Abstract
Recent structural and functional neuroimaging studies of adults suggest that efficient patterns of brain connectivity are fundamental to human intelligence. Specifically, whole brain networks with an efficient small-world organization, along with specific brain regions (i.e., Parieto-Frontal Integration Theory, P-FIT) appear related to intellectual ability. However, these relationships have not been studied in children using structural network measures. This cross-sectional study examined the relation between non-verbal intellectual ability and structural network organization in 99 typically developing healthy preadolescent children. We showed a strong positive association between the network’s global efficiency and intelligence, in which a subtest for visuo-spatial motor processing (Block Design, BD) was prominent in both global brain structure and local regions included within P-FIT as well as temporal regions involved with pattern and form processing. BD was also associated with rich club organization, which encompassed frontal, occipital, temporal, hippocampal, and neostriatal regions. This suggests that children’s visual construction ability is significantly related to how efficiently children’s brains are globally and locally integrated. Our findings indicate that visual construction and reasoning may make general demands on globally integrated processing by the brain.
Keywords: Intelligence, children, connectivity, network, diffusion tensor imaging
Introduction
The inter-relationship among localized and distributed brain regions has been conceptualized as an integrated network organized into many segregated subregions linked by axonal white matter tracts (Sporns et al., 2005). A robust finding from the network perspective is that the human brain is organized in a highly efficient way for integrated information transfer, in so called small-world topology (for a review, see Bullmore and Sporns, 2009). Moreover, recent neuroimaging studies have suggested that some brain regions including precuneus, posterior cingulate, and medial prefrontal cortex play a pivotal role as hubs or part of a structural core in the brain (Hagmann et al., 2008; Sporns et al., 2007) supporting fast communication between distributed regions. These key cortical hubs also are likely to be preferentially connected to each other forming a rich club (van den Heuvel and Sporns, 2011). Network organization undergoes rapid alterations in development with changes in axonal synaptic connectivity, white matter volume, and the thickness of corresponding cortical regions; see Vertes and Bullmore (2015) for a summary of developmental changes in network organization. In particular, structural maturation of white matter as well as cortical and subcortical areas is strongly associated with intellectual abilities from early childhood throughout adolescence (Shaw et al., 2006; Tamnes et al., 2010). However, the relationship of network properties derived from axonal white matter tracts such as network efficiency with intelligence during childhood has received little investigation.
Intelligence can be defined as the individual’s capacity for mental functioning across a variety of domains including reasoning, executive function, information processing speed, memory and spatial manipulation – so called, general intelligence (g). Efficient and economical information processing among the distributed brain regions along white matter fibers is thought to contribute to general intelligence capacity (Deary et al., 2010; Gray and Thompson, 2004). The parieto-frontal integration theory (P-FIT) postulates that the dorsolateral prefrontal cortex and the parietal cortex, including the arcuate fasciculus connecting those two regions, comprises an important neuronal network associated with efficient intellectual functioning (Jung and Haier, 2007). The P-FIT of intelligence has been supported by neuroimaging findings including studies of gray matter volumes (Colom et al., 2009), cortical thickness (Narr et al., 2007) and white matter tracts (Van Beek et al., 2014). A brain network perspective provides a quantitative model for elucidating the association between the efficiency of brain networks and intelligence (Cole et al., 2012; van den Heuvel et al., 2009). Network approaches to understanding adult intelligence reveal consistent positive associations between intellectual performance and network integrity characterized by diffusion tensor imaging (DTI; Fischer et al., 2014; Li et al., 2009), resting-state functional MRI (van den Heuvel et al., 2009), and the electroencephalogram (EEG; Langer et al., 2012). Since brain development in childhood is associated with large-scale changes in synaptic connectivity, gray matter thickness and myelination, these relationships could be quite different than those observed in the adult brain. For example, there is evidence that the association between cortical regions and intelligence must include consideration of the trajectory of brain development, in which the relations between brain systems and function are dynamic and are altered as a function of age (Shaw et al., 2006). While one imaging study for children showed no relation of functional brain networks with IQ (Wu et al., 2013), no neuroimaging studies have been performed to date to investigate the relations between children’s intellectual ability and whole-brain structural network properties.
In this study, we applied a graph theoretic network analysis to structural neuroimaging data acquired in typically developing children. This approach enabled characterization of global network associations with children’s intelligence scores, including the role of hub regions and specific local regions in brain network architecture. We tested the hypothesis that the perceptual reasoning index (PRI), representing individual’s nonverbal fluid reasoning skills, was associated with individual’s structural network organization. Based on network studies on adults, higher structural network integration was predicted to be significantly associated with higher perceptual reasoning abilities. In addition to this, we particularly focused on domain-specific associations derived from three PRI subtests (Block Design, Picture Concepts, and Matrix Reasoning), and examined the association between cortical network architecture and specific intellectual performance of preadolescent children.
Materials and Methods
Participants
The dataset of 99 typically developing healthy preadolescent children was collected from a subset of children participating in longitudinal developmental studies who were born at one of two hospitals in the greater Los Angeles area (UC Irvine Medical Center or Long Beach Memorial Medical Center) – Table 1. All children were between 6 and 11 years old (mean±SD: 7.80±1.22 years), right-handed (defined by the modified version of the Edinburgh Handedness Inventory; Oldfield, 1971), and were the result of a singleton pregnancy. This low risk sample of children was full-term at birth, had a stable neonatal course (median Apgar score = 9, range 7–10) and did not have congenital, chromosomal, or genetic anomalies. Further there was no evidence of intraventricular hemorrhage (determined by ultrasound), periventricular leukomalacia, and/or low-pressure ventriculomegaly in the newborn period. At the study entry, all children had normal neurological findings based on review of MRI scans by a neuroradiologist. All children were typically developing and in the appropriate grade for their age. Further, no emotional or physical problems were reported in a structured interview using the MacArthur Health and Behavior Questionnaire (HBQ; Armstrong and Goldstein, 2003). After providing a complete description of the study to all participants, written and verbal informed consent was obtained from a parent and affirmed assent was obtained from the children. The research protocol was approved by the Institutional Review Board for protection of human subjects.
