Abstract
A dual-nuclei radiofrequency coil array was constructed for phosphorus and proton magnetic resonance imaging and spectroscopy of the human brain at 7 Tesla. An eight-channel transceive degenerate birdcage phosphorus module was implemented to provide whole-brain coverage and significant sensitivity improvement over a standard dual-tuned loop coil. A nested eight-channel proton module provided adequate sensitivity for anatomical localization without substantially sacrificing performance on the phosphorus module. The developed array enabled phosphorus spectroscopy, a saturation transfer technique to calculate the global creatine kinase forward reaction rate, and single-metabolite whole-brain imaging with 1.4 cm nominal isotropic resolution in 15 min (2.3 cm actual resolution), while additionally enabling 1 mm isotropic proton imaging. This study demonstrates that a multi-channel array can be utilized for phosphorus and proton applications with improved coverage and/or sensitivity over traditional single-channel coils. The efficient multi-channel coil array, time-efficient pulse sequences, and the enhanced signal strength available at ultra-high fields can be combined to allow volumetric assessment of the brain and could provide new insights into the underlying energy metabolism impairment in several neurodegenerative conditions, such as Alzheimer’s and Parkinson’s diseases, as well as mental disorders such as schizophrenia.
Keywords: dual-tuned coil, transceive phased array, degenerate birdcage, creatine kinase reaction, FLORET pulse sequence
1. Introduction
Phosphorus (31P) magnetic resonance spectroscopy (MRS) is a unique tool for evaluating cerebral metabolism in vivo (Shulman et al., 2004). It allows direct detection and quantification of cerebral phospholipids, high-energy phosphates such as phosphocreatine (PCr) and adenosine triphosphate (ATP) (Ackerman et al., 1980), intracellular pH, and the rate constants of important reactions such as the creatine kinase (CK), and the ATP synthesis hydrolysis cycle (ATPase) (Alger and Shulman, 1984). 31P-MRS has provided new evidence of underlying bioenergetic abnormalities in many conditions including Alzheimer’s disease (Mandal et al., 2012), Parkinson’s disease (Hattingen et al., 2009; Hu et al., 2000), bipolar disorder (Sikoglu et al., 2013; Yuksel et al., 2015), schizophrenia (Smesny et al., 2007), and epilepsy (Hetherington et al., 2002). Unlike other techniques that measure cerebral metabolism such as positron emission tomography (PET) or carbon (13C) MRS, 31P-MRS does not require the injection of labeled precursors and is completely noninvasive (Chaumeil et al., 2009).
It is well established that energy demand in the brain is not uniform (Dudley et al., 2014; Zhu et al., 2012) and distribution of 31P containing metabolites varies in white and gray matter (Hetherington et al., 2001). Therefore, it is important to develop techniques able to resolve this spatial variation in health and disease. 31P-MRS data typically suffer from low signal-to-noise ratio (SNR) due to the low concentration of 31P containing metabolites in the brain and the low MR sensitivity associated with the 31P nucleus. As a result, 31P MR signal in the brain is about four orders of magnitude lower than 1H signal. As a result, undesirable compromises in spatial resolution and coverage have been required to perform 31P-MRS in reasonable acquisition times in standard magnets (< 3 Tesla). High-field magnets (≥ 3 Tesla) provide increased chemical shift dispersion, improved SNR per unit acquisition time through greater bulk magnetization, as well as shorter longitudinal relaxation time (T1), which have been parlayed into improved resolution and coverage (Lei et al., 2003b; Lu et al., 2013; Twieg et al., 1994). These benefits can be naturally extended to ultra-high field devices (≥ 7 Tesla) where 31P SNR efficiency is more than double that at 3 Tesla.
Significant SNR enhancement can also be achieved with improved radiofrequency (RF) coil detectors, which have transitioned from preceding single channel and volume coils to multi-element phased arrays. Owing to their dominant relevance in clinical MRI, proton (1H) arrays were the first to be developed during the previous two decades (Roemer et al., 1990; Wright and Wald, 1997). The advantages of phased arrays have been well documented and include extended coverage over an individual surface coil, SNR improvement over a volume coil, and the ability to perform accelerated imaging through parallel reception techniques (Griswold et al., 2002; Pruessmann et al., 1999; Sodickson and Manning, 1997).
More recently, phased arrays have been established for non-proton nuclei to benefit SNR starved 31P and 23Na applications (Avdievich and Hetherington, 2007; Brown et al., 2014; Brown et al., 2013; Kaggie et al., 2014; Lakshmanan et al., 2014; Moon et al., 2013; Shajan et al., 2015; van der Velden et al., 2014). Paradoxically, one of the main difficulties associated with dual-nuclei phased array development is that the ensemble detector is required to allow 1H imaging. The 1H module is essential for anatomical localization and is particularly useful for B0 shimming in high field MRI where reduced B0 uniformity can be unfavorable in 31P applications that demand differentiation of the multi-peak spectra. A dual-nuclei 31P/1H coil typically requires performance tradeoffs on one or both modules (Schnall et al., 1985). Various methods have been utilized in dual-nuclei volume or surface coils to minimize loss on the 31P module, while tolerating greater 1H loss due to its overwhelming baseline SNR advantage (Adriany and Gruetter, 1997; Alecci et al., 2006; Bottomley et al., 1989; Fitzsimmons et al., 1993; Lanz et al., 1997; Murphy-Boesch et al., 1994; Shen et al., 1997; Xie et al., 2007).
In this work, we expand on previous methods to develop a 31P/1H array for brain imaging at 7 T. The array consists of an eight-channel 31P module and separate eight-channel 1H module that embodied the underlying strategy to enhance 31P performance through a multi-channel phased array free of extraneous elements such as detuning circuits, fuses, inductors, and trap circuits. This strategy led to a 31P transmit/receive degenerate birdcage (DBC) structure that was paired with a “nested” eight-channel module to provide 1H imaging capability without significantly conceding 31P performance. We outline the dual-nuclei nested design strategy and provide benchmark measurements along with in vivo data collected using both standard and non-Cartesian pulse sequences.
2. Methods
2.1. Coil Design Strategy
The main objective for the 31P/1H head coil was to provide high 31P SNR, while secondary objectives included the generation of a uniform 31P transmit magnetic field (B1+) and a 1H module for anatomical localization and B0 shimming. Several strategies were utilized to accomplish these objectives. First, a 31P multi-coil array configuration was preferred over a volume coil because of well-known SNR advantages (Roemer et al., 1990; Wright and Wald, 1997). Second, it was sought to eliminate lossy elements such as detuning circuits, fuses and extraneous inductors from the 31P receive coils. Finally, the introduction of 1H coils was to be done in such a way that 31P coil efficiency was not conceded.
A transmit/receive array was pursued in order to satisfy the multi-coil array criteria while simultaneously eliminating lossy elements such as detuning circuits and fuses that are required in receive-only arrays. Further, a fully surrounding transmit/receive array with radial symmetry enables a circularly polarized B1+ field to be generated in the center of the object in a straightforward manner. One topology that matches these criteria is a degenerate birdcage, where modes collapse at the transition point between the high pass and low pass configurations (Cheng et al., 2003; Leussler et al., 1997; Lin et al., 2003; Nistler et al., 2006; Taracila et al., 2006; Tropp, 1992; Wong and Luh, 1999). A key benefit of the DBC is its ability to function as both a multi-coil array with a SNR advantage during signal reception and as a traditional birdcage with uniform B1+ during spin excitation. To eliminate inline high impedance “trap” circuits or diodes that have been utilized to achieve dual-resonance (Schnall et al., 1985), the DBC was designed to resonate at the 31P frequency only. To simultaneously provide 1H imaging capability, a “nested” strategy was utilized in which separate 1H coils were inserted concentric to the 31P coils (Brown et al., 2013; Fitzsimmons et al., 1987; Mispelter et al., 1989). The 31P and 1H coils are described in more detail in subsequent sections.
Meanwhile, the following analysis is provided to demonstrate the efficacy of the dual nuclei nested coil strategy. Coil impedance is given by Z = jωL+(jωC)−1, where j is the imaginary unit, ω is radial frequency, L is coil inductance, C is coil capacitance and the resistance is ignored. Consider a coil tuned to the proton frequency:Z(ω= ω1H)=0. When viewed at the lower x-nucleus frequency, its impedance is dominated by the large capacitive reactance and therefore approximates an open circuit:Z(ω= ωlow)∼(jωlowC)−1→∞. Of course, the degree to which the open circuit condition is approached is proportional to the ratio between the resonant frequencies of interest. For 31P applications, and the resulting impedance is in the 500 Ω range, which is sufficient to predominately disallow co-rotating (in-phase) current flow. Therefore, the performance of a neighboring 31P coil is principally preserved. Consider next a coil tuned to the x-nuclei frequency Z′(ω=ωlow)=0. When viewed at the proton frequency, its impedance is dominated by its inductive reactance and therefore can be thought of as a shield:Z′(ω=ω1H)∼jω1HL. This combination of behaviors can be leveraged in a concentric nested dual nuclei strategy; 1) the outer low frequency coil is not affected by the inner high frequency coil, while 2) the low frequency coil shields the high frequency coil to reduce its radiation loss and neighbor coupling (Lanz and Griswold, 2006), albeit at the expense of coverage and penetration due to counter-rotating (out-of-phase) current induced in the low frequency coil shield (Mispelter et al., 1989).
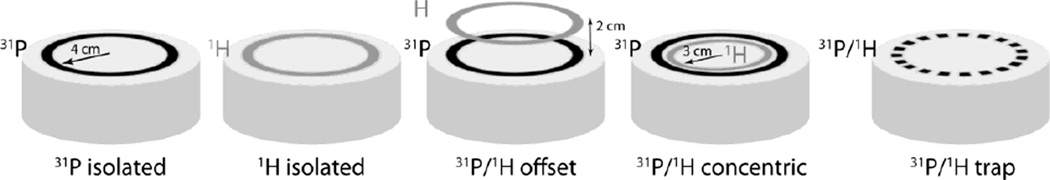
To evaluate the nested strategy in practice, several circular coils with 8 cm or 6 cm diameter were etched on 0.8 mm thick FR4 copper clad (1 oz/ft2) circuit boards. The coils were tuned to 297.2 MHz (1H resonant frequency at 7 Tesla) or 120.3 MHz (31P) using four (1H) or two (31P) distributed capacitors (series 5, Voltronics Corp., Salisbury MD). A 31P/1H dual-tuned “trap” coil was also realized using three distributed capacitors and one capacitor/inductor parallel circuit. The inductor was hand wound (air core) using 20 AWG wire a nd its in ductance was approximately 5 times less than that of the coil to achieve a desirable tradeoff between 31P and 1H performance (Schnall et al., 1985). Quality factor (Q) measurements were performed in the following baseline isolated as well as dual-nuclei configurations (see Figure 1): 1) isolated 31P coil, 2) isolated 1H coil, 3) “offset” 31P/1H coil pair; 4) concentric nested 31P/1H coil pair, and 5) trap circuit coil. The Q was measured with a network analyzer connected to a shielded double probe that was coupled lightly to the coil. Measurements were made with the coil in free space and loaded with a water phantom doped with 3.75g NiSO4 and 5g NaCl per 1000g dH2O.
