Abstract
Purpose
Cardiotoxic side effects of anthracyclines limit their use as effective chemotherapeutics. One model for the mechanism of anthracycline induced cardiotoxicity is attributed to the generation of intracellular reactive oxygen species (ROS). However this theory has been questioned because several cardioprotective strategies have included the use of antioxidants without significant clinical benefit. We sought to determine whether measurement of intracellular reactive oxygen species after anthracycline exposure in vivo and in vitro could provide a means for designing more effective antioxidant cardioprotective schemes.
Methods
Intracellular levels of ROS were assessed in peripheral blood mononuclear cells from leukemia bearing mice exposed to anthracyclines and in patients receiving anthracyclines. Comparison of cell death induction and ROS levels were also conducted in vitro in cardiomyocyte and leukemia lines. ROS blockade using antioxidants was conducted and effects on cell death were assessed.
Results
Elevated ROS in blood of mice and representative patient samples correlated with cardiomyocyte necrosis and decreased ejection fraction. In vitro, comparison of the cytotoxic effects of anthracyclines in acute leukemia cells and in cardiomyocytes revealed distinct kinetics of cell death induction and dependence upon oxidative stress. Although apoptotic cell death was observed in both acute leukemia cells and cardiomyocytes, the antioxidant N-acetylcysteine protected cardiomyocytes but not acute leukemia cells from anthracycline cytotoxicity.
Conclusions
Our findings point towards revisiting the use of NAC as a cardioprotective agent since it does not appear to interfere with the cytotoxic action of anthracyclines. NAC has been evaluated clinically for cardioprotective activity but future trials must ensure that adequate dose, scheduling and incorporation of markers of oxidative stress are included.
Keywords: Anthracyclines, cardiotoxicity, oxidative stress, cell death
Introduction
Anthracyclines are among the most effective drugs used for the treatment of oncologic diseases including leukemia, lymphoma and solid tumors (1). However, their use is tempered by dose-dependent cardiotoxicity in a subset of patients (2). Cardiac abnormalities such as systolic dysfunction develop in up to 60% of patients exposed to high dose anthracyclines alone or in combination with other treatment modalities (2, 3). Multiple factors, including genetic polymorphisms, may account for differences in the incidence, severity and timing of cardiotoxicity among patients exposed to anthracyclines (4, 5). However these risk factors are not considered in altering anthracycline dosing or in delivering cardioprotective interventions. While consideration of these risk factors is taken into account when prescribing long term follow up guidelines, prevention of cardiotoxic late effects remains challenging.
Understanding the mechanism of action of anthracyclines has potential to identify cardioprotective interventions. The cardiotoxic action of anthracyclines involves multiple mechanisms: inhibition of the topoisomerase II (Top2) enzyme (mainly Top2 alpha), thereby blocking DNA transcription and replication by causing DNA strand breaks and generation of iron mediated, and ROS generation (leading to mitochondrial DNA damage, lipid peroxidation, protein carbonylation, energy depletion and apoptosis) (6–9). These free radicals may continue to be generated even after treatment with doxorubicin has ceased, which provides an explanation for late onset cardiotoxicity (10) which occurs at least one year after completion of therapy and leads to irreversible heart damage and congestive heart failure (2, 11, 12).
Several strategies have been used to decrease anthracycline induced cardiotoxicity. These include limiting the cumulative dose, altering administration of anthracyclines, use of analogues of anthracyclines or special formulations (liposomes), and use of cardioprotectants concomitant with anthracycline administration (2, 13). The only FDA approved agent used for anthracycline cardiotoxicity is dexrazoxane (Zinecard, also known as ICRF-187). Studies have suggested that this agent works by antagonizing doxorubicin-induced DNA damage through its interference with Topoisomerase II beta (14) and also by reducing mitochondrial iron levels, which is associated with deleterious effects of doxorubicin in cardiomyocytes in vitro and are elevated in patients with doxorubicin induced cardiomyopathy (15). Despite these molecular insights into the role of free radicals in contributing to cardiotoxicity, the use of antioxidants as cardioprotective agents has been controversial. In many cases, antioxidants have shown protective effects in studies performed in vitro and in mouse models but have not shown similar effects in clinical trials. Study design and lack of monitoring of biomarkers for oxidative stress in clinical studies may provide an explanation for these disparate results (16). Here, we present data supporting a role for ROS measured in peripheral blood as a biomarker for cardiotoxic events in mice and in a cohort of pediatric cancer patients receiving anthracyclines. We also conducted mechanistic comparisons of cell death induction by anthracyclines in acute leukemia cells and cardiomyocytes and assessed the use antioxidants on cytotoxicity of anthracyclines in these models.
Material and Methods
Cell lines and culture conditions
Acute leukemia cells used included ML-1 (M5 human acute myelogenous leukemia derived from a patient that relapsed after initial T cell acute lymphoblastic leukemia), AML-3 (M4 human acute myelogenous leukemia) and Jurkat (human T cell acute lymphocytic leukemia). The H9C2 cardiomyocyte cell line (derived from embryonic rat heart tissue) was also used. All cell lines were obtained from the American Type Culture Collection (Rockville, MD). The acute leukemia lines were maintained in RPMI 1640 media, supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml of streptomycin, HEPES and 4 mM glutamine. H9C2 cells were cultured in high glucose Dulbecco-modified MEM, supplemented with 10% of fetal bovine serum, 100 U/ml of penicillin and 100 µg/ml of streptomycin and 4 mM glutamine. Cells were fed every 2–3 days, and sub-cultured once they reached 70–80% confluence to prevent loss of differentiation potential for no more than three months after thawing. All cells were maintained in a humidified atmosphere of 5% of CO2. Routine mycoplasma testing and DNA fingerprinting were conducted to ensure the cells were not contaminated and were verified.
Chemicals and reagents
Daunorubicin, reduced glutathione ethylester, N-acetylcysteine, reduced glutathione, propidium iodide (PI) were all purchased from Sigma Aldrich (St Louis, MO). Doxorubicin was purchased from LC Laboratories. Zvad-FMK, and caspase-3 substrate AC-DEVD-AMC were purchased from Enzo Life Sciences (Farmingdale, NY). Hydroethidium (HE) dye was obtained from Molecular Probes (Eugene, OR).