Table 1.
Demographic data of the study sample
| Number of children | 99 |
| Sex (% male) | 54 |
| Handedness (% right handed) | 100 |
| Gestational age at birth (weeks) | 39.4±1.3 (37–42.6) |
| Age at assessment (years) | 7.80±1.22 (6.1–10.6) |
| Birth weight (gram) | 3395.1±449.3 (2020–4561) |
| Maternal age (years) | 29.4±6.6 (16.2–43.2) |
| Maternal Education (%) | |
| Elementary or middle school | 7.1 |
| High school or equivalent | 33.3 |
| Associates or Vocational | 19.2 |
| Bachelors degree | 28.3 |
| Graduate degree | 12.1 |
| Annual Household Income (%) | |
| $0 – $30,000 | 14.1 |
| $30,001 – $60,000 | 27.2 |
| $60,001 – $100,000 | 25.4 |
| Over $100,000 | 33.3 |
| Child Race/Ethnicity (%) | |
| Hispanic | 33.3 |
| Non-Hispanic White | 32.3 |
| Asian | 5.1 |
| African American or Black | 4.0 |
| Multi-Ethnic | 25.3 |
| Bilingual Households (%) | 49.5 |
| Performance IQ (PRI§) | 105.67±15.8 (73–139) |
| Block design (BD) | 9.9±3.1 (3–16) |
| Picture concepts (PC) | 11.6±2.9 (3–18) |
| Matrix reasoning (MR) | 11.3±3.5(4–19) |
PRI – perceptual reasoning index.
Standardized intelligence assessment
To eliminate a possible bias associated with language in our diverse sample, children’s intelligence was assessed using the Perceptual Reasoning Index (PRI) of the Wechsler Intelligence Scale for Children (WISC-IV). The PRI does not require expressive language responses from the children and is relatively culturally-fair (Baron, 2004). Two of the three subscales (Matrix Reasoning and Block Design) have been shown to be excellent indicators of general intelligence (Baron, 2004; Wechsler, 2002). In this study, three subtests – Block Design, Picture Concepts, and Matrix Reasoning – were administered. Table 2 shows the correlations among these measures.
Table 2.
Correlations between IQ scores
| BD | PC | MR | |
|---|---|---|---|
| PRI | 0.81 | 0.73 | 0.89 |
| BD | 0.33 | 0.64 | |
| PC | 0.50 |
Abbreviations. PRI, Perceptual reasoning index; BD, Block design; PC, Picture concepts; MR, Matrix reasoning.
Block Design (BD)
Children were asked to re-create designs using several red-and-white blocks within a specified time limit. This task assesses visual perception and organization, the ability to process abstract visual stimuli (i.e., visual-spatial reasoning) and motor manipulation.
Picture Concepts (PC)
Two or three rows of pictures were presented and the child was instructed to choose one picture from each row that shared a common characteristic. This subtest measures abstract, categorical reasoning ability and the ability to recognize common features within nonverbal stimuli.
Matrix Reasoning (MR)
Children were presented with an incomplete matrix and asked to complete it by selecting the missing piece from five response choices. Four types of matrices were used in this task: continuous and discrete pattern completion, classification, analogical reasoning, and serial reasoning. Matrix reasoning provides a measure of visual information processing, abstract reasoning, and fluid intelligence.
Perceptual reasoning index (PRI)
Raw scores on each subtest were converted to scaled scores based on the child’s age. The three scaled scores were then summed and converted to the equivalent PRI score, representing the child’s overall ability for perceptual reasoning and organization.
MRI acquisition and preprocessing
Participants underwent whole brain MRI examination on a 3T Achieva system (Philips). Structural images were acquired using an inversion-recovery spoiled gradient recalled acquisition (IR-SPGR) sequence with the following parameters: 240×240 image matrix with 150 slices on the sagittal plane, 1×1×1 mm3 voxels, echo time = 3.35 ms, repetition time = 11 ms, inversion time = 1.1 s, turbo field echo (TFE) factor = 192 without SENSE (sensitivity encoding) acceleration, and flip angle 18°. Diffusion tensor images (DTI) were acquired using a spin-echo echo-planar imaging (SE-EPI) sequence. For each subject, DTI data covering the whole brain had the following parameters: 60 contiguous transverse slices, slice thickness = 2 mm, in-plane voxel size = 2×2 mm2, repetition time = 11.6 s, echo time = 55 ms, field-of-view = 224×224×120 mm, imaging matrix = 128×128, and acquisition time = 8 min 5 s. Diffusion gradients were applied along 32 non-collinear directions (b = 800 s/mm2) and one non-diffusion weighted image was acquired. During MR scans, the head motion of children was minimized with restraining foam pads around the head, and ear protection was also given to all children.
