Abstract
Peripheral arterial disease (PAD) is responsible for 20% of all US hospital admissions. Management of PAD has evolved over time to include many medical and transcatheter interventions in addition to the traditional surgical approach. Non-invasive interventions including supervised exercise programs and antiplatelets use are economically attractive therapies that should be considered in all patients at risk. While surgery offers so far a clinically and economically appropriate option, the improvement of percutaneous transluminal angioplasty (PTA) technique with the addition of drug-coated balloons offers a reasonably clinically and economically attractive alternative that will continue to evolve in the future.
Keywords: Cost- effectiveness, Peripheral vascular disease, Percutaneous transluminal angioplasty, Endovascular interventions, Bypass surgery
1. Introduction
Admissions for peripheral artery disease (PAD) have been increasing and are currently responsible for approximately 20% of all U.S. hospital admissions. Data analysis of over 2 million hospital admissions for PAD between 2001 and 2007 showed that the choice of treatment has dramatically changed, with a 78% increase in endovascular procedures, and a concomitant decrease in open bypass and amputations [1]. That trend was associated with a change in the distribution of cases among different specialties involved in performing them. Between 1998 and 2005, there was a 6-fold drop in peripheral procedures performed by interventional radiologists (5.6% of all cases in 2005), with a 3-fold increase for interventional cardiologists (29% of all cases), and a 2-fold increase for vascular surgeons (43% of all cases) [2]. The number of interventional laboratories that have the capability to do peripheral vascular interventions is rapidly growing, with many fellowship programs now offering additional training in these techniques. Advances in technology, use of bare metal stents and atherectomy, and intravascular imaging have helped to increase success and reduce complications. It is estimated by industry that peripheral interventions will grow an average of 8% per year over the next 4 years [3,4].
1.1. Cost effectiveness analysis and decision making
The primary goal of cost-effectiveness analysis is to evaluate different health care intervention options in common terms so that policy and other decision makers can be informed of the most efficient method of producing extra health benefits from among the alternative ways that health care dollars can be distributed. The metric used to assess incremental cost effectiveness is the Incremental Cost-effectiveness Ratio (ICER). An ICER is defined as the ratio of incremental costs to incremental health benefits of treatment 1 compared to treatment 2, or ICER = (C1 – C2)/ (HB1 – HB2); where C1 and C2 are cost for treatments 1 and 2, respectively and HB is the health benefit of treatments 1 and 2, respectively [5].
The ICER defines the cost that should be assumed for gaining one unit of output. In other words, if one of the alternatives is the usual practice, then it will tell us how much it will cost to gain a unit of outcome when moving from the usual practice to a new alternative. The health benefit may be measured in any sensible unit, such number of MIs averted, but most studies use the conventional option of measuring clinical benefits as either the number of added life-years (LYs) or quality adjusted life years (QALYs) [5,6]. Both of these approaches require estimation of life expectancy with and without the intervention being considered.
When assessing whether a treatment is cost effective, a requirement for threshold can arise when policy makers seek a benchmark to compare different treatments and judge different studies. In general, wealthier countries may be willing to pay more (i.e. accept higher threshold) for a given treatment than poorer countries [5,7]. In the United States, a cost-effectiveness ratio <$50,000 per LY or QALY is frequently regarded as economically attractive, in part because it approximates the cost of providing chronic hemodialysis to patients with renal failure, at a cost that meets willingness-to-pay through Medicare [5]. Conversely, a cost-effectiveness ratio of >$100,000 per added LY or QALY is frequently regarded as economically unattractive. The range between these two benchmarks is the gray zone in which there is no consensus on whether a treatment is economically acceptable [5]. While the benchmarks may be viewed as informative, there is actually no scientific basis for any threshold above which a treatment would be viewed as not cost effective.
2. Factors that impact the costs of peripheral vascular disease management
There are many factors that may impact the cost of vascular interventions. In a study assessing the cost of peripheral procedures at the Brigham and Women's hospital from 1990 through 1995, the cost of these interventions was noticed to be higher with advance age ($1345, P = 0.02), the presence of CAD ($1287, P = 0.05) and female gender ($1461, P = 0.03). The presence of complications was associated with a substantial increase in cost with additional cost estimated for fatal systemic complications of $11,675 (P = 0.004) and for nonfatal systemic complications of $9345 (P < 0.001) (Table 1) [8].
