Abstract
Objective
This study examined mean level differences in marijuana expectancies and the differential associations between expectancies and marijuana use for individuals with and without a history of Attention-Deficit/Hyperactivity Disorder (ADHD)
Background
Substance use expectancies are a widely studied risk factor for alcohol and other drug use. The relations between marijuana use expectancies and self-reported marijuana use have not been examined in young adults with ADHD, a population shown to be at risk for marijuana use.
Method
Participants were 306 (190 ADHD and 116 nonADHD) young adults (M age = 20.06, SD = 2.03) from the Pittsburgh ADHD Longitudinal Study (PALS) who provided data about marijuana use and marijuana use expectancies.
Results
Individuals in the ADHD group reported lower levels of social enhancement, tension reduction, and cognitive and behavioral impairment expectancies compared to individuals in the nonADHD group. Positive and negative marijuana use expectancies were associated with marijuana use frequency in the whole sample and statistically significant ADHD group by expectancy interactions were found. Sexual enhancement expectancies were more strongly associated with marijuana use frequency among individuals with ADHD histories while cognitive behavioral impairment expectancies were more strongly associated with marijuana use frequency among individuals without ADHD.
Conclusions
Marijuana use expectancies may be acquired, and operate differently, for individuals with and without ADHD histories. Although future research is needed to test this speculation, these differences may be associated with ADHD-related difficulties in higher order cognitive processes that affect the encoding and utilization of expectations regarding marijuana’s effects.
Keywords: Marijuana expectancies, ADHD, Marijuana
Introduction
Marijuana is the most frequently used illicit substance among individuals 12 and older (Substance Abuse and Mental Health Services Administration [SAMHSA], 2013) with use being most prevalent among individuals between the ages of 18-30 (American Psychiatric Association [APA], 2013). Marijuana use is not without consequence and may progress to a recognized substance use disorder (APA, 2013). In the United States it is estimated that 4.3 million people 12 or older meet criteria for a past year cannabis use disorder (CUD; SAMHSA, 2013). As such, there is a need to examine the factors that increase the risk for frequent and impairing marijuana use. The current study focuses on an increasingly studied risk factor for marijuana use, marijuana use expectancies, in a population at risk for experiencing marijuana use problems: individuals with a history of Attention-Deficit/Hyperactivity Disorder (ADHD).
ADHD and Marijuana Use
Prospective longitudinal studies have shown that a diagnosis of ADHD in childhood increases the risk for later substance use and substance use disorders including adolescent/young adult marijuana use (Barkley, Fischer, Edelbrock, & Smallish, 1990; Harty, Ivanov, Newcorn, & Halperin, 2011; Molina et al., 2013; Molina & Pelham, 2003) and CUD (Elkins, McGue, & Iacono, 2007; Molina et al., 2013). Results of a recent meta-analysis (Lee, Humphreys, Flory, Liu, & Glass, 2011) revealed that, compared to their nonADHD peers, individuals diagnosed with ADHD in childhood were approximately three times more likely to have reported ever using marijuana and 1.5 times more likely to have met criteria for a CUD. Relatively little research with this population has examined risk factors for marijuana use and CUD beyond ADHD symptom severity (Elkins et al., 2007), symptom persistence (Molina & Pelham, 2003), and co-occurring externalizing disorders such as Conduct Disorder (CD; Barkley et al., 1990; Biederman et al., 2008; Elkins et al., 2007; Harty et al., 2011). Moving beyond this descriptive research to examine the motivational and cognitive processes that may differ for individuals with ADHD is an important next step in understanding why such individuals may be at increased risk for experiencing marijuana use problems.
Substance Use Expectancies
Substance use expectancies are frequently studied cognitive risk factors that are understood as the anticipatory cognitions regarding the perceived outcome(s) of use. Although much of this work has been conducted with reference to alcohol use (for review see: Del Boca, Darkes, Goldman, & Smith, 2002; Goldman, 2002), there is a growing body of literature documenting associations between marijuana use expectancies, both positive and negative, and various marijuana-related outcomes. Among community and college populations, positive marijuana use expectancies have been associated with marijuana initiation (Malmberg et al., 2012) and frequency and severity of use (Hayaki et al., 2010). On the other hand, negative expectancies have been shown to be elevated among non-users (Schafer & Brown, 1991; Willner, 2001) and may operate as a protective factor (Kristjansson, Agrawal, Lynskey, & Chassin, 2012).
A large body of research has conceptualized expectancies as proximal mediators of distal risk factors such as family history of substance use disorder and personality vulnerability. Previous studies have shown that substance use expectancies partially account for the association between established risk factors (e.g.,family history of alcohol use disorders (Conway, Swendsen, & Merikangas, 2003; Sher, Walitzer, Wood, & Brent, 1991); sensation seeking (Kalichman, Weinhardt, DiFonzo, Austin, & Luke, 2002); behavioral dysregulation (Barnow et al., 2004) and substance use. For example, Barnow and colleagues reported that adolescents with externalizing behaviors reported more positive alcohol expectancies which in turn predicted alcohol quantity/frequency one year later (Barnow et al., 2004). Given these findings, adolescents with ADHD histories should theoretically be at risk of forming expectancies that are more positive, and less negative, than those reported by adolescents without ADHD histories. We test this hypothesis in the current study.
Research has also focused on the relation between expectancies and substance use being constrained or enhanced for certain individuals or under certain conditions. One recent example of this type of thinking is reflected in the dual process model of alcohol cognition (Stacy & Wiers, 2010) that identifies two cognitive systems: a rational, explicit system and an automatic, implicit system. Research has shown that for individuals with higher order cognitive processing deficits, implicit associations about alcohol are more strongly related to alcohol use behavior than explicit cognitions (Thush et al., 2008). Previous studies have repeatedly demonstrated that deficits in executive functions are frequently seen among individuals diagnosed with ADHD (for review see: Nigg, 2013). As such, individuals with ADHD may rely more on implicit cognitive processes and less on explicit substance use cognitions; an attenuated association between explicit expectancies and substance use may result. This hypothesis, however, has yet to be tested for explicit expectancies which are a mainstay of the literature on cognitive factors that contribute to substance use.