After the visual inspection for possible artifacts of MR data, further preprocessing of DTI scans was done using the FSL toolbox (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/, Version 5.0) and Diffusion Toolkit (http://trackvis.org). The preprocessing involved: (1) removal of non-brain regions, (2) distortion correction for the eddy current and subject head motion, (3) linear estimation of diffusion tensor at each voxel, (4) computation of the fractional anisotropy (FA) and principal eigenvector from diffusion tensor, (5) whole brain fiber tractography by FACT (Fiber Assignment by Continuous Tracking; Mori et al., 1999) algorithm, in which the tracts were extracted for voxels with FA>.2 and a smooth turn of <30°. Twenty seeds, uniformly distributed in each voxel, were used to generate a sufficient number of fiber tracts for the whole-brain (Cheng et al., 2012).
Structural network analysis
Children’s structural networks were constructed from graph theory, in which a mathematical representation of a network is constructed using a set of nodes and their connections (Bullmore and Sporns, 2009). In this study, gray matter regions – i.e., 90 cortical and subcortical areas from automated anatomical labeling (AAL) atlas – were used as nodes (Tzourio-Mazoyer et al., 2002), and the axonal streamlines derived from tractography formed the connections (termed ‘edge’). Edges were deemed present if there were at least three streamlines between any pairs of two nodes (Lo et al., 2010) minimizing the effect of false-positive connections (Mori, 2006). The level of structural connectivity was defined by the averaged FA values of interconnecting fiber tracts reflecting the integrity of the interregional white matter connections (Kim et al., 2014; van den Heuvel and Sporns, 2011; Wen et al., 2011), resulting in 90×90 undirectional weighted connectivity matrix.
The topological properties of children’s brain networks were computed using representative network measures related to network segregation, network integration, and their optimal balance (Brain Connectivity Toolbox, https://sites.google.com/site/bctnet; Rubinov and Sporns, 2010). In the human brain, a functionally specialized brain region comprises densely interconnected single neurons, neuronal populations, or cortical areas forming local communities of nodes (Sporns, 2011). The communities within a network are often referred to as clusters or modules, and network segregation represents the degree to which a network can be decomposed into such communities. Clustering coefficient (γ) and modularity (Q) are two representative measures for global network segregation. The clustering coefficient was computed as the likelihood that the neighbors of a node are interconnected to each other (Watts and Strogatz, 1998), indexing the prevalence of clustered connections are prevalent around each node within a network. Furthermore, highly clustered nodes are likely to form a distinct community (termed ‘module’). The modularity represents the degree to which a network can be optimally partitioned into distinct subcommunities where each module has densely interconnected nodes and where such modules have relatively fewer connections between them (Newman, 2006). Meanwhile, network integration implies that such segregated communities should communicate with each other in an optimal way, where shorter connection distances between nodes indicate potentially more direct communication within the network. Characteristic path length (λ) represents one aspect of network integration and has been defined by the average distance between nodes (Watts and Strogatz, 1998). Global efficiency (E) also reflects integrative properties of a network, which is computed from the inverse of the connection distances and less influenced by the disconnected or isolated nodes compared to the path length (Rubinov and Sporns, 2010). Moreover, a network can be classified as a regular (higher clustering with longer path length), random (lower clustering with shorter path lengths), or small-world (high-clustering with shorter paths) network. The extent to which a given network forms a small-world network (σ) was computed as a ratio of clustering coefficient and characteristic path length. Because some network measures (i.e., clustering coefficient, characteristic path length, and small-worldness) are highly dependent on the number of nodes and edges in the network, we used normalized measures (i.e., γ, λ, and σ) which were defined by comparison to distributions comprising 1,000 constrained null (i.e., random) networks retaining the connection weights as well as the number of nodes, edges, and degree sequences of individual networks (Maslov and Sneppen, 2002). Next, we tested whether the computed network characteristics were associated with children’s perceptual reasoning ability (i.e., PRI). Subsequently, the subtests of the PRI (BD, PC, and MR) also were investigated to determine which domain of perceptual intelligence is most correlated with aspects of children’s brain networks. In this study, partial correlation coefficients (r) were computed controlling for age and sex as possible confounding variables. Potential variability of each connectivity matrix was also considered using the number and strength of connections in the matrix as additional regressors for correlation coefficients. A significance level of p<0.05 was used to investigate the relations between network measures and intelligence scores. Additionally, for the local network measures computed at each node (e.g., path length and local efficiency), significant correlations were defined using a multiple correction by false discovery rate (FDR) to avoid Type I statistical errors.
Rich club analysis
In the human brain, some cortical regions have substantially higher numbers of connections to other brain regions, suggesting the presence of hubs (Sporns et al., 2007). Studies examining the connectivity among hub regions have demonstrated the existence of cores (Hagmann et al., 2008) as well as the presence of rich club organization (van den Heuvel and Sporns, 2011). Rich club organization is present in a network when highly connected regions are more likely to be interconnected to one another than expected by chance (van den Heuvel and Sporns, 2011). In this study, the rich club organization of children’s brains was investigated based on previous studies (Kim et al., 2014; van den Heuvel et al., 2013). First, the weighted rich club coefficient Φw(k) was computed as where the number of connections (E>k) and the sum of weights (W>k) were represented for nodal degrees (=the number of connections at a node) larger than k, and all non-zero elements of connectivity matrix were sorted by their weights, giving a vector of . For comparison across individuals, Φw(k) was typically normalized by the averaged values from 1000 random networks preserving the network size, weights, and degree distribution, giving the normalized rich club coefficient (Opsahl et al., 2008). As a function of degree k, rich club regime was defined if is significant in >75% individuals for children (van den Heuvel and Sporns, 2011; van den Heuvel et al., 2013). Subsequently, all connections in each connectivity matrix for children were categorized into rich club connections (i.e., the links among rich club nodes), feeder connections (linking rich club and non-rich club nodes), and local connections that link non-rich club nodes (van den Heuvel and Sporns, 2011). We also examined whether the connectivity of rich club, feeder, and local nodes was associated with the PRI and its subscales (BD, PC, and MR). Partial correlation coefficients (significant for p<0.05) were computed controlling for children’s age and sex, as well as the number and strength of connectivity matrix.