Cell viability and cell death assays
Viability was quantified by trypan blue exclusion using a Vi-cell analyzer (Beckman Coulter, Brea, CA). For analysis of percent of cells with subdiploid amounts of DNA, indicative of apoptotic DNA fragmentation, cells were harvested and then stained with propidium iodide (PI) solution (50 µ/ml PI, 0.1% Triton X and 0.1% of sodium citrate) for a minimum of 4 hours. Samples were read on the FL-3 channel of a flow cytometry (FACSCalibur, Becton Dickinson, Franklin Lakes, NJ). Subdiploid population was quantified using CellQuest software (BD Bioscience, Franklin Lakes, NJ).
Caspase-3 activity
Cells were harvested and pelleted. Pellets were resuspended with 50 µL of PBS for every 1 million cells and frozen on dry ice and plated onto a 96 well opaque plate in duplicate. For time course experiments, cells were continuously added to plates stored at −80 °C so that all samples were processed together. DEVD-AMC buffer was prepared by mixing 100 mM HEPES, 10% sucrose, 5mM DTT, 0.0001 % IGEPAL and 0.1 CHAPS and the solution pH was adjusted at 7.25. Once all samples were collected, they were allowed to thaw and 50 µM DEVD-AMC was added to the buffer at a dilution of 1:500. To each well containing 50 µL of cell suspension 150 µL of the mixture of DEVD-AMC Buffer + DEVD was added. The 96-well plate was then incubated for 3 hours at room temperature in the dark. Plates were read on the SpectraMax GeminiEM using 355 nm excitation and 460 nm wavelengths for emission. Fluorescence generated by the cleavage of the fluorogenic peptide is proportional to caspase-3 activity of each sample.
Quantification of cellular glutathione (GSH) levels
GSH standards were prepared prior to harvesting cells. Equal numbers of harvested cells were aliquoted to new tubes and 50 µM of monochlorobimane (mBCL) was added to each sample, except for the unstained control. Cells were incubated with mBCL for 15 minutes at 37°C. After incubation, the reaction was stopped by adding trichloroacetic acid to a final concentration of 5%. Samples were collected and an equal volume of methylene chloride was added, followed by centrifugation at 3500 RPM for 2 minutes. After centrifugation, 200 µl from the aqueous phase was collected in a well of a white opaque 96 well plate. Fluorescence was measured using a microplate reader (Spectra Gemini EM, Molecular Devices, Inc. Sunnyvale CA) with excitation 398 nm and emission of 488 nm. GSH level was calculated using linear regression from the standard curve.
Evaluation of effects of anthracyclines in vivo
Female SCID/NCr (BALB/c) background) mice were purchased from NCI-Frederick (Frederick, MD, U.S.A.). Mice were allowed to acclimate to their new environment for a minimum of 1 week and were housed at a maximum of 5 mice per cage in a barrier facility with regulated temperature and light cycles. Animal experiments were performed in accordance with the MD Anderson Institutional Animal Care and Use Committee and approved by the American Association for Laboratory Animal Science (AALAS). Mice were divided into 2 groups of 5 mice each. Doxorubicin (6 mg/kg) or PBS was administered via tail vein injection every other week for three weeks. Mice were euthanized on day 36, and hearts were removed and placed in formaldehyde. Paraffin slides were prepared for H & E staining and were examined by an experienced veterinarian pathologist, who was blinded to the treatment regimen. Assessment of the myocardium for signs of histologic damage was based on the guidelines established by Bertazzoli et al for evaluation of doxorubicin cardiotoxicity in the mouse (17). For each mouse, upon euthanization, blood samples were obtained and Ficoll density centrifugation was used to isolate mononuclear cells for measurement of intracellular superoxide levels.
Measurement of ROS levels and cardiac function in patient samples
After informed consent was obtained according to a protocol approved by the University of Texas MD Anderson’s Institutional Review Board which conforms to the ethical guidelines of the Declaration of Helsinki, blood samples were obtained from 5 pediatric patients before and after treatment with doxorubicin. Mononuclear cells were isolated by Ficoll density centrifugation. Dichlorofluoroscein staining was used to measure intracellular peroxides in patient samples. Samples were washed in PBS and resuspended with H2DCFDA solution and incubated for 30 minutes at 37 degrees Celsius in the dark. Samples were centrifuged and resuspended in PBS to be read on the FL-1 channel of the flow cytometer. Mean fluorescence of each sample was calculated using CellQuest software. Consistent with standard of care guidelines, echocardiograms were performed on patients and left ventricular ejection fraction values were obtained prior to treatment and after exposure to doxorubicin.
Statistical analyses
The data obtained in the in vitro experiments represents the mean with the standard error of the mean (SEM) from 3 independent experiments performed using GraphPad Prism version 6.0 for Windows, Graph Pad Software, La Jolla, California, USA (www.graphpad.com). The Student t-test was performed to determine statistical significant differences between samples. A p-value less than 0.05 was considered statistically significant.