Figure 1.
Illustration of various single and dual tuned coil arrangements. The corresponding Q values are listed in Table 2.
2.2. 31P Degenerate Birdcage
The nested dual nuclei strategy was initially put into practice using an eight leg DBC with 20 cm length and 28 cm diameter that was constructed on FR4 circuit boards with 12 mm conductor width (see Figure 2). One difficulty associated with a DBC is that inductive coupling between segments prevents complete degeneracy that could undermine transmit efficiency (Cheng et al., 2003) (note that coupling can be mitigated during signal reception using preamplifier decoupling). Tapered legs were implemented to effectively reduce the size of the individual segments and hence the means for inductive coupling, albeit at the expense of coverage in the z-direction.
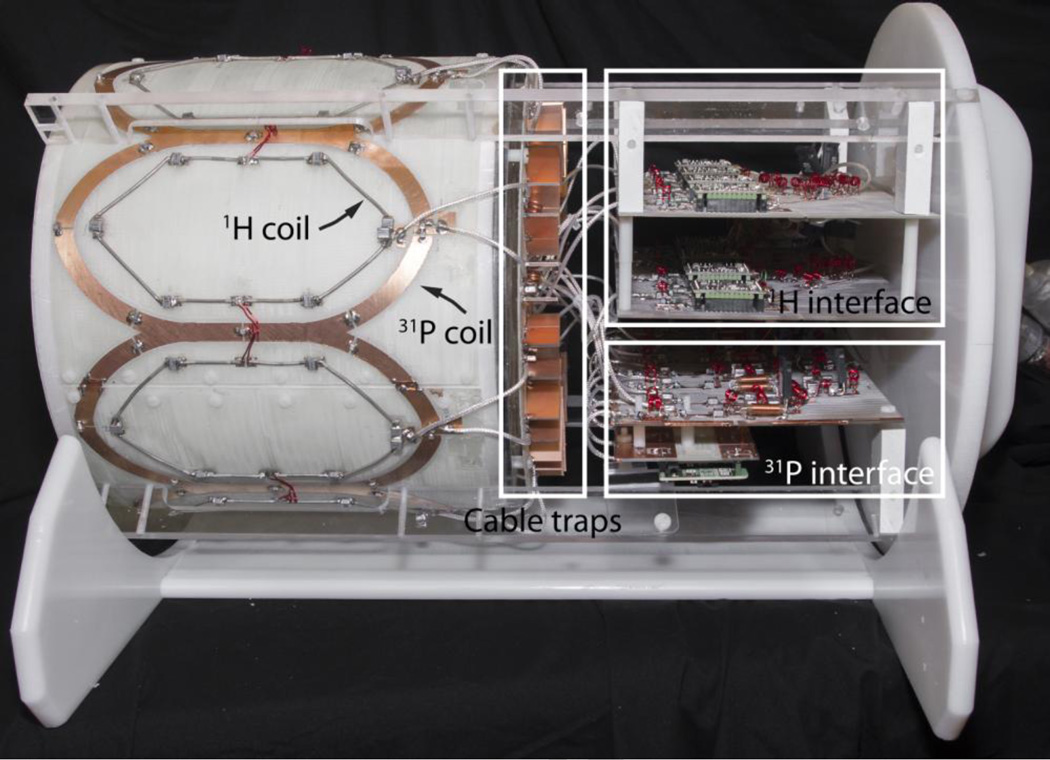
Figure 2.
Photograph of the 31P/1H 16-channel array and interface with the protective cover removed. Overlays outline the cable trap and interface boards that accommodate the power dividers, transmit/receive switches, and preamplifiers.
The means for achieving degeneracy wherein the modes of the birdcage collapse at the desired frequency was achieved through an iterative process (Alagappan et al., 2007). Briefly, 1) individual coil segments were tuned to the Larmor frequency (120.3 MHz for 31P at 7 Tesla) while the other segments were disabled, 2) two neighboring coil segments were tuned and isolated by adjusting the ratio between the end ring and leg capacitors while the other segments were disabled, and 3) step two was repeated for all segment pairs (typically it is necessary to iterate this step at least twice to compensate for perturbations caused by adjustments to the system). DBC segments were capacitively matched to 50 Ω (see the electrical schematic in Figure 3) while loaded with a water based gel phantom (Duan et al., 2014) whose anatomical structure and dielectric properties (Gabriel et al., 1996) were designed to mimic those of the human head.
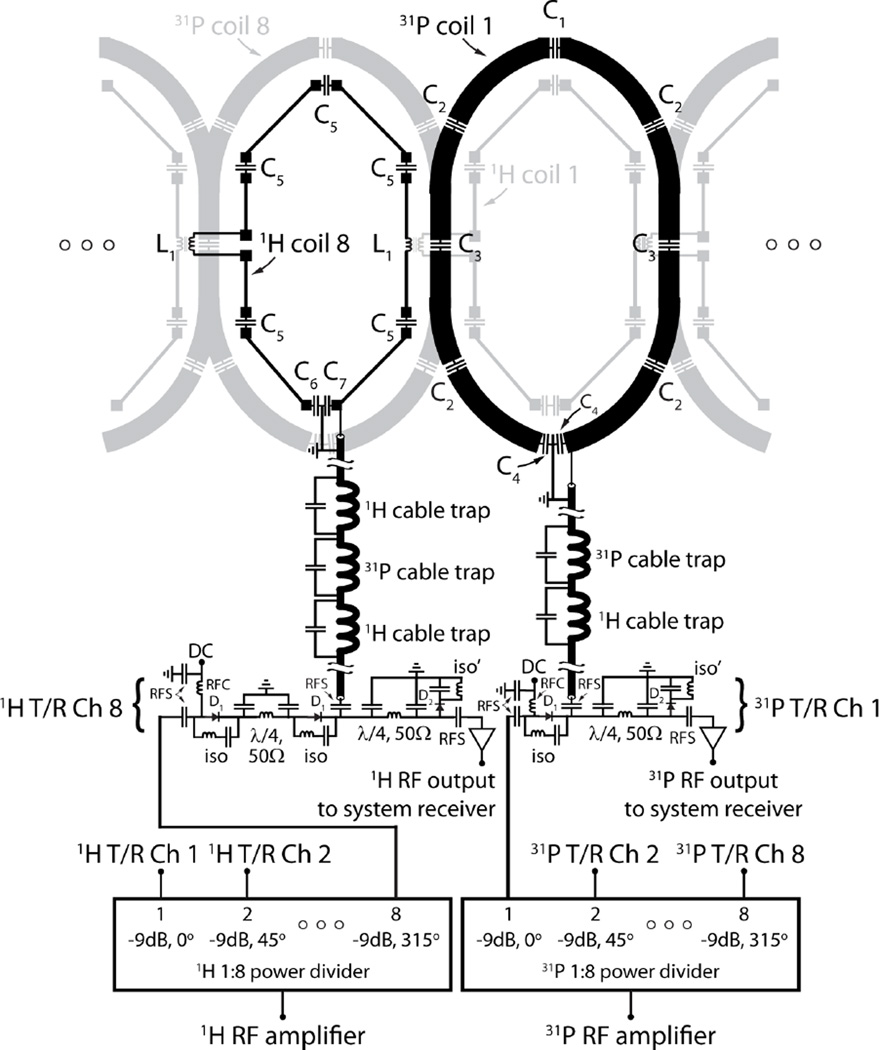
Figure 3.
Flattened two-dimensional schematic diagram of the 31P/1H coil array and interface. For simplicity one 31P and one 1H coil of the 16-channel nested array are highlighted in black while neighboring elements are displayed in light gray. The status of the DC bias is forward in transmit mode and reverse in receive mode. In receive mode, components D1 and iso provide isolation between the RF amplifier and preamplifiers in receive mode, while the iso’ components minimize insertion loss between the coils and preamplifiers. The λ/4, 50Ω circuit help balance the transmit path and improve isolation. Typical component values are; C1 = 40 pF, C2 = 56 pF, C3 = 27 pF, C4 = 75 pF, C5 = 5.3 pF, C6 = 30 pF, C7 = 36 pF, L1 = 24 nH, RFC: radiofrequency choke (1 to 10 uH), and RFS: radiofrequency short (330 to 1000 pF).
To generate a circularly polarized B1+ field, the DBC was driven through an eight-way power splitter consisting of three stages of Wilkinson power dividers and quadrature hybrids arranged to provide outputs with 45° phase offsets that corresponded to the azimuthal angle of the eight birdcage segments. Individual power splitter outputs were connected to transmit/receive switches to protect the preamplifiers during 31P transmission. The transmit/receive switches utilized a quarter-wavelength-based design similar to that previously outlined in (Shajan et al., 2011). The interface electronics shared a common ground that was broken by individual cable traps that were connected to each DBC port; the cable traps were tuned to block common mode current at both 31P and 1H (297.2 MHz) frequencies on the coaxial cable shield. Preamplifier decoupling was accomplished by selecting the coaxial cable length such that the low input impedance of the preamplifier was translated into an inductance that formed a parallel resonant circuit with the DBC match capacitor (Roemer et al., 1990).
The dual frequency cable traps were formed by bridging two individual inductors with distinct capacitors; 3 turns of semi-rigid coaxial cable with 6 mm diameter bridged by a 27 pF capacitor for the 31P trap and a 2-turn inductor bridged by a 3.3 pF capacitor for the 1H trap. Note that cable traps were included at both frequencies to counteract ground loops that may form in the complex system consisting of a multitude of coils and coaxial cables. The operating frequencies of the back-to-back 31P and 1H cable traps were sufficiently separated to avoid resonant frequency splitting. Each trap was surrounded on three sides with copper “fences” that formed a partial shield to reduce interaction between neighboring traps tuned to the same frequency.
