Abstract
Antigen-specific, T cell hybridomas are useful to study the cellular, molecular and functional events, but their generation is a lengthy process. Thus, there is a need to develop robust methods to generate the hybridoma clones rapidly in a short period of time. To this end, we have demonstrated a novel approach using major histocompatibility complex (MHC) class II dextramers to generate T cell hybridomas for an autoantigen, proteolipid protein (PLP) 139-151. Using MHC class II dextramers assembled with PLP 139-151 as screening and sorting tools, we successfully obtained mono antigen-specific clones within seven to eight weeks. In conjunction with other T cell markers, dextramers permitted phenotypic characterization of hybridoma clones for their antigen specificity in a single step by flow cytometry. Importantly, we achieved successful fusions using dextramer+ cells sorted by flow cytometry as a starting population, resulting in direct identification of multiple antigen-specific clones. Characterization of selected clones led us to identify chemokine receptor, CCR4+ to be expressed consistently, but their cytokine-producing ability was variable. Our work provides a proof-of principle that the antigen-specific, CD4 T cell hybridoma clones can be generated directly using MHC class II dextramers. The availability of hybridoma clones that bind dextramers may serve as useful tools for various in vitro and in vivo applications.
Keywords: T cell hybridoma, MHC class II dextramers, autoreactive T cells
1. Introduction
T cell hybridomas are useful tools for studying antigen-specific cellular, molecular, and functional events at a monoclonal level [1–6]. Unlike primary T cell clones, which may eventually lose their antigen specificity over a period of time if left unstimulated, T cell hybridomas can maintain antigen specificity for extended periods of time because of their inherent ability to grow continuously in cultures [2, 5, 6].
T cell hybridomas are generated through fusion of antigen-sensitized effector T cells with mouse thymoma-derived BW5147 αβ T cell receptor (TCR)−/− cells, leading to expression of only the TCRs specific to the fused T cells [2, 5–7]. However, the screening procedure for antigen specificity is a laborious process [2]. Generally, CD4 T cell hybridomas are screened based on cytokine secretion in response to antigen stimulations, and individual clones are then obtained mainly by limiting dilution cloning (LDC) [2, 6, 8]. Other methods include dispersion in soft agar, micromanipulation and flow cytometry [2]. All these methods require expertise with the individual screening systems, making them both less practical and less desirable. For example, LDC is a labor-intensive, time-consuming procedure, because the cells need to be diluted to a low density (2 to 3 cells/ml), followed by screening of individual clones for their antigen specificity [2, 9]. At least 25 such clones must be verified repeatedly to obtain a single antigen-specific clone, and the entire process to derive antigen-specific hybridoma clones can take up to three months or longer, or, the fusions may need to be repeated [2, 6]. Thus, there is a critical need to develop robust methods to derive T cell hybridoma clones quickly and efficiently in a shorter period of time.
We previously reported creation of the next generation of major histocompatibility complex (MHC) class II tetramers, designated “dextramers,” which enabled us to detect and enumerate the precursor frequencies of autoreactive, antigen-specific CD4 T cells in a variety of systems [10–12]. Structurally, dextramers contain dextran molecules (polymers of glucose), each carrying up to seven streptavidin moieties to which multiple biotinylated MHC/peptide monomers can be assembled. Thus, dextramers can engage more TCRs than that could be achieved with tetramers[12]. We demonstrated that MHC class II dextramers exhibited greater sensitivity and specificity than could be achieved with MHC class II tetramers in several autoantigens, such as proteolipid protein (PLP) 139-151, myelin oligodendrocyte glycoprotein 35–55, and cardiac myosin heavy chain-α 334–352 [12]. Using PLP 139-151 dextramers as screening tools, we have devised a novel approach for rapidly deriving antigen-specific T cell hybridoma clones within 7 to 8 weeks. Importantly, dextramer-assisted sorting of antigen-specific clones allowed us to evaluate the expression of T cell markers, TCRs, and their vβ-usage in a single step by flow cytometry, including cytokine secretion and chemokine receptor expression.
2. Materials and Methods
2.1. Ethics statement
Five-to-six-week-old female SJL/J (H-2s) mice were procured from the Jackson Laboratory (Bar Harbor, ME, USA) and maintained in accordance with the animal protocol guidelines of the University of Nebraska-Lincoln, Lincoln, NE, USA. The study was conducted in accordance with the National Institutes of Health guidelines for the use of experimental animals, and the protocols were specifically approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee (permit number: A3459-01; protocol # 999).
2.2. Peptide Synthesis and Immunization Procedures
PLP 139-151 (HSLGKWLGHPDKF) and Theiler’s murine encephalomyelitis virus (TMEV) 70-86 (WTTSQEAFSHIRIPLP), were synthesized on 9-fluorenylmethyloxycarbonyl chemistry (Neopeptide, Cambridge, MA, USA). TMEV 70-86 peptide previously has been shown to bind MHC class II allele, IAs molecule in SJL mice [12–16]. Thus, we used TMEV 70-86 as a control peptide for PLP 139-151 in all assays. The peptides were HPLC-purified (>90%), identity-confirmed by mass spectroscopy, and dissolved in sterile 1x PBS prior to use. Peptide emulsions involving PLP 139-151 were prepared in complete Freund’s adjuvant (CFA) containing Mycobacterium tuberculosis (M.tb, 1 mg/ml) H37RA extract (Difco Laboratories, Detroit, MI, USA), and administered subcutaneously into SJL mice (100 μg/mouse; n=3) [15]. At termination, animals were euthanized using a CO2 chamber prefilled with 2% CO2.
2.3. Generation of MHC Class II Dextramers
Dextramer reagents comprised of IAs/PLP 139-151 and IAs/TMEV 70-86 (control) were generated as described previously [12]. We have used IAs/TMEV 70-86 dextramers as controls to ascertain TCR-binding specificity of IAs/PLP 139-151 dextramers, in all dextramer staining reactions [12]. Briefly, the α and β constructs of IAs allele along with the peptide of interest was expressed together using baculovirus expression systems in SF9 insect cells (Invitrogen, Carlsbad, CA). Soluble MHC class II monomers of IAs were then purified, concentrated, and biotinylated using biotin ligase (25 μg/10 nmol of substrate; Avidity, Denver, CO) [12, 14, 15]. The biotinylated monomers were assembled to fluorophore conjugated dextran molecules (kindly provided by Immudex, Copenhagen, Denmark) at a molar ratio of 20:1 in 1x Tris HCl 0.05 M, pH 7.2, by incubating in the dark for 30 minutes at room temperature (RT) [12]. The dextramer reagents were aliquoted and stored at 4°C until use.