Results
Global network measures
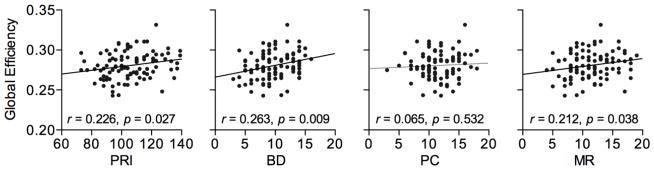
Consistent with previous studies on the global network organization of children (Cao et al., 2013; Kim et al., 2014), the structural brain networks of children showed a small-world organization – i.e., σ>1 (mean±SD=1.42±0.13) resulting from higher network clustering (γ>1; 1.53±0.15) and relatively shorter path length (λ≈1; 1.08±0.02) compared to the random networks (all p<0.05; one-sample t-test). The association between brain network organization and children’s perceptual intelligence was first examined by correlating the PRI with the global network measures. PRI was significantly correlated with the network’s global efficiency E (r=0.226, p=0.027 in Fig. 1). Thus, brain networks had greater global integration in children with higher PRI (intelligence) scores. Subsequently, scores on the three subtests (BD, PC, and MR) of the PRI were tested to investigate domain-specific relationships of intelligence to the global network characteristics. Global efficiency was significantly and positively correlated with BD (r=0.263, p=0.009) and MR (r=0.212, p=0.038) scores in children, correcting for individual variations in overall connectivity number and strength. No significant correlations were found between PRI subtest scores and the other global network measures (Supplementary Fig. 1).
Figure 1.
Partial correlations between global network efficiency and intelligence scores with perceptual reasoning index (PRI) and its subtests: block design (BD), picture concepts (PC), and matrix reasoning (MR). Significant positive correlations (solid line) were found in BD and MR as well as PRI (p<0.05, after controlling for age, sex, and overall connectivity differences – i.e., the number and strength of connections).
Node-specific network measures
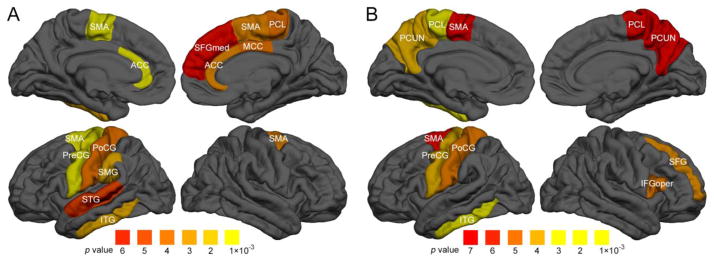
To further determine which specific brain regions showed the association between local network organization and intelligence, the path length and the local efficiency of each individual node were correlated with PRI, BD, PC, and MR. Fig. 2 represents the correlation coefficients of regions that showed a significant correlation between BD scores and path length and local efficiency (p<0.05, FDR corrected). In general, the pattern of correlations supported a key role for the P-FIT model, although correlations with the inferior and superior temporal gyri suggest an important role for these regions as well. Prominent negative associations between the BD score and path length were found in the left precentral gyrus (Brodmann area 4, r=−0.405, p=5×10−5), the supplementary motor area (Brodmann area 6, left: r=−0.371, p=2×10−4, right: r=−0.297, p=3×10−3), the anterior cingulate cortex (Brodmann area 32, left: r=−0.338, p=7×10−4, right: r=−0.296, p=3×10−3), the left inferior temporal gyrus (Brodmann area 20, r=−0.298, p=3×10−3), the left supramaginal gyrus (Brodmann area 40, r=−0.292, p=4×10−3), the left postcentral gyrus (Brodmann area 1–3, r=−0.292, p=4×10−3), the right middle cingulate gyrus (Brodmann area 24, r=−0.288, p=4×10−3), the right paracentral lobule (Brodmann area 5, r=−0.287, p=5×10−3), the left superior temporal gyrus (Brodmann area 22, r=−0.282, p=5×10−3), and the right medial superior frontal gyrus (Brodmann area 9, r=−0.279, p=6×10−3). In addition, local efficiency at each node was positively correlated with children’s BD score in the left inferior tempo gyrus (Brodmann area 20, r=0.375, p=2×10−4), the left paracentral lobule (Brodmann area 5, r=0.330, p=1×10−3), the left precentral gyrus (Brodmann area 4, r=0.324, p=1×10−3), the right superior frontal gyrus (Brodmann area 46, r=0.305, p=3×10−3), the left precuneus (Brodmann area 7, r=0.304, p=3×10−3), and the right inferior frontal gyrus (Pars opercularis, Brodmann area 44, r=0.298, p=3×10−3). No associations with local efficiency and path length were found for PRI or PC and MR subscales.
Figure 2.