Substance Use Expectancies and ADHD
Few studies have specifically examined substance use expectancies among individuals with ADHD. Additionally, these studies have only examined alcohol use expectancies, and results have been inconsistent. For example, in a study of alcohol expectancies among college students, Dattilo, Murphy, Van Eck, and Flory (2013) found that ADHD symptoms moderated the relationship between positive alcohol-related expectancies and alcohol use problems. For students with higher ADHD symptom scores, positive expectancies were more strongly associated with alcohol use. However, ADHD symptoms were self-reported and may have tapped different and potentially milder vulnerabilities from those usually studied in individuals diagnosed in childhood (Barkley, Murphy, & Fischer, 2008). Poor insight is a well-established characteristic of many individuals with ADHD (Hoza, Pelham, Owens, & Pillow, 2002). Recent work from our group (Pedersen, Harty, Pelham, Gnagy, & Molina, 2014) found a pattern of results opposite to the Dattilo findings. Adolescents with a documented history of ADHD in childhood had lower levels of both positive and negative alcohol expectancies compared to individuals without ADHD and, consistent with the dual process model of alcohol cognition (Stacy & Wiers, 2010), negative alcohol expectancies were less related to alcohol use for individuals with, compared to individuals without, ADHD. Given the relative paucity of research and the equivocal nature of the findings thus far, further research is needed that utilizes samples well-characterized in their ADHD histories, and that extends the substance of interest beyond alcohol to include the most widely used illicit drug: marijuana.
Purpose of the current study
The current study sought to examine, for the first time, explicit marijuana use expectancies reported by young adults with and without well-documented ADHD histories. In a sample comprehensively assessed as children and followed to adulthood, we tested whether marijuana expectancies differed for young adults with, and without, ADHD histories, and whether the expectancy-marijuana use association was significantly attenuated in the ADHD group.
Materials and Methods
Participants
Individuals with ADHD
Adults with ADHD histories were diagnosed in childhood with DSM-III-R or DSM-IV ADHD at the ADD Clinic, Western Psychiatric Institute and Clinic, in Pittsburgh, PA between 1987 and 1996. Average age at initial evaluation was 9.40 years old (SD = 2.27 years, range = 5.0-16.92). Ninety percent of children were diagnosed in their elementary school-aged years (ages 5–12). Participants were selected for longitudinal follow-up due to their diagnosis of ADHD and participation in a summer treatment program for children with ADHD.
Diagnostic information for individuals with ADHD was collected in childhood using standardized parent and teacher DSM-III-R and DSM-IV symptom rating scales (DBD; Pelham, Gnagy, Greenslade, & Milich, 1992) and a standardized semi-structured diagnostic interview administered to parents by a Ph.D. level clinician. Two Ph.D. level clinicians independently reviewed all ratings and interviews to confirm DSM diagnoses and when disagreement occurred, a third clinician reviewed the file and the majority decision was used. Exclusion criteria for follow-up was assessed in childhood and included a full-scale IQ < 80, a history of seizures or other neurological problems, and/or a history of pervasive developmental disorder, schizophrenia, or other psychotic disorders.
Of those eligible for follow-up in the Pittsburgh ADHD Longitudinal Study (PALS; n = 516), 70.5% (n = 364) participated (M = 8.35 years after childhood diagnosis, S.D. = 2.79). A minority could not be located (n = 23); 129 refused or failed to participate. Participating individuals were compared with nonparticipating individuals with ADHD on demographic and diagnostic variables collected in childhood. Only one of 14 comparisons was statistically significant at the p < .05 significance level. Participants had a slightly lower average CD symptom rating as indicated by a composite of parent and teacher ratings (participants M = .43, non-participants M = .53, Cohen’s d = .30). At the first PALS follow-up interview, which occurred on a rolling basis between 1999 and 2003, mean age was 17.75 yrs, S.D. = 3.39 years, range = 11 to 25 (three subjects were 26-28 years old).
NonADHD comparison group
Individuals without ADHD were recruited into the PALS at the same time as individuals diagnosed with ADHD were re-contacted to enroll in the follow-up study. NonADHD comparison participants were recruited on a rolling basis to ensure demographic similarity to the individuals with ADHD as a group. They were recruited from the greater Pittsburgh area from several sources including pediatric practices serving patients from diverse socioeconomic backgrounds (40.8%), advertisements in local newspapers and the university hospital staff newsletter (27.5%), local universities and colleges (20.8%), and other methods (10.9%) such as Pittsburgh Public Schools and word of mouth. Individuals who met DSM-III-R criteria for ADHD (presence of 8 or more symptoms reported by either the parent or young adult), currently or historically, were excluded. NonADHD comparison participants with subthreshold ADHD symptomatology, or with other psychiatric disorders, were retained. There were no statistically significant differences between the 364 individuals with ADHD histories and 240 nonADHD comparison participants on age, sex, ethnicity/race, and highest parent education.
Subsample for the current study
For the current study, data were selected for individuals ages 18 and older at the first PALS follow-up interview, resulting in 306 participants (190 ADHD and 116 nonADHD) with a mean age of 20.6 years old (SD = 2.03, range = 18-28). As shown in Table 1, there were no statistically significant differences between individuals with and without childhood ADHD on age, sex, and ethnic/racial minority status. A greater proportion of individuals with ADHD reported living with their parents, χ2 (1) = 13.01, p < .01, at the time of the interview.
Table 1.
Demographic characteristics of the sample.
| nonADHD | ADHD | |
|
|
||
| N | 116 | 190 |
| Demographic Matching Variables | ||
| Age (M, SD) | 19.81 (1.71) | 20.21 (2.19) |
| Gender (% Male) | 86.9 | 84.9 |
| Racial Minority (%) | 13.5 | 18.1 |
| Living with parents (%)** | 47.9 | 68.4 |
Note.