2.4. Generation of Antigen-Sensitized Primary T Cells
Ten days post-immunization with PLP 139-151, the draining lymph nodes (mandibular, axillary, inguinal, and popliteal) were collected and single cell suspensions were prepared. Lymph node cells (LNC) were stimulated with PLP 139-151 (20 μg/ml) at a density of 5×106 cells/ml for two days in clone medium (RPMI medium supplemented with 10% fetal bovine serum [FBS], 1 mM sodium pyruvate, 4 mM L-glutamine, 1x each of non-essential amino acids and vitamin mixture, and 100 U/ml penicillin-streptomycin [Lonza, Walkersville, MD]) [14, 15, 17]. After two days, the cultures were supplemented with clone medium containing interleukin (IL)-2 (hereafter called IL-2 medium) and maintained for an additional two days. Viable lymphoblasts were harvested on day 4 and maintained in IL-2 medium until fusion. In some experiments, LNC obtained from immunized mice were expanded with concanavalin-A (Con-A; 1 μg/ml) at a density of 2×106 cells/ml for two days before fusion [18].
2.5. Fusion with BW5147 αβ −/− Cells
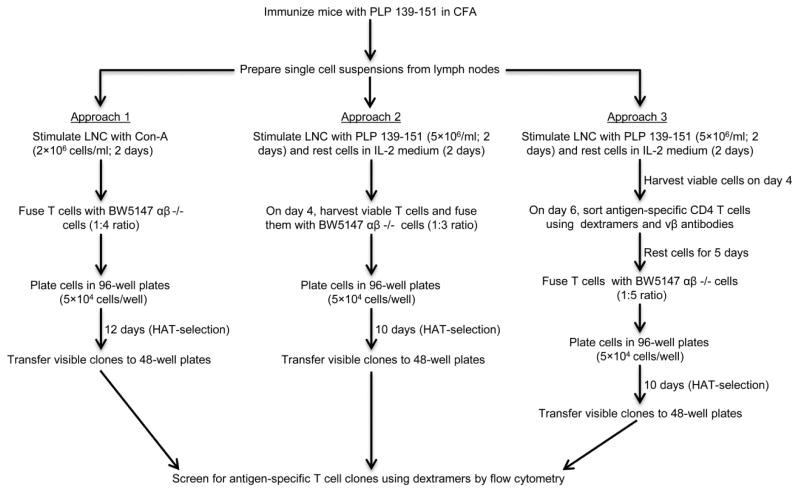
Three approaches were adopted for the generation of antigen-specific T cell hybridoma clones (Figure 1).
Figure 1. Approaches to the derivation of T cell hybridomas.
Approach 1. LNC from immunized mice were expanded with Con-A for two days, then fused with BW5147 αβ −/− cells.
2.5.1. Approach 1: Derivation of T cell hybridomas using Con-A-stimulated T cells generated in immunized mice
LNC stimulated with Con-A were harvested after 48 hours, and cells were washed twice with DMEM (1x DMEM [HyClone laboratories, South Logan, UT] containing 10% FBS, 1 mM sodium pyruvate, 7.5 mM L-glutamine, 0.66 M L-Arginine [Fisher BioReagents, Fair Lawn, NJ], 0.27 M L-Asparagine [MP Biomedicals, LLC Solon, OH], 24 mM sodium bicarbonate [Sigma-Aldrich, St. Louis, MO], 10 mM HEPES [Roche Life Sciences, Indianapolis, IN], 100 U/ml penicillin–streptomycin, 0.05 mM β-Mercaptoethanol [PMD Biosciences, La Jolla, CA]). Cells were then mixed with BW5147 αβ −/− cells at a ratio of 1:4, washed once, and fused as described earlier [5, 6, 19, 20]. The tube containing the cell pellet was placed in a 37° C water bath, and 0.4 ml of 50% polyethylene glycol (PEG) in 75 mM HEPES (Roche Life Sciences) was gently added in a circular motion over a 1-minute period. After stirring the pellet for an additional minute, a total of 10 ml of pre-warmed DMEM with 10% FBS (hereafter called hybridoma medium) was delivered, 1 ml during the first minute, followed by another ml during the second minute, and the rest (8 ml) during the next 2 minutes (1 ml/15 seconds) as the mixture was gently stirred continuously [5, 6, 19]. After washing with hybridoma medium, cells were plated in 96-well plates at a density of 3.6×105 cells/ml (5×104 cells/140μl/well; Figure 1). Approach 2. LNC from immunized mice were stimulated with the PLP 139-151 for two days, and the cultures were then supplemented with IL-2 medium. On day 4, viable lymphoblasts were harvested and fused with BW5147 αβ −/− cells. Approach 3. LNC obtained from immunized mice were cultured with PLP 139-151 for two days and the cells were supplemented with IL-2 medium. Viable cells were harvested on day 4, and the cells were rested in IL-2 medium for two days. On day 6, cells were stained with dextramers (IAs/PLP 139-151 and control), followed by a panel of selected TCR vβ antibodies; the double positive cells were then sorted by flow cytometry. After resting in IL-2 medium for 5 days, cells were fused. Finally, after fusions, cells were plated in 96-well plates, and, after two rounds of HAT selection, visible clones were transferred to 48-well plates between days 10 to 12. Cells were then switched to HT medium and screened for antigen specificity using dextramers by flow cytometry
2.5.2. Approach 2: Generation of T cell hybridomas using antigen-stimulated T cells generated in vitro
Viable lymphoblasts were harvested on day 4 poststimulation with PLP 139-151 and fused with BW5147 αβ −/− cells at a ratio of 1:3; the cells were plated as above in 96-well plates (Figure 1).
2.5.3. Approach 3: Generation of T cell hybridomas using the MHC class II dextramer+ cells sorted by flow cytometry
Viable lymphoblasts were harvested on day 4 poststimulation with PLP 139-151 and rested in IL-2 medium for two days. Cells were then stained with the dextramers (IAs/PLP 139-151 and control dextramers) in dextramer-staining medium (IL-2 medium containing 2.5% FBS, pH 7.63) at RT for 2 hours [12]. After washing, PLP 139-151 dextramer-stained cells were incubated with a cocktail of antibodies for the predominant TCR vβs expressed by PLP-reactive cells [21] (vβ 2, 3, 4, 6, 14 and 17a; BD Pharmingen, San Diego, CA), and anti-CD4 (eBioscience, San Diego, CA). Cells were then washed and the live CD4+ dextramer+ vβ+ cells were sorted by flow cytometry (FACSAria, BD Biosciences, San Jose, CA). Cells were washed and rested in IL-2 medium for 5 days, at which time dextramers bound to the cells were no longer detectable by flow cytometry, leading us to fuse these cells with BW5147 αβ −/− cells at a ratio of 1:5 in 0.2 ml of PEG. The fused cells were plated as above in 96-well plates (Figure 1).