2.3. 1H Eight Channel Array
We begin by reiterating that a coil tuned to the 1H frequency has a large negative reactance at the 31P frequency. This characteristic is harmonious with the nested design approach because the 1H coils are expected to have minimal impact on the 31P DBC. To simultaneously achieve 1H B0 shimming and anatomical localization, an array of eight 1H transmit/receive coils was constructed. It should be noted that the 1H coils were tuned subsequent to the 31P DBC, which is important because the 31P coil is perceived as a shield at the 1H frequency and has a significant impact on the 1H coil resonance. To utilize the shielding effect, the 1H coils were inserted concentric to the 31P DBC segments, which can reduce radiation loss and coupling between neighboring coils (Lanz and Griswold, 2006), albeit at the possible expense of coverage and B1 penetration due to counter-rotating currents induced in the 31P DBC.
The 1H coils were octagonal in shape with an arc length of 7.7 cm and head/foot length of 15 cm at their widest points. To reduce eddy currents induced by the 31P DBC, the 1H coils were constructed using 12 AWG copper wire, which can be advantageous compared to etched copper traces (Kumar et al., 2009; Wiggins et al., 2009). The coils were tuned and matched while loaded with the head phantom described above, and neighboring coils were inductively decoupled. The 1H array was interfaced through an eight-way power splitter and transmit/receive switch apparatus in an analogous manner to that described for the 31P DBC (see Figure 3).
Unloaded and loaded Q values were measured on all 16 31P/1H coil elements in situ in the developed array. To evaluate the impact of module interaction, separate sets of measurements were performed on one module (31P or 1H) while the other was resonant or open-circuited. Additionally, the scattering parameter (S) matrix was measured by directly coupling to a given pair of coil elements while all other elements were resonant and terminated with 50 Ω.
2.4. Coil performance
3D electromagnetic models of the 31P and 1H arrays were constructed in CST Microwave Studio (Framingham, MA) to numerically analyze the coil. The coil models included realistic geometric representations of the actual copper conductors, while cable traps, coaxial cables, and other interface electronics were excluded. Coupling between the 31P and 1H arrays were accounted for by performing simulations at either the 31P or 1H frequency while the other array was resonant. The maximum specific absorption rate averaged over 10 g of tissue (SAR10g) was calculated in a multi-structure virtual family “Duke” body model (Christ et al., 2010) to establish a safe operating regime below the local limit of 10 W/kg set by the International Electrotechnical Commission (IEC document 60601-2–33 2010). To accelerate the simulation process a co-simulation framework was implemented (Kozlov and Turner, 2009) and the Duke model was truncated in a transverse plane below the lungs.
All imaging experiments were performed on a whole-body 7 Tesla scanner (MAGNETOM, Siemens Medical Solutions, Erlangen, Germany). An anatomically realistic head phantom filled with distilled water and doped with 42 mM Pi was used to characterize coil performance. In order to provide improved SNR for coil performance measurements, the phantom was prepared with roughly 10-times greater 31P concentration compared to that in the brain. The phantom’s electrical properties (conductivity = 0.58 S/m and relative permittivity = 80) were similar to those in the brain (Gabriel et al., 1996) and therefore provided a realistic representation of in vivo magnetic field patterns, though the phantom’s higher permittivity and lack of tissue boundaries are expected to accentuate interference patterns.
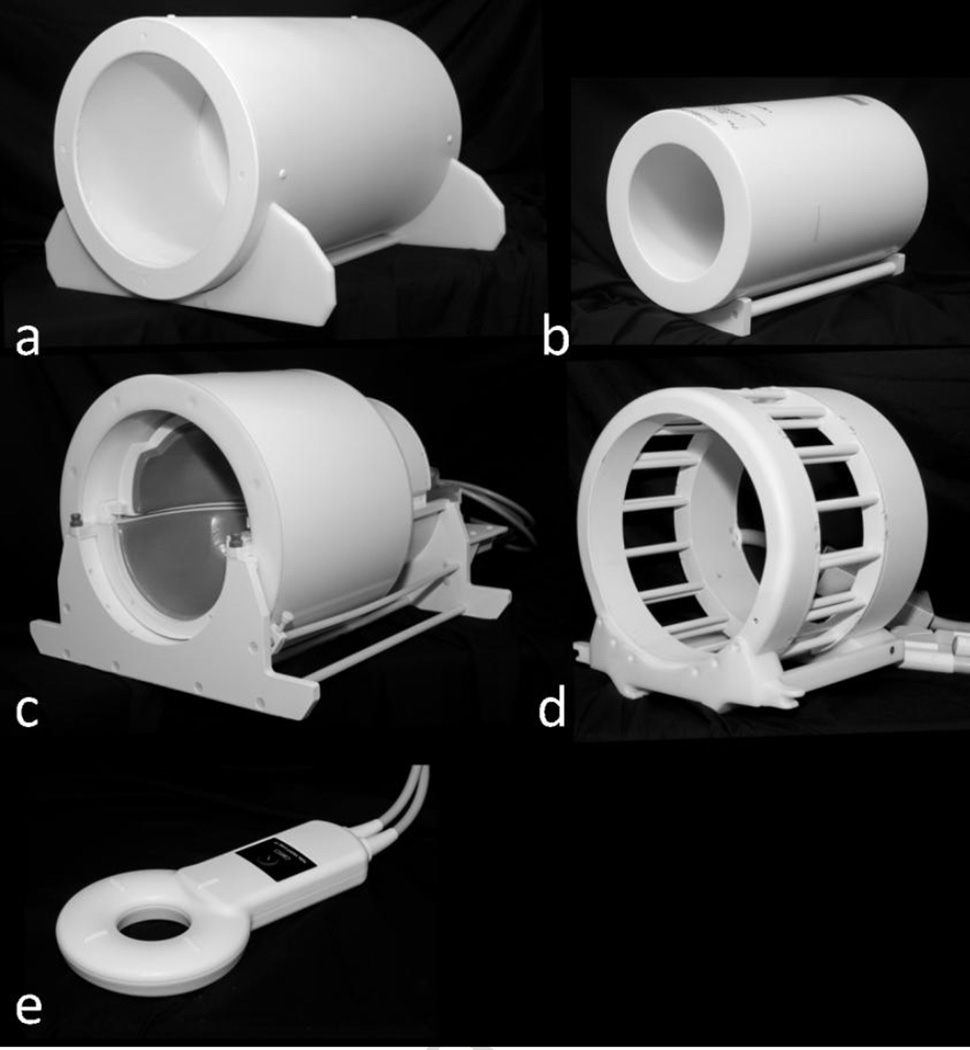
The B1+ field generated by the 31P/1H array was measured using the method described in (Breton et al., 2010). Because the pulse sequence was not available for 31P B1+ mapping, the measurement was performed by scaling the period of a sine curve fitted to the pixel-wise signal intensities of a series of gradient echo images that were collected with a range of imaging pulse amplitudes (parameters are listed in Table 1). For comparison, benchmark measurements were provided by a variety of commercial coils available at our Center (see Figure 4): 1) conventional single-nuclei 1H birdcage head coil (∼27 cm diameter, “Siemens 7.0T Tim Head Coil”, Invivo Corp., Gainesville, FL), 2) state-of-the-art single-nuclei 1H head array (“24 Channel Duyn Array”, Nova Medical, Wilmington, MA), 3) dual-tuned single-channel surface coil (∼12 cm diameter, “1H/31P TxRx Loop Coil”, Quality Electrodynamics, Mayfield, OH), and 4) dual-tuned 31P/1H knee birdcage (∼19 cm diameter, “31P/1H Knee Coil 7T”, Rapid Biomedical, Rimpar, Germany). Knee coil measurements were performed with a cylindrical phantom (14.5 cm diameter, 42 mM Pi) due to its inability to accommodate the head phantom.
Table 1.
Pulse sequence parameters.
| Description | B1+ mapping | SNR measurement | Anatomical | Metabolic Imaging |
Spectroscopy | ||
|---|---|---|---|---|---|---|---|
| Pulse sequence | 2D GRE | 2D GRE | T1w MP-RAGE | FLORET* | 3D CSI | ||
| Nucleus or metabolite | 31P | 1H | 31P | 1H | 1H | PCr γ- ATP |
31P |
| Acquired voxel size (mm2) | 7.8×7.8 | 2.0×2.0 | 7.8×7.8 | 2.0×2.0 | 1.0×1.0 | 14.0×14.0 | 16.7×16.7 |
| Slice thickness (mm) | 50.0 | 5.0 | 50.0 | 5.0 | 1.0 | 14.0 | 16.7 |
| TE (ms) | 6.4 | 1.2 | 6.4 | 3.5 | 2.2 | 0.2 | - |
| TI (ms) | - | - | - | - | 1100 | - | - |
| TR (ms) | 10000 | 15000 | 10000 | 3500 | 2250 | 5000 2000 | 1500 |
| Nominal flip angle (°) | - | - | 50 | 10 | 9 | 75 75 | 35 |
| Number of slices | 1 | 1 | 1 | 1 | 176 | 32 | 16 |
| Field-of-view (mm2) | 500×500 | 256×256 | 500×500 | 256×256 | 216×256 | 445×445 | 200×200 |
| Number of signal averages | 1 | 1 | 1 | 1 | 1 | 6 15 | 10 |
| Acquisition time (s) | 632 | 15 | 632 | 338 | 486 | 900 900 | 2124 |
Other FLORET parameters were: 3 hubs at 45°, 10 interleaves per hub, ADC duration = 12 ms, and dwell time = 10 µs.
Figure 4.
Photographs of the five coils examined in this study: (a) the developed 31P/1H 16-channel array, (b) 31P/1H knee birdcage coil manufactured by Rapid Biomedical, (c) 1H 24-channel Duyn array by Nova Medical, (d) 1H head birdcage coil by Invivo Corp, and (e) 31P/1H single-channel loop by Quality Electrodynamics.
To determine the receive sensitivity, signal maps were acquired with a gradient echo pulse sequence, and noise data were acquired with the same sequence and the RF pulse amplitude set to zero. Raw SNR maps were calculated from the signal and noise measurements using the optimal array combination method (Kellman and McVeigh, 2005; Roemer et al., 1990). To account for the spatially dependent B1+ and finite repetition time (TR), the raw SNR maps were scaled according to where α is the flip angle and T1 is the predetermined relaxation time (1H T1 = 2.4 s and 31P T1 = 5.8 s).