Results
In vivo exposure to anthracyclines is accompanied by cytotoxic changes in cardiac tissue and increased ROS levels in peripheral blood monocytes
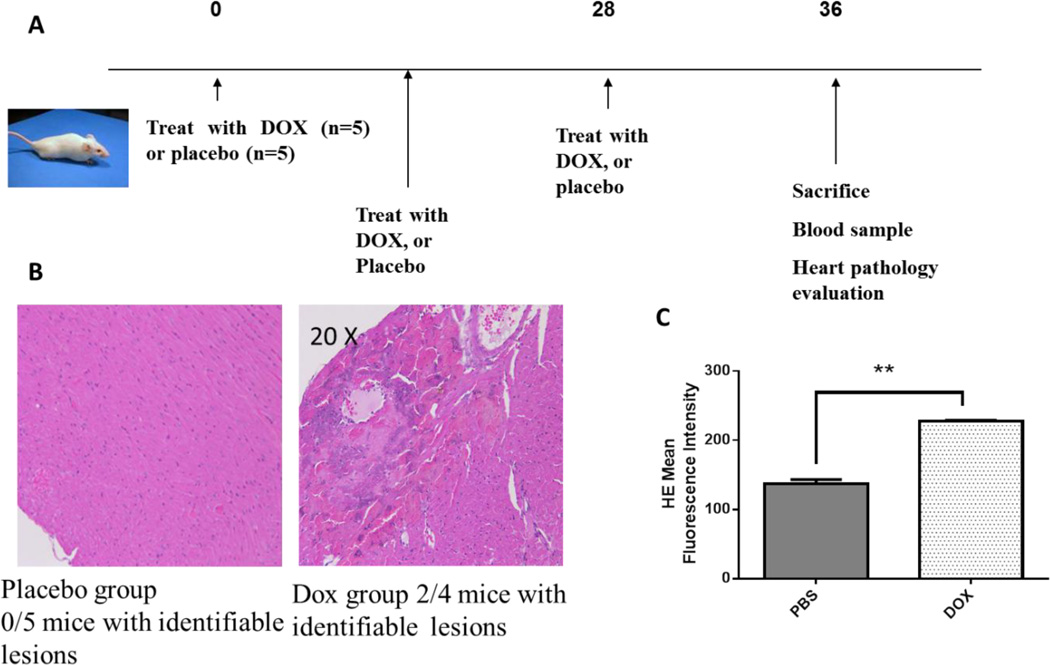
Cardiotoxicity has been associated with exposure to anthracyclines in mice and humans, and ROS have been implicated in the mechanism of action. However few studies have examined whether ROS in peripheral blood mononuclear cells can be followed as a surrogate for cardiotoxic events. To address this question, we adopted a previously published model for in vivo exposure to the anthracycline, doxorubicin. As depicted in Figure 1A, mice were exposed to 6 mg/kg doxorubicin on day 0, 14 and 28 and then sacrificed on day 36. Evaluation of cardiac tissue harvested from these mice by a veterinary pathologist blinded to experimental conditions revealed that 2/4 mice had histologic lesions of the heart that mainly consisted of striation and signs of necrosis (Fig. 1B). One of the doxorubicin treated mice died on day 32, and we were unable to assess cardiac abnormalities. In contrast, in the PBS treated group no histologic lesions could be identified in the cardiac tissue of five mice evaluated. Peripheral blood mononuclear cells were harvested from the tail veins of mice and ROS levels were quantified by measurement of intracellular superoxide levels using dihydroethidium staining and subsequent flow cytometric detection. A significantly higher level of intracellular superoxide was seen in doxorubicin treated mice compared to PBS treated mice (Fig. 1C). Collectively, these findings confirm the cytotoxic effects of anthracyclines on heart tissue in vivo and also that increased ROS levels elicited by anthracycline treatment are measurable in peripheral blood.
Figure 1. Anthracyclines induce cytotoxic changes in cardiac tissue and increase ROS levels in monocytes. Doxorubicin cumulative dose is correlated with ROS levels.
A) Mice were treated with placebo (n=5) or DOX (n=5) on days 0, 14 and 28 and were sacrificed at day 36. B) Hematoxylin and Eosin staining of the left ventricular myocardium was performed. In the placebo group (left) 0/5 mice had identifiable histologic lesions in the heart. In the DOX treated group (right) 2/4 mice had histologic lesions consistent of change in striation and signs of necrosis. C) ROS levels were quantified by HE assay (measure of superoxide levels) and results expressed as Mean Fluorescence Intensity. DOX treated mice showed more than double ROS levels compared to PBS treated mice and this finding was statistically significant. ** p < 0.01.
Changes in ROS levels correlate with alterations of cardiac functions in patient samples
Pediatric sarcoma patients are routinely treated with anthracyclines, and through an IRB-approved protocol, we collected peripheral blood from four patients before and after doxorubicin exposure. Intracellular peroxide levels in peripheral blood was assessed in three osteosarcoma patients and in one patient diagnosed with unclassified sarcoma. Patients also had baseline echocardiograms prior to start therapy with doxorubicin and after treatment with doxorubicin. In three out of four patients, no significant effect on cardiac function was observed as measured by ejection fraction (EF) after doxorubicin exposure. However one patient (# 2) had a significant decrease in ejection fraction (Table 1). The same patient also had nearly a 3-fold increase in ROS levels: the largest increase seen in this limited cohort. These findings point to the possibility that spikes in ROS in peripheral blood of patients could be a biomarker for compromised cardiac function in patients exposed to anthracyclines.
Table 1. Patient with the highest increase in ROS levels had significant drop in ejection fraction.
DCF staining (measure peroxydes) was measured in 4 patients with different oncologic diagnosis prior to treatment (DCF1) and after treatment with DOX (DCF2). Staining was quantified by flow cytometry measurement of mean fluorescence intensity. Echocardiograms were performed at baseline and Ejection Fraction (EF) was recorded then (Pretx EF) and after treatment with DOX (Posttx EF). Patient 2 had near 3-fold increase in DCF staining with a significant drop in EF. Patients 1,3 and 4 did not have significant changes in ROS levels or in EF.