Table 1.
Cost-effectiveness of non-invasive versus invasive management of peripheral vascular disease.
| Author | Comparison | Primary outcome | Results | Cost effectiveness |
|---|---|---|---|---|
| Treesak et al. [12] | Exercise vs. PTA vs. none. | Absolute claudication distance at 3 and 6 months. | At 3 months, PTA was more effective than exercise therapy and resulted in an additional 38 m. At 6 months, however, exercise was more effective than PTA, resulting in an additional 137 m walked. | At 3 months, PTA was more effective than exercise therapy and resulted in an additional cost of $6719, for an ICER of $177/m. At 6 months, however, exercise was more effective than PTA, resulting in less costs ($61 less per m gained). |
| Van Asselt AD [13] | Supervised Exercise Therapy (SET) vs. Walking advice (WA). | Walking distance | Median walking distance was 620 m for exercise vs. 400 m for walking advice group. | Mean total costs were higher for SET than for WA (3407 versus 2304 Euros). ICER for cost per extra m was € 4.08 and € 28,693 per QALY. |
| Chen et al [17] | Aspirin vs. aspirin and clopidogrel in patients with cardiovascular disease | Composite of death, myocardial infarction and stroke | Adding clopidogrel use was associated with a reduction in the primary outcome at 28 months (6.9 vs. 7.9%, P = 0.048) | ICER was $36,343 per LY |
| Squires et al [18] | Naftidrofuryl oxalate vs. cilostazol | Logarithm mean of maximal walking distance | Cilostazol was superior in increasing the mean of maximal walking (0.181 to 0.762) vs. (0.108 to 0.337). | Naftidrofuryl oxalate, had an ICER of around £6070 per QALY gained when compared with no vasoactive drug, whereas cilostazol was associated with ICER of >£ 20,000 per QALY gained when compared with no vasoactive drug [18]. |
Abbreviations: PTA = percutaneous transluminal angioplasty; SET = supervised exercise therapy; WA = walking advice; ICER = incremental cost-effectiveness ratio; QALY = quality adjusted life years; LY = life year.
The extent and severity of PAD also have a substantial impact on the cost of treatment. While treatment of a patient with stage IIa PAD by Fontaine criteria (i.e., with a pain-free walking distance of more than 200 m) costs about $650 per year, treatment of a patient with stage IV PAD (defined by ischemic tissue necrosis) costs $9353 per year. Similarly critical leg ischemia (PAD stages III–IV) is on average $4478 more expensive than the treatment of intermittent claudication (PAD stage II disease) [8,9]. This also holds true for the costs of a specific therapeutic/invasive procedure; for example, the costs for percutaneous transluminal angioplasty (PTA) are much greater for patients with critical ischemia and tissue necrosis than for patients with disabling claudication secondary to higher complication rates and longer hospital stays [10]. Another consideration is that amputation has been shown to be about twice as expensive as a limb salvage strategy with either interventional or surgical methods and for both acute and chronic limb threatening ischemia [11].
3. Cost effectiveness of noninvasive therapy
3.1. Cost effectiveness of exercise programs
Exercise therapy was shown to be associated with improve symptoms and increased walking distance in PAD. A cost-effectiveness analysis comparing an exercise program to PTA showed that although at 3 months, PTA was more effective than exercise therapy and resulted in an additional 38 m, it did that at an additional cost of $6719, for an ICER of $177/m. At 6 months, however, exercise was more effective than PTA, resulting in an additional 137 m walked, and costs less ($61 less per meter gained). Therefore, exercise rehabilitation at 6 months is more effective and costs less than plain PTA, and is therefore cost saving [12]. However, it should be noticed that in this study exercise program was compared to PTA before the introduction of new techniques including drug-eluting stents (DES), drug-coated balloons (DCB) and woven nitinol stenting.