2.5. In vivo spectroscopy and imaging
The study was approved by our internal review board and human subjects were scanned after providing informed written consent. The efficacy of the array was investigated in vivo using several techniques. On the proton module, acquisitions were performed using a magnetization prepared rapid acquisition gradient echo (MP-RAGE) pulse sequence (Table 1). Prior to 31P experiments, the free induction decay signal was measured over a range of pulse amplitudes and fit to a sine curve to determine the nominal flip angle. Performance of the 31P module was displayed using three methods: 1) a product 3D chemical shift imaging (CSI) sequence that utilized elliptical k-space sampling. Post acquisition processing included zero-filling the free induction decay data from 1024 to 8192 points and applying a 30-Hz exponential line broadening filter. 2) Spectra were also acquired using a previously developed unlocalized progressive saturation transfer (ST) sequence (Parasoglou et al., 2014a) in order to evaluate volumetric suppression of γ-ATP using non-adiabatic saturation pulses and estimate the global CK forward exchange rate in the head (Horska and Spencer, 1997; Spencer et al., 1988). Saturation time (tsat) was incremented by varying the number of Gaussian pulses (each 50 ms duration and 60 Hz bandwidth) irradiated at the γ-ATP frequency. Spoiler gradients (magnitude 20 mT/m and duration 5 ms) were inserted during the 7 ms delay between two consecutive pulses to destroy remaining transverse magnetization. The number of Gaussian pulses defined tsat in each experiment (eight experiments total, tsat min = 0, and tsat max = 6.8s). 3) Images of single metabolites (PCr and γ-ATP) were acquired using a spectrally selective 3D non-Cartesian fermat looped, orthogonally encoded trajectories (FLORET) pulse sequence with 1.4 cm isotropic resolution (Madelin et al., 2014; Pipe et al., 2011).
3. Results
3.1. Coil Benchmark Measurements
Q measurements for a variety of single and dual-nuclei coil configurations are given in Table 2. Importantly, the Q of the 31P coil was practically unaffected (< 5%) by the presence of the 1H coil in the offset and concentric dual-nuclei arrangements. Conversely, the offset 31P coil shielded the 1H coil, resulting in a slightly higher unloaded Q value (likely due to reduced radiation loss) but also a 10-fold increase in loaded Q due to shielding from the sample and thus a significantly reduced Q ratio. The concentric arrangement partially restored the Q ratio of the 1H coil, although counter-rotating currents generated in the 31P coil prevented optimal performance. Finally, the trap method results in loss at both frequencies.
Table 2.
Qvalues at the 31P and 1H resonant frequencies for a variety of demonstrational single and dual tuned coil configurations as illustrated in Figure 1. Values are listed as Qu:QL.
|
31P Isolated |
1H Isolated |
31P/1H Offset |
31P/1H Concentric |
31P/1H Trap* |
|
|---|---|---|---|---|---|
| 31P | 440:34 | - | 447:33 | 418:31 | 346:35 |
| 1H | - | 245:9 | 270:105 | 315:47 | 190:67 |
The ratio of the trap inductance to coil inductance was approximately 1:5.
Similar to the demonstrational results above, Q measurements on the in situ 31P array suggested that the 1H array had little impact on 31P performance; Qu : QL = 301±25 : 66±2 with the 1H coils open-circuited and 274±10 : 62±3 with the 1H coils resonant. For the 1H array, the unloaded Q was higher in the presence of the 31P array but the Q ratio was lower; 139±16 : 46±4 with the 31P coils open-circuited and 287±11 : 202±10 with the 31P coils resonant. The Q ratio of the 1H coils were reduced when the 31P coils were introduced due to counter-rotating (out-of-phase) currents induced in the shield-like 31P elements (Dabirzadeh and McDougall, 2009). Shielding had the advantage of reducing radiation loss as evidenced by the higher unloaded Q value. This, however, comes at the expense of shielding the 1H coils’ magnetic field from the loading tissue, which reduced the Q ratio compared to an isolated 1H coil.
The S-parameter and noise correlation matrices are summarized in Table 3. The 31P DBC elements were well matched to 50Ω (≤ −16 dB), while decoupling was marginally adequate (< −9 dB) between both nearest and next-nearest neighbors. Coupling between 1H coils was easily controlled (< −18 dB) due in part to the shielding effect of the 31P elements. Noise correlation values for both 31P and 1H modules were in the expected range (Roemer et al., 1990), suggesting that common mode currents on the coaxial cables were suppressed and preamplifier feedback loops were absent.
Table 3.
Scattering (S) and noise correlation measurements for the developed array. The measurements were performed while the array was loaded with a tissue equivalent gel phantom. Values are listed as mean ± standard deviation with the worst case or maximum value in parentheses.
| S (dB) | Noise correlation | |
|---|---|---|
| 31P Channel | ||
| Diagonal elements | −17.2 ± 2.5 (−16.0) | 1 |
| Adjacent neighbors | −12.3 ±6.9 (−9.7) | 0.18 ±0.11 (0.39) |
| Next nearest neighbors | −12.9 ±9.1 (−9.1) | 0.09± 0.10 (0.29) |
| Distant neighbors | −18.0 ±4.1 (−13.6) | 0.16 ±0.08 (0.30) |
| 1H Channel | ||
| Diagonal elements | −20.3 ±3.2 (−16.3) | 1 |
| Adjacent neighbors | −21.6 ±2.3 (−18.8) | 0.06 ±0.05 (0.14) |
| Next nearest neighbors | −25.7 ±7.6 (−20.2) | 0.05 ±0.10 (0.29) |
| Distant neighbors | −33.3 ±6.0 (−22.3) | 0.07 ±0.05 (0.16) |
Loss through the receive paths between the coil ports to preamplifier inputs (including coaxial cables, cable traps and transmit/receive switch) were less than 0.55 dB on the 31P channels and 0.75 dB on the 1H channels. Loss through the transmit paths including the power dividers, transmit/receive switches, cable traps, and coaxial cables was approximately 10.0 dB on the 31P channels and 10.4 dB on the 1H channels (compared to 9 dB for an ideal system with eight-way power division). Preamplifier protection in transmit mode was greater than 35 dB on both 31P and 1H channels. For 1 W input power the calculated maximum SAR10g in the Duke head model was 0.47 W/kg for the 31P array and 0.37 W/kg for the 1H array. In practice, the transmit power was restricted according to the simulated values with a two-fold safety buffer below the 10 W/kg limit set by the IEC.
3.2. Phantom Measurements
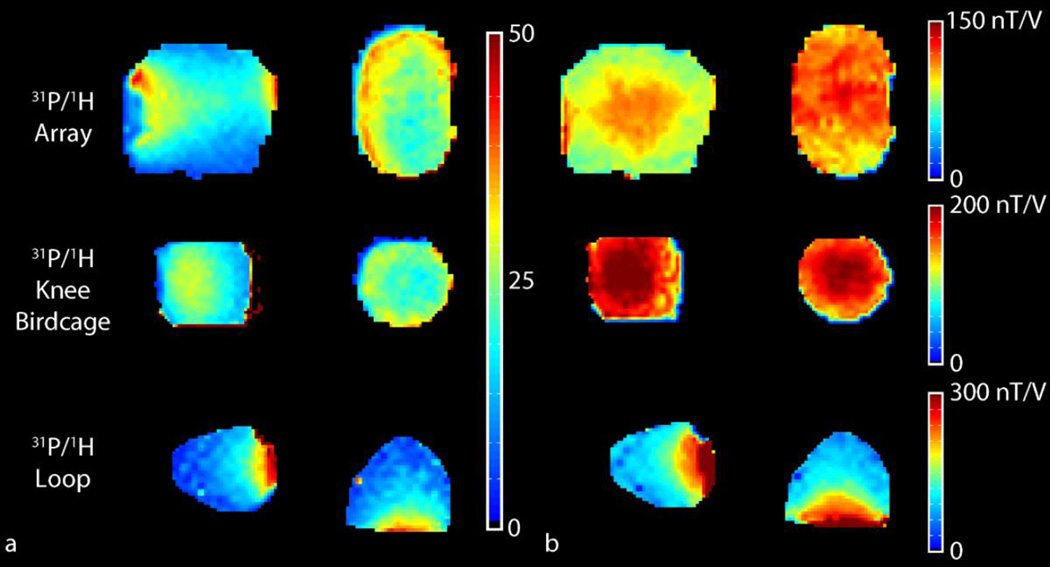
B1+ and SNR maps measured in the 42 mM Pi phantom using the 31P modules of the developed array and single channel reference coil are shown in Figure 5 and are summarized in Table 4. The central and peripheral SNR provided by the 31P/1H head array was 22 and 42, respectively. Owing to its smaller diameter and volume receive configuration, the 31P/1H knee birdcage provided higher central SNR (29) but did not provide an advantage in the periphery. In comparison, the central and peripheral SNR for the 31P/1H loop coil was 12 and 63, respectively. Central transmit efficiency for the developed 31P/1H head array and 31P/1H knee birdcage were 123.1 and 215.6 nT/V, respectively. The developed array provided better than 80% transmit uniformity in the masked regions in Figure 5b (defined as 1 minus the standard deviation divided by the mean B1+).
Figure 5.
31P SNR (a) and B1+ maps (b) in the 42mM Pi head and knee phantoms in the central sagittal (left column) and transverse planes (right column) for the developed array (top row) and two commercially available coils. B1+ maps were smoothed using a two dimensional median filter with a 3×3 kernel. The results are summarized in Table 4. The maps were masked to remove regions with low SNR or B1+.
Table 4.
