Abstract
Visuomotor adaptation with prism glasses is a paradigm often used to understand how the motor system responds to visual perturbations. Both reaching and walking adaptation have been documented, but not directly compared. Because the sensorimotor environment and demands are different between reaching and walking, we hypothesized that characteristics of prism adaptation, namely rates and after-effects, would be different during walking compared to reaching. Furthermore, we aimed to determine the impact of age on motor adaptation. We studied healthy younger and older adults who performed visually-guided reaching and walking tasks with and without prism glasses. We noted age effects on visuomotor adaptation, such that older adults adapted and re-adapted slower compared to younger adults, in accord with previous studies of adaptation in older adults. Interestingly, we also noted that both groups adapted slower and showed smaller after-effects during walking prism adaptation compared to reaching. We propose that walking adaptation is slower because of the complex multi-effector and multi-sensory demands associated with walking. Altogether, these data suggest that humans can adapt various movement types but the rate and extent of adaptation is not the same across movement types nor across ages.
Keywords: Visuomotor adaptation, aging, after-effects, gait adaptation, prisms
Introduction
A majority of daily walking involves navigation of complex environments and is highly dependent on visual guidance. Humans can flexibly adapt their walking patterns to visual distortions, which are easily created with gaze-shifting prism glasses. In this paradigm, individuals rapidly alter motor output based on trial-to-trial feedback, eventually establishing a new visuomotor mapping. While many studies of human prism adaptation focus on the upper extremity [1-4], adaptation is also observed during saccades [5, 6], lower extremity movements [7] and walking [8-10]. Some have compared movement types in the context of generalization or how the type of movement or task generalizes to another [7, 8, 10]. However, no study has yet to determine if adaptation is similar in rate and extent across different adapted tasks, or if the type of movement influences how it is adapted (e.g. upper limb movements are adapted faster than lower limb movements). It is obvious that the demands associated with upper extremity movements and walking are quite different. Based on the model of visuomotor coordination proposed by Redding and Wallace [11], we propose that walking adaptation involves many more subsystems than reaching adaptation, resulting in slower error-correction processes. The behavioral consequence of this is slower adaptation during walking. In order to support or refute this hypothesis, we herein compare adaptation of reaching to adaptation of walking.
A secondary aim of this paper was to determine the effects of aging on motor adaptation of reaching and walking. Normal aging involves a myriad of changes in the nervous system that affect visuomotor adaptation, including degradation of sensory receptors and atrophy of the frontal cortex and cerebellum [12, 13]. Older adults respond poorly to changes in their environment, which may underlie the high incidence of falls and movement-related injuries in this population. Indeed, existing data indicate that older adults adapt slower to visual perturbations but show similar if not larger after-effects compared to younger adults [14, 15]. Strategic control processes, which are important during adaptation but not for expression of after-effects, are thought to be impaired in older adults and account for slower adaptation. However, the available literature has focused primarily on upper-extremity adaptation in older adults. The additional challenges, mainly balance and coordination, during walking may further impair older adults’ ability to adapt their walking pattern, but this has not been studied.
In this experiment, we evaluated visuomotor adaptation to prism glasses in healthy older and younger adults during reaching and walking. Our goal was to examine the effects of both age and motor task on the properties of visuomotor adaptation. In accord with previous studies, we predicted older adults would adapt slower but have similar after-effects compared to younger adults during both tasks. Furthermore, we postulated that because walking is more demanding than reaching, adaptation rates during walking would be slower compared to reaching for all participants.
Materials and Methods
Participants
Young (n = 15, 7 male, mean age 25.0 ± 5.83 years) and old (n = 18, 9 male, mean age 70.1 ± 7.27 years) adults participated. Younger adults were recruited from the student cohort at the Washington University School of Medicine Program in Physical Therapy. Older adults were recruited using a volunteer database provided by the Department of Psychology at Washington University. All participants had normal neurological function, 20/40 vision or better without the aid of glasses, and were not cognitively impaired (Mini-mental status exam ≥ 26). Participants provided written consent before participation and were compensated for their time, travel, and effort. All procedures were approved by the Human Research Protection Office at Washington University School of Medicine in St. Louis.