Regions with significant correlations (highlighted in red, orange and yellow coloration) between local network measures and intelligence scores. Children’s block design (BD) scores showed (A) significant negative associations with network path length at each node and (B) positive associations with local efficiency (p<0.05, FDR corrected after covarying with age, sex, and the number and strength of connections). ACC, anterior cingulate cortex; IFGoper, inferior frontal gyrus (pars opercularis); ITG, inferior temporal gyrus; MCC, middle cingulate cortex; PCL, paracentral lobule; PCUN, precuneus; PoCG, postcentral gyrus; PreCG, precentral gyrus; SFG, superior frontal gyrus; SFGmed, medial superior frontal gyrus; SMA, supplementary motor area; SMG, supramarginal gyrus; STG, superior temporal gyrus.
Rich club organization
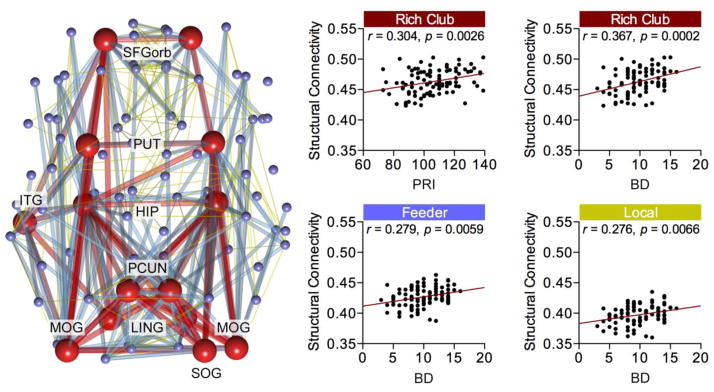
Rich club organization with densely connected cortical regions was evident in the brain networks of children, in which the nodes with degree k>25 showed normalized rich club coefficients – with p<0.05 in >75% children after application of 10,000-times permutation test (Kim et al., 2014). Observed rich club regions were comparable to those detected in previous rich club studies carried out in adults (van den Heuvel and Sporns, 2011) and children (Ball et al., 2014), and include bilateral precuneus, hippocampus, putamen, lingual cortex, middle occipital cortex, superior frontal (orbital) cortex, left inferior temporal cortex, and right superior occipital regions (Fig. 3), The mean structural connectivity, measured by averaged FA values for rich club connections, showed significant positive correlation with children’s PRI (r=0.304, p=0.0026). Notably, the BD score showed significant correlations with rich-club (r=0.367, p=0.0002), feeder (r=0.279, p=0.0059), and local connections (r=0.276, p=0.0066), suggesting the level of structural connectivity is preferentially related to the children’s ability for visuo-motor spatial reasoning. No significant correlations were found between the structural connectivity and PC and MR scores (all p>0.05, FDR corrected).
Figure 3.
Rich club connectivity. Significant rich club nodes (red spheres in the left diagram) were found in the wide range of degree k>25 including the superior frontal (orbital) gyrus (SFGorb), putamen (PUT), hippocampus (HIP), inferior temporal gyrus (ITG), precuneus (PCUN), lingual gyrus (LING), middle occipital gyrus (MOG), and superior occipital gyrus (SOG). Structural connectivity, as measured by averaged fractional anisotropy (FA) value of fiber tracts, was positively correlated among rich club nodes with perceptual reasoning index (PRI), while children’s block design (BD) score was correlated for rich club, feeder (blue), and local nodes (yellow) (p<0.05 with covariates of age, sex, and the number and strength of connections).
Discussion
The main finding of this study was a strong association between specific intellectual abilities of children and the level of integration of their structural network organization. Our results showed that children with high scores on measures of perceptual reasoning, especially Block Design (BD) and Matrix Reasoning (MR), exhibited significantly greater global efficiency of structural brain networks (Fig. 1). This finding supports the notion that superior performance of children on a test of intelligence is associated with the efficient transfer of local information among all nodes in the network (Sporns, 2012). Further, because the BD score also reflects visuo-spatial motor processing ability (Mervis et al., 1999), these results may suggest that more efficiently integrated networks are related to a higher level of visual-spatial reasoning, sensorimotor integration and every spatial problem activities (Groth-Marnat and Teal, 2000). Naturally, however, a BD score alone is not a robust measure of a specific cognitive domain, so further research is necessary to understand relationships with specific versus global constructs and processes.
The present structural findings that higher cognitive ability was related to both global and local network properties extend previous functional neuroimaging studies which reported that greater cognitive processing abilities were associated with network communication capacities arising from both whole brain (Langer et al., 2012; van den Heuvel et al., 2009) and specific brain regions (e.g., prefrontal cortex; Cole et al., 2012). The present results also extend the diffusion tensor imaging and tractography findings of Li et al. (2009) who showed that characteristic path length and global efficiency were significantly correlated with higher intelligence in adults (mean age=23.8 years, range=17–33 years). To our knowledge, this is the first study to demonstrate similar findings in children. These findings in children are especially important because the relationship between brain network topology and intelligence is known to be age-dependent. For example, findings from a functional network study revealed a negative association between age and global brain integration (Achard and Bullmore, 2007) were supported by a structural network study showing significant correlations between structural network properties and general intelligence for elderly people (>75 years; Fischer et al., 2014). Accordingly, further understanding the relation between network properties and intellectual ability with cognitive functions during development, when these networks are formed, will be important to understand the neural systems involved in emerging cognitive processes in childhood. The association of network efficiency with higher performance PRI scores (especially, for BD) found in the current study in children aged 6–11 years suggests that non-verbal cognitive performance of children may benefit from increasing integrity (i.e., more optimal communication among distributed brain regions) within the global brain structure.