Summary of B1+ and SNR measurements in the 42 mM Pi head phantom.
| SNR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Center (nT/V) | B1+ Sagittal uniformity |
Transverse uniformity |
Center | Maximum | ||||||
| Nucleus | 31P | 1H | 31P | 1H | 31P | 1H | 31P |
1H (×103) |
31P |
1H (× 103) |
| 31P/1H Array | 123.1 | 65.5 | 0.81 | 0.48 | 0.87 | 0.64 | 22 | 4.2 | 42 | 9.5 |
| 31P/1H Knee | ||||||||||
| Birdcage | 215.6 | 144.6 | 0.90 | 0.51 | 0.86 | 0.50 | 29 | 5.1 | 29 | 5.3 |
| 31P/1H Loop | 72.6 | 40.4 | - | - | - | - | 12 | 2.6 | 63 | 13.4 |
| 1H 24-ch Duyn | - | 18.5 | ||||||||
| Array | - | 89.6 | - | 0.66 | - | 0.68 | - | 6.9 | ||
| 1H Head Birdcage | - | 93.1 | - | 0.72 | - | 0.59 | - | 3.8 | - | 3.8 |
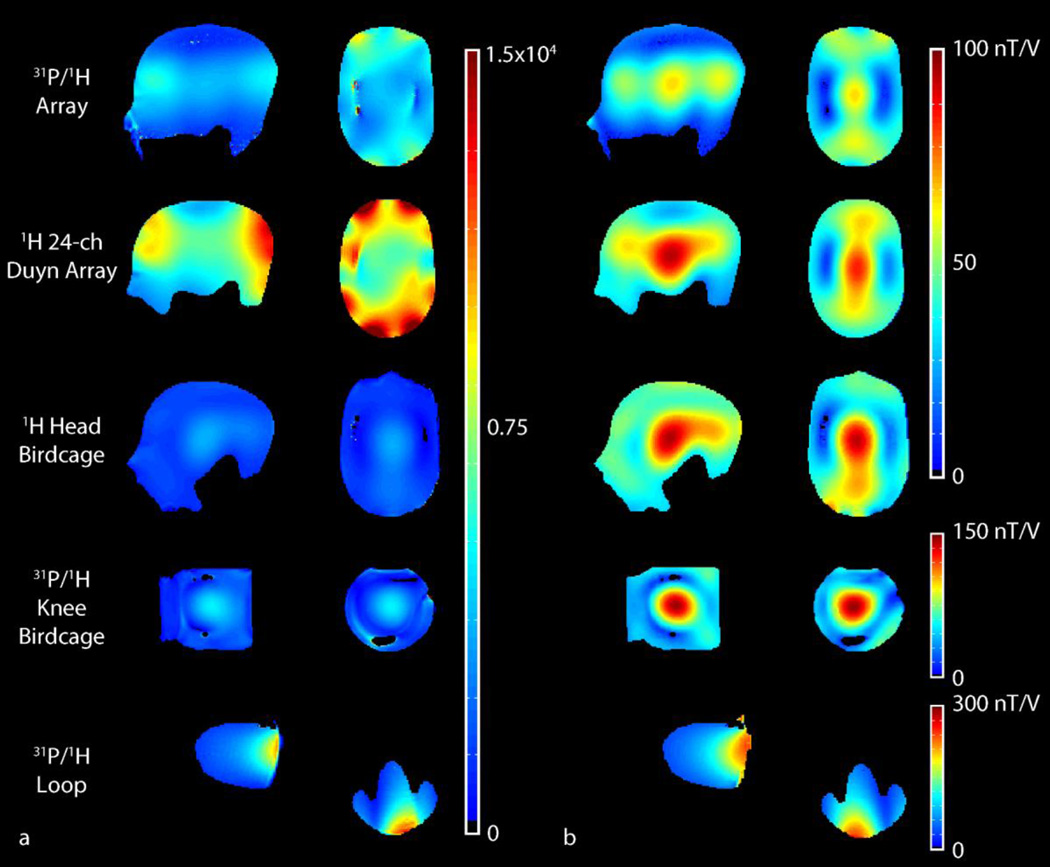
The transmit efficiency and SNR of the 1H module of the developed array was 65.5 nT/V and 4.2×103 in the center of the phantom, respectively (Figure 6). Out-of-phase currents generated in the shield-like 31P array likely contributed to a reduction in 1H transmit efficiency compared to the single-nuclei 24-channel Duyn array, which delivered 89.6 nT/V and a central SNR of 6.9×103. Nonetheless, the developed array achieved superior 1H SNR in both the center and periphery compared to the conventional 1H head birdcage.
Figure 6.
1H SNR (a) and B1+ maps (b) in the 42mM Pi head and knee phantoms in the central sagittal (left column) and transverse planes (right column) for the developed array (top row) and four commercially available coils. The results are summarized in Table 4. The maps were masked to remove regions with low SNR or B1+.
3.3. In Vivo Measurements
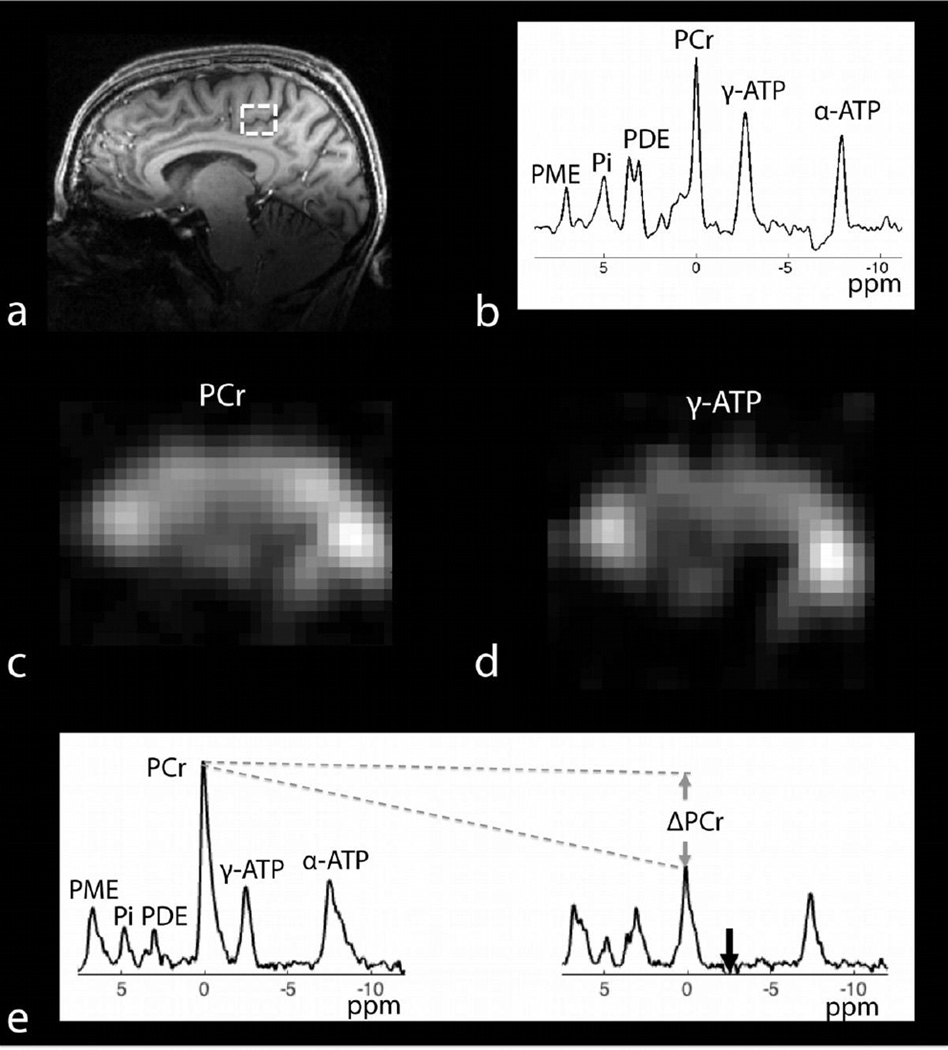
31P spectra acquired with a product 3D–CSI sequence and isotropic resolution of 16.7 mm is shown in Figure 7b. The SNR of PCr across all voxels was 30.5 ± 7.5 (mean ± standard deviation). Ratios of PCr to γ-ATP after correcting for T1 relaxation (Lei et al., 2003b) ranged from 1.4 ± 0.1 in central regions of the brain to approximately 5 in voxels with muscle tissue. Intracellular pH was estimated to be 7.0 ± 0.1 using the chemical shift of Pi (Petroff et al., 1985). Spectroscopic images of PCr and γ-ATP derived from the 3D–CSI dataset are shown in Figure 7c,d.
Figure 7.
31P/1H data set: a) anatomical 1H MP-RAGE in the sagittal plane, b) typical 31P-CSI spectrum from a 4.66 mL voxel whose location is outlined in (a), spectroscopic images of (c) PCr and (d) γ-ATP in the sagittal plane derived from the 3D–CSI data, e) global 31P spectra (4-averages, TR = 12 s) acquired in the absence (tsat = 0, left) and presence (tsat = 6.8 s, right) of γ-ATP saturation. Complete saturation of γ-ATP is observed despite the use of non-adiabatic saturation pulses. Eight spectra were acquired by varying tsat in order to estimate the global CK forward reaction rate.
Global 31P spectra acquired in the absence (tsat = 0 s) and presence (tsat = 6.8 s) of saturation of γ-ATP are shown in Figure 7e. Complete saturation of γ-ATP is observed despite the use of non-adiabatic saturation pulses. By acquiring a series of spectra at different tsat the global pseudo first-order forward rate constant of the CK reaction was estimated to be 0.22 s-1 by fitting the PCr signal to a Bloch equation modified to account for chemical exchange in a two-pool PCr and γ-ATP system (Horska and Spencer, 1997).
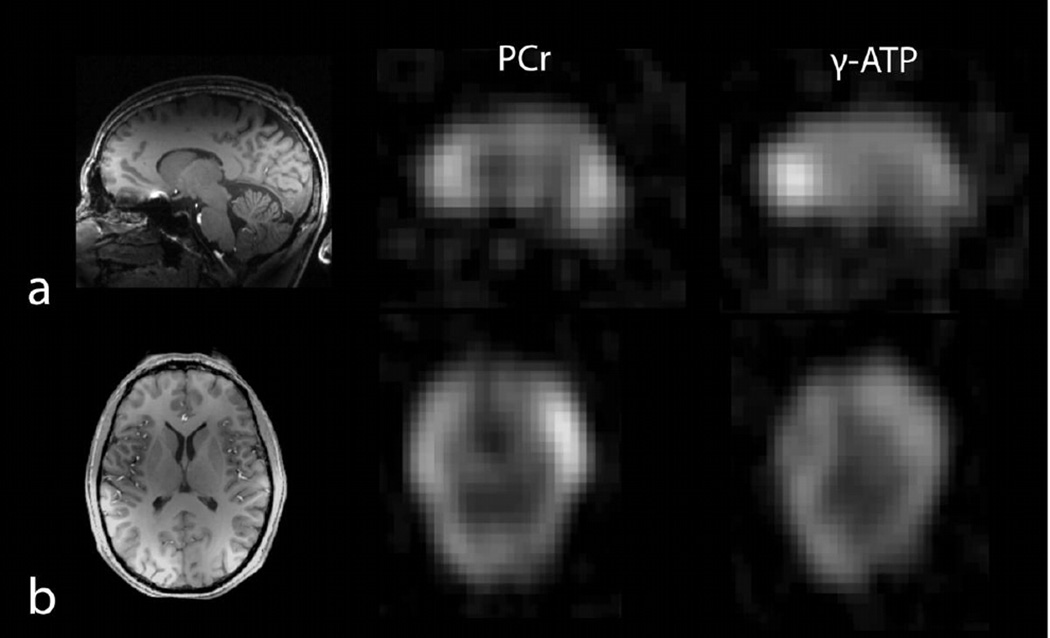
Single metabolite images acquired using a spectrally selective 3D–FLORET sequence are shown in Figure 8. The pseudo-SNR of the PCr image was 20.1 ± 4.5, which was calculated as the mean signal divided by the standard deviation of the noise background. The ratio of PCr-to-γ-ATP was 1.5 ± 0.1, which was in good agreement with that derived from the CSI measurement.