Regardless of the approach used, cells in 96-well plates were supplemented with 2x hypoxanthine/aminopterin/thymidine (HAT; Sigma-Aldrich) medium (DMEM/20% FBS and 2x HAT) 24 hours after fusion and on day 7 postfusion. Between days 10 and 12, visible clones were transferred from 96-well plates to 48-well plates (Figure 1). The cells were then gradually deprived of HAT medium by replacing with hypoxanthine/thymidine (HT; Sigma-Aldrich) medium (DMEM/20% FBS and 1x HT) [5, 6, 19].
2.6. Screening of T Cell Hybridomas for Antigen-Specificity and TCR vβ-Usage and Derivation of Antigen-Specific T Cell Hybridoma Clones
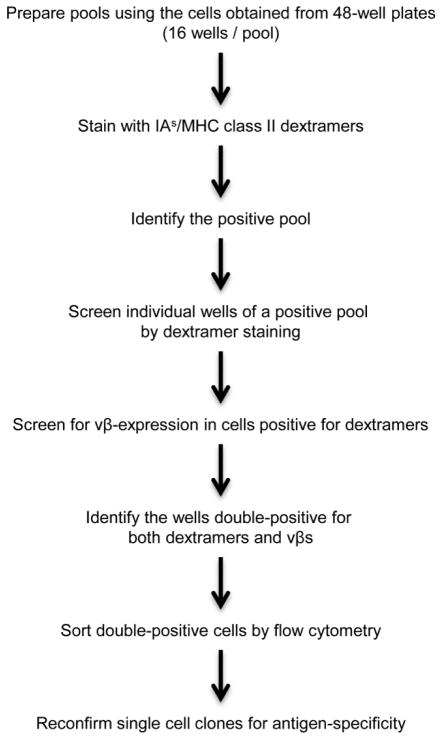
The T cell hybridoma clones were screened for antigen specificity 10 to 12 days after the cells were plated in 96-well plates (Figure 2). Pools of clones were prepared, each pool represented by cells obtained from 16 wells in a 48-well plate. Cells were washed and stained with IAs/PLP 139-151 and control dextramers in hybridoma medium at RT for 1 hour [12]. The cells were then stained with anti-CD3, anti-CD4, and 7-aminoactinomycin-D (7-AAD) and acquired by flow cytometry. The pools positive for CD3, CD4, and PLP 139-151 dextramers within the live populations (7-AAD−) were analyzed using Flow Jo software (Tree Star, Ashland, OR). The next day, individual wells from the positive pools were screened again by dextramer staining as above. Because each well could contain more than one clone with different vβs, cells in each well were then stained with a panel of anti-mouse TCR vβ antibodies as indicated, along with anti-CD3, anti-CD4, and 7-AAD. After being washed and acquired by flow cytometer, vβ+ CD3+ CD4+ T cell hybridoma clones were analyzed in the live (7-AAD−) population. Finally, by using PLP 139-151 dextramers, and vβ antibodies, cells positive for both were identified. To obtain single clones, cells stained as above with the specific anti-TCR vβ were sorted with one cell per well into 96-well plates containing hybridoma medium. Plates were incubated until visible clones were observed, which occurred between days 10 to 14 postsorting. Positive clones were gradually expanded and reascertained for dextramer positivity. Clones were labeled appropriately and preserved in liquid nitrogen until further use.
Figure 2. Screening of T cell hybridomas for antigen specificity.
Pools of cells representing 16 wells in 48-well plates were prepared, and the cells were stained with IAs/MHC class II dextramers (PLP 139-151 and control). Pools that were positive for PLP 139-151 dextramers were identified, then individual wells containing the dextramer+ cells were identified. Cells in these wells were tested for both TCR vβs and dextramers; the double-positive cells were sorted by flow cytometry with one cell per well; and their antigen-specificity was reconfirmed.
2.7. Measurement of Proliferative Responses
To assess the functional responsiveness of T cell clones, we used tritiated 3[H]-thymidine-incorporation assay. Briefly, hybridoma clones were cultured in clone medium in the presence of irradiated (3000 rads) syngeneic LNC/splenocytes pulsed with PLP 139-151 as antigen-presenting cells at a ratio of 1: 5 (1× 104 : 5× 104) for 20 hours. After pulsing with 3[H]-thymidine for 16 hours, proliferative responses were measured as counts per minute.
2.8. Analysis of Chemokine Receptor Expression
The surface expression of chemokine receptors (CCRs) was analyzed using antibodies for CCR4, CCR5, or CCR6, anti-CD4, and 7-AAD. After washing, cells were acquired by flow cytometry, and percentages of chemokine receptor-expressing cells were determined in the live (7-AAD−) population using Flow Jo software. The clones of anti-chemokine receptor antibodies used were CCR-4 (2G12), CCR-5 (HM-CCR5) and CCR-6 (29-2L17), all from BioLegend, San Diego, CA.
2.9. Cytokine Measurement
The ability of T cell hybridomas to secrete different cytokines was assessed by intracellular staining [15, 17, 22–24]. Cells were stimulated with phorbol 12-myristate-13 acetate (PMA, 10 ng/ml) and ionomycin (150 ng/ml, Sigma-Aldrich) for 5 hours in the presence of 1 mM monensin (Golgi stop, BD Pharmingen). After staining with anti-CD4 and 7-AAD, cells were fixed, permeabilized, and stained with anti-cytokine antibodies or with the respective isotype controls. After washing, the cells were acquired by flow cytometry, and the frequencies of cytokine-producing cells were determined in the live (7-AAD−) CD4 population using Flow Jo software. The clones of anti-cytokine antibodies used were: IL-2 (JES6-5H4), interferon (IFN)-γ (XMG1.2), IL-4 (11B11), IL-10 (JES5-16E3), IL-17A (eBio17B7), and tumor necrosis factor (TNF)-α (MP6-XT22), all from eBioscience.
3. Results and Discussion
We demonstrated the utility of using MHC class II dextramers to create CD4 T cell hybridomas for PLP 139-151 that induce experimental autoimmune encephalomyelitis in SJL (H-2s) mice. The use of MHC class II dextramers offered few advantages: 1). The dextramers could be used as screening tools to verify the antigen specificity of potential clones as early as 10 to 12 days after fusion. In conjunction with other T cell markers (CD3, CD4, CD8, and TCR/vβs) the use of dextramers allowed us to identify antigen-specific clones by flow cytometry in a single step. 2). After identifying positive clones, we could derive mono antigen-specific clones by sorting the cells with one cell per well using dextramers. 3). Unlike the traditional method that requires a large number of antigen-sensitized T cells (up to 5×107 cells), successful fusions could be achieved with as few as ~7.6×105 dextramers+ cells. Overall, the time required to obtain clones can be cut down significantly, and all steps from immunizations to cloning single cells can be finished in 7–8 weeks.
