Abstract
Deficits in reward anticipation are putative mechanisms for multiple psychopathologies. Research indicates that these deficits are characterized by reduced left (relative to right) frontal electroencephalogram (EEG) activity and blood oxygenation level-dependent (BOLD) signal abnormalities in mesolimbic and prefrontal neural regions during reward anticipation. Although it is often assumed that these two measures capture similar mechanisms, no study to our knowledge has directly examined the convergence between frontal EEG alpha asymmetry and functional magnetic resonance imaging (fMRI) during reward anticipation in the same sample. Therefore, the aim of the current study was to investigate if and where in the brain frontal EEG alpha asymmetry and fMRI measures were correlated in a sample of 40 adults. All participants completed two analogous reward anticipation tasks – once during EEG data collection and the other during fMRI data collection. Results indicated that the two measures do converge and that during reward anticipation, increased relative left frontal activity is associated with increased left anterior cingulate cortex (ACC)/medial prefrontal cortex (mPFC) and left orbitofrontal cortex (OFC) activation. This suggests that the two measures may similarly capture PFC functioning, which is noteworthy given the role of these regions in reward processing and the pathophysiology of disorders such as depression and schizophrenia.
Keywords: reward anticipation, functional magnetic resonance imaging, electroencephalography, psychopathology
Abnormal reward processing is a hallmark feature of several psychopathologies (Beck et al., 2009; Figee et al., 2011; Scheres et al., 2007), but is most often implicated in major depressive disorder (MDD) and schizophrenia (Choi et al., 2013; Gard et al., 2007; Pizzagalli et al., 2009a; Wacker et al., 2009; see Bylsma et al., 2008 for a review). For instance, numerous studies have shown that relative to healthy controls, individuals with MDD and schizophrenia exhibit reduced reward expectancies (Pause et al., 2003; Premkumar et al. 2008), abnormal physiological reactivity to reward (Pizzagalli et al., 2009a; De Leeuw et al., 2015), and attenuated positive affect during reward attainment (Berenbaum et al., 1992; Kring & Barch, 2014); as well as broad processing deficits of positive stimuli (Dalili et al., 2014).
The heterogeneity of these findings underscores the fact that reward processing is a broad construct and can be divided into at least two distinct, temporal components – reward anticipation and reward consummation (Berridge & Robinson, 2003; Gard et al., 2006). It has long been argued that reduced reward anticipation (i.e., a diminished tendency to expect and/or approach rewards,), in particular, is a core symptom of several psychopathologies including depression and schizophrenia (Davidson, 1998; Meehl, 1975). This premise has been supported by several behavioral and psychophysiological studies (Kring & Barch, 2014; Shankman et al., 2013) and recently, data has directly suggested that the broad reward-related abnormalities seen in these disorders are primarily driven by deficits in reward anticipation and not reward consummation (Engel, Fritscke, & Lincoln, 2013; Sherdell et al., 2012).
To date, the neural processes underlying reduced reward anticipation have been empirically examined in several ways, which has unfortunately led to relatively separate literatures. For decades, researchers have used asymmetry in electroencephalogram (EEG) activity between right and left frontal brain regions (i.e., increased activity in the left frontal region relative to the right frontal region; Davidson, 1994, 1998) as an indicator of reward sensitivity (and approach motivation more broadly). Power in the alpha band of the EEG signal has been argued to reflect an inverse measure of brain activity that captures both state and trait affective processes (Allen et al., 2004; Hagemann et al., 2005; Henriques & Davidson, 1991). Using this measure, it has repeatedly been demonstrated that individuals with MDD and individuals at risk for MDD display reduced relative left frontal activity during rest (Stewart et al., 2010; Tomarken et al., 2004; Shankman & Klein, 2003) and during anticipation of rewards (Shankman et al., 2007, 2013) compared with healthy controls. Although the EEG asymmetry literature is smaller in schizophrenia, some studies have also reported reduced relative left frontal activity in those with schizophrenia relative to controls (Horan et al., 2014). As such, abnormal frontal EEG asymmetry has been proposed as a potential psychophysiological indicator of reduced reward anticipation (Shankman et al., 2013; Stewart et al., 2011).