| Patient | Diagnosis | Gender | Age (yrs) |
Pretx ROS levels |
Posttx ROS levels * |
Pretx EF |
Posttx EF |
|---|---|---|---|---|---|---|---|
| 1 | Osteosarcoma | M | 16 | 26.61 | 42.7 | 55–60% | 55–60% |
| 2 | Osteosarcoma | F | 16 | 30.24 | 89.71 | 60–65% | 44% |
| 3 | Osteosarcoma | M | 18 | 23.11 | 27.41 | > 70% | 60–65% |
| 4 | Unclassified sarcoma | F | 4 | 26.51 | 49.32 | 59% | 53% |
Examination of anthracycline induced cell death in cardiomyocytes and cancer cells
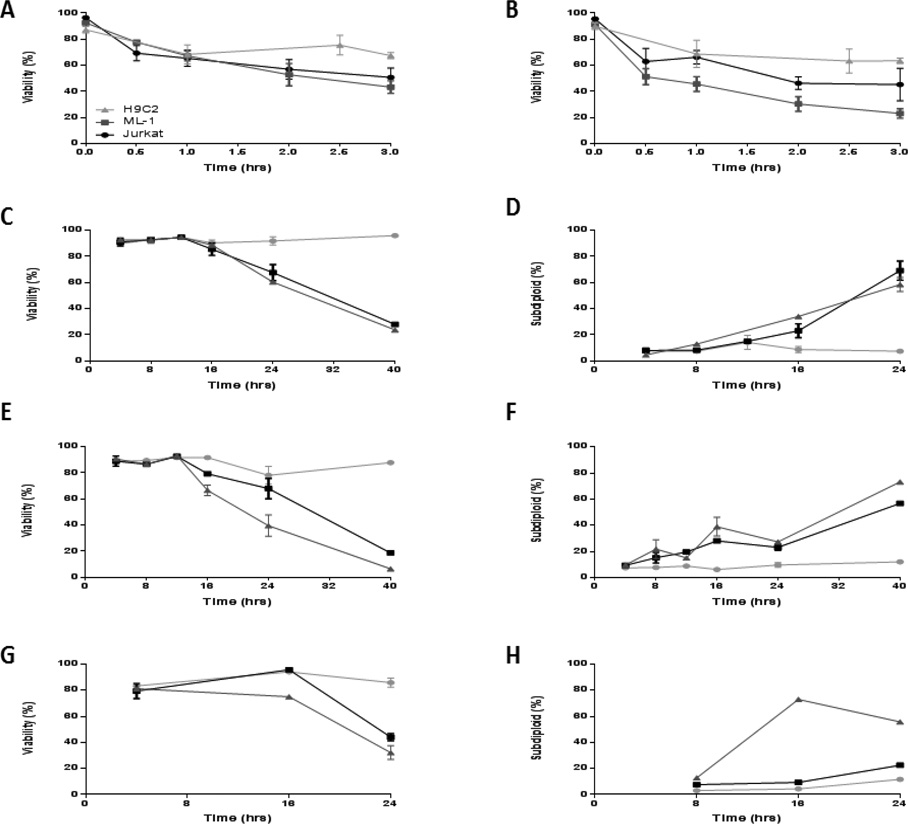
In order to determine the relationship between elevated ROS and cytotoxicity, we undertook experiments in cell lines. Dose response experiments using doxorubicin and daunorubicin were conducted in Jurkat and ML-1 cells (acute leukemia) and in H9C2 (cardiomyocyte) cells. Viability and DNA fragmentation were assessed by trypan blue exclusion and propidium iodide staining, respectively. Acute leukemia cells appeared to be slightly more sensitive than H9C2 cells to anthracyclines but this was not statistically significant (Figures 3A and 3B), therefore acute leukemia cells and cardiomyocytes showed similar sensitivity to anthracyclines at a fixed (24 h) time point. In order to address whether the kinetics of anthracycline induced cytotoxicity were different, time course experiments were performed at a range of times spanning 4 h to 40 h in the three cell lines. In general, viability and DNA fragmentation trends were similar, but differences in sensitivity at later time points were apparent between the leukemia lines and cardiomyocyte cell line. Viability decreased at 24 hours in all 3 cell lines with a preceding or concomitant increase in DNA fragmentation at that time (Figures 2 C–H).
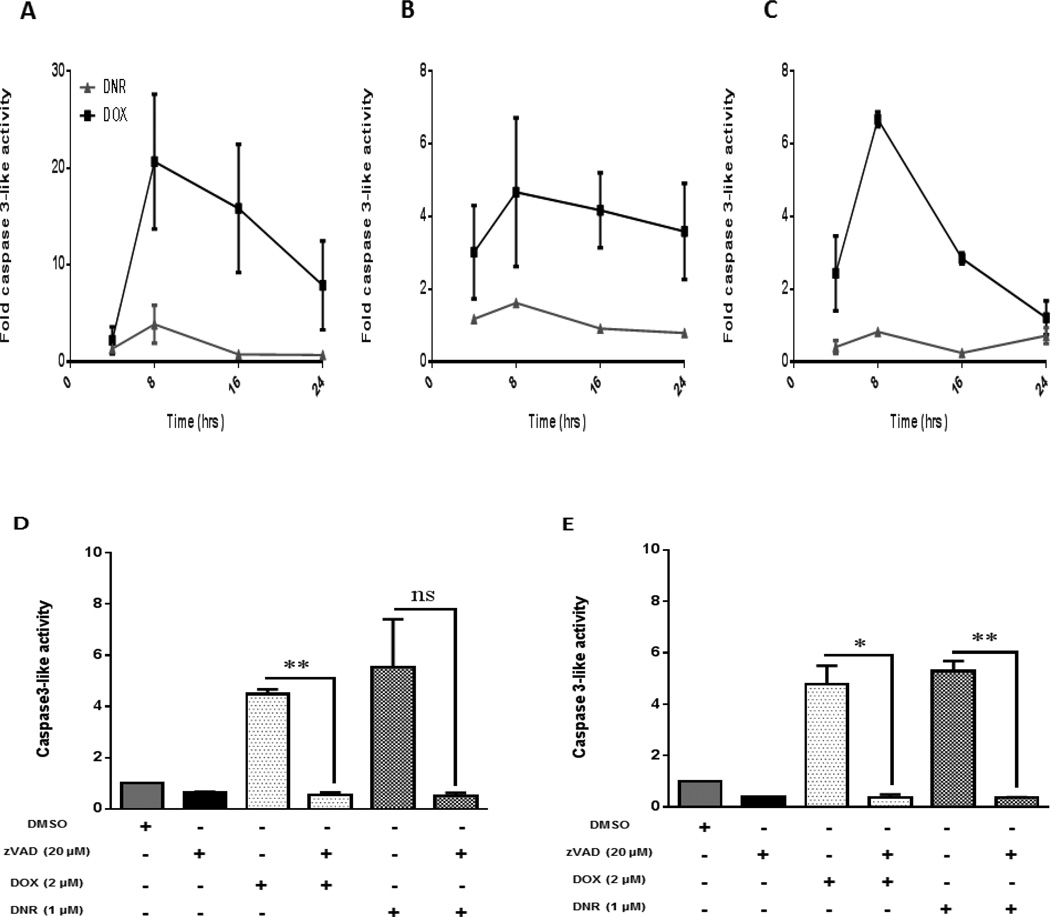
Figure 3. Caspase-3 activation precedes changes in viability and DNA fragmentation in acute leukemia cells and cardiomyocytes.