A supervised exercise program seems to be superior to simply motivating patients to exercise. The supervised exercise therapy (SET) in the Exercise Therapy in Peripheral Arterial Disease (EXITPAD) study was shown be more effective than ‘go home and walk’ advice (WA) for patients with intermittent claudication in regards of walking distance (620 m SET vs. 400 m WA) and quality of life (QALYs: 0.71 SET vs. 0.67 WA) making it cost effective with an ICER for cost per extra meter on the 12-month treadmill test of € 4.08 [13].
3.2. Cost effectiveness of antiplatelet agents
The use of antiplatelet agents was shown to be effective in reducing the risk of vascular occlusion in a wide range of patients with PAD. A meta-analysis of 8000 patients built from 46 randomized trials of anti-platelet therapy versus control and 14 randomized trials comparing one antiplatelet regimen with another showed that antiplatelet therapy (chiefly aspirin alone or aspirin plus dipyridamole) produced a highly significant (P < 0.0001) reduction in vascular occlusion, with the largest absolute reductions among patients at highest risk of occlusion and smaller but still significant absolute reductions among lower risk patients. Also, antiplatelet therapy in patients with PAD produced a significant 25% reduction (P = 0.002) in the incidence of vascular events (non-fatal MI, non-fatal stroke, or vascular death) [14]. Giving the compelling evidence of the benefit of aspirin in patients with PAD, no economic assessment was performed to compare its use to placebo.
The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial that originally randomized 15,603 patients with either clinically evident cardiovascular disease or multiple risk factors for cardiovascular disease to receive clopidogrel plus low-dose aspirin or low-dose aspirin alone showed no significant difference in the composite of MI, stroke, or cardiac death [15]. However, a subgroup analysis including 9478 patients with manifest cardiovascular disease (prior MI, stroke, or symptomatic PAD) of the CHARISMA trial showed a significant reduction in the incidence of the composite of cardiovascular death, MI, or stroke with the use DAPT (7.3 vs. 8.8%; HR = 0.83, 95% CI [0.72–0.96]; P < 0.01) as well as a reduction in hospitalizations for ischemia (11.4 vs. 13.2%; HR = 0.86; 95% CI [0.76–0.96]; P = 0.008) [16].
Economic evaluation of this subgroup showed that DAPT use was associated with $2607 higher cost with projected life expectancy increased by an average of 0.072 years. The use of DAPT, therefore, appeared to be an economically attractive option with ICER of $36,343 per LY gained [17]. Even after adjusting for discount rate, life expectancy projections, post-event costs, and indirect costs from lost productivity; the ICER remained <$50,000/life-year deeming DAPT to be cost-effective.
3.3. Cost effectiveness of vasoactive agents
Vasoactive agents including cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate are used for symptomatic relief in patients with intermittent claudication. A review and analysis of the results of 26 RCTs showed that both naftidrofuryl oxalate and cilostazol appear to be effective treatments with significant improvement in the logarithm mean of maximal walking distance from 0.181 to 0.762 and 0.108 to 0.337, respectively for this patient population. Naftidrofuryl oxalate, however, seemed to be the only treatment that is likely to be considered cost-effective with ICER of around £6070 per QALY gained when compared with no vasoactive drug, whereas cilostazol was associated with ICER of >£20,000 per QALY gained when compared with no vaso-active drug [18].
4. Cost effectiveness of percutaneous transluminal angioplasty versus bypass surgery
The first data on the comparative cost effectiveness of PTA vs. bypass surgery come from the Revascularization for Femoro-popliteal Disease trial that studied the impact of both strategies on 65-year-old men with disabling claudication or chronic critical limb ischemia secondary to femoro-popliteal stenosis. Initial angioplasty increased quality-adjusted life expectancy by 2 to 13 months in patients with disabling claudication and by 1 to 4 months in patients with chronic critical ischemia and resulted in decreased lifetime expenditures compared with bypass surgery in both groups. A Markov model economic estimation based on this study using a maximum threshold cost of US$50,000 per QALY showed that PTA was cost effective when compared with vein bypass for lesions that could be treated with a better than 30% 5-year patency [10].