Tasks and Procedures
Participants completed 70 visually-guided reaching and walking trials in the Locomotor Control Laboratory at Washington University School of Medicine in St. Louis. Each task was divided into three phases: Baseline (10 trials), Adaptation (40 trials), and Post-Adaptation (20 trials).
For the reaching task, participants reached and pointed to a visual target with their dominant arm as quickly as possible using a laser pointer. Participants stood 1.6 m from a large piece of paper hung on a wall. A 5 cm × 5 cm crosshair served as the target and was positioned at each participant’s shoulder height. After each reach, the experimenter marked the position of the reach end-point on the paper to allow feedback regarding reach accuracy. During Baseline, reaching occurred without vision (eyes closed). During Adaptation, participants reached while wearing eyeglass frames containing 30-diopter rightward-shifting prism lenses (Fresnel Prism and Lens Co, Bloomington, MN). They also wore modified, lens-free safety goggle frames over the prisms to obscure peripheral vision and ensure gaze was directed through the prism lenses. Eyes remained open throughout Adaptation phase. For Post-Adaptation, prisms were removed and reaching was completed without vision. For all trials, participants viewed their performance after each reach before completing the next trial.
The walking task required participants to walk forward on a path to a visual target on the floor (white piece of tape, 0.3 m long). Participants were instructed to stop with the arches of their feet resting in the middle of the tape. After each trial, the participant turned around and completed the next trial in the opposite direction. Walking was completed with the same phases and vision restrictions as in the reaching task. In addition, participants were fitted with a platform extending forward from the chest to limit vision of the feet and target during Adaptation. Participants were instructed to first look at the target then look straight ahead while walking. However, we ensured that each participant was able to view the position of the feet relative to the target after each Adaptation trial. Walking position was measured using an 8-camera motion capture system (Motion Analysis Corp, Santa Rosa, CA). Reflective markers were placed bilaterally on the greater trochanters and on the left scapula (offset marker). The midpoint of the pelvis markers was used to represent walking trajectory.
Data Analysis
Reaching errors were calculated by measuring the horizontal distance from reach end-point to center of the target. Absolute error was converted to an angular error using trigonometric calculations. Data measured using motion capture were processed for discontinuities and digitally low-pass Butterworth filtered (cut-off of 6 Hz). Walking errors were calculated from the difference in walking trajectory endpoint and center of walking target. These distances were also converted to angular errors. We defined rightward errors as positive and leftward errors as negative.
Trial-to-trial angular error curves for each phase were plotted for each task, and then averaged across all participants. We analyzed four characteristics of prism adaptation: magnitude of the adaptation (Madap), magnitude of the after-effect (Mae), rate of adaptation (Radap) and rate of Post-Adaptation (Rpost). Madap was defined as the difference in angular error between the first Adaptation trial and the average of the last five Adaptation trials. Mae was defined as the angular error during the first Post-Adaptation trial [2]. Although Mae is simply a magnitude, we present it as negative to indicate direction of the error and not to confuse it with Madap. Adaptation and Post-Adaptation curves were fitted by a monotonic exponential function, allowing for estimation of the curve decay constant. We used built-in Matlab (R2011b, Mathworks Inc., Natick, MA) data fitting functions to fit curves during Adaptation and Post-Adaptation phases to the form y = A*exp(−b*t)+c, where A is a scaling constant, b is the decay constant, t is the trial number, and c is the horizontal asymptote. Radap and Rpost were defined as 1/b for the exponential fit of Adaptation and Post-Adaptation curves, respectively. We limited the range of b to 0.025-1 for Adaptation fits and 0.05-1 for Post-Adaptation fits, which translates to a range of 1-40 for Radap and 1-20 for Rpost. These ranges reflect the minimum and maximum possible adaptation rates given the number of trials in each phase. Goodness-of-fit was determined by visual inspection in conjunction with R2 values. Several fits from each group fit poorly to the exponential function, resulting in inaccurate parameter estimates. Specifically, three reaching Adaptation (1 old, 2 young), three walking Adaptation (1 old, 2 young), 1 reaching Post-Adaptation (old) and six walking Post-Adaptation (3 young, 3 old) were deemed poor fits. We excluded these from analysis of Radap and Rpost. (Subsequent analyses showed their inclusion did not change interpretation of the data). Finally, to quantify trial-to-trial variability, we calculated the standard deviation of the last five trials of each phase.