In more recent years, with the significant advancement of neuroscience techniques, there has been a proliferation of research on the neural correlates of reduced reward anticipation using functional magnetic resonance imaging (fMRI). These studies have most often implicated the mesolimbic dopaminergic pathway in reward anticipation (Haber & Knutson, 2010), which originates in the ventral tegmental area (VTA) and projects to the nucleus accumbens (NAcc) of the ventral striatum, the dorsal striatum, amygdala, and medial prefrontal cortex (Knutson et al., 2001; Tsurugizawa et al., 2012). Several fMRI studies have demonstrated that individuals with MDD and schizophrenia display reduced activation in mesolimbic regions during reward anticipation relative to healthy controls (Grimm et al., 2014; Smoksi et al., 2009; Pizzagalli et al., 2009b). It is important to note, however, that there have been some mixed findings in this literature. For instance, two separate studies have found that compared with controls, individuals with depression exhibit enhanced anterior cingulate cortex (ACC) activation during anticipation of reward, yet relatively normal mesolimbic activation (Gorka et al., 2014; Knutson et al., 2008). Taken together, this literature suggests that specific patterns of fMRI activation may also be psychophysiological indicators of reward anticipation deficits.
As was briefly mentioned above, although EEG and fMRI are two complimentary brain mapping techniques, the findings from these literatures have rarely been synthesized and we know very little about the convergence of EEG and fMRI measures of dysfunctional reward anticipation. It is currently unclear if individuals that display reduced relative left frontal activity also exhibit mesolimbic and/or ACC abnormalities during anticipation of reward. In other words, are the two measures capturing the same neural mechanisms albeit in different ways? Alternatively, one could speculate that the two measures have little convergence and reflect different disease processes, or that that they converge in different areas of the brain pointing to potentially novel or overlooked reward processing clinical targets. Previously, source-localization studies have suggested that frontal EEG asymmetry at rest is mediated by left dorsolateral prefrontal cortex (DLPFC) and orbitofrontal cortex (OFC) activation (Pizzagalli et al., 2005), not ventral striatum or ACC. This speaks to the possibility that the two measures have different correlates; however, there is a need to directly test this hypothesis.
The question of method convergence has recently become more salient with the advent of the National Institute on Mental Health’s (NIMH) Research Domain Criteria (RDoC) initiative (Insel et al., 2010; Cuthbert & Kozak, 2013), which seeks to examine constructs such as reward anticipation across multiple units of analysis (e.g., genes, molecules, circuits, physiology, behavior). Implicit in the initiative is that there should be convergence across units of analysis of a given domain or construct, such that fMRI indicators of reduced reward anticipation should converge with EEG indictors of reduced reward anticipation. This question remains to be tested though. One of the ways to establish convergence is to examine if and where in the brain fMRI blood oxygenation level-dependent (BOLD) signal and frontal EEG asymmetry during anticipation of reward are correlated in the same sample.
Thus far, very few studies have investigated these associations. One noteworthy example is a study by Wacker et al. (2009) which examined the associations between anhedonia, resting EEG delta activity, and fMRI during reward feedback (i.e., monetary gains) in a sample of healthy adults. Results indicated that anhedonia was positively associated with resting EEG delta current density (i.e., low resting activity) in the rostral ACC (rACC) and negatively associated with ventral striatum BOLD signal during reward feedback. Moreover, resting rACC delta activity was negatively associated with ventral striatum responses during reward feedback. This suggests that these two measures of reward functioning were indeed correlated. However, Wacker et al. (2009) measured EEG activity in the delta frequency band, rather than the more commonly investigated alpha band (Davidson, 2004). EEG data was also collected at rest and not during actual reward anticipation. Because EEG asymmetry is sensitive to changes in affective state (Allen et al., 2004), a potentially more useful comparison is the convergence of EEG asymmetry and fMRI measures during analogous laboratory tasks that directly elicit reward anticipation.
Although not a measure of EEG asymmetry, there have been several prior studies that have examined the convergence between EEG scalp-recorded event-related potentials (ERPs) and fMRI measures during laboratory reward tasks. For instance, Carlson et al. (2011) reported that during monetary gains (i.e., reward consummation), feedback negativity (i.e., FN - an ERP indicator of reward sensitivity) was positively correlated with fMRI activation in the ventral striatum and medial prefrontal cortex (mPFC). Similar studies have also reported a relation between the FN and BOLD response (e.g., Hauser et al., 2014; Becker et al., 2014). In addition, Plichta et al. (2013) found that during reward anticipation, the ERP component, contingent negative variation (CNV) was associated with thalamus fMRI BOLD response and the strength of connectivity from the supplementary motor area (SMA) to the ventral striatum and thalamus. It is important to stress that ERP measures are conceptually and methodologically different than EEG asymmetry measures; nevertheless, these studies provide initial evidence to suggest that separate brain mapping techniques, assessed during affective states, can exhibit convergence in specific areas of the brain.