The Bypass versus Angioplasty in Severe Ischemia of the Leg (BASIL) trial randomized 452 patients with severe limb ischemia due to infrainguinal disease to receive either a surgery-first or an angioplasty-first strategy. After 1 year, the two strategies did not differ significantly in amputation-free survival (71% bypass vs. 68% PTA; adjusted HR = 0.73, 95% CI [0.49–1.07]) [19]. However, 5 years of follow-up of the BASIL trial showed that bypass surgery was associated with better overall survival (47% bypass vs. 41% PTA; adjusted HR = 0.61; 95% CI [0.50–0.75]; P < 0.009) and a non-significant difference in amputation free survival (38% bypass vs. 37% PTA; adjusted HR = 0.85; 95% CI [0.5–1.07]; P = 0.108) [20].
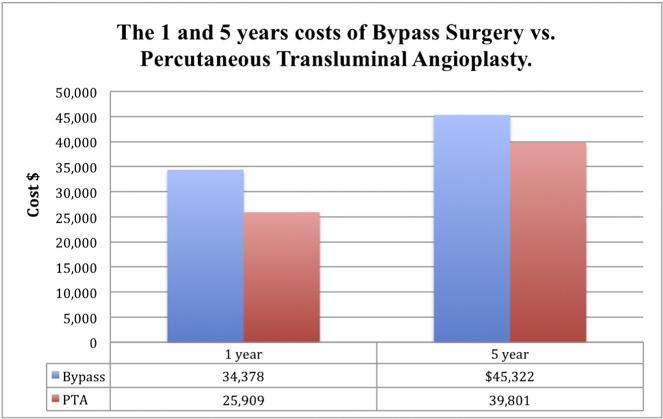
Economic assessment showed that for the first year, the hospital costs associated with a surgery-first strategy were $8469 higher ($34,378 bypass vs. $25,909 PTA). However, at the end of the 5-year follow up and secondary to increased costs subsequently incurred by the need for repeat revascularization including surgery in the PTA patients, this difference decreased to $5521 ($45,322 bypass vs. $39,801 PTA) and was no longer significant. Although the bypass strategy was associated with better survival, it was not economically attractive with ICER of $184,492 per QALY gained [20]. Fig. 1 shows the 1 and 5 year costs of bypass vs. PTA.
Fig. 1.
The 1 and 5 years costs of bypass surgery vs. percutaneous transluminal angioplasty.
It should be noticed that both studies were comparing surgical options to plain PTA. There is no randomized data that compare surgery with more advanced endovascular interventions including atherectomy, drug-eluting stents (DES), drug-coated balloons (DCB) and woven nitinol stenting. Furthermore, the only available economic assessment comparing surgery to PTA is based on either estimation models or from small trials. This lack and disparity in economic findings lead to variability in clinical with regards to angioplasty vs. bypass selection.
5. Cost effectiveness of technological advances in endovascular interventions
Although PTA alone was originally introduced as a treatment alternative to surgical revascularization, it was associated with increased incidence of acute recoil and high rate of restenosis. Subsequent development of stent technology has demonstrated a lower incidence of target lesion revascularization (TLR) [21–23]. More recently, drug-coated balloons (DCB) have emerged as a revascularization strategy that holds the promise of reducing TLRs further, while avoiding stent-related related risks such as in-stent restenosis and stent fracture and maintaining all therapeutic options for subsequent intervention [24].
There are two studies that compared the economics of all three modalities. The first study was a British economic evaluation of the cost-effectiveness comparing PTA with no bail-out stenting, PTA with bail-out drug-eluting stents, drug-coated balloons, primary bare metal stents, primary drug-eluting stents, endovascular brachytherapy, stent-grafts and cryoplasty in patients with intermittent claudication and critical leg ischemia. The cost and QALY were favorable for drug-coated balloon (DCB) over all other strategies in both intermittent claudication group (cost: £12 668 vs. £13 032–£17 578 and QALY: 6 · 120 vs. 5 · 931–6.081) and critical limb ischemia (cost: £49 890 vs. £54 775– £58 097 and QALY 3 · 402 vs. 2 · 988–3 · 297). Using £100000 as a higher cut-off for calculation, the use of drug-coated balloons seemed to be more economically attractive by having both lower lifetime costs and greater effectiveness. The probability of drug-coated balloons being cost-effective was at least 58.3% for patients with intermittent claudication and at least 72.2% for patients with critical leg ischemia [25]. Table 2 shows the British Cost-effectiveness analysis Of Different Interventional Peripheral Procedures.