To examine the effects of age and task on the four adaptation variables, we used a mixed-effects ANOVA with between-groups effect of Group (Young vs. Old) and within-groups effect of Task (Reaching vs. Walking) using SPSS v21 (IBM Corp, Chicago IL). Because walking speed may affect magnitude or rate of adaptation and after-effects, we included walking speed as a covariate in the ANOVA model. We also performed a 3- way ANOVA (Task-Phase-Group) to compare changes in variability across the experiment. If a main effect was present, post-hoc t-tests were used to compare group differences within each task. Statistics were considered significant if p<0.05.
Results
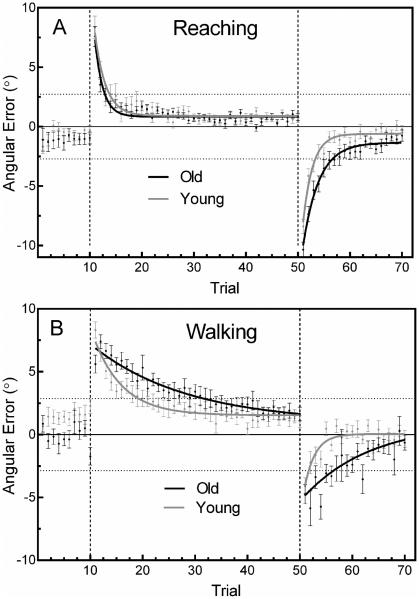
In this experiment, older and younger adults reached and walked to a visual target while wearing gaze-shifting prism glasses. Both groups exhibited normal prism adaptation curves and large negative after-effects following removal of the prisms, indicating participants achieved true spatial realignment. Average walking speed was not different between the groups (Old = 0.74 ± 0.18 m/s, Young = 0.73 ± 0.09 m/s; independent samples t-test, p = 0.88), nor was speed different between phases. Figure 1 shows group mean trial-to-trial angular errors for each phase during reaching and walking. In both tasks, Baseline errors were similar across tasks and groups (mean Baseline error during reaching: Old = −1.08 ± 0.18°, Young = −0.64 ± 0.52°; during walking: Old = − 0.12 ± 0.82°, Young = 1.48 ± 0.44°), and were within the target boundaries denoted by the horizontal dotted lines (± 2.8°).
Figure 1.
Mean trial-to-trial angular errors during reaching (A) and walking (B). Data points represent the mean error for a single trial across participants. Continuous lines are the exponential best fit to the mean data. Horizontal dotted lines indicate the boundaries of the reaching or walking target. Vertical dashed lines separate the phases of the task: Baseline-left, Adaptation-middle, Post-Adaptation-right. Error bars are ± SEM.
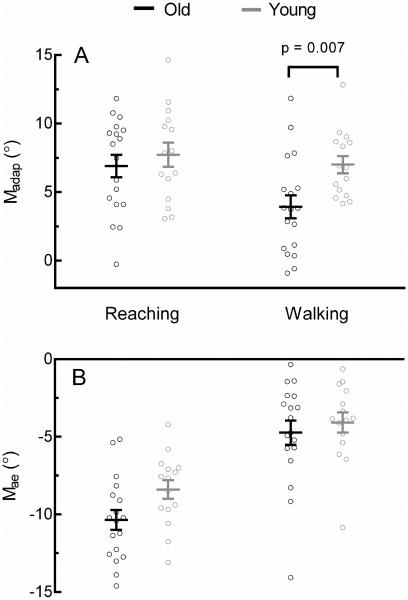
Figure 2 shows individual and mean values for Madap and Mae. Madap was similar between groups during reaching (Young = 7.72 ± 0.88°, Old = 6.90 ± 0.81°) but greater in the young group during walking (Young = 7.00 ± 0.63°, Old = 3.93 ± 0.84°) (Figure 2A). Table 1 summarizes the ANOVAs for all four adaptation measures. There were significant main effects of Task (Reaching > Walking) and Group (Young > Old) for Madap. Mae was slightly greater in the old group during reaching (Old = −10.36 ± 0.63°, Young = −8.39 ± 0.60) but similar between groups during walking (Old = −4.73 ± 0.78°, Young = −4.08 ± 0.65°) (Figure 2B). Task was also a significant main effect in the ANOVA of Mae (Reaching > Walking) however Group was not significant.