The regional associations indicated that shorter path lengths and increased local efficiency were significantly related to the frontal lobes – SFG (Brodmann area 46), SFGmed (Brodmann area 9), and IFGoper (Brodmann area 44), parietal lobes – PCUN (Brodmann area 7) and SMG (Brodmann area 40), and temporal lobes – STG (Brodmann area 22) and ITG (Brodmann area 20), including ACC (Brodmann area 32). Our findings are consistent with a previous review of structural and task-related functional neuroimaging studies reporting that the integration of distributed networks, in particular involving the parietal and frontal regions, contributes to individual differences in intelligence (P-FIT; Jung and Haier, 2007). Interestingly, we found there were strong associations between the BD score of children in the left motor-related areas – e.g., PreCG (BA4), PoCG (BA1-3), and the SMA (BA6). This suggests that the global network integration for visuo-spatial motor processing is likely to be associated with improved motor ability of children manipulating physical blocks. Moreover, the left hemisphere (in our right handed sample) might be more important for the lateralized cognitive task performance in children than the right hemisphere (Deary et al., 2010).
By assessing structural connectivity in the preadolescent children in the current study, we found significant rich club organization (Fig. 3) similar to findings in previous studies of children (Grayson et al., 2014; Kim et al., 2014) and adults (van den Heuvel and Sporns, 2011). Because the rich club has a central position within a brain network, it potentially exerts major control over the efficient global transfer of neural information (van den Heuvel and Sporns, 2013). Our finding suggests that higher intellectual ability can be characterized by increased structural integrity of this central aggregation of brain regions in children, potentially implicated in promoting global communication capacity in the cognitive performance tasks. Another important finding is the significant positive correlations between the level of structural connectivity and the BD score, indicative of the preferential association in a specific sub-domain of the PRI. The significant associations between BD and structural connectivity regardless of rich-club, feeder and local nodes suggest that the network integration may additionally reflect spatial problem-solving with manipulative abilities important in everyday spatial tasks (Groth-Marnat and Teal, 2000). For example, the rapid motor performance during BD might require the integrative functioning of rich club (e.g., putamen; Wymbs et al., 2012) and non-rich club (e.g., supplemental motor area; Gerloff et al., 1998) in that higher BD scores are from the rapid finger execution within a limited time.
Recent studies have reported that general intelligence is associated with the level of white mater tract integrity measured by FA (Penke et al., 2012a; Penke et al., 2012b), regional cortical thickness (Karama et al., 2009; Karama et al., 2011), and functional activations during a cognitive task (Basten et al., 2015) across widespread brain regions including subcortical structures. Our network findings are consistent with these previous studies in that the network associations with intelligence were found in the distributed local brain regions (Fig. 2), included in the P-FIT model, with subcortical areas as central rich club nodes (Fig. 3) as well as in the global network organization (Fig. 1). The present results also suggest the ‘efficient’ network-wide transfer of neural information among distal brain regions via white matter tracts are responsible for the complex cognitive functions (Bressler and Menon, 2010) supporting intellectual function (Glascher et al., 2010).
It has been reported that the association between intelligence and neural correlates from structural and functional brain imaging is dependent on the age and sex (Schmithorst, 2009; Schmithorst and Holland, 2006, 2007; Schmithorst et al., 2008). In this study, no significant differences were found in the structural connectivity and global network properties between male and female children, suggesting that small-world organization of brain with a high level of network segregation and global integration is present regardless of sex (Supplementary Fig. 2). However, the association between reasoning abilities (i.e., PRI, BD, and MR as in Fig. 1) and the level of network integrity was strongest for network efficiency in males and for network path length for females, suggesting subtle sex differences in the relation of network integration to intelligence. Furthermore, positive correlations between global network efficiency and BD and PRI scores in this study were largest in younger children, suggesting different trajectories of the brain network underlying intelligence across the children’s age (Supplementary Fig. 3) as proposed in Shaw et al. (2006). In future studies, it would be helpful to investigate the effect of age on a sample with broader age range.
Our study has limitations, similar to those of other DTI-based studies using tractography techniques for the structural connectivity (Fischer et al., 2014; Kim et al., 2014; Li et al., 2009), including the fact that there are many different definitions and methods for defining optimal structural connectivity, selection of nodes constituting the brain network, and the removal of false-positive network connections. Other limitations include the constraints and biases of deterministic fiber tracking, and the relatively low number of diffusion gradients (=32) in the present study. However, previous studies have reported that structural network and rich club organization metrics are largely consistent regardless of the connectivity definition (Lo et al., 2010; van den Heuvel and Sporns, 2011) though the optimal formulation for the number of network nodes and the optimal tracking strategy for fiber tractography are unknown. We also found that the correlation patterns in this study between global network measures and intelligence scores were grossly consistent even with a different parcellation template – e.g., Harvard-Oxford atlas; Supplementary Fig. 4; c.f., de Reus and van den Heuvel (2013). Finally, our study focused only on the performance component of intelligence. In general, verbal and performance intelligence are highly correlated (Deary et al., 2010); however, further investigation of the association between brain’s network organization and comprehensive measures of cognitive abilities will be essential for understanding the neural architecture underlying the development of intelligence in children.