A) Jurkat B) ML-1 and C) H9C2 cells were treated at 4, 8, 16 and 24 hours with DOX and DNR. Mean fold increase of fluorescence (DEVD-AMC) was measured by spectrofluorometry as an indication of caspase-3 activity (results were normalized to control). D) Jurkat and E) ML-1 cells were pretreated with pancaspase inhibitor Z-vad-fmk (20 µM) and then exposed to anthracyclines for 24 hours. Caspase-3 activity was quantified by measurement of mean fold increase of fluorescence (DEVD-AMC) by spectrofluorometry. Results were normalized to control and represent the mean ± SEM of 3 different experiments Mean ± SEM of 3 different experiments are represented. *p<0.05, **p<0.01, ns= not significant.
Figure 2. Acute leukemia cells are slightly more sensitive to anthracyclines. Viability decreases and DNA fragmentation increases at 24 hours in acute leukemia cells and cardiomyocytes.
Jurkat (derived from patient with T-cell ALL) and ML-1 (derived from a patient with M5 AML) and cardiomyocyte H9C2 cell lines were treated with increasing doses of A) DOX and B) DNR (0.5 µM–3 µM) for 24 hours. Cell viability was quantified by trypan blue exclusion using a Vi-cell analyzer. Subsequently, Jurkat (C and D), ML-1 cells (E and F) were treated with DOX (2 µM) and DNR (1 µM) and H9C2 (G and H) were treated with 25 µM of DOX and DNR for different time points and viability and DNA fragmentation were assessed, respectively. The results represent the mean ± SEM of 3 different experiments.
We next tried to establish a temporal relationship between cell death and apoptosis as assessed by caspase-3 activation. Acute leukemia cell lines (Jurkat and ML-1) and the cardiomyocyte cell line H9C2 were treated at different time points (4, 8, 16 and 24 hours) with the anthracyclines doxorubicin and daunorubicin. Caspase-3 activity assay was performed and results obtained were normalized to control. In all cell lines, doxorubicin elicited caspase-3 activation with a peak effect at 8 hours. Daunorubicin did not elicit major caspase-3 activation in these cell lines, suggesting that an alternate mode of cell death or reliance on different caspase family members may be triggered by daunorubicin exposure (Figures 3 A–C). To further clarify the question of whether cell death in acute leukemia cell lines is a caspase dependent event, we pretreated the cell lines with the pan-caspase inhibitor z-vad-fmk at a dose of 20 µM for at least 30 minutes and then treated the cells with doxorubicin and daunorubicin for 24 hours and measured caspase-3 activity. For acute leukemia cells treated with either doxorubicin or daunorubicin, z-vad-fmk was protective against caspase activation (Figures 3D and E).
Kinetics of ROS alterations and cell death induction in anthracycline treated acute leukemia cells and cardiomyocytes
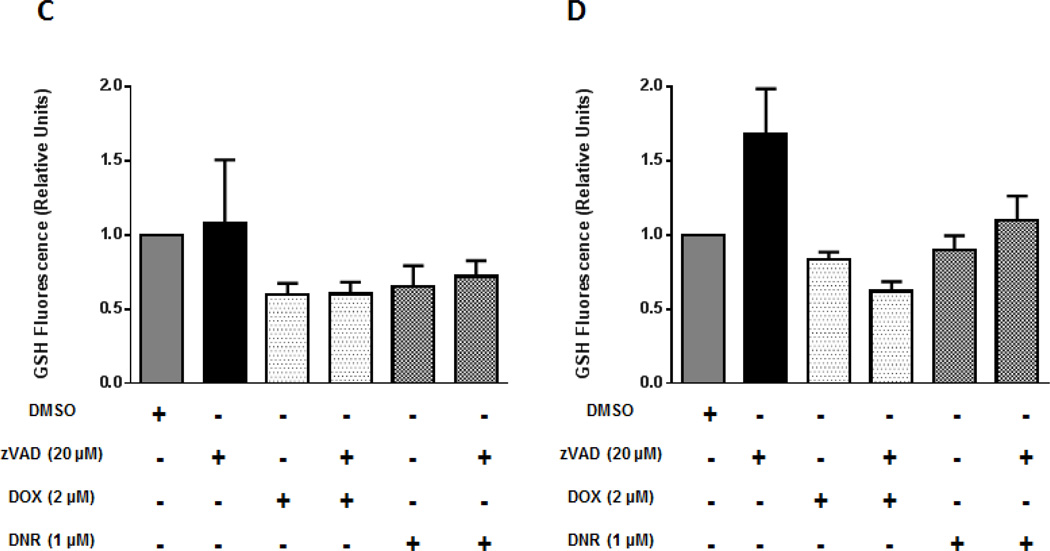
In order to understand the relationship between anthracycline induced cell death and ROS alterations, GSH levels were examined because the fluorescence of the anthracyclines in cell lines was found to interfere with the fluorescence emitted by the HE and DCF probes. GSH levels were measured at the height of caspase activation: 8h and did not significantly change in any of the three cell lines (Figures 4A and 4B). In order to establish if blockade of caspase activation affects GSH changes in acute leukemia cells we pretreated the cell lines with a pancaspase inhibitor z-VAD-fmk at a dose of 20 µM for at least 30 minutes and then treated the cells with doxorubicin and daunorubicin for 24 hours and quantified GSH levels. For acute leukemia cells, GSH levels were not changed in the presence of the caspase inhibitor, are therefore independent of caspase regulation in these cell lines (Figures 4C and 4D). Taken together, these findings suggest that manipulation of ROS levels in acute leukemia cells should not affect the cytotoxicity of anthracyclines in these cell lines.
Figure 4. Glutathione levels (GSH) do not significantly change before cell death in anthracycline treated acute leukemia cells and cardiomyocytes. GSH events are independent of caspase regulation in acute leukemia cells.
Jurkat, ML-1 and H9C2 cells were treated at 8 hours with A) DOX and B) DNR and GSH levels were measured by spectrofluorometry. Results were normalized to control. Jurkat and ML-1 cells were pretreated with pancaspase inhibitor Z-vad-fmk (20 µM) and then exposed to anthracyclines for 24 hours. GSH levels were measured by spectrofluorometry and results were normalized to control. Results represent the mean ± SEM of 3 different experiments.