As we reported previously, dextramers were found to be helpful in detecting the rare antigen-specific, autoreactive CD4 T cells, which was not possible using tetramers, both ex vivo, and in situ within target organs such as hearts and brains [10–12]. Unlike MHC class II tetramers that contain no more than four biotinylated MHC/peptide complexes because only a single streptavidin moiety is available, a large number of MHC/peptide complexes (up to 20) can be assembled to each dextran backbone in dextramers, because multiple streptavidin moieties (6 to 7) are available for binding the biotinylated MHC molecules [12, 25]. As a result, large aggregates of MHC/peptide complexes can be generated through dextramers, leading to enhanced avidity for binding to T cells by engaging multiple TCRs. Thus, using dextramers could increase detection sensitivity by at least 5-fold, as compared with use of tetramers [12]. Although derivation of T cell hybridomas for PLP 139-151 has been reported previously [26, 27], we took advantage of the availability of dextramer reagents for this antigen to demonstrate their utility for deriving antigen-specific T cell hybridomas quickly and efficiently, by exploring three different approaches (Figure 1).
3.1. Approach 1: Derivation of T cell hybridomas using Con-A-stimulated T cells generated in immunized mice
Con-A is traditionally used as a mitogen to stimulate unprimed polyclonal T cell populations that permit assessment of the overall immunocompetence in physiological and clinical conditions [28–30]. Here, we used this reagent to expand antigen-sensitized T cells ex vivo with an expectation that they may get enriched upon activation, thus allowing their availability to be more in number for fusions. We used LNC sensitized with PLP 139-151 from immunized mice, and, after expansion with Con-A for two days, cells were fused, expecting that viable clones could be directly screened by dextramers for their antigen specificity. The fused cells were plated in ten 96-well plates (10X96 = 960 wells), and, after 12 days, successful fusions as indicated by viability/growth of cells were noted in 30% of the wells (288/960X100). We then made 18 pools, each representing cells from 16 wells for staining with PLP 139-151 dextramers (Figure 2). Contrary to our expectations, we did not detect the pools positive for staining with PLP 139-151 dextramers. Such an outcome was not surprising, however, given the low frequencies of antigen-sensitized T cells present in the ex vivo LNC populations [31]. We had previously determined the frequency of PLP 139-151-specific cells to be 0.12% of total CD4 T cells in immunized SJL mice [12]. We had predicted that Con-A-stimulation might sufficiently facilitate the expansion of antigen-specific T cells to allow us to obtain a few clones, but this was not the case. Alternatively, this approach may still prove to be successful if CD4 T cells enriched from immunized animals are used as a starting population for fusions, but we did not investigate this possibility.
3.2. Approach 2: Generation of T cell hybridomas using antigen-stimulated T cells generated in vitro
Here, we fused the effector T cells generated from PLP 139-151 immunized mice with BW5147 αβ−/− cells [2, 5, 6]. LNC were obtained from immunized SJL mice, and after stimulating the cells with PLP 139-151 and resting them in IL-2 medium, viable lymphoblasts were used for fusions (Figure 1). We used dextramer reagents (IAs/PLP 139-151, and control) for screening and cloning antigen-specific hybridomas as a novel strategy in a step-wise fashion (Figure 2).
3.2.1. Step 1
To generate PLP 139-151-specific hybridomas, eight 96-well plates were plated with the fused cells (8×96 = 768 wells). Successful fusions, indicated by viability/growth of cells, were noted in 62.5% of the wells (480/768×100). We then made 30 pools, each consisting of cells from 16 wells, and tested them for staining with PLP 139-151 dextramers, where TMEV 70-86 dextramers were used as controls. We randomly picked 8 pools that were positive for PLP 139-151 dextramers. Dextramer staining was then repeated individually using cells from each of the 128 wells (8 pools x 16). This analysis led us to identify at least 10 wells that were positive for PLP 139-151 dextramers (10/128 = 7.8%) (Table 1).
Table 1.
Evaluation of TCR vβ-usage in PLP 139-151 dextramer+ CD4+ T cell hybridoma clones
| Well ID | Dextramer staining | Panel of TCR vβ antibodies used
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| vβ2 | vβ3 | vβ4 | vβ5.1/5.2 | vβ6 | vβ7 | vβ8.1/8.2 | vβ8.3 | vβ9 | vβ10b | vβ11 | vβ12 | vβ13 | vβ14 | vβ17a | ||
| A9 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
| A17 | + | + | − | + | − | − | − | − | − | − | − | − | − | − | − | + |
| A31 | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| D19 | + | + | − | + | − | − | − | − | − | − | − | − | − | − | + | + |
| D26 | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
| E48 | + | + | − | + | − | − | − | − | − | − | − | − | − | − | − | + |
| H2 | + | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − |
| H15 | + | + | − | + | − | − | − | − | − | − | − | − | − | − | − | + |
| I14 | + | − | + | − | − | + | − | − | − | − | − | − | − | − | − | + |
| I29 | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
3.2.2. Step 2
In this step, cells in the dextramer+ wells were screened for TCR vβ-usage. Since, dextramer+ cells in each well could contain more than one clone, potentially with different vβ-usage, we decided to first screen each of the 10 wells of PLP 139-151-dextramer+ clones with a panel of vβ antibodies. The selected vβ panels were based on the published literature and our own data [17, 21, 32, 33]. Through these analyses we identified wells showing positive staining for up to six vβs for PLP 139-151 (vβ2, vβ3, vβ4, vβ6, vβ14 and vβ17a; Table 1). Of note, for analysis of vβ-expressing dextramer+ cells, it was critical to stain with dextramers first, followed by staining with vβ antibodies [21]. Staining with the above reagents in reverse order allows detection of only vβ+ cells, and as such, the proportion of dextramer+ cells can be absent or significantly reduced, indicating that vβ-antibodies can sterically hinder dextramers from binding to TCRs.
3.2.3. Step 3
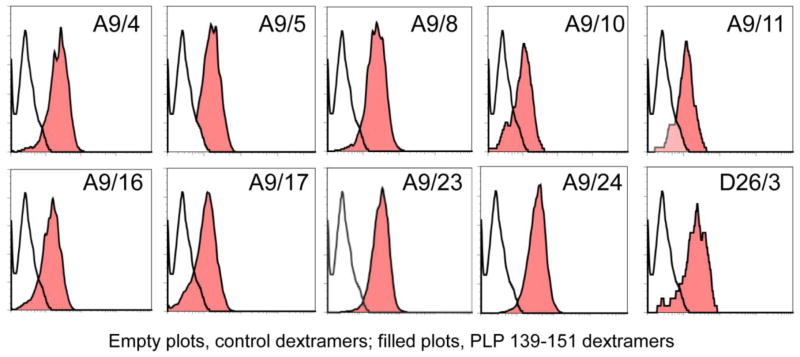
After identifying the wells positive for both dextramers and specific vβs, we sought to provide a proof-of principle that single cell clones can be obtained by flow cytometric sorting of dextramer+ cells. To this end, we selected two wells (A9 and D26; Table 1) and sorted the cells positive for PLP 139-151 dextramers and anti-vβ17a or anti-vβ2 respectively with a single cell per well into one 96-well plate for each. We obtained a total of 31 viable clones, and rescreening of these by dextramer staining led us to identify at least ten clones to be highly positive for PLP 139-151 dextramers (Figure 3).