= p < .01
Procedure
Interviews with all participants were conducted in the ADD program offices by post-baccalaureate research staff. Interviewers were not blind to recruitment source (i.e., presence or absence of ADHD), but they were trained to avoid bias in data collection. Moreover, many of the PALS questionnaires were completed privately by participants which helped to minimize interviewer contamination. Informed consent was obtained and all participants were assured confidentiality of all disclosed material except in cases of impending danger or harm to self or others. Privacy was reinforced with a DHHS Certificate of Confidentiality. Where distance prevented participant travel, information was collected using mail and telephone correspondence; home visits were offered as need dictated. Self-report questionnaires were completed either with paper and pencil or with web-based versions on a closed-circuit internet page.
Measures
Marijuana expectancies were assessed with an adapted version of the Comprehensive Effects of Alcohol questionnaire (CEOA; Fromme, Stroot, & Kaplan, 1993). The CEOA assesses respondents’ beliefs about changes in self as a function of alcohol. The current study substituted “marijuana” for “alcohol.” The expected effect portion of the revised measure begins with the stem “If I were under the influence from smoking marijuana” followed by 38 concluding phrases (e.g., I would be outgoing, I would have difficulty thinking). Responses were on a 4 point scale (1 = disagree, 4 = agree). Positive expectancies consisted of four subscales: Social Enhancement (8 items, α =.88), Tension Reduction (3 items, α =.83), Courage, (5 items, α =.84) and Sexual Enhancement (4 items, α =.79). Negative expectancies consisted of three subscales: Cognitive and Behavioral Impairment (9 items, α =.91), Risk and Aggression (5 items, α =.78), and Negative Self-Perception (4 items, α =.76). (All alphas from current sample.)
Marijuana use was evaluated with a substance use self-report questionnaire (SUQ; Molina & Pelham, 2003) that is an adaptation of existing measures, including the Health Behavior Questionnaire (Jessor, Donovan, & Costa, 1989) and National Household Survey of Drug Abuse interview (1992). The SUQ includes lifetime exposure and quantity/frequency questions. Participants were classified as marijuana users if they reported any lifetime use (yes/no). Participants were asked to report their age at initial use as well as marijuana use frequency over the previous 3 months. Lifetime presence of CUD was assessed with the Structured Clinical Interview for DSM-III-R (First, Gibbon, Spitzer, & Williams, 1997) adapted for DSM-IV.
Statistical Analyses
Between group t-tests or chi square tests were used to characterize the sample, marijuana expectancies, frequency of marijuana use, and CUD. Separate hierarchical linear and logistic regressions were used to test the associations between ADHD history (yes/no), the seven marijuana use expectancy subscales, and marijuana use frequency and CUD. All predictor variables were mean centered and analyses controlled for gender and age. For the hierarchical linear and logistic regressions, gender, age, and ADHD group were entered in the first step, main effects for the individual expectancy subscales were entered in the second step, and the ADHD by expectancy interaction term was entered in the third step. Procedures for testing and interpreting interactions were based on Aiken and West (1991).
Results
Marijuana Use Descriptives
As can be seen in Table 2, the ADHD and nonADHD groups reported similar rates of having ever tried marijuana, marijuana use frequency, and ratings of CUD. However, individuals with ADHD histories reported an earlier age of initial use compared to the nonADHD group. Among those reporting marijuana use in the last 3 months there was a trend toward an ADHD group difference in marijuana use frequency, with the ADHD group reporting more frequent use than the nonADHD group.
Table 2.
Marijuana Use Descriptives
| nonADHD | ADHD | |
|
|
||
| N | 116 | 190 |
| Descriptives | ||
| Ever Tried (%) | 63.79 | 68.42 |
| Age of Initial Use (M, SD)* | 16.05 (1.94) | 15.36 (2.65) |
| Any Past 3-Month Use (%) | 42.24 | 42.63 |
| Past 3-Month Use Frequency (M, SD) ^+ | 2.84 (1.18) | 3.28 (1.39) |
| CUD (%) | 19.46 | 23.31 |
Note.
= p ≤ .05,
= p = .06,
= a rating of 2 = “Once a week or less”, 3 = “A couple of times a week”, among users in the past three months. ADHD group differences were also not present for any use of marijuana in the past 12 months (M=2.22, SD=1.30 for nonADHD vs. 2.42, SD=1.56 for ADHD).
Marijuana Expectancies
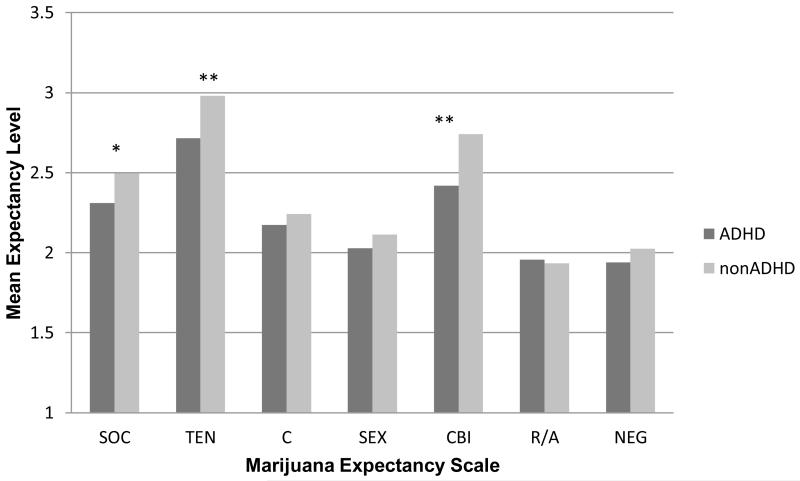
Compared to individuals without ADHD, individuals with a history of ADHD had significantly lower mean levels for three of the seven marijuana expectancy subscales (See Figure 1). Among positive expectancies, they had lower levels of Social Enhancement, t(306) = −1.95, p = .05, and Tension Reduction, t(306) = −2.44, p = .02. For negative expectancies, individuals with ADHD reported lower levels of Cognitive and Behavioral Impairment expectancies, t(306) = −3.37, p < .01.