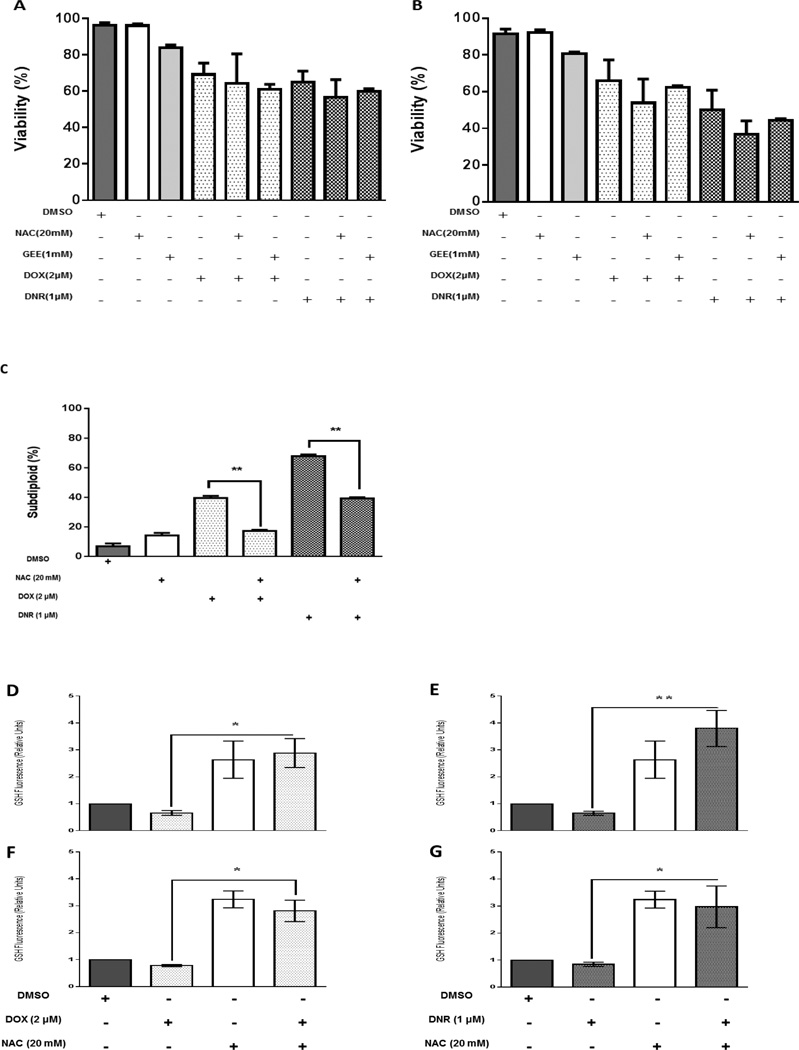
NAC protects H9C2 cells from anthracycline cytotoxicity without interfering with the cytotoxicity of anthracyclines on acute leukemia cells
To further probe the role of GSH in anthracycline induced cytotoxicity, we pretreated acute leukemia cells Jurkat and ML-1 with the antioxidant N-acetylcysteine (NAC) or glutathione ethyl ester (GEE), which either indirectly or directly increase GSH levels. H9c2 cells were pretreated with NAC. Cells were then treated with doxorubicin or daunorubicin for 24 hours. Neither of the antioxidants interfered with the cytotoxicity of anthracyclines in acute leukemia cells (Figures 5A and 5B). However in H9c2 cells, NAC was significantly protective against anthracycline induced DNA fragmentation (Figure 5C). These data indicate that NAC is protective of anthracycline-induced cytotoxicity in cardiomyocytes without interfering with the antileukemic activity.
Figure 5. NAC and GEE do not interfere with anthracycline cytotoxicity in acute leukemia cells. NAC protects H9C2 cells from anthracycline cytotoxicity. NAC increases GSH levels in acute leukemia cells treated with anthracyclines.
A) Jurkat and B) ML-1 cells were pretreated with NAC and GEE and then exposed to DOX and DNR for 24 hours. Viability was assessed. C) H9C2 cells were pretreated with NAC and subsequently exposed to DOX and DNR for 24 hours and DNA fragmentation was assessed. The results represent the mean ± SEM of 3 different experiments D, E) Jurkat and F, G) ML-1 cells were pretreated with N-acetylcysteine (NAC), and then exposed to DOX and DNR. GSH levels were measured by quantification of fluorescence by spectrofluorometry. The results represent the mean ± SEM of 3 different experiments, *p<0.05, **p<0.01.
NAC increased GSH levels in acute in acute leukemia cells treated with anthracyclines
We next sought to evaluate the effect of antioxidants on GSH levels in acute leukemia cells treated with anthracyclines. Jurkat and ML-1 cells were pretreated with the antioxidant N-acetylcysteine (NAC) and after 30 minutes were exposed to doxorubicin and daunorubicin. Anthracyclines decreased GSH levels in acute leukemia cells and pretreatment with NAC significantly counteracted that decrease (Figures 5 D–G), effectively boosting GSH levels in both acute leukemia lines.
Discussion
Anthracyclines have been linked to ROS generation in cancer cells and cardiomyocytes in this and in previous studies (17–19). However, few in vivo studies have actually captured ROS levels as a surrogate marker in peripheral blood. Our work in a limited number of patients, suggests that increased ROS may be associated with changes in cardiac function (specifically reduced ejection fraction). A previously published study performed in patients receiving treatment with the anthracycline epirubicin for different types of cancer suggested that an impairment in cardiac contractility was the earliest finding related to cardiotoxicity secondary to epirubicin and that this was associated with high levels of ROS and markers of inflammation (20). These changes occurred even before changes in troponin levels were observed. Therefore, incorporating ROS level measurement in patients may prove to be useful for monitoring of cardiotoxic effects of anthracyclines in earlier stages and to implement treatment modifications that include cardioprotective agents or dose modification.