Figure 3. Generation of antigen-specific, single cell clones by flow cytometry.
To obtain single cell clones, cells were stained with IAs/dextramers (PLP 139-151 and control), CD4 and vβ17a or vβ2 antibodies and 7-AAD. Through flow cytometric analysis, PLP 139-151 dextramer+ vβ+ cells were sorted with one cell per well, and after expansion, their antigen-specificity was reconfirmed by dextramer staining as described above.
3.3. Approach 3: Generation of T cell hybridomas using the MHC class II dextramer+ cells sorted by flow cytometry
In this approach, we asked whether dextramer+ T cells could be used as a starting population for fusing the cells, expecting the dextramer+ clones to be antigen specific, thus avoiding the multi-step procedure described in approach 2. We addressed this question by sorting the antigen-specific CD4 T cells from LNC cultures derived from SJL mice immunized with PLP 139-151. For sorting PLP 139-151-specific cells, we used PLP 139-151 or control dextramers, anti-CD4, and a mixture of anti-vβs (vβ2, 3, 4, 6, 14 and 17a). We sorted a total of 7.6×105 dextramer+vβ+ cells by flow cytometry, and after resting the cells in IL-2-medium for 5 days, the cells were fused with BW5147 αβ−/− cells, followed by plating in one 96-well plate. We noted successful fusions as indicated by viable clones in 78 wells (78/96X100 = 81.3%). A total of 16 pools, each represented by cells from 3 to 5 wells, were then stained with PLP 139-151 dextramers, leading us to identify 10 positive pools. Cells from each of 50 wells (10 pools x 5) were then screened for staining with dextramers leading us to identify atleast 14 wells that were positive for dextramers (14/50 = 28%), which were then examined for vβ-expression using selected TCR vβ antibodies, as shown in Table 2. Expectedly, cells in each well showed positive staining for more than one vβ, suggesting the presence of multiple clones in a given well (Table 2). Thus, we have provided a proof of principle that antigen-specific T cell hybridoma clones can be obtained using dextramer+ cells as a starting population for fusions.
Table 2.
Derivation of PLP 139-151-specific hybridoma clones using PLP 139-151 dextramer+ cells sorted by flow cytometry as the starting population for fusions
| Well ID | Dextramer staining | Panel of TCR vβ antibodies used
|
|||||
|---|---|---|---|---|---|---|---|
| vβ2 | vβ3 | vβ4 | vβ6 | vβ14 | vβ17a | ||
| A3 | + | + | − | − | − | − | + |
| A9 | + | + | − | + | − | − | + |
| A13 | + | + | − | − | − | + | − |
| A18 | + | − | − | + | − | − | + |
| A22 | + | − | − | + | − | + | − |
| A36 | + | + | − | − | + | − | − |
| A38 | + | − | − | + | − | − | − |
| A47 | + | − | − | − | − | − | − |
| B2 | + | − | − | + | − | − | + |
| B11 | + | − | − | + | − | − | + |
| B12 | + | + | − | − | − | − | + |
| B13 | + | − | − | + | − | − | + |
| B16 | + | + | − | + | + | − | − |
| B27 | + | − | − | − | − | − | + |
Overall, the relative successful cell-fusion frequencies obtained from all the three approaches were in the order of approach 3 (81.3%) followed by approach 2 (62.5%), and approach 1 (30%). Similarly, screening of these fusions, also led us to identify 28% of wells to contain antigen (PLP 139-151)-specific hybridoma clones with approach 3 as opposed to 7.8% with approach 2, and none with approach 1.
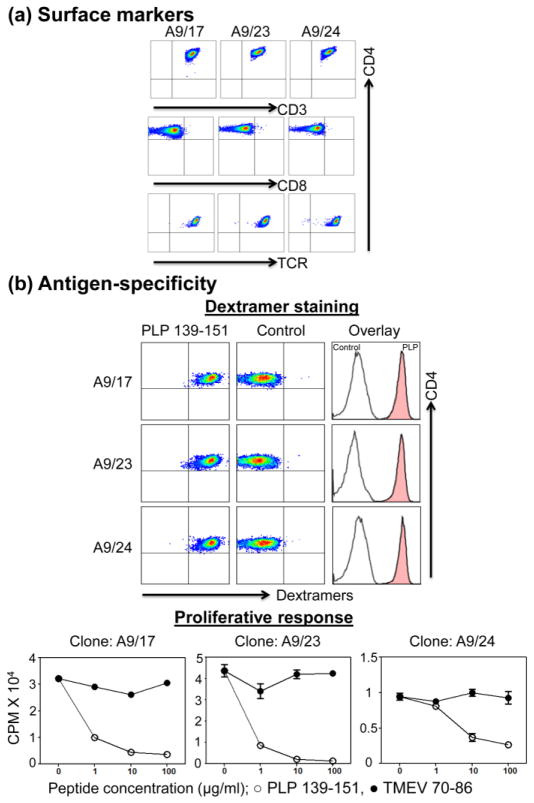
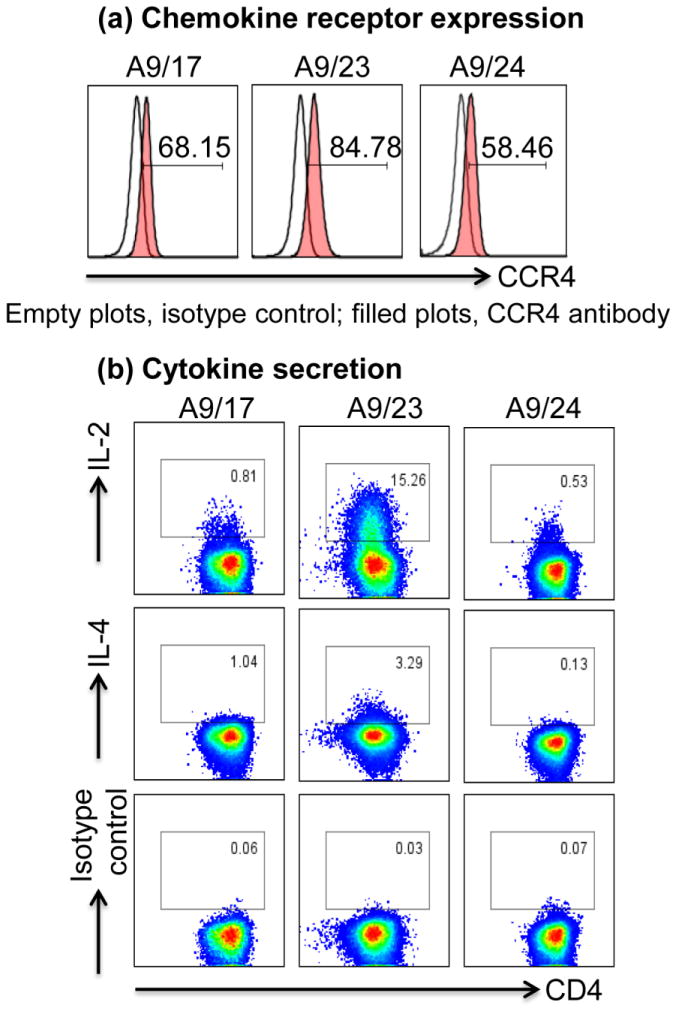
After generating the T cell hybridomas for PLP 139-151, we sought to characterize their phenotypes. We selected three clones (A9/17, A9/23, and A9/24; Figure 3); all of them showed the expression of CD3, CD4 and TCR (Figure 4a: top panel). We then evaluated antigen specificity based on dextramer staining and proliferative responses. Expectedly, all PLP 139-151-specific clones could be stained with the corresponding dextramers but not with control dextramers (Figure 4b: top panel). Next, we asked whether PLP 139-151-specific clones can functionally respond to antigens. Using 3[H]-thymidine incorporation assay, it was clear that all PLP 139-151-specific clones responded to PLP 139-151 stimulation, but the net outcome was reduced proliferative response likely due to cell death as reported by others [26, 34–36], which occurred only with PLP 139-151 but not with the control antigen (Figure 4b: bottom panel). Thus, we identified functionally responsive clones for PLP 139-151. Finally, we asked whether the functionalities of antigen-specific T cell hybridomas can be distinguished based on chemokine receptor