Figure 2.
Individual (circles) and mean (line) Madap (A) and Mae (B) during reaching and walking. Madap was smaller during walking and in older adults, while Mae was greater during walking compared to reaching. Error bars are ± SEM. See Table 1 for ANOVA results. P-values represent post-hoc comparisons
Table 1.
ANOVA summary for adaptation variables
|
Main Effects
|
Interaction
|
Post-hoc (Young vs. Old)
|
|||
|---|---|---|---|---|---|
|
Measure
|
Task |
Group |
Reach |
Walk |
|
| Madap | 6.06 (0.02) | 5.58 (0.03) | 2.26 (0.14) | 0.69 (0.50) | 2.82 (0.01) |
| Mae | 59.9 (<0.001) | 3.93 (0.06) | 1.04 (0.32) | N/A | N/A |
| Radap | 12.2 (0.002) | 3.04 (0.09) | 4.00 (0.06) | N/A | N/A |
| Rpost | 6.88 (0.02) | 6.08 (0.02) | 4.98 (0.04) | 1.36 (0.18) | 2.44 (0.02) |
Values are F-statistic from ANOVA model and t-statistic for post-hoc tests; p-values are given in parentheses Bolded text indicates significance; N/A: Not applicable; no main effect present
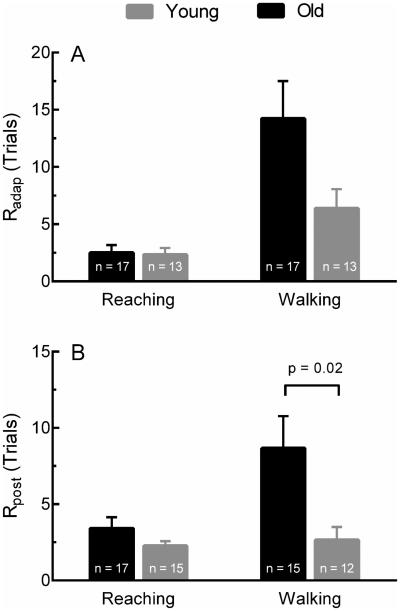
Decay rates of the Adaptation and Post-Adaptation curves revealed further differences between groups. Figure 3 shows the mean Radap and Rpost during both tasks. Radap was similar between groups during reaching (Old = 2.51 ± 0.66 trials, Young = 2.33 ± 0.59 trials) but was greater in older adults during walking (Old = 14.23 ± 3.28 trials, Young = 6.38 ±1.68 trials) (Figure 3A). The ANOVA of Radap showed a significant main effect of Task (Walking >Reaching) while Group and Task*Group interaction did not reach significance. Further differences were observed in the estimate of Rpost. Older adults (8.67 ± 2.09 trials) had greater Rpost compared to younger adults (2.66 ± 0.85 trials) during walking (Figure 3B). Here, the ANOVA showed significant effects of Task, Group and Group*Task interaction. Overall, younger adults de-adapted faster compared to older adults for both tasks, but this difference was pronounced during walking.
Figure 3.
Mean estimated Radap (A) and Rpost (B) during reaching and walking. Both groups adapted (Radap) slower during walking compared to reaching, and this difference was pronounced in older adults. Rpost was slower on average during walking compared to reaching, and older adults re-adapted slower than younger adults, particularly during the walking task. Error bars are ± SEM. See Table 1 for ANOVA results. P-values represent post-hoc comparisons.