ROS modulation has been shown in the present study not to affect the cytotoxic action of anthracyclines in acute leukemia cells therefore we propose that counteracting ROS with the use of antioxidants can be a viable strategy to provide cardioprotection in patients treated with anthracyclines without interfering with the antileukemic action of these agents. Previous studies have shown antioxidants such as vitamin C to be protective against anthracycline induced cardiotoxicity in animals, without reducing the anticancer activity of anthracyclines (21). However, these results could not be extrapolated to humans (22). Lacking from the human studies were biomarkers of ROS, thereby limiting our understanding of whether vitamin C was truly acting as an antioxidant. This is an important point, since reports of vitamin C actually causing oxidative stress rather than preventing it, appear to be reliant upon dose and duration of exposure.
Consistent with our in vitro findings, NAC has also shown to have protective effects in animal models of acute cardiotoxicity (23), however these results have not been reproduced in human clinical trials. In one randomized trial assessing the prevention of doxorubicin cardiomyopathy by NAC, patients received 75 mg/m2 intravenously of doxorubicin every 4 weeks either alone (control group with 30 patients) or preceded 1 hour by 5.5 g/m2 of NAC (intervention group with 24 patients). Cardiac function of all patients were tested before treatment via electrocardiograms and ejection fractions were measured at rest and after exercise and these parameters were again reassessed when the patients had received total cumulative doses of doxorubicin of 300–350 mg/m2 and 500–550 mg/m2. The study concluded that oral NAC was not effective as cardioprotective agent against doxorubicin-induced cardiotoxicity in the scheduled and dose used in the mentioned study (24). It is important to mention that the schedule of NAC administration may not have been optimal as this agent was used one hour before doxorubicin administration whereas it is known that ROS can be generated for long periods of time after anthracycline exposure. While the study used the measurement of ejection fraction as an endpoint it did not include the use of markers of oxidative stress which would have determined whether NAC was exerting an effect on lowering ROS levels.
The EPOCH trial (Evaluation of short-term use of N-acetylcysteine as a strategy for prevention of anthracycline-induced cardiomyopathy) was a prospective study that included 103 patients and pretreated 50 of them with 1200 mg of NAC orally every 8 hours starting before and ending after the intravenous infusion of anthracycline in all chemotherapy cycles. The primary outcome was changes in left ventricular ejection fraction, which did not differ between the two groups (25). Again, no markers of oxidative stress were used and the authors acknowledged the possibility of inadequate dosing and length of administration of NAC.
From these and other previous studies performed we can conclude that there are multiple possible reasons why studies with antioxidants that have demonstrated protective effects on cardiomyocytes in vitro and in vivo, could not demonstrate similar effects when tested on patients. Relevant explanations include the choice of endpoints and trial duration, the selection of the dose and intervention (16). Multiple studies that have assessed the use of antioxidants in patients have not evaluated biomarkers of oxidative stress, instead focusing on assessment of clinical endpoints like electrocardiographic changes. Though echocardiographic changes are important they may miss subtle changes in cardiac function between groups, and differences in group may not become apparent until years later since late cardiotoxicity may develop years after patients are off therapy. Also the duration of antioxidant based intervention may not have been optimized. By measuring biomarkers of oxidative stress, dosing and schedule of antioxidant administration may be guided.
Taken together we postulate that by designing an antioxidant intervention for anthracycline cardiotoxicity with appropriate selection of agent, dosing, schedule and with the use of biomarkers of oxidative stress as endpoints, benefit may be seen in a clinical setting. As numbers of cancer survivors who have been treated with anthracyclines increase, the potential benefits of NAC or other antioxidants to prevent cardiac damage may provide significant improvement in quality of life and healthcare costs for this growing population.
Acknowledgments
Funding
This study was supported by a research grant from Riders for the Cure, and a gift from David Herr & Family. J.F. and D.E. were supported by Division of Pediatrics funding for the Pediatric Hematology and Oncology Fellowship. Funding to J.C. from the NIH/NCI RO1 CA115811 is gratefully acknowledged. This work was also supported by the NIH/NCI under award number P30CA016672 and used the Research Animal Core Facility.
Footnotes
Conflict of Interest
J.C. has received research support from Celgene and Nereus Pharmaceuticals.
References
- 1.Kizek R, Adam V, Hrabeta J, Eckschlager T, Smutny S, Burda JV, et al. Anthracyclines and ellipticines as DNA-damaging anticancer drugs: Recent advances. Pharmacol Therapeut. 2012 Jan;133(1):26–39. doi: 10.1016/j.pharmthera.2011.07.006. PubMed PMID: WOS: 000299979600003. English. [DOI] [PubMed] [Google Scholar]
- 2.Wouters KA, Kremer LC, Miller TL, Herman EH, Lipshultz SE. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. British journal of haematology. 2005 Dec;131(5):561–578. doi: 10.1111/j.1365-2141.2005.05759.x. PubMed PMID: 16351632. [DOI] [PubMed] [Google Scholar]