expression and cytokine synthesis (Figure 5). By testing for chemokine receptors CCR4, CCR5, and CCR6 as the putative markers for Th2, Th1, and Th17 phenotypes [37, 38], respectively, only CCR4 was found to be present in all PLP 139-151-specific clones (Figure 5a). However, testing for their cytokine-producing ability by intracellular staining revealed that one PLP 139-151-specific clone (A9/23) was positive for IL-2 and IL-4, whereas two other clones (A9/17 and A9/24) showed only marginal production of IL-2 (Figure 5b). None of the clones showed any detectable staining for other cytokines (proinflammatory cytokines IFN-γ, IL-17A, and TNF-α and the anti-inflammatory cytokine IL-10; data not shown) indicating that the cytokine-producing ability of T cell hybridomas is largely restricted to T cell growth factors. Similar observations have been made with T cell hybridoma clones specific to various other antigens [2, 39].
Figure 4. Phenotypic and functional characterization of selected T cell hybridomas.
(a) Surface markers. Three T cell hybridoma clones, specific for PLP 139-151 were selected, and expression of CD3, CD4, CD8, and TCRs was characterized by flow cytometry. (b) Antigen specificity. Antigen specificity of the selected clones was tested by dextramer staining, and proliferative responses as evaluated by flow cytometry and 3[H]-thymidine incorporation assay, respectively. The experimental details are as described in the methods section. PLP 139-151: specific antigen; TMEV 70-86: control antigen. Representative data from three to four individual experiments are shown.
Figure 5. Characterization of T cell hybridomas based on the expression of chemokine receptors and cytokines.
Three T cell hybridoma clones, specific to PLP 139-151 were evaluated for surface expression of chemokine receptors and cytokine secretion by intracellular staining as described in the methods section. Top panel (a) indicates expression of CCR4, and bottom panel (b) represents cytokine-producing CD4+ cells. Representative data from three individual experiments are shown.
4. Conclusions
In summary, we have demonstrated the utility of MHC class II dextramers for cloning antigen-specific CD4 T cell hybridomas using PLP 139-151 as an autoantigen. We have previously reported that dextramers are expected to bind T cells bearing high-affinity TCRs and therefore propose that the hybridoma clones obtained by using dextramers might carry such TCRs. However, in spite of enhanced detection sensitivity, dextramers cannot detect the complete repertoire of antigen-specific T cells as we previously demonstrated [12]. Thus, we cannot conclude that dextramer− clones are not antigen-specific, and the clones possibly expressing the low-affinity TCRs might be undetected by dextramers. Nonetheless, if MHC class II dextramer-bound T cells are to be interpreted as T cells bearing high-affinity TCRs, then the availability of dextramer-detectable hybridoma clones can serve as useful tools for various applications, such as screening peptide libraries to identify antigenic epitopes, epitope mimics, altered peptide ligands, and chemical analogues of activating ligands; studies related to antigen processing and presentation pathways; production of TCR-specific monoclonal antibodies; in vivo tracking of antigen-specific T cells; and other molecular and functional studies that involve interactions with TCRs [2, 40–43]. One caveat to keep in mind about dextramer-assisted generation of T cell hybridomas is that the availability of MHC dextramers for antigens of interest is absolutely critical, but their generation per se may be a time-consuming process. Should the dextramer reagents be made available, then derivation of T cell hybridomas can be accomplished rapidly in a limited timeframe of 7–8 weeks.
Highlights.
Achieved successful hybridoma fusions using dextramer-positive cells sorted by flow cytometry as a starting population resulting in direct identification of multiple antigen-specific clones.
Demonstrated that MHC class II dextramers can be used as screening and sorting tools to generate mono antigen-specific clones rapidly within 7 to 8 weeks.
In conjunction with other T cell markers, dextramers permitted phenotypic characterization of antigen-specific clones in a single step by flow cytometry.
Acknowledgments
This work was supported by the National Institutes of Health (HL114669). CM is a recipient of a postdoctoral research fellowship grant awarded by the Myocarditis Foundation, NJ.