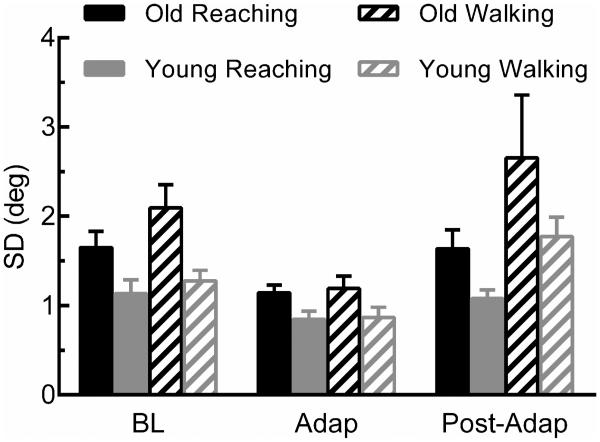
Movement variability (standard deviation) across the experimental phases is shown in Figure 4. Variability was greater during walking (1.67 ± 0.15°) compared to reaching (1.27 ± 0.07°), as indicated by a main effect of Task; F(31,1) = 4.64, p = 0.04) and was associated with the experimental phase (F(31,2) = 10.2, p < 0.001), such that Baseline and Post-Adaptation phases were more variable than the Adaptation phase. To add, older adults tended to have greater variability overall (Old = 1.73 ± 0.14°, Young = 1.16 ± 0.06°, main effect of Group; F(31,1) = 9.44, p = 0.004). In total, variability was significantly altered by task conditions and age of the participant.
Figure 4.
Mean standard deviation shown for each group, task, and across each experimental phase. SD represents the average standard deviation of the last five trials of the respective phase. Error bars are ± SEM. Main effects from ANOVA: Task (p = 0.04), Phase ( p < 0.001) and Group (p= 0.004).
Discussion
We observed age effects on prism adaptation during reaching and walking, where older adults adapted and re-adapted slower during both tasks. Numerous studies comparing older and younger adults show aging affects adaptation but not after-effects [14-16]. These results support the idea that two main processes regulate motor adaptation: strategic control and sensory recalibration. Strategic control is the ability to use cognitive strategies or prior knowledge to reduce movement errors, while sensory recalibration is an intrinsic property of the nervous system to respond to changes in one’s environment [11, 13]. For the prism adaptation paradigm used herein, sensory recalibration occurs during both Adaptation and Post-Adaptation and slowly reconciles motor output with visual and proprioceptive feedback. However, strategic control occurs only during the early phases of Adaptation and Post-Adaptation as participants seek to quickly reduce movement errors. As was thought previously, aging likely affects strategic control more so than sensory recalibration since older adults exhibit slower adaptation rates but normal after-effects. Our data support this and show that strategic control may also impact Post-Adaptation given the slower Rpost observed in older adults. During Post-Adaptation, initial large errors drive similar adaptive processes as used during Adaptation. In this situation, strategic control is essential because vision was permitted only at the start and end of Post-Adaptation trials, requiring participants to use explicit information about their starting and ending positions to correct movements. Altogether, these results point to age-related slowing of visuomotor adaptation but no changes in total realignment during multiple motor tasks.
The novel result of this study was the task-specific effects on characteristics of prism adaptation. All four variables (Madap, Mae, Radap, and Rpost) were significantly different between reaching and walking. Madap was smaller during walking, showing the visual perturbation caused greater errors during reaching compared to walking. We propose several potential explanations for these changes.
One major difference between tasks was movement duration; walking trials were considerably longer than reaching trials. Because of longer trial durations, one may expect within-trial adjustments would lead to faster, not slower, adaptation rates during walking. While this may have occurred on the first several trials, accounting for lower Madap during walking, the overall error reduction rate was still significantly slower during walking relative to reaching. Previous studies indicate that movement duration also impacts the magnitude of visual and proprioceptive adaptation [4, 17]. It is not clear if the relative contribution of visual versus proprioceptive adaptation differed between tasks as this was not specifically measured. Therefore, we cannot rule out that smaller after-effects were due to less proprioceptive or visual adaptation achieved during walking compared to reaching. Future studies could compare visual and proprioceptive adaptation in comparison to overall after-effect magnitudes in each task.