Figure 1.
Marijuana expectancy ratings as a function of ADHD group.
Note: * = p < .05; ** = p = .01; SOC = social enhancement, TEN = tension reduction, C = courage, SEX = sexual enhancement, CBI = cognitive and behavioral impairment, R/A = risk taking and aggression, NEG = negative self-perception
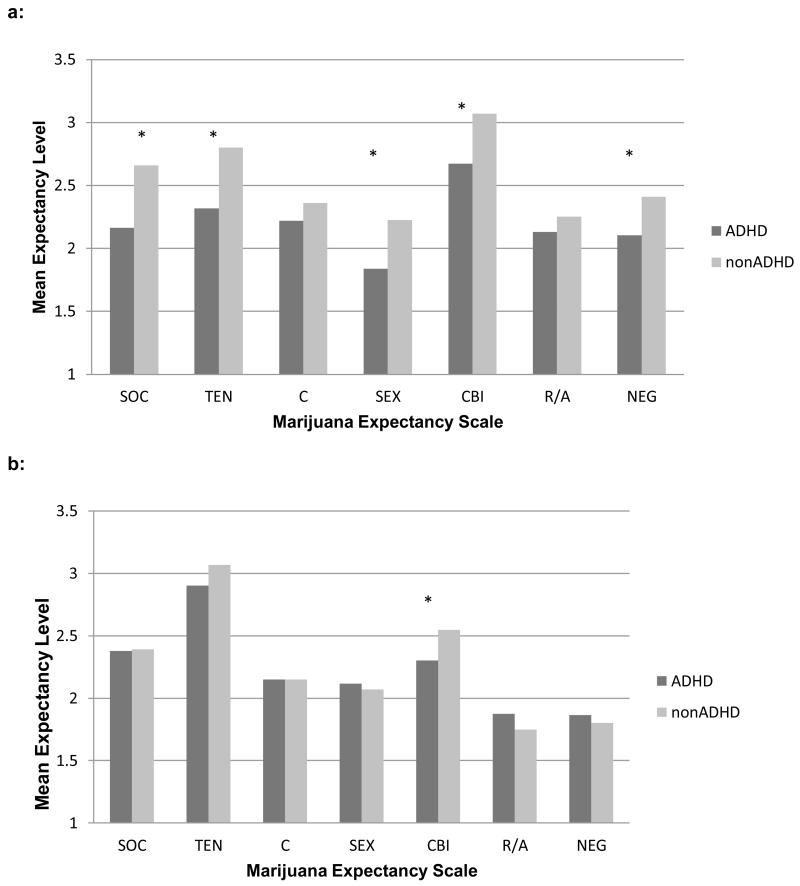
Due to the effects of direct experience on expectancies (e.g., Goldman, Del Boca, & Darkes, 1999), we re-analyzed the data separately for individuals who never used marijuana (ADHD = 60, nonADHD = 42) and for those with any lifetime marijuana use (ADHD = 130, nonADHD = 74). Among non-users (see Figure 2a), individuals with ADHD, when compared to individuals without ADHD histories, reported lower levels of Social Enhancement , t(100) = −2.95, p < .01, Tension Reduction, t(100) = −2.72, p = .01, Sexual Enhancement, t(100) = −2.35, p = .02, Cognitive and Behavioral Impairment, t(100) = −2.54, p = .01, and Negative Self-Perception expectancies, t(100) = −2.01, p = .04.
Figure 2.
Marijuana expectancy ratings among marijuana non-users (a) and users (b).
Note: * = p < .05; SOC = social enhancement, TEN = tension reduction, C = courage, SEX = sexual enhancement, CBI = cognitive and behavioral impairment, R/A = risk taking and aggression, NEG = negative self-perception
In contrast (see Figure 2b), only one ADHD group difference was statistically significant for individuals reporting lifetime marijuana use histories. Users with ADHD histories expected less Cognitive and Behavioral Impairment from marijuana use compared to users without ADHD histories, t(202) = −2.21, p = .03.