To date, no study to our knowledge has assessed correlations between frontal EEG alpha asymmetry and fMRI BOLD signal during reward anticipation in the same sample. Given that both of these measures have been used to assess reward anticipation deficits, it is useful for studies to elucidate the magnitude and location of convergence across these two neural measures. Thus, the primary aim of the current study is to examine the correlation between EEG alpha asymmetry and fMRI measures of reward anticipation by having participants complete two analogous reward anticipation tasks approximately one week apart – once during collection of EEG asymmetry and the other during fMRI BOLD signal. Because there is limited existing data on EEG-fMRI convergence, the aims of the study are relatively exploratory; however, we hypothesized that the two measures would be correlated such that reduced relative left frontal activity would relate to reduced ventral striatum (NAcc included) and vmPFC/ACC activation during reward anticipation, as these neural regions have often been implicated in reward functioning.
Methods
Participants
The sample included 40 adults who were recruited from the community and enrolled in a larger study on emotional processes (Shankman et al., 2013). Participant demographics and clinical characteristics are presented in Table 1. As part of the larger study aims, participants were in one following diagnostic groups: 1) current MDD (n=9), 2) current MDD with comorbid panic disorder (PD) (n=13), or 3) no lifetime history of psychopathology (n=18). Although examining the impact of diagnosis is not an aim of the current study, having individuals with a range of internalizing psychopathology provides variability in our EEG and fMRI measures, as prior studies suggest that depression and anxiety impact neural responding to rewards (e.g., Forbes et al., 2006). This notably improves our statistical ability to detect associations between individual differences on the two measures. Current and lifetime diagnoses were made using the Structured Clinical Interview for DSM-IV (SCID; First et al., 1996). Individuals were excluded from the larger study if they had a lifetime diagnosis of a psychotic disorder, bipolar disorder, or dementia; were unable to read or write in English; had a history of head trauma with loss of consciousness; or were left-handed. All methods were approved by the University of Illinois-Chicago Institutional Review Board.
Table 1.
Participant demographics and clinical characteristics
| Demographics | Mean (SD) or % |
|---|---|
| Age (years) | 31.4 (12.1) |
| Sex (% female) | 72.5% |
| Race (% Caucasian) | 47.5% |
| Clinical Variables | |
| Current Major Depressive Disorder | 55.0% |
| Current Panic Disorder | 32.5% |
| HRSD Total Score | 15.9 (13.7) |
| BAI Total Score | 10.1 (10.8) |
| EEG Asymmetry during Reward Anticipation | 0.17 (0.51) |
Note. HRSD = Hamilton Rating Scale for Depression (Hamilton, 1960); BAI = Beck Anxiety Inventory (Beck et al., 1988); EEG = electroencephalography.
Procedure
EEG Reward Task and Data Collection
EEG and fMRI data collection occurred on two separate days, approximately one week apart. On Day 1, participants completed the EEG protocol. After providing written informed consent, participants were seated in an electrically shielded, sound-attenuated booth and completed a computerized slot machine task previously used to probe sensitivity to reward anticipation (Shankman et al., 2007, 2013). The task consisted of three reels which ‘spun’ for 11-seconds and then landed on a result. There were 60 total spins, divided into two possible outcomes of 30 trials each - reward condition (R) in which participants won money if the reels landed on three pieces of fruit and no incentive condition (NI) in which participants did not win or lose money. Participants began the game with $2 and were told the specific condition (R or NI) prior to each trial. During R conditions, participants won between $0.50 and $3.00 on each trial. Trials were presented in a pseudorandom order. There were never more than two consecutive trials of the same outcome. Half of the trials in each condition “landed” on three pieces of fruit and thus during R conditions, participants won money 50% of the time. Total task time was approximately 20-minutes. All participants were given their winnings of ~$12 in cash at the end of the task.
