessina AC. Identification of the etiology of primary aldosteronism with adrenal vein sampling in patients with equivocal computed tomography and magnetic resonance findings: Results in 104 consecutive cases. J Clin Endocrinol Metab. 2001;86:1083–1090. doi: 10.1210/jcem.86.3.7287. [DOI] [PubMed] [Google Scholar]
- 36.Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004;136:1227–1235. doi: 10.1016/j.surg.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 37.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-qaly threshold. N Engl J Med. 2014;371:796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 38.Braithwaite RS, Meltzer DO, King JT, Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 39.Gaddam K, Corros C, Pimenta E, Ahmed M, Denney T, Aban I, Inusah S, Gupta H, Lloyd SG, Oparil S, Husain A, Dell’Italia LJ, Calhoun DA. Rapid reversal of left ventricular hypertrophy and intracardiac volume overload in patients with resistant hypertension and hyperaldosteronism: A prospective clinical study. Hypertension. 2010;55:1137–1142. doi: 10.1161/HYPERTENSIONAHA.109.141531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Linden P, Steichen O, Zinzindohoue F, Plouin PF. Blood pressure and medication changes following adrenalectomy for unilateral primary aldosteronism: A follow-up study. J Hypertens. 2012;30:761–769. doi: 10.1097/HJH.0b013e328350225d. [DOI] [PubMed] [Google Scholar]
- 41.Sawka AM, Young WF, Thompson GB, Grant CS, Farley DR, Leibson C, van Heerden JA. Primary aldosteronism: Factors associated with normalization of blood pressure after surgery. Ann Intern Med. 2001;135:258–261. doi: 10.7326/0003-4819-135-4-200108210-00010. [DOI] [PubMed] [Google Scholar]
- 42.Young WF., Jr Primary aldosteronism – treatment options. Growth Horm IGF Res. 2003;13(Suppl A):S102–108. doi: 10.1016/s1096-6374(03)00064-9. [DOI] [PubMed] [Google Scholar]
- 43.Reimel B, Zanocco K, Russo MJ, Zarnegar R, Clark OH, Allendorf JD, Chabot JA, Duh QY, Lee JA, Sturgeon C. The management of aldosterone-producing adrenal adenomas–does adrenalectomy increase costs? Surgery. 2010;148:1178–1185. doi: 10.1016/j.surg.2010.09.012. discussion 1185. [DOI] [PubMed] [Google Scholar]
- 44.Judd E, Calhoun DA. Apparent and true resistant hypertension: Definition, prevalence and outcomes. J Hum Hypertens. 2014;28:463–468. doi: 10.1038/jhh.2013.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