Predicting Marijuana Use Outcomes
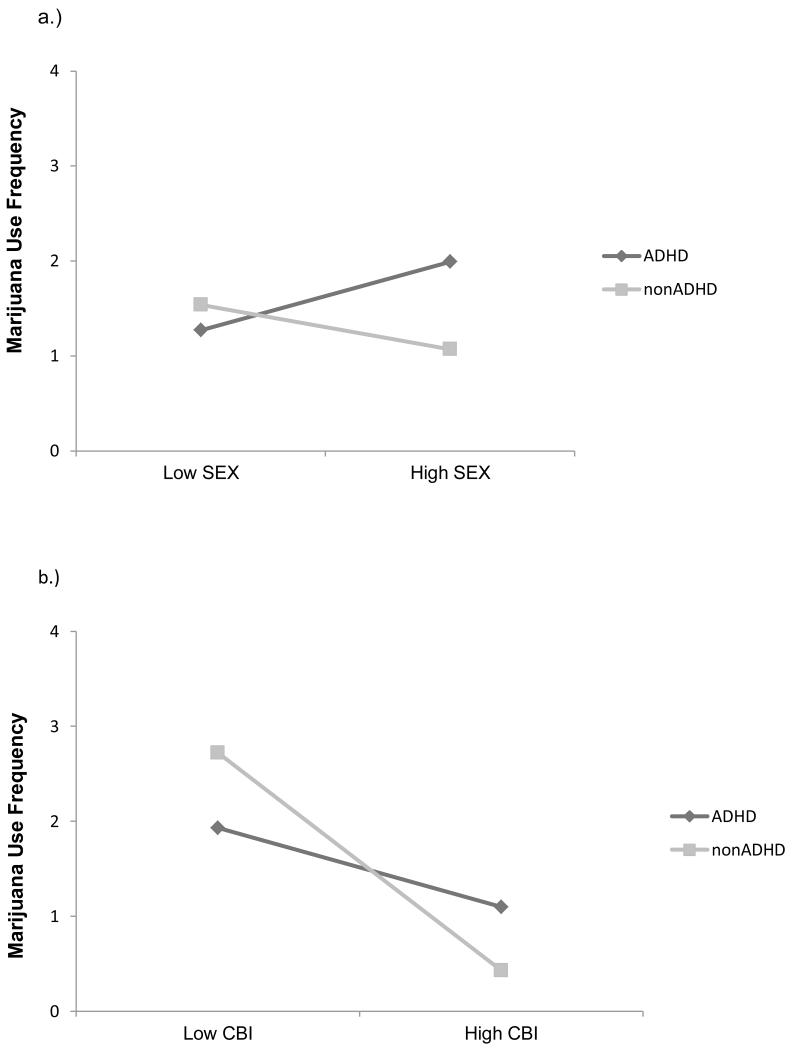
The regression analyses testing ADHD, expectancies, and the ADHD-by-expectancy interactions in relation to frequency of marijuana use revealed significant main effects for: Social Enhancement, b = .05, SE = .01, p = .01, Tension Reduction, b = .13, SE = .06, p < .01, Sexual Enhancement, b = .07, SE = .03, p = .02, and Cognitive and Behavioral Impairment, b = −.03, SE = .01, p < .01. The other expectancy subscales were not significantly related to marijuana use frequency. Significant interactions were found between ADHD group and Sexual Enhancement expectancies, b = −.11, SE = .05, p = .04, and ADHD group and Cognitive and Behavioral Impairment expectancies, b = −.06, SE = .02, p = .01. For the Sexual Enhancement by ADHD interaction, higher sexual enhancement expectancies were associated with more frequent marijuana use for the ADHD group, b =.07, p = .04, but not for the nonADHD group, b = −.04, p = .34 (see Figure 3a). For the Cognitive and Behavioral Impairment expectancies by ADHD interaction (see Figure 3b), higher cognitive and behavioral impairment expectancies were more strongly associated with less frequent marijuana use for the nonADHD than for the ADHD group. However, both groups exhibited slopes that were significantly different from zero with higher cognitive and behavioral impairment expectancies being associated with less frequent marijuana use for both the nonADHD group, b = −.09, p < .01 and the ADHD group, b = −.03, p = .01. In all analyses, after accounting for other predictor variables, age, gender, and ADHD group were not significantly associated with marijuana use.
Figure 3.
Graph of the two-way interaction between ADHD group status and: a.) Sexual enhancement expectancies (SEX) and b.) Cognitive behavioral impairment expectancies (CBI).
Note: (2a): SEX = sexual enhancement expectancies; (2b): CBI = cognitive and behavioral impairment expectancies.
Cognitive and behavioral impairment expectancy ratings were significantly associated with lifetime CUD, p <.01; OR =.93 (CI = .88 – .95). Individuals with higher levels of cognitive and behavioral impairment expectancies were less likely to meet criteria for a CUD compared to individuals with lower levels of these cognitions. No significant interactions between ADHD and expectancy subscales were found in relation to CUD.
Discussion
Previous research has suggested that marijuana expectancies function uniquely in certain populations and that individual differences in expectancy acquisition and utilization may be important for understanding marijuana use vulnerability. Despite being at increased risk for marijuana use problems, research has not directly studied marijuana expectancies among individuals with ADHD. Results from this study indicate for the first time that individuals with ADHD endorse lower levels of marijuana use expectancies for both negative (cognitive and behavioral impairment) and positive (social enhancement and tension reduction) expectations compared to individuals without ADHD. In addition, specific marijuana expectancies (sexual enhancement and cognitive and behavioral impairment) were differentially associated with marijuana use for the ADHD versus nonADHD group. These results have important implications for understanding the role of cognitions in ADHD-related risk of marijuana use and point to new research possibilities that may have treatment implications for this population.
One school of thought with regard to high risk populations, such as children with ADHD, is that they should be more likely to endorse positive marijuana use expectancies as a result of their elevated impulsivity levels and associated reward-seeking tendencies (Smith & Anderson, 2001). We found, instead, lower levels of expectancy endorsements in the ADHD group. This finding is important for multiple reasons. First, it extends our prior similar findings for alcohol use expectancies (Pedersen et al., 2014) to those for marijuana, suggesting that ADHD may be associated with a decreased awareness of the subjective effects of psychoactive substances with different psychoactive profiles. These results also suggest that the dual process model offered by Stacey and Weirs (2010), in which implicit cognitive processes, as compared to explicit cognitions, are more strongly associated with substance use behaviors among individuals with executive functioning difficulties, may explain the role of marijuana expectancies for individuals with ADHD.
Further supporting the applicability of this theory, we found that expectancies for the negative effects of marijuana use on cognitive and behavioral performance were less strongly associated with marijuana use for the ADHD versus nonADHD group. Thus, in addition to reporting that marijuana was less likely to cause impairments such as difficulty thinking and slowed responses, a disconnect between these explicitly reported cognitions and marijuana use was evident for the ADHD group relative to the nonADHD group. This finding has important theoretical and clinical implications.