The smaller Mae observed during walking may be a direct result of adaptation rate, given that all participants adapted reaching movements faster than walking. As a result, they performed a greater number of correctly adapted reaches compared to correctly adapted walks during Adaptation. Previous work by Fernandez-Ruiz and Diaz showed a positive correlation between number of trials performed after complete adaptation and Mae [2]. In our data, we did not find any relationship between Mae and Radap, however. Thus, the smaller after-effect observed during walking is not likely only due to the fewer number of walking trials completed after complete adaptation.
Factors related to sensorimotor integration may also explain our results. There may be differences in sensory weighting during reaching and walking [18]. Prism adaptation causes a re-weighting of sensory input such that visual feedback dominates proprioceptive and/or vestibular feedback. While vestibular input is present during both tasks, it is much more important for walking control than for reaching. The extra information provided by the vestibular system may have caused interference that slowed the sensory recalibration process during walking [19]. Another potential rationale is the contrast in motor demands between tasks. Walking requires dynamic control of balance and all four extremities, while reaching requires static control of balance and movement of one extremity. Based on Redding and Wallace’s model of prism adaptation, the nervous system integrates signals from multiple sensory-motor subsystems to achieve spatial realignment [11]. During walking, there are many more active subsystems compared to reaching. Although they suggest that these subsystems are controlled in parallel, there may be some cost associated with operating many subsystems simultaneously. If the cost is time-related, it would result in more walking trials (i.e. slower Radap) to reach accordance between visual input and motor output. This is slightly counter-intuitive, given that walking is assumed to be an automatic motor program. However, in our task, walking was probably under more voluntary control because participants walked with a goal in mind, and adjusted their walking accordingly. Overall, walking may require multiple effector-specific motor commands, resulting in prolonged adaptation rates.
In this study, we show the effects of both age and motor task on properties of visuomotor adaptation to prism glasses. Similar to previous reports we found that older adults adapted slower to visual perturbations. Despite these differences, the after-effect magnitude was similar between older and younger adults, suggesting that strategic control is more impacted by age than is sensory recalibration. Finally, while we show task-dependent effects on rates of adaptation and de-adaptation, additional work is needed to elucidate the relationship between the motor task and processes underlying visuomotor adaptation.
We compared visuomotor adaptation during reaching and walking in young and older adults
Older adults overall adapted slower than younger adults during both tasks
Walking adaptation and re-adaptation was generally slower than during reaching for both groups
Both aging and the nature of the motor task can affect properties of visuomotor adaptation
Acknowledgments
The authors thank Richard Nagel, Katherine Seidler, and Martha Hessler for assistance with data collection. This work was supported by the Washington University Institute for Clinical and Translational Science (UL1 TR000448 and TL1 TR00049), the Parkinson’s and Movement Disorders Foundation, and the Washington University Program in Physical Therapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have no conflicts to disclose.
Contributor Information
Samuel T. Nemanich, Email: nemanichs@wusm.wustl.edu.
Gammon M. Earhart, Email: earhartg@wusm.wustl.edu.