Conclusion
The findings in this study indicate that typically developing preadolescent children with higher intellectual ability as measured by the Perceptual Reasoning Index have brain networks that are more highly integrated at both global and local levels. Moreover, a subtest for visuo-spatial motor processing (i.e., Block Design), which is strongly associated with general intelligence (g), was most robustly correlated with global network structure as well as local brain regions and brain’s rich club. This suggests that visual construction and reasoning may make general demands on globally integrated processing by the brain.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3:e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JM, Goldstein LH. Manual for the MacArthur Health and Behavior Questionnaire (HBQ 1.0) University of Pittsburgh; Chicago: 2003. [Google Scholar]
- Ball G, Aljabar P, Zebari S, Tusor N, Arichi T, Merchant N, Robinson EC, Ogundipe E, Rueckert D, Edwards AD, Counsell SJ. Rich-club organization of the newborn human brain. Proc Natl Acad Sci U S A. 2014;111:7456–7461. doi: 10.1073/pnas.1324118111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron IS. Neuropsychological Evaluation of the Child. Oxford University Press Inc; New York: 2004. [Google Scholar]
- Basten U, Hilger K, Fiebach CJ. Where smart brains are different: A quantitative meta-analysis of functional and structural brain imaging studies on intelligence. Intelligence. 2015;51:10–27. [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Cao Q, Shu N, An L, Wang P, Sun L, Xia MR, Wang JH, Gong GL, Zang YF, Wang YF, He Y. Probabilistic diffusion tractography and graph theory analysis reveal abnormal white matter structural connectivity networks in drug-naive boys with attention deficit/hyperactivity disorder. J Neurosci. 2013;33:10676–10687. doi: 10.1523/JNEUROSCI.4793-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Wang Y, Sheng J, Sporns O, Kronenberger WG, Mathews VP, Hummer TA, Saykin AJ. Optimization of seed density in DTI tractography for structural networks. J Neurosci Methods. 2012;203:264–272. doi: 10.1016/j.jneumeth.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Yarkoni T, Repovs G, Anticevic A, Braver TS. Global connectivity of prefrontal cortex predicts cognitive control and intelligence. J Neurosci. 2012;32:8988–8999. doi: 10.1523/JNEUROSCI.0536-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin G, van den Heuvel MP. The ontogeny of the human connectome: development and dynamic changes of brain connectivity across the life span. Neuroscientist. 2013;19:616–628. doi: 10.1177/1073858413503712. [DOI] [PubMed] [Google Scholar]
- Colom R, Haier RJ, Head K, Alvarez-Linera J, Quiroga MA, Shih PC, Jung RE. Gray matter correlates of fluid, crystallized, and spatial intelligence: Testing the P-FIT model. Intelligence. 2009;37:124–135. [Google Scholar]
- de Reus MA, van den Heuvel MP. The parcellation-based connectome: limitations and extensions. Neuroimage. 2013;80:397–404. doi: 10.1016/j.neuroimage.2013.03.053. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci. 2010;11:201–211. doi: 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- Fischer FU, Wolf D, Scheurich A, Fellgiebel A. Association of structural global brain network properties with intelligence in normal aging. PLoS One. 2014;9:e86258. doi: 10.1371/journal.pone.0086258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG. The role of the human motor cortex in the control of complex and simple finger movement sequences. Brain. 1998;121 (Pt 9):1695–1709. doi: 10.1093/brain/121.9.1695. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascher J, Rudrauf D, Colom R, Paul LK, Tranel D, Damasio H, Adolphs R. Distributed neural system for general intelligence revealed by lesion mapping. Proc Natl Acad Sci U S A. 2010;107:4705–4709. doi: 10.1073/pnas.0910397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Thompson PM. Neurobiology of intelligence: science and ethics. Nat Rev Neurosci. 2004;5:471–482. doi: 10.1038/nrn1405. [DOI] [PubMed] [Google Scholar]
- Grayson DS, Ray S, Carpenter S, Iyer S, Dias TG, Stevens C, Nigg JT, Fair DA. Structural and functional rich club organization of the brain in children and adults. PLoS One. 2014;9:e88297. doi: 10.1371/journal.pone.0088297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth-Marnat G, Teal M. Block design as a measure of everyday spatial ability: A study of ecological validity. Perceptual and Motor Skills. 2000;90:522–526. doi: 10.2466/pms.2000.90.2.522. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci. 2007;30:135–154. doi: 10.1017/S0140525X07001185. discussion 154–187. [DOI] [PubMed] [Google Scholar]
- Karama S, Ad-Dab’bagh Y, Haier RJ, Deary IJ, Lyttelton OC, Lepage C, Evans AC, 1 BDCG. Positive association between cognitive ability and cortical thickness in a representative US sample of healthy 6 to 18 year-olds (vol 37, pg 145, 2009) Intelligence. 2009;37:431–442. doi: 10.1016/j.intell.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Colom R, Johnson W, Deary IJ, Haier R, Waber DP, Lepage C, Ganjavi H, Jung R, Evans AC Brain Development Cooperative G. Cortical thickness correlates of specific cognitive performance accounted for by the general factor of intelligence in healthy children aged 6 to 18. Neuroimage. 2011;55:1443–1453. doi: 10.1016/j.neuroimage.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Davis EP, Sandman CA, Sporns O, O’Donnell BF, Buss C, Hetrick WP. Longer gestation is associated with more efficient brain networks in preadolescent children. Neuroimage. 2014;100:619–627. doi: 10.1016/j.neuroimage.2014.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer N, Pedroni A, Gianotti LR, Hanggi J, Knoch D, Jancke L. Functional brain network efficiency predicts intelligence. Hum Brain Mapp. 2012;33:1393–1406. doi: 10.1002/hbm.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu Y, Li J, Qin W, Li K, Yu C, Jiang T. Brain anatomical network and intelligence. PLoS Comput Biol. 2009;5:e1000395. doi: 10.1371/journal.pcbi.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CY, Wang PN, Chou KH, Wang J, He Y, Lin CP. Diffusion tensor tractography reveals abnormal topological organization in structural cortical networks in Alzheimer’s disease. J Neurosci. 2010;30:16876–16885. doi: 10.1523/JNEUROSCI.4136-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov S, Sneppen K. Specificity and stability in topology of protein networks. Science. 2002;296:910–913. doi: 10.1126/science.1065103. [DOI] [PubMed] [Google Scholar]