Stacy and Wiers (2010) posit that the difficulties in utilizing explicit cognitions to guide behavior are due, in part, to cognitive processing deficits. In a study examining the relationship between implicit and explicit cognitions and working memory in the prediction of alcohol use, Thush et al. (2008) showed that implicit cognitions (measured indirectly using tasks that do not require self-reflection) were more related to alcohol use for individuals with lower working memory. Studies in nonADHD samples examining non-substance using behaviors (sex and eating) have suggested that individuals low in working memory capacity may be less likely to utilize explicit cognitions to regulate their behaviors (Hofmann, Gschwendner, Friese, Wiers, Schmitt, 2008). The results of several meta analyses indicate that, relative to typically developing peers, individuals diagnosed with ADHD often exhibit deficits in working memory (Kasper, Alderson, & Hudec, 2012; Martinussen, Hayden, Hogg-Johnson, & Tannock, 2005). Thus, although not directly tested in the current study, deficient working memory may be responsible for the diminished association between marijuana use and expectations of cognitive and behavioral impairment.
Although speculative, feedback loops between expectancy formation and working memory-related processing of social cues may be deficient. Working memory has been shown to be involved in successfully navigating the social environment, and deficits in working memory among individuals with ADHD have been associated with impairments in this area (Kofler et al., 2011). Difficulties in working memory may negatively affect the encoding, storage, and retrieval of information necessary to effectively process information about marijuana use (e.g., its consequences) in the social environment. For example, although marijuana use was not included, Matthys, Cuperus, and Engeland (1999) found that individuals with ADHD encoded fewer social cues and generated fewer responses when compared to their nonADHD peers. Thus, deficits in working memory for individuals with ADHD may result in a reduced ability to effectively process the association between marijuana consumption and its effects (at least the ones that result in social feedback). This lack of association may then result in reduced expectancy formation (positive and negative) for marijuana use.
These interpretations are somewhat complicated by our finding of stronger associations in the ADHD versus nonADHD group between the sexual enhancement expectancies and marijuana use. Although these expectancies were reported at lower absolute levels by those with ADHD histories, the association between these beliefs (e.g., enjoying sex more and performing better) and marijuana use was positive and significant in the ADHD group but not in the nonADHD group. Some research has shown that individuals with ADHD may be more sensitive to rewards (for review see: Luman, Tripp, & Scheres, 2010). Although speculative, one possible interpretation is that access and utilization of expectancies regarding intensely pleasurable activities may be enhanced for this group due to elevated reward sensitivity. Interestingly, Lee and Humphreys (2014) recently reported that children with ADHD symptoms and possible genetic predisposition to behaviors that include reward sensitivity (via the 7-repeat allele of the DRD4 gene) were especially likely to report expectancies of “wild and crazy” behavior following alcohol use. Whether these beliefs translate into expectancies of sexual enhancement following experience with substances remains to be studied.
Our findings should be interpreted in the context of the marijuana use levels in our sample. We did not find overall group differences in lifetime use of marijuana. More than half of our young adults had tried marijuana and a significant minority had used it recently. The absence of group differences may reflect the developmentally limited higher rate of marijuana use in the United States for young adults regardless of their childhood risk profile. Among the smaller subgroup who reported recent use, frequency of use was somewhat higher in the ADHD versus nonADHD group. Also, individuals in the ADHD group initiated marijuana use at a younger age compared to their nonADHD peers. Given the established association of early initiation of marijuana use with later internalizing and externalizing behaviors (Fergusson, Horwood, & Swain-Campbell, 2002) and substance use disorders (for review see: Chen, Storr, & Anthony, 2009), this finding suggests a possible earlier-starting trajectory of marijuana use that may lead to enduring problems in the ADHD group. It remains to be seen whether this tendency is observed in the ADHD group as they approach their fourth decade of life.
The results of this study must be viewed within the context of several methodological considerations. This study sought to examine marijuana use expectancies among young adults with and without ADHD and the degree to which expectancy domains were differentially associated with marijuana use outcomes. As such, inclusion of the well-established co-occurrence of conduct problems in our analyses was not pertinent to our study aims (although it is highly relevant in other ADHD-related pathways to substance use, see Molina & Pelham, 2014). Additionally, the PALS participants were referred in childhood to a clinic for ADHD and may not be representative of all children with ADHD. Also, our measure of marijuana use expectancies was adapted from an alcohol expectancies questionnaire and it is possible that not all marijuana use expectancy domains were assessed. There was, however, overlap with the major expectancy categories reported for an established marijuana use expectancy questionnaire (MEEQ; Schafer & Brown, 1991). Similar to other studies (Aarons, Brown, Stice, & Coe, 2001; Clark, Ringwalt, & Shamblen, 2011; Hayaki et al., 2010), both positive and negative expectancies were associated with marijuana use. As such, we are confident that this adapted measure captured important marijuana use expectancy domains. Lastly, marijuana expectancies and marijuana use were assessed at the same time point in adulthood so we are unable to examine the developmental process of expectancies and if this differs for individuals with ADHD. This is an important next step in understanding why individuals with ADHD are at increased risk for marijuana use and CUD.
Conclusion
Compared to those without ADHD, individuals with childhood ADHD reported lower levels of negative, as well as positive, marijuana use expectancies. Additionally, marijuana expectancies were found to relate to marijuana use differently for individuals with and without ADHD. Such differences suggest that the explicit cognitions regarding the positive and negative consequences of marijuana use operate differently for this population. The differences highlighted in this study may be evidence of difficulties in higher order cognitive processes; these difficulties, such as those seen in working memory processes, may negatively affect the encoding and utilization of explicit marijuana use expectations. Laboratory based studies aimed at further understanding the degree to which individuals with ADHD perceive and experience impairments and benefits of marijuana use are needed. It is also notable that across all measurements, individuals diagnosed with ADHD endorsed lower rates of perceived cognitive and behavioral impairment resulting from marijuana use. Future studies may wish to further explore the perceived positive and negative cognitive and behavioral consequences of marijuana use among those diagnosed with ADHD.