References
- [1].Block HJ, Bastian AJ. Cerebellar involvement in motor but not sensory adaptation. Neuropsychologia. 2012;50:1766–75. doi: 10.1016/j.neuropsychologia.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fernandez-Ruiz J, Diaz R. Prism adaptation and aftereffect: specifying the properties of a procedural memory system. Learn Mem. 1999;6:47–53. [PMC free article] [PubMed] [Google Scholar]
- [3].Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain. 1996;119:1183–98. doi: 10.1093/brain/119.4.1183. Pt 4. [DOI] [PubMed] [Google Scholar]
- [4].Redding GM, Wallace B. Prism exposure aftereffects and direct effects for different movement and feedback times. J Mot Behav. 2000;32:83–99. doi: 10.1080/00222890009601362. [DOI] [PubMed] [Google Scholar]
- [5].Bekkering H, Abrams RA, Pratt J. Transfer of saccadic adaptation to the manual motor system. Human Movement Science. 1995;14:155–64. [Google Scholar]
- [6].Bultitude JH, Van der Stigchel S, Nijboer TC. Prism adaptation alters spatial remapping in healthy individuals: evidence from double-step saccades. Cortex. 2013;49:759–70. doi: 10.1016/j.cortex.2012.01.008. [DOI] [PubMed] [Google Scholar]
- [7].Savin DN, Morton SM. Asymmetric generalization between the arm and leg following prism-induced visuomotor adaptation. Exp Brain Res. 2008;186:175–82. doi: 10.1007/s00221-007-1220-9. [DOI] [PubMed] [Google Scholar]
- [8].Alexander MS, Flodin BW, Marigold DS. Prism adaptation and generalization during visually guided locomotor tasks. J Neurophysiol. 2011;106:860–71. doi: 10.1152/jn.01040.2010. [DOI] [PubMed] [Google Scholar]
- [9].Michel C, Vernet P, Courtine G, Ballay Y, Pozzo T. Asymmetrical after-effects of prism adaptation during goal oriented locomotion. Exp Brain Res. 2008;185:259–68. doi: 10.1007/s00221-007-1152-4. [DOI] [PubMed] [Google Scholar]
- [10].Morton SM, Bastian AJ. Prism adaptation during walking generalizes to reaching and requires the cerebellum. J Neurophysiol. 2004;92:2497–509. doi: 10.1152/jn.00129.2004. [DOI] [PubMed] [Google Scholar]
- [11].Redding GM, Wallace B. Strategic calibration and spatial alignment: a model from prism adaptation. J Mot Behav. 2002;34:126–38. doi: 10.1080/00222890209601935. [DOI] [PubMed] [Google Scholar]
- [12].Freiherr J, Lundstrom JN, Habel U, Reetz K. Multisensory integration mechanisms during aging. Front Hum Neurosci. 2013;7:863. doi: 10.3389/fnhum.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].King BR, Fogel SM, Albouy G, Doyon J. Neural correlates of the age-related changes in motor sequence learning and motor adaptation in older adults. Front Hum Neurosci. 2013;7:142. doi: 10.3389/fnhum.2013.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Buch ER, Young S, Contreras-Vidal JL. Visuomotor adaptation in normal aging. Learn Mem. 2003;10:55–63. doi: 10.1101/lm.50303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fernandez-Ruiz J, Hall C, Vergara P, Diiaz R. Prism adaptation in normal aging: slower adaptation rate and larger aftereffect. Brain Res Cogn Brain Res. 2000;9:223–6. doi: 10.1016/s0926-6410(99)00057-9. [DOI] [PubMed] [Google Scholar]
- [16].Bock O. Components of sensorimotor adaptation in young and elderly subjects. Exp Brain Res. 2005;160:259–63. doi: 10.1007/s00221-004-2133-5. [DOI] [PubMed] [Google Scholar]
- [17].Redding GM, Wallace B. Effects of movement duration and visual feedback on visual and proprioceptive components of prism adaptation. J Mot Behav. 1994;26:257–66. doi: 10.1080/00222895.1994.9941681. [DOI] [PubMed] [Google Scholar]
- [18].Huitema RB, Brouwer WH, Mulder T, Dekker R, Hof AL, Postema K. Effect of ageing on the ability to adapt to a visual distortion during walking. Gait Posture. 2005;21:440–6. doi: 10.1016/j.gaitpost.2004.05.007. [DOI] [PubMed] [Google Scholar]
- [19].Kennedy PM, Carlsen AN, Inglis JT, Chow R, Franks IM, Chua R. Relative contributions of visual and vestibular information on the trajectory of human gait. Exp Brain Res. 2003;153:113–7. doi: 10.1007/s00221-003-1633-z. [DOI] [PubMed] [Google Scholar]