Table 2.
The British cost-effectiveness analysis of different interventional peripheral procedures.
| Costs (£) |
QALYs |
|||
|---|---|---|---|---|
| IC | CLI | IC | CLI | |
| DCB | 12 668 | 49 890 | 6 · 120 | 3 · 402 |
| PTA with bail-out DES | 13 032 | 52 335 | 6 · 081 | 3 · 297 |
| PTA with bail-out BMS | 14 637 | 55 199 | 5 · 956 | 3 · 047 |
| PTA without bail-out stent | 14 787 | 56 539 | 5 · 931 | 2 · 988 |
| PTA with primary BMS | 15 030 | 54775 | 5 · 989 | 3 · 144 |
| PTA with primary DES | 15 692 | 55012 | 5 · 993 | 3 · 157 |
| Endovascular Brachytherapy | 15 891 | 55 928 | 5 · 984 | 3 · 134 |
| Stent-graft | 16171 | 55 852 | 5 · 989 | 3 · 144 |
| Cryoplasty | 17 578 | 58 097 | 5 · 934 | 3 · 003 |
Abbreviations: QALY = quality adjusted life years; IC = intermittent claudication; CLI = critical limb ischemia; DCB = drug-coated balloon; PTA = percutaneous transluminal angioplasty; DES = drug eluting stent; BMS = bare metal stent.
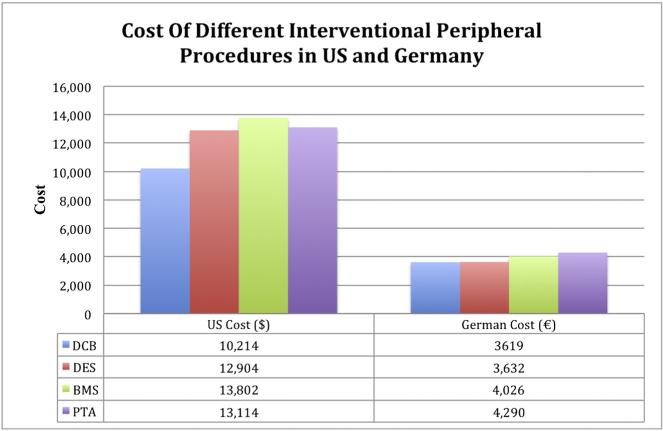
Another economic analysis was performed on a cohort of 2406 patients from 13 trials comparing the economic impact of the use of BMS, DES, or DCB compared to PTA alone based on the 2013 reimbursement rates of the U.S. Medicare and the German statutory sickness fund perspectives. Results showed a TLR rate of 14.3%, 19.3%, 28.1%, and 40.3% for DCB, DES, BMS, and PTA, respectively. The drug-eluting strategies had a lower projected budget impact over 24 months compared to BMS and PTA in both the U.S. Medicare (DCB: $10,214; DES: $12,904; PTA $13,114; BMS $13,802) and German public health care systems (DCB €3619; DES €3632; BMS €4026; PTA €4290) [26]. Interestingly, many hospitals in the US may not be interested in a strategy as DCB, even so it is more cost effective, as such a strategy is associated with a higher initial cost with lower need for further procedures in the future. Fig. 2 shows the cost of different interventional peripheral procedures in both the U.S. Medicare and German public health care systems.
Fig. 2.
The cost of different interventional peripheral procedures in both the U.S. Medicare and German public health care systems.
However both economic evaluations are estimation models derived from observational cohort. No data comparing the clinical and cost effectiveness of these different technologies are available yet. Furthermore no data are available to compare DCB, DES and BMS with other available technologies including atherectomy and woven nitinol stenting. Many clinical trials are either ongoing or in development to evaluate the efficacy of these different technologies in the periphery and the results of these trials will guide future decision making when managing peripheral vascular disease.