Footnotes
No commercial relationships to declare
References
- Aarons GA, Brown SA, Stice E, Coe MT. Psychometric evaluation of the marijuana and stimulant effect expectancy questionnaires for adolescents. Addict Behav. 2001;26(2):219–236. doi: 10.1016/s0306-4603(00)00103-9. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage Publications; Newbury Park: 1991. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed. Author; Washington, DC: 2013. [Google Scholar]
- Arria AM, Caldeira KM, O’Grady KE, Vincent KB, Fitzelle DB, Johnson EP, Wish ED. Drug exposure opportunities and use patterns among college students: results of a longitudinal prospective cohort study. 2008 doi: 10.1080/08897070802418451. doi: 10.1080/08897070802418451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1990;29(4):546–557. doi: 10.1097/00004583-199007000-00007. doi: 10.1097/00004583-199007000-00007. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Murphy KR, Fischer M. Adult ADHD: What the science says. Guilford; New York: 2008. [Google Scholar]
- Barnow S, Schultz G, Lucht M, Ulrich I, Preuss UW, Freyberger HJ. Do alcohol expectancies and peer delinquency/substance use mediate the relationship between impulsivity and drinking behaviour in adolescence? Alcohol Alcohol. 2004;39(3):213–9. doi: 10.1093/alcalc/agh048. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Dolan C, Hughes S, Mick E, Monuteaux MC, Faraone SV. The long-term longitudinal course of oppositional defiant disorder and conduct disorder in ADHD boys: findings from a controlled 10-year prospective longitudinal follow-up study. Psychol Med. 2008;38(7):1027–1036. doi: 10.1017/S0033291707002668. doi: 10.1017/S0033291707002668. [DOI] [PubMed] [Google Scholar]
- Chen CY, Storr CL, Anthony JC. Early-onset drug use and risk for drug dependence problems. Addictive Behaviors. 2009;34(3):319–322. doi: 10.1016/j.addbeh.2008.10.021. doi: DOI 10.1016/j.addbeh.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark HK, Ringwalt CL, Shamblen SR. Predicting adolescent substance use: the effects of depressed mood and positive expectancies. Addict Behav. 2011;36(5):488–493. doi: 10.1016/j.addbeh.2011.01.018. doi: 10.1016/j.addbeh.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Conway KP, Swendsen JD, Merikangas KR. Alcohol expectancies, alcohol consumption, and problem drinking: the moderating role of family history. Addict Behav. 2003;28(5):823–836. doi: 10.1016/s0306-4603(02)00265-4. [DOI] [PubMed] [Google Scholar]
- Dattilo L, Murphy KG, Van Eck K, Flory K. Do ADHD symptoms moderate the relation between positive alcohol expectancies and alcohol-related outcomes? Atten Defic Hyperact Disord. 2013;5(2):93–104. doi: 10.1007/s12402-012-0098-y. doi: 10.1007/s12402-012-0098-y. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Darkes J, Goldman MS, Smith GT. Advancing the expectancy concept via the interplay between theory and research. Alcohol Clin Exp Res. 2002;26(6):926–935. [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch Gen Psychiatry. 2007;64(10):1145–1152. doi: 10.1001/archpsyc.64.10.1145. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97(9):1123–1135. doi: 10.1046/j.1360-0443.2002.00103.x. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM–IV Axis II Personality Disorders (SCID–II) American Psychiatric Publishing; Arlington, VA: 1997. [Google Scholar]
- Fromme K, Stroot E, Kaplan D. Comprehensive effects of alcohol: Development and psychometric assessment of a new expectancy questionnaire. Psychological Assessment. 1993;5 [Google Scholar]
- Goldman MS. Expectancy and risk for alcoholism: the unfortunate exploitation of a fundamental characteristic of neurobehavioral adaptation. Alcohol Clin Exp Res. 2002;26(5):737–746. [PubMed] [Google Scholar]
- Harty SC, Ivanov I, Newcorn JH, Halperin JM. The impact of conduct disorder and stimulant medication on later substance use in an ethnically diverse sample of individuals with attention-deficit/hyperactivity disorder in childhood. J Child Adolesc Psychopharmacol. 2011;21(4):331–339. doi: 10.1089/cap.2010.0074. doi: 10.1089/cap.2010.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaki J, Hagerty CE, Herman DS, de Dios MA, Anderson BJ, Stein MD. Expectancies and marijuana use frequency and severity among young females. Addictive Behaviors. 2010;35(11):995–1000. doi: 10.1016/j.addbeh.2010.06.017. doi: DOI 10.1016/j.addbeh.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W, Gschwendner T, Friese M, Wiers RW, Schmitt M. Working memory capacity and self-regulatory behavior: toward an individual differences perspective on behavior determination by automatic versus controlled processes. J Pers Soc Psychol. 2008;95(4):962–977. doi: 10.1037/a0012705. doi: 10.1037/a0012705. [DOI] [PubMed] [Google Scholar]
- Hoza B, Pelham WE, Jr., Dobbs J, Owens JS, Pillow DR. Do boys with attention-deficit/hyperactivity disorder have positive illusory self-concepts? J Abnorm Psychol. 2002;111:268–278. doi: 10.1037//0021-843x.111.2.268. [DOI] [PubMed] [Google Scholar]
- Jessor R, Donovan JE, Costa FM. Health Behavior Questionnaire. Institute of Behavioral Science, University of Colorado; Boulder: 1989. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Marijuana use continues to rise among U.S. teens, while alcohol use hits historic lows. University of Michigan News Service. 2011 Retrieved from http://www.monitoringthefuture.org/data/11data.html#2011data-drugswebsite.