- 3.Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA : the journal of the American Medical Association. 2013 Jun 12;309(22):2371–2381. doi: 10.1001/jama.2013.6296. PubMed PMID: 23757085. Pubmed Central PMCID: 3771083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco JG, Sun CL, Landier W, Chen L, Esparza-Duran D, Leisenring W, et al. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes--a report from the Children's Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 May 1;30(13):1415–1421. doi: 10.1200/JCO.2011.34.8987. PubMed PMID: 22124095. Pubmed Central PMCID: 3383117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. The New England journal of medicine. 1995 Jun 29;332(26):1738–1743. doi: 10.1056/NEJM199506293322602. PubMed PMID: 7760889. [DOI] [PubMed] [Google Scholar]
- 6.Sawyer DB. Anthracyclines and heart failure. The New England journal of medicine. 2013 Mar 21;368(12):1154–1156. doi: 10.1056/NEJMcibr1214975. PubMed PMID: 23514294. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nature medicine. 2012 Nov;18(11):1639–1642. doi: 10.1038/nm.2919. PubMed PMID: 23104132. [DOI] [PubMed] [Google Scholar]
- 8.Appel JM, Nielsen D, Zerahn B, Jensen BV, Skagen K. Anthracycline-induced chronic cardiotoxicity and heart failure. Acta Oncol. 2007;46(5):576–580. doi: 10.1080/02841860601156165. PubMed PMID: WOS:000248027300001. English. [DOI] [PubMed] [Google Scholar]
- 9.Lipshultz SE, Cochran TR, Franco VI, Miller TL. Treatment-related cardiotoxicity in survivors of childhood cancer. Nature reviews Clinical oncology. 2013 Dec;10(12):697–710. doi: 10.1038/nrclinonc.2013.195. PubMed PMID: 24165948. [DOI] [PubMed] [Google Scholar]
- 10.Lebrecht D, Setzer B, Ketelsen UP, Haberstroh J, Walker UA. Time-dependent and tissue-specific accumulation of mtDNA and respiratory chain defects in chronic doxorubicin cardiomyopathy. Circulation. 2003 Nov 11;108(19):2423–2429. doi: 10.1161/01.CIR.0000093196.59829.DF. PubMed PMID: 14568902. [DOI] [PubMed] [Google Scholar]
- 11.Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Annals of internal medicine. 1996 Jul 1;125(1):47–58. doi: 10.7326/0003-4819-125-1-199607010-00008. PubMed PMID: 8644988. [DOI] [PubMed] [Google Scholar]
- 12.Sterba M, Popelova O, Vavrova A, Jirkovsky E, Kovarikova P, Gersl V, et al. Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection. Antioxidants & redox signaling. 2013 Mar 10;18(8):899–929. doi: 10.1089/ars.2012.4795. PubMed PMID: 22794198. Pubmed Central PMCID: 3557437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lotrionte M, Palazzoni G, Abbate A, De Marco E, Mezzaroma E, Di Persio S, et al. Cardiotoxicity of a non-pegylated liposomal doxorubicin-based regimen versus an epirubicin-based regimen for breast cancer: the LITE (Liposomal doxorubicin-Investigational chemotherapy-Tissue Doppler imaging Evaluation) randomized pilot study. International journal of cardiology. 2013 Aug 10;167(3):1055–1057. doi: 10.1016/j.ijcard.2012.10.079. PubMed PMID: 23174173. [DOI] [PubMed] [Google Scholar]
- 14.Lyu YL, Kerrigan JE, Lin CP, Azarova AM, Tsai YC, Ban Y, et al. Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer research. 2007 Sep 15;67(18):8839–8846. doi: 10.1158/0008-5472.CAN-07-1649. PubMed PMID: 17875725. [DOI] [PubMed] [Google Scholar]
- 15.Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV, et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. The Journal of clinical investigation. 2014 Feb 3;124(2):617–630. doi: 10.1172/JCI72931. PubMed PMID: 24382354. Pubmed Central PMCID: 3904631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman M, Bostick RM, Kucuk O, Jones DP. Clinical trials of antioxidants as cancer prevention agents: past, present, and future. Free radical biology & medicine. 2011 Sep 1;51(5):1068–1084. doi: 10.1016/j.freeradbiomed.2011.05.018. PubMed PMID: 21683786. [DOI] [PubMed] [Google Scholar]
- 17.Sawyer DB, Fukazawa R, Arstall MA, Kelly RA. Daunorubicin-induced apoptosis in rat cardiac myocytes is inhibited by dexrazoxane. Circulation research. 1999 Feb 19;84(3):257–265. doi: 10.1161/01.res.84.3.257. PubMed PMID: 10024299. [DOI] [PubMed] [Google Scholar]
- 18.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochemical pharmacology. 1999 Apr 1;57(7):727–741. doi: 10.1016/s0006-2952(98)00307-4. PubMed PMID: 10075079. [DOI] [PubMed] [Google Scholar]
- 19.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological reviews. 2004 Jun;56(2):185–229. doi: 10.1124/pr.56.2.6. PubMed PMID: 15169927. [DOI] [PubMed] [Google Scholar]
- 20.Mercuro G, Cadeddu C, Piras A, Dessi M, Madeddu C, Deidda M, et al. Early epirubicin-induced myocardial dysfunction revealed by serial tissue Doppler echocardiography: correlation with inflammatory and oxidative stress markers. The oncologist. 2007 Sep;12(9):1124–1133. doi: 10.1634/theoncologist.12-9-1124. PubMed PMID: 17914082. [DOI] [PubMed] [Google Scholar]
- 21.Shimpo K, Nagatsu T, Yamada K, Sato T, Niimi H, Shamoto M, et al. Ascorbic acid and adriamycin toxicity. The American journal of clinical nutrition. 1991 Dec;54(6 Suppl):1298S–1301S. doi: 10.1093/ajcn/54.6.1298s. PubMed PMID: 1962586. [DOI] [PubMed] [Google Scholar]
- 22.Barry E, Alvarez JA, Scully RE, Miller TL, Lipshultz SE. Anthracycline-induced cardiotoxicity: course, pathophysiology, prevention and management. Expert opinion on pharmacotherapy. 2007 Jun;8(8):1039–1058. doi: 10.1517/14656566.8.8.1039. PubMed PMID: 17516870. [DOI] [PubMed] [Google Scholar]
- 23.Gottlieb JA, Baker LH, Quagliana JM, Luce JK, Whitecar JP, Jr, Sinkovics JG, et al. Chemotherapy of sarcomas with a combination of adriamycin and dimethyl triazeno imidazole carboxamide. Cancer. 1972 Dec;30(6):1632–1638. doi: 10.1002/1097-0142(197212)30:6<1632::aid-cncr2820300632>3.0.co;2-s. PubMed PMID: 4663966. [DOI] [PubMed] [Google Scholar]
- 24.Myers C, Bonow R, Palmeri S, Jenkins J, Corden B, Locker G, et al. A Randomized Controlled Trial Assessing the Prevention of Doxorubicin Cardiomyopathy by N-Acetylcysteine. Semin Oncol. 1983;10(1):53–56. PubMed PMID: WOS:A1983QJ47500010. English. [PubMed] [Google Scholar]
- 25.Jo SH, Kim LS, Kim SA, Kim HS, Han SJ, Park WJ, et al. Evaluation of Short-Term Use of N-Acetylcysteine as a Strategy for Prevention of Anthracycline-Induced Cardiomyopathy: EPOCH Trial - A Prospective Randomized Study. Korean circulation journal. 2013 Mar;43(3):174–181. doi: 10.4070/kcj.2013.43.3.174. PubMed PMID: 23613694. Pubmed Central PMCID: 3629243. [DOI] [PMC free article] [PubMed] [Google Scholar]