List of Abbreviations
- MHC
major histocompatibility complex
- PLP
proteolipid protein
- TCR
T cell receptor
- LDC
limiting dilution cloning
- TMEV
theiler’s murine encephalomyelitis virus
- CFA
complete Freund’s adjuvant
- M.tb
Mycobacterium tuberculosis
- RT
room temperature
- LNC
lymph node cells
- IL
interleukin
- FBS
fetal bovine serum
- Con-A
concanavalin-A
- PEG
polyethylene glycol
- HAT
hypoxanthine/aminopterin/thymidine
- HT
hypoxanthine/thymidine
- 7-AAD
7-aminoactinomycin-D
- 3[H]
tritiated
- CCRs
chemokine receptors
- PMA
phorbol 12-myristate-13 acetate
- INF
interferon
- TNF
tumor necrosis factor
Footnotes
Conflict of interest and author declaration
We wish to confirm that there are no known conflicts of interest associated with this publication. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kubota K, Iwabuchi K. Phenotypic changes in growth-arrested T cell hybrids: a possible avenue to produce functional T cell hybridoma. Front Immunol. 2014;5:229. doi: 10.3389/fimmu.2014.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rock KL. Functional T cell hybridomas. Hybridoma Technology in the Biosciences and Medicine. 1985;100:527–544. [Google Scholar]
- 3.Rock KL, Benacerraf B. MHC-restricted T cell activation: analysis with T cell hybridomas. Immunological reviews. 1983;76:29–57. doi: 10.1111/j.1600-065x.1983.tb01096.x. [DOI] [PubMed] [Google Scholar]
- 4.Schrader JW, Clark-Lewis I. The use of T cell hybridomas in the biochemical and biological characterization of multiple regulatory factors produced by T cells. Current topics in microbiology and immunology. 1982;100:221–229. doi: 10.1007/978-3-642-68586-6_25. [DOI] [PubMed] [Google Scholar]
- 5.White J, Kappler J, Marrack P. T cell protocols: Development and Activation. In: Kearse KP, editor. Methods Mol Biol. Vol. 134. Ottawa, NJ: Humana press Inc; 2000. pp. 185–193. [DOI] [PubMed] [Google Scholar]
- 6.Kruisbeek AM. Production of mouse T cell hybridomas. Curr Protoc Immunol. 2001;Chapter 3(Unit 3):14. doi: 10.1002/0471142735.im0314s24. [DOI] [PubMed] [Google Scholar]
- 7.Kubota K. A novel functional T cell hybridoma recognizes macrophage cell death induced by bacteria: a possible role for innate lymphocytes in bacterial infection. J Immunol. 2006;176(12):7576–7588. doi: 10.4049/jimmunol.176.12.7576. [DOI] [PubMed] [Google Scholar]
- 8.Peterson DA, DiPaolo RJ, Kanagawa O, Unanue ER. Quantitative analysis of the T cell repertoire that escapes negative selection. Immunity. 1999;11(4):453–462. doi: 10.1016/s1074-7613(00)80120-x. [DOI] [PubMed] [Google Scholar]
- 9.Lefkovits I, Waldmann H. Limiting dilution analysis of the cells of immune system I. The clonal basis of the immune response. Immunology today. 1984;5(9):265–268. doi: 10.1016/0167-5699(84)90137-3. [DOI] [PubMed] [Google Scholar]
- 10.Massilamany C, Gangaplara A, Jia T, Elowsky C, Kang G, Riethoven JJ, Li Q, Zhou Y, Reddy J. Direct staining with major histocompatibility complex class II dextramers permits detection of antigen-specific, autoreactive CD4 T cells in situ. PloS one. 2014;9(1):e87519. doi: 10.1371/journal.pone.0087519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massilamany C, Gangaplara A, Jia T, Elowsky C, Li Q, Zhou Y, Reddy J. In situ detection of autoreactive CD4 T cells in brain and heart using major histocompatibility complex class II dextramers. Journal of visualized experiments : JoVE. 2014;(90):e51679. doi: 10.3791/51679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massilamany C, Upadhyaya B, Gangaplara A, Kuszynski C, Reddy J. Detection of autoreactive CD4 T cells using major histocompatibility complex class II dextramers. BMC Immunol. 2011;12:40. doi: 10.1186/1471-2172-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorenz RG, Tyler AN, Allen PM. T cell recognition of bovine ribonuclease. Self/non-self discrimination at the level of binding to the I-Ak molecule. J Immunol. 1988;141(12):4124–4128. [PubMed] [Google Scholar]
- 14.Massilamany C, Gangaplara A, Chapman N, Rose N, Reddy J. Detection of cardiac myosin heavy chain-alpha-specific CD4 cells by using MHC class II/IA(k) tetramers in A/J mice. J Immunol Methods. 2011;372(1–2):107–118. doi: 10.1016/j.jim.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Massilamany C, Steffen D, Reddy J. An epitope from Acanthamoeba castellanii that cross-react with proteolipid protein 139-151-reactive T cells induces autoimmune encephalomyelitis in SJL mice. J Neuroimmunol. 2010;219(1–2):17–24. doi: 10.1016/j.jneuroim.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Reddy J, Bettelli E, Nicholson L, Waldner H, Jang MH, Wucherpfennig KW, Kuchroo VK. Detection of autoreactive myelin proteolipid protein 139-151-specific T cells by using MHC II (IAs) tetramers. J Immunol. 2003;170(2):870–877. doi: 10.4049/jimmunol.170.2.870. [DOI] [PubMed] [Google Scholar]
- 17.Massilamany C, Gangaplara A, Steffen D, Reddy J. Identification of novel mimicry epitopes for cardiac myosin heavy chain-alpha that induce autoimmune myocarditis in A/J mice. Cell Immunol. 2011;271(2):438–449. doi: 10.1016/j.cellimm.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Massilamany C, Marciano-Cabral F, Rocha-Azevedo B, Jamerson M, Gangaplara A, Steffen D, Zabad R, Illes Z, Sobel RA, Reddy J. SJL mice infected with Acanthamoeba castellanii develop central nervous system autoimmunity through the generation of cross-reactive T cells for myelin antigens. PloS one. 2014;9(5):e98506. doi: 10.1371/journal.pone.0098506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canaday DH. Production of CD4+ and CD8+ T Cell Hybridomas. In: Endert PV, editor. Antigen Processing Methods and Protocols. Humana Press; 2013. pp. 297–308. [Google Scholar]
- 20.Kohler G, Lefkovits I, Elliott B, Coutinho A. Derivation of hybrids between a thymoma line and spleen cells activated in a mixed leukocyte reaction. Eur J Immunol. 1977;7(11):758–761. doi: 10.1002/eji.1830071103. [DOI] [PubMed] [Google Scholar]