- Kalichman SC, Weinhardt L, DiFonzo K, Austin J, Luke W. Sensation seeking and alcohol use as markers of sexual transmission risk behavior in HIV-positive men. Ann Behav Med. 2002;24(3):229–235. doi: 10.1207/S15324796ABM2403_08. [DOI] [PubMed] [Google Scholar]
- Kasper LJ, Alderson RM, Hudec KL. Moderators of working memory deficits in children with attention-deficit/hyperactivity disorder (ADHD): a meta-analytic review. Clin Psychol Rev. 2012;32(7):605–617. doi: 10.1016/j.cpr.2012.07.001. doi: 10.1016/j.cpr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Bolden J, Sarver DE, Raiker JS, Alderson RM. Working memory deficits and social problems in children with ADHD. J Abnorm Child Psychol. 2011;39(6):805–817. doi: 10.1007/s10802-011-9492-8. doi: 10.1007/s10802-011-9492-8. [DOI] [PubMed] [Google Scholar]
- Kristjansson SD, Agrawal A, Lynskey MT, Chassin LA. Marijuana expectancies and relationships with adolescent and adult marijuana use. Drug Alcohol Depend. 2012;126(1-2):102–110. doi: 10.1016/j.drugalcdep.2012.04.024. doi: 10.1016/j.drugalcdep.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clinical Psychology Review. 2011;31(3):328–341. doi: 10.1016/j.cpr.2011.01.006. doi: DOI 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Humphreys KL. Interactive association of dopamine receptor (DRD4) genotype and ADHD on alcohol expectancies in children. Experimental and Clinical Psychopharmacology. 2014;22(2):100–109. doi: 10.1037/a0035338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luman M, Tripp G, Scheres A. Identifying the neurobiology of altered reinforcement sensitivity in ADHD: a review and research agenda. Neurosci Biobehav Rev. 2010;34(5):744–754. doi: 10.1016/j.neubiorev.2009.11.021. doi: 10.1016/j.neubiorev.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Malmberg M, Kleinjan M, Vermulst AA, Overbeek G, Monshouwer K, Lammers J, Engels RC. Do substance use risk personality dimensions predict the onset of substance use in early adolescence? A variable- and person-centered approach. J Youth Adolesc. 2012;41(11):1512–1525. doi: 10.1007/s10964-012-9775-6. doi: 10.1007/s10964-012-9775-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2005;44(4):377–384. doi: 10.1097/01.chi.0000153228.72591.73. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- Matthys W, Cuperus JM, Engeland HV. Deficient social problem-solving in boys with ODD/CD, with ADHD, and with both disorders. J Am Acad Child Adolesc Psychiatry. 1999;38(3):311–321. [PubMed] [Google Scholar]
- Molina BS, Hinshaw SP, Eugene Arnold L, Swanson JM, Pelham WE, Hechtman L, Marcus S. Adolescent substance use in the multimodal treatment study of Attention-Deficit/Hyperactivity Disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. J Am Acad Child Adolesc Psychiatry. 2013;52(3):250–263. doi: 10.1016/j.jaac.2012.12.014. doi: 10.1016/j.jaac.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BS, Pelham WE., Jr. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol. 2003;112(3):497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE., Jr. Attention-Deficit/Hyperactivity Disorder and risk of substance use disorder: Developmental considerations, potential pathways, and opportunities for research. Annual Review of Clinical Psychology. 2014;10:607–639. doi: 10.1146/annurev-clinpsy-032813-153722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. Attention deficits and hyperactivity-impulsivity: What have we learned, what next? Development and Psychopathology. 2013;25:1489–1503. doi: 10.1017/S0954579413000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SL, Harty SC, Pelham WE, Gnagy EM, Molina BS. Differential associations between alcohol expectancies and adolescent alcohol use as a function of childhood ADHD. J Stud Alcohol Drugs. 2014;75(1):145–152. doi: 10.15288/jsad.2014.75.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Jr., Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry. 1992;31(2):210–218. doi: 10.1097/00004583-199203000-00006. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Roizen NJ, Blondis TA, Irwin M, Rubinoff A, Kieffer J, Stein MA. Psychiatric and developmental disorders in families of children with attention-deficit hyperactivity disorder. Arch Pediatr Adolesc Med. 1996;150(2):203–208. doi: 10.1001/archpedi.1996.02170270085013. [DOI] [PubMed] [Google Scholar]
- Schafer J, Brown SA. Marijuana and cocaine effect expectancies and drug use patterns. J Consult Clin Psychol. 1991;59(4):558–565. doi: 10.1037//0022-006x.59.4.558. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Walitzer KS, Wood PK, Brent EE. Characteristics of children of alcoholics: putative risk factors, substance use and abuse, and psychopathology. J Abnorm Psychol. 1991;100(4):427–448. doi: 10.1037//0021-843x.100.4.427. [DOI] [PubMed] [Google Scholar]
- Smith GT, Anderson KG. Adolescent risk for alcohol prob- lems as acquired preparedness: A model and suggestions for intervention. In: Monti PM, Colby SM, O’Leary TA, editors. Adolescents, alcohol, and substance abuse: Reaching teens through brief interventions. Guilford Press; New York, NY: 2001. pp. 109–141. [Google Scholar]
- Stacy AW, Wiers RW. Implicit cognition and addiction: a tool for explaining paradoxical behavior. Annu Rev Clin Psychol. 2010;6:551–575. doi: 10.1146/annurev.clinpsy.121208.131444. doi: 10.1146/annurev.clinpsy.121208.131444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . National Household Survey on Drug Abuse. Author; Rockville, MD: 1992. Retrieved from http://www.icpsr.umich.edu/icpsrweb/SAMHDA/studies/6887. [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. Author; Rockville, MD: 2013. NSDUH Series H-44, HHS Publication No. (SMA) 12-4713. [PubMed] [Google Scholar]
- Thush C, Wiers RW, Ames SL, Grenard JL, Sussman S, Stacy AW. Interactions between implicit and explicit cognition and working memory capacity in the prediction of alcohol use in at-risk adolescents. Drug Alcohol Depend. 2008;94(1-3):116–124. doi: 10.1016/j.drugalcdep.2007.10.019. doi: 10.1016/j.drugalcdep.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. A view through the gateway: expectancies as a possible pathway from alcohol to cannabis. Addiction. 2001;96(5):691–703. doi: 10.1046/j.1360-0443.2001.9656915.x. doi: 10.1080/09652140020039062. [DOI] [PubMed] [Google Scholar]