Figure 8.
1H MP-RAGE and single metabolite 31P images (2.74 mL voxel, 15 min acquisition per metabolite) using a spectrally selective 3D–non uniform FLORET sequence: a) sagittal 1H MP-RAGE (left) and coregistered images of PCr (middle), and γ-ATP (right). b) axial 1H MP-RAGE and co-registered PCr and γ-ATP images.
1H MP-RAGE images acquired with the developed array enable straightforward co-registration with 31P data. The sagittal image in Figure 7a shows excellent grey/white matter contrast in the frontal, parietal, and occipital lobes, while the transverse image in Figure 8b illustrates good coverage and uniformity.
4. Discussion and Conclusion
In this work we have demonstrated that a 31P transmit/receive DBC structure paired with a “nested” 8-channel 1H array can provide high quality 31P and 1H spectroscopy and imaging data with volumetric coverage at 7 Tesla. This new coil design was combined with efficient pulse sequences, such as FLORET, to demonstrate mapping of important 31P containing metabolites in the brain at relatively high spatial resolution. We were able to map PCr and γ-ΑTP at 1.4 cm isotropic nominal voxel size. The point spread function of the sampling scheme was estimated to be 1.6 voxels, resulting in an actual resolution of 2.3 cm. To the best of our knowledge, such resolution has only been reported previously at 9.4 Tesla using a mono-tuned volume coil (Lu et al., 2013).
In several previous brain studies, exchange rates and metabolic fluxes of important reactions such as CK and ATPase have been mapped using surface coils that have limited coverage and require power-demanding adiabatic preparation pulses (Lei et al., 2003a). Zhu et al. (Zhu et al., 2012), were the first to acquire high-resolution metabolic fluxes using a 31P-MT approach, which employed 3D–CSI and a dual-tuned volume coil. The 31P DBC coil described here provided whole-brain coverage that was combined with a spectrally selective pulse sequence to study the CK reaction in a manner similar to that described previously by our group in the skeletal muscle (Parasoglou et al., 2013a, b; Parasoglou et al., 2014b). Despite the use of non-adiabatic saturation pulses, γ -ATP was completely saturated (Figure 7) throughout the head due to the relatively homogenous B1+ generated by the DBC.
The essential goal of the developed coil was to enable 31P MR imaging and spectroscopy of the brain with good resolution in a reasonable acquisition time. The first stage of the design utilized a 31P transmit/receive DBC to satisfy several requirements. In receive mode, the DBC was configured as an eight-channel phased array to provide full coverage and SNR advantages over a surface or volume coil. In transmit mode, a fixed RF shim was applied to the DBC to produce a circularly polarized field similar to that of a standard birdcage. Combining the transmit and receive functionalities eliminated a separate transmit structure, as well as lossy components such as fuses and detuning circuits that are required in receive-only coils. For example, popular fuses rated from 315 to 800 mA have resistances of about 0.2 to 1.6 Ω at 120 MHz (395 series; Littelfuse; Chicago, IL). However, we point out that advantages associated with the transceive configuration are tempered by losses associated with transmit/receive switches and distant preamplifiers (∼0.55 dB loss on the 31P receive path). Further, while preamplifier decoupling was utilized to counteract residual coupling between 31P DBC segments in receive mode, coupling likely reduced DBC transmit efficiency compared to a standard birdcage.
Another challenge in dual-nuclei coil design is to provide 1H imaging capability. Of course, an ideal dual-nuclei coil matches the performance on both the low and high frequency modules to that of two independent single-nuclei coils. The co-planar design utilized in this work, where 1H coils are nested within 31P elements, provided improved 1H SNR over a traditional birdcage coil without sacrificing 31P performance. The 1H module provided adequate SNR for 1 mm isotropic MP-RAGE imaging while its B0 shimming capability preserved the excellent spectral resolution available at 7 Tesla as evident in the dual-peak PDE spectra (Figure 8). Going forward, advanced B0 correction techniques are expected to be useful for 31P quantification at ultra-high fields (Mirkes et al., 2014).
Furthermore, although a multi-channel array substantially improves SNR over a volume coil, its spatially varying B1− field (and to a lesser extent B1+) can make 31P quantification less straightforward. Because of the low SNR associated with 31P applications, subject-specific B1 maps are generally not a viable option and may not be necessary given the relatively small variation of head sizes compared to the wavelength associated with the 31P operating frequency at 7 Tesla. An alternative that overcomes this complexity in vivo is to assume a constant γ-ATP level, which allows PCr quantification by calculating the ratio of the two metabolites. However, it is unknown if γ-ATP is constant in certain pathological states. The phantom replacement method may also be pursued (Atkinson et al., 2011; Jansen et al., 2006), in which pre-scans are performed on a variety of phantoms in order to relate the spatially dependent signal intensity to concentration.
We point out that the 31P DBC structure was implemented with eight channels on a cylindrical former, whereas substantial sensitivity gains have been previously demonstrated with 32 and 96 channel 1H head arrays at 3 Tesla using tight-fitting domed structures (de Zwart et al., 2002; de Zwart et al., 2004; Wiggins et al., 2009; Wiggins et al., 2006) (which can be expected to perform similar to 7 Tesla 31P arrays due to their comparable operating frequencies). These designs are appealing for future implementation, although an irregular domed coil structure in a dual-nuclei array may necessitate the abandonment of the DBC transceive arrangement, as it would be difficult to find azimuthal symmetries that are preferred for its operation.
On the 1H module, the nested approach gave rise to counter-rotating 1H currents on the 31P element While this shielding reduced radiation loss, it also contributed to reduced transmit efficiency (about 70% that of the 1H Duyn array and standard 1H head birdcage). A potential improvement could include 1H current “blocking” trap circuits in the 31P DBC, an approach that has been shown to reduce counter-rotating currents with minimal loss of performance on the x-nuclei channel (Dabirzadeh and McDougall, 2009; Klomp et al., 2006). (Note that blocking traps serve a different function than the “intermediate” trap circuits introduced to provide dual-resonance on a single coil element (Schnall et al., 1985).) Additionally, the elements’ tapered geometry combined with the shielding effect of the 31P elements led to compromised coverage in the head/foot direction. This was evident in sagittal views of the B1+, SNR, and MP-RAGE acquisitions, in which either inferior regions such as the cerebellum or superior regions such as the central sulcus were difficult to visualize depending on subject positioning (Figure 7 and Figure 8). Coverage is a common problem for coils operating at high frequency due to the conflicting desires to reduce parasitic and radiation loss. Alternative solutions include dual-row coils (Kozlov and Turner, 2011; Shajan et al., 2013) and half-wavelength electric dipoles (Raaijmakers et al., 2011; Shajan et al., 2015; Wiggins et al., 2012), both of which could conceivably provide extended coverage in a dual-nuclei array.
31P-MR has provided evidence of underlying energy metabolism impairment in several neurodegenerative conditions, such as Alzheimer’s (Brown et al., 1989) and Parkinson’s (Hoang et al., 1998) diseases, as well as mental disorders such as schizophrenia (Smesny et al., 2007). Relatively high-resolution 31P-MR with complete brain coverage can be achieved with an efficient multi-channel coil array, time-efficient pulse sequences, and the enhanced signal strength at ultra-high fields. This combination of hardware and software improvements will allow simultaneous assessment of a variety of brain regions and could provide new insights into the patterns of disease progression.
Highlights.
A degenerate birdcage array provided high SNR and a uniform B1+ field for 31P MRI.
Single metabolite imaging was demonstrated with a 3D non-Cartesian acquisition.
γ-ATP was saturated with non-adiabatic pulses to estimate the CK forward reaction rate.
A nested 1H array enabled straightforward image co-registration.