- 21.Massilamany C, Thulasingam S, Steffen D, Reddy J. Gender differences in CNS autoimmunity induced by mimicry epitope for PLP 139-151 in SJL mice. J Neuroimmunol. 2011;230(1–2):95–104. doi: 10.1016/j.jneuroim.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Evavold BD, Sloan-Lancaster J, Allen PM. Antagonism of superantigen-stimulated helper T-cell clones and hybridomas by altered peptide ligand. Proc Natl Acad Sci U S A. 1994;91(6):2300–2304. doi: 10.1073/pnas.91.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakajima A, Seroogy CM, Sandora MR, Tarner IH, Costa GL, Taylor-Edwards C, Bachmann MH, Contag CH, Fathman CG. Antigen-specific T cell-mediated gene therapy in collagen-induced arthritis. J Clin Invest. 2001;107(10):1293–1301. doi: 10.1172/JCI12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilde DB, Roberts K, Sturmhofel K, Kikuchi G, Coligan JE, Shevach EM. Mouse autoreactive gamma/delta T cells. I. Functional properties of autoreactive T cell hybridomas. Eur J Immunol. 1992;22(2):483–489. doi: 10.1002/eji.1830220229. [DOI] [PubMed] [Google Scholar]
- 25.Batard P, Peterson DA, Devevre E, Guillaume P, Cerottini JC, Rimoldi D, Speiser DE, Winther L, Romero P. Dextramers: new generation of fluorescent MHC class I/peptide multimers for visualization of antigen-specific CD8+ T cells. J Immunol Methods. 2006;310(1–2):136–148. doi: 10.1016/j.jim.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Donermeyer DL, Beisel KW, Allen PM, Smith SC. Myocarditis-inducing epitope of myosin binds constitutively and stably to I-Ak on antigen-presenting cells in the heart. The Journal of experimental medicine. 1995;182(5):1291–1300. doi: 10.1084/jem.182.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuchroo VK, Greer JM, Kaul D, Ishioka G, Franco A, Sette A, Sobel RA, Lees MB. A single TCR antagonist peptide inhibits experimental allergic encephalomyelitis mediated by a diverse T cell repertoire. J Immunol. 1994;153(7):3326–3336. [PubMed] [Google Scholar]
- 28.Stone KD, Feldman HA, Huisman C, Howlett C, Jabara HH, Bonilla FA. Analysis of in vitro lymphocyte proliferation as a screening tool for cellular immunodeficiency. Clin Immunol. 2009;131(1):41–49. doi: 10.1016/j.clim.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Kruisbeek AM, Shevach E, Thornton AM. Proliferative assays for T cell function. Curr Protoc Immunol. 2004;Chapter 3(Unit 3):12. doi: 10.1002/0471142735.im0312s60. [DOI] [PubMed] [Google Scholar]
- 30.Krammer PH, Marcucci F, Waller M, Kirchner H. Heterogeneity of soluble T cell products. I. Precursor frequency and correlation analysis of cytotoxic and immune interferon (IFN-gamma)-producing spleen cells in the mouse. Eur J Immunol. 1982;12(3):200–204. doi: 10.1002/eji.1830120306. [DOI] [PubMed] [Google Scholar]
- 31.Jenkins MK, Moon JJ. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J Immunol. 2012;188(9):4135–4140. doi: 10.4049/jimmunol.1102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuchroo VK, Sobel RA, Laning JC, Martin CA, Greenfield E, Dorf ME, Lees MB. Experimental allergic encephalomyelitis mediated by cloned T cells specific for a synthetic peptide of myelin proteolipid protein. Fine specificity and T cell receptor V beta usage. J Immunol. 1992;148(12):3776–3782. [PubMed] [Google Scholar]
- 33.Whitham RH, Kotzin BL, Buenafe AC, Weinberg AD, Jones RE, Hashim GA, Hoy CM, Vandenbark AA, Offner H. Treatment of relapsing experimental autoimmune encephalomyelitis with T cell receptor peptides. Journal of neuroscience research. 1993;35(2):115–128. doi: 10.1002/jnr.490350202. [DOI] [PubMed] [Google Scholar]
- 34.Shi YF, Sahai BM, Green DR. Cyclosporin A inhibits activation-induced cell death in T-cell hybridomas and thymocytes. Nature. 1989;339(6226):625–626. doi: 10.1038/339625a0. [DOI] [PubMed] [Google Scholar]
- 35.Shi YF, Szalay MG, Paskar L, Sahai BM, Boyer M, Singh B, Green DR. Activation-induced cell death in T cell hybridomas is due to apoptosis. Morphologic aspects and DNA fragmentation. J Immunol. 1990;144(9):3326–3333. [PubMed] [Google Scholar]
- 36.Waldner H, Whitters MJ, Sobel RA, Collins M, Kuchroo VK. Fulminant spontaneous autoimmunity of the central nervous system in mice transgenic for the myelin proteolipid protein-specific T cell receptor. Proc Natl Acad Sci U S A. 2000;97(7):3412–3417. doi: 10.1073/pnas.97.7.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Islam SA, Luster AD. T cell homing to epithelial barriers in allergic disease. Nat Med. 2012;18(5):705–715. doi: 10.1038/nm.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu M, Fang H, Hwang ST. Cutting edge: CCR4 mediates antigen-primed T cell binding to activated dendritic cells. J Immunol. 2001;167(9):4791–4795. doi: 10.4049/jimmunol.167.9.4791. [DOI] [PubMed] [Google Scholar]
- 39.Hagiwara H, Yokota T, Luh J, Lee F, Arai K, Arai N, Zlotnik A. The AKR thymoma BW5147 is able to produce lymphokines when stimulated with calcium ionophore and phorbol ester. J Immunol. 1988;140(5):1561–1565. [PubMed] [Google Scholar]
- 40.Canaday DH, Gehring A, Leonard EG, Eilertson B, Schreiber JR, Harding CV, Boom WH. T-cell hybridomas from HLA-transgenic mice as tools for analysis of human antigen processing. J Immunol Methods. 2003;281(1–2):129–142. doi: 10.1016/j.jim.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Depta JP, Altznauer F, Gamerdinger K, Burkhart C, Weltzien HU, Pichler WJ. Drug interaction with T-cell receptors: T-cell receptor density determines degree of cross-reactivity. J Allergy Clin Immunol. 2004;113(3):519–527. doi: 10.1016/j.jaci.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 42.Green DR, Mahboubi A, Nishioka W, Oja S, Echeverri F, Shi Y, Glynn J, Yang Y, Ashwell J, Bissonnette R. Promotion and inhibition of activation-induced apoptosis in T-cell hybridomas by oncogenes and related signals. Immunological reviews. 1994;142:321–342. doi: 10.1111/j.1600-065x.1994.tb00895.x. [DOI] [PubMed] [Google Scholar]
- 43.Zumla A, McCormack A, George A, Batchelor R, Lechler R. Use of a murine T-cell hybridoma expressing human T-cell receptor alpha- and beta-gene products as a tool for the production of human T-cell receptor-specific monoclonal antibodies. Human immunology. 1992;35(3):141–148. doi: 10.1016/0198-8859(92)90098-8. [DOI] [PubMed] [Google Scholar]