6. Discussion
In contrast to the field of coronary interventions, most data in regards of cost effectiveness of different therapies in peripheral vascular disease are derived from stimulation models based on observational cohort or from small randomized controlled trials. Multiple randomized clinical trial are expected to be reported in the near future that would help guiding the management of PVD using cost effective and meaningful therapies. Many considerations should be kept in mind while assessing the medical economics of PAD management. First, most of the therapies explored in this field, especially invasive interventions including both endovascular and surgical therapy are mainly directed towards symptoms control or less commonly limb salvage. Therefore, the assessment of the cost-effectiveness of these therapies will be directed towards ICER for QALY added rather than ICER per LY added. Second, while most patients presenting with PAD are complaining of symptoms related to their PAD, their overall prognosis is mainly based on the risk of the overall atherosclerosis and the associated major cardiovascular events. Therefore, non-invasive interventions including smoking cessation, exercise programs, antiplatelets therapy, strict diabetes control and lipid management may offer an impact on survival in addition to any expected impact of the quality of symptoms and the combination of these non-invasive interventions should be implemented in management plan for patients with any degree of PAD. Third, although this combination of aggressive multi-level non-invasive intervention mentions earlier is not compared to individual therapies, this combination will probably offer an economically dominant strategy over all options. Finally, although the cost effectiveness assessment of PTA vs. surgery was not conclusive, the continuous improvement in endovascular therapy over time will enhance the notion that endovascular interventions are becoming more attractive options both clinically and economically that will be favored by patients and health care providers. Among endovascular options, DCB seems very promising clinically and economically as it reduces TLRs, while avoiding stent-related risks and maintaining all therapeutic options for subsequent intervention at an economically attractive cost. Additional studies comparing DCB with DES and newer technologies like woven nitinol stents will provide more insight to guide a clinically and economically effective management of PVD.
Footnotes
Funding Source: Funded in part by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number U54-GM104941 (PI: Binder-Macleod).
References
- 1.Hong MS, Beck AW, Nelson PR. Emerging national trends in the management and outcomes of lower extremity peripheral arterial disease. Ann Vasc Surg. 2011;25:44–54. doi: 10.1016/j.avsg.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Eslami MH, Csikesz N, Schanzer A, Messina LM. Peripheral arterial interventions: trends in market share and outcomes by specialty, 1998–2005. J Vasc Surg. 2009;50:1071–8. doi: 10.1016/j.jvs.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Leon MB. Sweeping changes in the dynamic interventional global landscape: TCT 2011. San Francisco, CA: 2011. http://www.tctmd.com. [Google Scholar]
- 4.Faxon DP, Williams DO. The changing face of interventional cardiology. Circ Cardiovasc Interv. 2012;5:325–7. doi: 10.1161/CIRCINTERVENTIONS.112.971671. [DOI] [PubMed] [Google Scholar]
- 5.Mark DB, Hlatky MA. Medical economics and the assessment of value in cardiovascular medicine: part I. Circulation. 2002;106:516–20. doi: 10.1161/01.cir.0000021407.93752.7b. [DOI] [PubMed] [Google Scholar]
- 6.Wright JC, Weinstein MC. Gains in life expectancy from medical interventions—standardizing data on outcomes. N Engl J Med. 1998;339:380–6. doi: 10.1056/NEJM199808063390606. [DOI] [PubMed] [Google Scholar]
- 7.Gilder SS. London letter. Can Med Assoc J. 1971;104:473–81. [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen RM, de Vries SO, Cullen KA, Donaldson MC, Hunink MG. Cost-identification analysis of revascularization procedures on patients with peripheral arterial occlusive disease. J Vasc Surg. 1998;28:617–23. doi: 10.1016/s0741-5214(98)70085-0. [DOI] [PubMed] [Google Scholar]