Acknowledgement
The authors thank Jerzy Walczyk for construction of the coil housing. The Center for Advanced Imaging Innovation and Research (CAI2R, www.cai2r.net) at New York University School of Medicine is supported by NIH/NIBIB grant number P41 EB017183.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman JJ, Grove TH, Wong GG, Gadian DG, Radda GK. Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature. 1980;283:167–170. doi: 10.1038/283167a0. [DOI] [PubMed] [Google Scholar]
- Adriany G, Gruetter R. A half-volume coil for efficient proton decoupling in humans at 4 tesla. J Magn Reson. 1997;125:178–184. doi: 10.1006/jmre.1997.1113. [DOI] [PubMed] [Google Scholar]
- Alagappan V, Nistler J, Adalsteinsson E, Setsompop K, Fontius U, Zelinski A, Vester M, Wiggins GC, Hebrank F, Renz W, Schmitt F, Wald LL. Degenerate mode band-pass birdcage coil for accelerated parallel excitation. Magn Reson Med. 2007;57:1148–1158. doi: 10.1002/mrm.21247. [DOI] [PubMed] [Google Scholar]
- Alecci M, Romanzetti S, Kaffanke J, Celik A, Wegener HP, Shah NJ. Practical design of a 4 Tesla double-tuned RF surface coil for interleaved 1H and 23Na MRI of rat brain. J Magn Reson. 2006;181:203–211. doi: 10.1016/j.jmr.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Alger JR, Shulman RG. NMR studies of enzymatic rates in vitro and in vivo by magnetization transfer. Q Rev Biophys. 1984;17:83–124. doi: 10.1017/s0033583500005266. [DOI] [PubMed] [Google Scholar]
- Atkinson IC, Lu A, Thulborn KR. Clinically constrained optimization of flexTPI acquisition parameters for the tissue sodium concentration bioscale. Magn Reson Med. 2011;66:1089–1099. doi: 10.1002/mrm.22908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdievich NI, Hetherington HP. 4 T Actively detuneable double-tuned 1H/31P head volume coil and four-channel 31P phased array for human brain spectroscopy. J Magn Reson. 2007;186:341–346. doi: 10.1016/j.jmr.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley PA, Hardy CJ, Roemer PB, Mueller OM. Proton-decoupled, Overhauser-enhanced, spatially localized carbon-13 spectroscopy in humans. Magn Reson Med. 1989;12:348–363. doi: 10.1002/mrm.1910120307. [DOI] [PubMed] [Google Scholar]
- Breton E, McGorty K, Wiggins GC, Axel L, Kim D. Image-guided radio-frequency gain calibration for high-field MRI. NMR Biomed. 2010;23:368–374. doi: 10.1002/nbm.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GG, Levine SR, Gorell JM, Pettegrew JW, Gdowski JW, Bueri JA, Helpern JA, Welch KM. In vivo 31P NMR profiles of Alzheimer’s disease and multiple subcortical infarct dementia. Neurology. 1989;39:1423–1427. doi: 10.1212/wnl.39.11.1423. [DOI] [PubMed] [Google Scholar]
- Brown R, Lakshmanan K, Madelin G, Chang G, Sodickson DK, Regatte RR, Wiggins GC. Design and Application of a Nested Multi-Channel Sodium Proton Knee Array at 3T. ISMRM, Milan, Italy. 2014:4880. [Google Scholar]
- Brown R, Madelin G, Lattanzi R, Chang G, Regatte RR, Sodickson DK, Wiggins GC. Design of a nested eight-channel sodium and four-channel proton coil for 7T knee imaging. Magn Reson Med. 2013;70:259–268. doi: 10.1002/mrm.24432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumeil MM, Valette J, Guillermier M, Brouillet E, Boumezbeur F, Herard AS, Bloch G, Hantraye P, Lebon V. Multimodal neuroimaging provides a highly consistent picture of energy metabolism, validating 31P MRS for measuring brain ATP synthesis. Proc Natl Acad Sci U S A. 2009;106:3988–3993. doi: 10.1073/pnas.0806516106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YC, Eagan TP, Chmielewski T, Flock J, Kang MC, Kidane TK, Shvartsman Sh M, Brown RW. A degeneracy study in the circulant and bordered-circulant approach to birdcage and planar coils. MAGMA. 2003;16:103–111. doi: 10.1007/s10334-003-0009-5. [DOI] [PubMed] [Google Scholar]
- Christ A, Kainz W, Hahn EG, Honegger K, Zefferer M, Neufeld E, Rascher W, Janka R, Bautz W, Chen J, Kiefer B, Schmitt P, Hollenbach HP, Shen J, Oberle M, Szczerba D, Kam A, Guag JW, Kuster N. The Virtual Family--development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys Med Biol. 2010;55:N23–N38. doi: 10.1088/0031-9155/55/2/N01. [DOI] [PubMed] [Google Scholar]
- Dabirzadeh A, McDougall MP. Trap Design for Insertable Second-Nuclei Radiofrequency Coils for Magnetic Resonance Imaging and Spectroscopy. Concepts in Magnetic Resonance Part B (Magnetic Resonance Engineering) 2009;35B:121–132. [Google Scholar]
- de Zwart JA, Ledden PJ, Kellman P, van Gelderen P, Duyn JH. Design of a SENSE-optimized high-sensitivity MRI receive coil for brain imaging. Magn Reson Med. 2002;47:1218–1227. doi: 10.1002/mrm.10169. [DOI] [PubMed] [Google Scholar]
- de Zwart JA, Ledden PJ, van Gelderen P, Bodurka J, Chu R, Duyn JH. Signal-to-noise ratio and parallel imaging performance of a 16-channel receive-only brain coil array at 3.0 Tesla. Magn Reson Med. 2004;51:22–26. doi: 10.1002/mrm.10678. [DOI] [PubMed] [Google Scholar]
- Duan Q, Duyn JH, Gudino N, de Zwart JA, van Gelderen P, Sodickson DK, Brown R. Characterization of a dielectric phantom for high-field magnetic resonance imaging applications. Med Phys. 2014;41:102303. doi: 10.1118/1.4895823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley J, Chu W-J, Fugate EM, Lee J-H. Tissue dependent metabolism in the human brain suggested by quantitative phosphorus-31 MRSI. Journal of Spectroscopy and Dynamics. 2014;4 [Google Scholar]
- Fitzsimmons JR, Beck BL, Brooker HR. Double resonant quadrature birdcage. Magn Reson Med. 1993;30:107–114. doi: 10.1002/mrm.1910300116. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons JR, Brooker HR, Beck B. A transformer-coupled double-resonant probe for NMR imaging and spectroscopy. Magn Reson Med. 1987;5:471–477. doi: 10.1002/mrm.1910050510. [DOI] [PubMed] [Google Scholar]
- Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys Med Biol. 1996;41:2271–2293. doi: 10.1088/0031-9155/41/11/003. [DOI] [PubMed] [Google Scholar]
- Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47:1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- Hattingen E, Magerkurth J, Pilatus U, Mozer A, Seifried C, Steinmetz H, Zanella F, Hilker R. Phosphorus and proton magnetic resonance spectroscopy demonstrates mitochondrial dysfunction in early and advanced Parkinson’s disease. Brain. 2009;132:3285–3297. doi: 10.1093/brain/awp293. [DOI] [PubMed] [Google Scholar]
- Hetherington HP, Pan JW, Spencer DD. 1H and 31P spectroscopy and bioenergetics in the lateralization of seizures in temporal lobe epilepsy. J Magn Reson Imaging. 2002;16:477–483. doi: 10.1002/jmri.10177. [DOI] [PubMed] [Google Scholar]
- Hetherington HP, Spencer DD, Vaughan JT, Pan JW. Quantitative (31)P spectroscopic imaging of human brain at 4 Tesla: assessment of gray and white matter differences of phosphocreatine and ATP. Magn Reson Med. 2001;45:46–52. doi: 10.1002/1522-2594(200101)45:1<46::aid-mrm1008>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Hoang TQ, Bluml S, Dubowitz DJ, Moats R, Kopyov O, Jacques D, Ross BD. Quantitative proton-decoupled 31P MRS and 1H MRS in the evaluation of Huntington’s and Parkinson’s diseases. Neurology. 1998;50:1033–1040. doi: 10.1212/wnl.50.4.1033. [DOI] [PubMed] [Google Scholar]
- Horska A, Spencer GS. Correctly accounting for radiofrequency spillover in saturation transfer experiments: application to measurement of the creatine kinase reaction rate in human forearm muscle. MAGMA. 1997;5:159–163. doi: 10.1007/BF02592247. [DOI] [PubMed] [Google Scholar]
- Hu MT, Taylor-Robinson SD, Chaudhuri KR, Bell JD, Labbe C, Cunningham VJ, Koepp MJ, Hammers A, Morris RG, Turjanski N, Brooks DJ. Cortical dysfunction in non-demented Parkinson’s disease patients: a combined (31)P-MRS and (18)FDG-PET study. Brain. 2000;123(Pt 2):340–352. doi: 10.1093/brain/123.2.340. [DOI] [PubMed] [Google Scholar]
- Jansen JF, Backes WH, Nicolay K, Kooi ME. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology. 2006;240:318–332. doi: 10.1148/radiol.2402050314. [DOI] [PubMed] [Google Scholar]
- Kaggie JD, Hadley JR, Badal J, Campbell JR, Park DJ, Parker DL, Morrell G, Newbould RD, Wood AF, Bangerter NK. A 3 T sodium and proton composite array breast coil. Magn Reson Med. 2014;71:2231–2242. doi: 10.1002/mrm.24860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellman P, McVeigh ER. Image reconstruction in SNR units: a general method for SNR measurement. Magn Reson Med. 2005;54:1439–1447. doi: 10.1002/mrm.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klomp DW, Renema WK, van der Graaf M, de Galan BE, Kentgens AP, Heerschap A. Sensitivity-enhanced 13C MR spectroscopy of the human brain at 3 Tesla. Magn Reson Med. 2006;55:271–278. doi: 10.1002/mrm.20745. [DOI] [PubMed] [Google Scholar]
- Kozlov M, Turner R. Fast MRI coil analysis based on 3-D electromagnetic and RF circuit co-simulation. J Magn Reson. 2009;200:147–152. doi: 10.1016/j.jmr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Kozlov M, Turner R. Analysis of RF transmit performance for a multi-row multi-channel MRI loop array at 300 and 400 MHz. Asia-Pacific Microwave Conference, Melbourne, Australia. 2011:1190–1193. doi: 10.1109/IEMBS.2011.6090101. [DOI] [PubMed] [Google Scholar]
- Kumar A, Edelstein WA, Bottomley PA. Noise figure limits for circular loop MR coils. Magn Reson Med. 2009;61:1201–1209. doi: 10.1002/mrm.21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan K, Brown R, Madelin G, Boada FE, Wiggins G. ISMRM. Milan, Italy: 2014. An 8 Channel Transmit Receive Sodium & Nested 8 Channel Transmit Receive Proton Coil for 3.0 T Brain Imaging; p. 4879. [Google Scholar]
- Lanz T, Griswold M. Concentrically Shielded Surface Coils - A New Method for Decoupling Phased Array Elements. ISMRM. 2006:217. [Google Scholar]
- Lanz T, von Kienlin M, Behr W, Haase A. Double-tuned four-ring birdcage resonators for in vivo 31P–nuclear magnetic resonance spectroscopy at 11.75 T. MAGMA. 1997;5:243–246. doi: 10.1007/BF02594587. [DOI] [PubMed] [Google Scholar]