- Mervis CB, Robinson BF, Pani JR. Visuospatial construction. Am J Hum Genet. 1999;65:1222–1229. doi: 10.1086/302633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S. Introduction to diffusion tensor imaging. Elsevier; Amsterdam; Boston, MA: 2006. [Google Scholar]
- Muftuler LT, Davis EP, Buss C, Solodkin A, Su MY, Head KM, Hasso AN, Sandman CA. Development of white matter pathways in typically developing preadolescent children. Brain Res. 2012;1466:33–43. doi: 10.1016/j.brainres.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, Gurbani M, Toga AW, Bilder RM. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb Cortex. 2007;17:2163–2171. doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- Newman ME. Modularity and community structure in networks. Proc Natl Acad Sci U S A. 2006;103:8577–8582. doi: 10.1073/pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opsahl T, Colizza V, Panzarasa P, Ramasco JJ. Prominence and control: the weighted rich-club effect. Phys Rev Lett. 2008;101:168702. doi: 10.1103/PhysRevLett.101.168702. [DOI] [PubMed] [Google Scholar]
- Penke L, Maniega SM, Bastin ME, Hernandez MC, Murray C, Royle NA, Starr JM, Wardlaw JM, Deary IJ. Brain-wide white matter tract integrity is associated with information processing speed and general intelligence. Mol Psychiatry. 2012a;17:955. doi: 10.1038/mp.2012.127. [DOI] [PubMed] [Google Scholar]
- Penke L, Maniega SM, Bastin ME, Valdes Hernandez MC, Murray C, Royle NA, Starr JM, Wardlaw JM, Deary IJ. Brain white matter tract integrity as a neural foundation for general intelligence. Mol Psychiatry. 2012b;17:1026–1030. doi: 10.1038/mp.2012.66. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ. Developmental sex differences in the relation of neuroanatomical connectivity to intelligence. Intelligence. 2009;37:164–173. doi: 10.1016/j.intell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Functional MRI evidence for disparate developmental processes underlying intelligence in boys and girls. Neuroimage. 2006;31:1366–1379. doi: 10.1016/j.neuroimage.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Sex differences in the development of neuroanatomical functional connectivity underlying intelligence found using Bayesian connectivity analysis. Neuroimage. 2007;35:406–419. doi: 10.1016/j.neuroimage.2006.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls. Human Brain Mapping. 2008;29:696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Sporns O. Networks of the brain. MIT Press; Cambridge, Mass: 2011. [Google Scholar]
- Sporns O. Discovering the human connectome. MIT Press; Cambridge, Mass: 2012. [Google Scholar]
- Sporns O, Honey CJ, Kotter R. Identification and classification of hubs in brain networks. PLoS One. 2007;2:e1049. doi: 10.1371/journal.pone.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Kotter R. The human connectome: A structural description of the human brain. PLoS Comput Biol. 2005;1:e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Zwi JD. The small world of the cerebral cortex. Neuroinformatics. 2004;2:145–162. doi: 10.1385/NI:2:2:145. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Walhovd KB, Westlye LT, Due-Tonnessen P, Fjell AM. Intellectual abilities and white matter microstructure in development: a diffusion tensor imaging study. Hum Brain Mapp. 2010;31:1609–1625. doi: 10.1002/hbm.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Beek L, Ghesquiere P, Lagae L, De Smedt B. Left fronto-parietal white matter correlates with individual differences in children’s ability to solve additions and multiplications: a tractography study. Neuroimage. 2014;90:117–127. doi: 10.1016/j.neuroimage.2013.12.030. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. Rich-club organization of the human connectome. J Neurosci. 2011;31:15775–15786. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends Cogn Sci. 2013;17:683–696. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O, Collin G, Scheewe T, Mandl RC, Cahn W, Goni J, Hulshoff Pol HE, Kahn RS. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry. 2013;70:783–792. doi: 10.1001/jamapsychiatry.2013.1328. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE. Efficiency of functional brain networks and intellectual performance. J Neurosci. 2009;29:7619–7624. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes PE, Bullmore ET. Annual Research Review: Growth connectomics - the organization and reorganization of brain networks during normal and abnormal development. J Child Psychol Psychiatry. 2015;56:299–320. doi: 10.1111/jcpp.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. The Psychological Corporation; San Antonio, TX: 2002. [Google Scholar]
- Wen W, Zhu W, He Y, Kochan NA, Reppermund S, Slavin MJ, Brodaty H, Crawford J, Xia A, Sachdev P. Discrete neuroanatomical networks are associated with specific cognitive abilities in old age. J Neurosci. 2011;31:1204–1212. doi: 10.1523/JNEUROSCI.4085-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Taki Y, Sato K, Hashizume H, Sassa Y, Takeuchi H, Thyreau B, He Y, Evans AC, Li X, Kawashima R, Fukuda H. Topological organization of functional brain networks in healthy children: differences in relation to age, sex, and intelligence. PLoS One. 2013;8:e55347. doi: 10.1371/journal.pone.0055347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymbs NF, Bassett DS, Mucha PJ, Porter MA, Grafton ST. Differential recruitment of the sensorimotor putamen and frontoparietal cortex during motor chunking in humans. Neuron. 2012;74:936–946. doi: 10.1016/j.neuron.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.