- 9.Kugler CF, Rudofsky G. The challenges of treating peripheral arterial disease. Vasc Med. 2003;8:109–14. doi: 10.1191/1358863x03vm478ra. [DOI] [PubMed] [Google Scholar]
- 10.Hunink MG, Wong JB, Donaldson MC, Meyerovitz MF, de Vries J, Harrington DP. Revascularization for femoropopliteal disease. A decision and cost-effectiveness analysis. JAMA. 1995;274:165–71. [PubMed] [Google Scholar]
- 11.Singh S, Evans L, Datta D, Gaines P, Beard JD. The costs of managing lower limb-threatening ischaemia. Eur J Vasc Endovasc Surg. 1996;12:359–62. doi: 10.1016/s1078-5884(96)80257-7. [DOI] [PubMed] [Google Scholar]
- 12.Treesak C, Kasemsup V, Treat-Jacobson D, Nyman JA, Hirsch AT. Cost-effectiveness of exercise training to improve claudication symptoms in patients with peripheral arterial disease. Vasc Med. 2004;9:279–85. doi: 10.1191/1358863x04vm570oa. [DOI] [PubMed] [Google Scholar]
- 13.Van Asselt AD, Nicolaï SP, Joore MA, Prins MH, Teijink JA, Exercise Therapy in Peripheral Arterial Disease Study Group Cost-effectiveness of exercise therapy in patients with intermittent claudication: supervised exercise therapy versus a ‘go home and walk’ advice. Eur J Vasc Endovasc Surg. 2011;41:97–103. doi: 10.1016/j.ejvs.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Antiplatelet Trialists’ Collaboration Collaborative overview of randomised trials of antiplatelet therapy—II: maintenance of vascular graft or arterial patency by antiplatelet therapy. BMJ. 1994;308:159–68. [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–17. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt DL, Flather MD, Hacke W, Berger PB, Black HR, Boden WE, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49:1982–8. doi: 10.1016/j.jacc.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Bhatt DL, Dunn ES, Shi C, Caro JJ, Mahoney EM, et al. Cost-effectiveness of clopidogrel plus aspirin versus aspirin alone for secondary prevention of cardiovascular events: results from the CHARISMA trial. Value Health. 2009;12:872–9. doi: 10.1111/j.1524-4733.2009.00529.x. [DOI] [PubMed] [Google Scholar]
- 18.Squires H, Simpson E, Meng Y, Harnan S, Stevens J, Wong R, et al. A systematic review and economic evaluation of cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for the treatment of intermittent claudication in people with peripheral arterial disease. Health Technol Assess. 2011;15:1–210. doi: 10.3310/hta15400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–34. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 20.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: an intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J Vasc Surg. 2010;51:5S–17S. doi: 10.1016/j.jvs.2010.01.073. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser C, Brunner-La Rocca HP, Buser PT, Bonetti PO, Osswald S, Linka A, et al. Incremental cost-effectiveness of drug-eluting stents compared with a third-generation bare-metal stent in a real-world setting: randomised Basel Stent Kosten Effektivitats Trial (BASKET). Lancet. 2005;366:921–9. doi: 10.1016/S0140-6736(05)67221-2. [DOI] [PubMed] [Google Scholar]
- 22.Duda SH, Bosiers M, Lammer J, Scheinert D, Zeller T, Oliva V, et al. Drug-eluting and bare nitinol stents for the treatment of atherosclerotic lesions in the superficial femoral artery: long-term results from the SIROCCO trial. J Endovasc Ther. 2006;13:701–10. doi: 10.1583/05-1704.1. [DOI] [PubMed] [Google Scholar]
- 23.Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011;4:495–504. doi: 10.1161/CIRCINTERVENTIONS.111.962324. [DOI] [PubMed] [Google Scholar]
- 24.Werk M, Albrecht T, Meyer DR, Ahmed MN, Behne A, Dietz U, et al. Paclitaxel-coated balloons reduce restenosis after femoro-popliteal angioplasty: evidence from the randomized PACIFIER trial. Circ Cardiovasc Interv. 2012;5:831–40. doi: 10.1161/CIRCINTERVENTIONS.112.971630. [DOI] [PubMed] [Google Scholar]
- 25.Kearns BC, Michaels JA, Stevenson MD, Thomas SM. Cost-effectiveness analysis of enhancements to angioplasty for infrainguinal arterial disease. Br J Surg. 2013;100:1180–8. doi: 10.1002/bjs.9195. [DOI] [PubMed] [Google Scholar]
- 26.Pietzsch JB, Geisler BP, Garner AM, Zeller T, Jaff MR. Economic analysis of endovascular interventions for femoropopliteal arterial disease: a systematic review and budget impact model for the United States and Germany. Catheter Cardiovasc Interv. 2014;84:546–54. doi: 10.1002/ccd.25536. [DOI] [PubMed] [Google Scholar]