- Lei H, Ugurbil K, Chen W. Measurement of unidirectional Pi to ATP flux in human visual cortex at 7 T by using in vivo 31P magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 2003a;100:14409–14414. doi: 10.1073/pnas.2332656100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Zhu XH, Zhang XL, Ugurbil K, Chen W. In vivo 31P magnetic resonance spectroscopy of human brain at 7 T: an initial experience. Magn Reson Med. 2003b;49:199–205. doi: 10.1002/mrm.10379. [DOI] [PubMed] [Google Scholar]
- Leussler C, Stimma J, Roschmann P. ISMRM. Toronto, Canada: 1997. The bandpass birdcage resonator modified as a coil array for simultaneous MR acquisition; p. 176. [Google Scholar]
- Lin F, Kwong K, Huang I, Belliveau J, Wald L. Degenerate mode birdcage volume coil for sensitivity-encoded imaging. Magn Reson Med. 2003;50:1107–1111. doi: 10.1002/mrm.10632. [DOI] [PubMed] [Google Scholar]
- Lu A, Atkinson IC, Zhou XJ, Thulborn KR. PCr/ATP ratio mapping of the human head by simultaneously imaging of multiple spectral peaks with interleaved excitations and flexible twisted projection imaging readout trajectories at 9.4 T. Magn Reson Med. 2013;69:538–544. doi: 10.1002/mrm.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madelin G, Kline R, Walvick R, Regatte RR. A method for estimating intracellular sodium concentration and extracellular volume fraction in brain in vivo using sodium magnetic resonance imaging. Sci Rep. 2014;4:4763. doi: 10.1038/srep04763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal PK, Akolkar H, Tripathi M. Mapping of hippocampal pH and neurochemicals from in vivo multi-voxel 31P study in healthy normal young male/female, mild cognitive impairment, and Alzheimer’s disease. J Alzheimers Dis 31 Suppl. 2012;3:S75–S86. doi: 10.3233/JAD-2012-120166. [DOI] [PubMed] [Google Scholar]
- Mirkes CC, Hoffmann J, Shajan G, Pohmann R, Scheffler K. High-resolution quantitative sodium imaging at 9.4 tesla. Magn Reson Med. 2014 doi: 10.1002/mrm.25096. [DOI] [PubMed] [Google Scholar]
- Mispelter J, Tiffon B, Quiniou E, Lhoste JM. Optimization of 13C-1H double coplanar surface-coil design for WALTZ-16 decoupling sequence. JMR. 1989;82:622–628. [Google Scholar]
- Moon CH, Kim JH, Zhao T, Bae KT. Quantitative (23) Na MRI of human knee cartilage using dual-tuned (1) H/(23) Na transceiver array radiofrequency coil at 7 tesla. J Magn Reson Imaging. 2013;38:1063–1072. doi: 10.1002/jmri.24030. [DOI] [PubMed] [Google Scholar]
- Murphy-Boesch J, Srinivasan R, Carvajal L, Brown TR. Two configurations of the four-ring birdcage coil for 1H imaging and 1H–decoupled 31P spectroscopy of the human head. J Magn Reson B. 1994;103:103–114. doi: 10.1006/jmrb.1994.1017. [DOI] [PubMed] [Google Scholar]
- Nistler J, Kurth R, Vester M, Renz W. ISMRM. Seattle, WA: 2006. B1 inhomogenisation using a multichannel transmit array; p. 2566. [Google Scholar]
- Parasoglou P, Xia D, Chang G, Regatte RR. 3D–mapping of phosphocreatine concentration in the human calf muscle at 7 T: comparison to 3 T. Magn Reson Med. 2013a;70:1619–1625. doi: 10.1002/mrm.24616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasoglou P, Xia D, Chang G, Regatte RR. Dynamic three-dimensional imaging of phosphocreatine recovery kinetics in the human lower leg muscles at 3T and 7T: a preliminary study. NMR Biomed. 2013b;26:348–356. doi: 10.1002/nbm.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasoglou P, Xia D, Chang G, Regatte RR. Three-dimensional saturation transfer (3)(1)P-MRI in muscles of the lower leg at 3.0 T. Sci Rep. 2014a;4:5219. doi: 10.1038/srep05219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasoglou P, Xia D, Regatte RR. Feasibility of mapping unidirectional Pi-to-ATP fluxes in muscles of the lower leg at 7.0 Tesla. Magn Reson Med. 2014b doi: 10.1002/mrm.25388. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Prichard JW, Behar KL, Alger JR, den Hollander JA, Shulman RG. Cerebral intracellular pH by 31P nuclear magnetic resonance spectroscopy. Neurology. 1985;35:781–788. doi: 10.1212/wnl.35.6.781. [DOI] [PubMed] [Google Scholar]
- Pipe JG, Zwart NR, Aboussouan EA, Robison RK, Devaraj A, Johnson KO. A new design and rationale for 3D orthogonally oversampled k-space trajectories. Magn Reson Med. 2011;66:1303–1311. doi: 10.1002/mrm.22918. [DOI] [PubMed] [Google Scholar]
- Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–962. [PubMed] [Google Scholar]
- Raaijmakers AJ, Ipek O, Klomp DW, Possanzini C, Harvey PR, Lagendijk JJ, van den Berg CA. Design of a radiative surface coil array element at 7 T: the single-side adapted dipole antenna. Magn Reson Med. 2011;66:1488–1497. doi: 10.1002/mrm.22886. [DOI] [PubMed] [Google Scholar]
- Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magn. Reson. Med. 1990;16:192–225. doi: 10.1002/mrm.1910160203. [DOI] [PubMed] [Google Scholar]
- Schnall MD, Subramanian VH, Leigh JS, Chance B. A new double-tuned probe for concurrent 1H and 31P NMR. J Magn Reson. 1985;65:122–129. [Google Scholar]
- Shajan G, Hoffmann J, Budde J, Adriany G, Ugurbil K, Pohmann R. Design and evaluation of an RF front-end for 9.4 T human MRI. Magn Reson Med. 2011;66:596–604. doi: 10.1002/mrm.22808. [DOI] [PubMed] [Google Scholar]
- Shajan G, Kozlov M, Hoffmann J, Turner R, Scheffler K, Pohmann R. A 16-channel dual-row transmit array in combination with a 31-element receive array for human brain imaging at 9.4 T. Magn Reson Med. 2013 doi: 10.1002/mrm.24726. [DOI] [PubMed] [Google Scholar]
- Shajan G, Mirkes C, Buckenmaier K, Hoffmann J, Pohmann R, Scheffler K. Three-layered radio frequency coil arrangement for sodium MRI of the human brain at 9.4 Tesla. Magn Reson Med. 2015 doi: 10.1002/mrm.25666. [DOI] [PubMed] [Google Scholar]
- Shen GX, Boada FE, Thulborn KR. Dual-frequency, dual-quadrature, birdcage RF coil design with identical B1 pattern for sodium and proton imaging of the human brain at 1.5 T. Magn Reson Med. 1997;38:717–725. doi: 10.1002/mrm.1910380507. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: implications for neuroimaging. Trends Neurosci. 2004;27:489–495. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Sikoglu EM, Jensen JE, Vitaliano G, Liso Navarro AA, Renshaw PF, Frazier JA, Moore CM. Bioenergetic measurements in children with bipolar disorder: a pilot 31P magnetic resonance spectroscopy study. PLoS One. 2013;8:e54536. doi: 10.1371/journal.pone.0054536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smesny S, Rosburg T, Nenadic I, Fenk KP, Kunstmann S, Rzanny R, Volz HP, Sauer H. Metabolic mapping using 2D 31P–MR spectroscopy reveals frontal and thalamic metabolic abnormalities in schizophrenia. Neuroimage. 2007;35:729–737. doi: 10.1016/j.neuroimage.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Sodickson DK, Manning WJ. Simultaneous acquisition of spatial harmonics (SMASH): ultra-fast imaging with radiofrequency coil arrays. Magn Res Med. 1997;38:591–603. doi: 10.1002/mrm.1910380414. [DOI] [PubMed] [Google Scholar]
- Spencer RG, Balschi JA, Leigh JS, Jr, Ingwall JS. ATP synthesis and degradation rates in the perfused rat heart. 31P–nuclear magnetic resonance double saturation transfer measurements. Biophys J. 1988;54:921–929. doi: 10.1016/S0006-3495(88)83028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taracila V, Petropoulos P, Eagan T, Brown R. Image uniformity improvement for birdcage like volume coils at 400 MHz using multichannel excitation. Concepts Magn Reson Part B 29B. 2006:153–160. [Google Scholar]
- Tropp J. ISMRM. Berlin, Germany: 1992. Mutual inductance in birdcage resonators; p. 4009. [Google Scholar]
- Twieg DB, Hetherington HP, Ponder SL, den Hollander J, Pohost GM. Spatial resolution in 31P metabolite imaging of the human brain at 4.1 T. J Magn Reson B. 1994;104:153–158. doi: 10.1006/jmrb.1994.1069. [DOI] [PubMed] [Google Scholar]
- van der Velden TA, Italiaander M, van der Kemp WJ, Raaijmakers AJ, Schmitz AM, Luijten PR, Boer VO, Klomp DW. Radiofrequency configuration to facilitate bilateral breast 31P MR spectroscopic imaging and high-resolution MRI at 7 Tesla. Magn Reson Med. 2014 doi: 10.1002/mrm.25573. [DOI] [PubMed] [Google Scholar]
- Wiggins GC, Polimeni JR, Potthast A, Schmitt M, Alagappan V, Wald LL. 96-Channel receive-only head coil for 3 Tesla: design optimization and evaluation. Magn Reson Med. 2009;62:754–762. doi: 10.1002/mrm.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins GC, Triantafyllou C, Potthast A, Reykowski A, Nittka M, Wald LL. 32-channel 3 Tesla receive-only phased-array head coil with soccer-ball element geometry. Magn Reson Med. 2006;56:216–223. doi: 10.1002/mrm.20925. [DOI] [PubMed] [Google Scholar]
- Wiggins GC, Zhang B, Lattanzi R, Chen G, Sodickson D. ISMRM. Melbourne, Australia: 2012. The Electric Dipole Array: An Attempt to Match the Ideal Current Pattern for Central SNR at 7 Tesla; p. 541. [Google Scholar]
- Wong E, Luh W. ISMRM. Philadelphia, PA: 1999. A multimode, single frequency birdcage coil for high sensitivity multichannel whole volume imaging; p. 165. [Google Scholar]
- Wright SM, Wald LL. Theory and application of array coils in MR spectroscopy. NMR Biomed. 1997;10:394–410. doi: 10.1002/(sici)1099-1492(199712)10:8<394::aid-nbm494>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Xie Z, Xu D, Kelley DA, Vigneron DB, Zhang X. ISMRM Workshop on Advances in High Field MR. Pacific Grove, California, USA: 2007. Dual-frequency Volume Microstrip Coil with Quadrature Capability for 13C/1H MRI/MRS at 7T. Poster 41. [Google Scholar]
- Yuksel C, Du F, Ravichandran C, Goldbach JR, Thida T, Lin P, Dora B, Gelda J, O’Connor L, Sehovic S, Gruber S, Ongur D, Cohen BM. Abnormal high-energy phosphate molecule metabolism during regional brain activation in patients with bipolar disorder. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.13. [DOI] [PubMed] [Google Scholar]
- Zhu XH, Qiao H, Du F, Xiong Q, Liu X, Zhang X, Ugurbil K, Chen W. Quantitative imaging of energy expenditure in human brain. Neuroimage. 2012;60:2107–2117. doi: 10.1016/j.neuroimage.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]