During the reward task, EEG data were recorded from Ag/AgCl electrodes in a 64-channel stretch-lycra electrode cap (Compumedics Neuroscan 4.4, Charlotte, NC). The frontal pole (AFZ) was used as the ground electrode and the online reference was near the vertex (between CZ and CPZ). Electrode impedances were under 5,000 ohms, and homologous sites (e.g., F7/F8) were within 1,500 ohms of each other. Data were recorded through a Neuroscan Synamp2 data acquisition system at a gain of 10K (5K for eye channels) with a bandpass of DC-200 Hz. Data were acquired and digitized continuously at a rate of 1000 Hz. EEG data were re-referenced offline using the average activity of all scalp electrodes.
fMRI Reward Task and Data Collection
On Day 2, participants completed the fMRI protocol. They were instructed to abstain from caffeine and tobacco for at least two hours prior to their session. During the scan, participants completed a modified version of the slot task described above. The fMRI task was a computerized slot machine paradigm with two conditions – reward (R) and no incentive (NI). In both conditions, a ring of geometric shapes would ‘spin’ on the screen and then ‘land’ on a result. Cues on the screen explicitly indicated whether the present trail was an R or NI condition. In the R condition, participants won money if it landed on an image of a birthday cake. This occurred approximately 50% of trails (consistent with the EEG slot task). When it landed on something else (e.g., square, circle), they did not lose any money. In the NI condition, participants did not win or lose money regardless of the outcome. The task included 2 runs of 32 trials that were equally divided into R and NI conditions. Each time, the reels would spin for 4–6 seconds and participants viewed the result for 3-seconds. R and NI conditions were presented in 30-second blocks, and the total task time was approximately 12-minutes. Between conditions a fixation cross was presented for 10-seconds to allow the fMRI BOLD signal to return to baseline. At the end of the task, all participants were given their winnings of ~$17 in cash.
Functional MRI data was collected using a 3T GE magnetic resonance scanner at the University of Illinois Medical Center. Functional images were acquired using a gradient-echo echo-planar images (2s TR, 25ms TE, 82° flip, 64×64 matix, 200 mm FOV, 3mm slice thickness, 0mm gap, with 40 axial slices). A high-resolution, T1-weighted anatomical scan was also acquired in the same axial orientation (25° flip; 512×512 matrix, 220mm FOV; 1.5mm slice thickness; 120 axial slices).
Data Processing and Analyses
EEG data during the 11-second reward anticipation phase were segmented into consecutive 1.024-second epochs every 0.512-seconds (50% overlap). Post re-referencing and baseline correction, each epoch was visually inspected by hand and rejected if there was evidence of excessive ocular, mechanical, or other artifact. After artifact rejection, 64% ±20.6 of epochs in the R condition and 60% ±21.1 of epochs in the NI condition remained. The number of remaining epochs in each condition did not differ by diagnostic group (p’s > 0.39; R condition: Controls = 66.1% ±22.1, MDD-only = 65.4% ±20.4, MDD and PD = 60.1% ± 20.0; NI condition: Controls = 64.9% ±22.0, MDD-only = 54.4% ±23.3, MDD and PD = 56.1% ±18.1). Data across the entire epoch was tapered by a Hanning window to suppress spectral side lobes. Artifact-free data were recovered in adjacent (overlapping) epochs and power spectra were computed offline from EEG data by using a fast Fourier transform. The average absolute alpha power was computed for each electrode site and then natural log transformed to normalize the data. Consistent with previous studies (e.g., Bruder, Fong, Tenke, & Leite, 1997), we defined the alpha band as 7.81–12.70 Hz and used it as an inverse measure of regional brain activity. Frontal asymmetry scores were computed for the R and NI conditions by subtracting power at the F7(left) frontal electrode from power at the homologous F8 (right) electrode, so that the higher values for the asymmetry score reflect greater activity in left relative to right frontal regions. EEG asymmetry values across the sample were normally distributed (M= 0.17±0.51, range: −0.92 – 1.42, skew: 0.18, kurtosis: 0.35).
fMRI data from all 40 participants met criteria for high quality and scan stability with minimum motion correction (i.e., 3 mm or less displacement in any one direction). Functional data were analyzed using Statistical Parametric Mapping software (SPM8, Wellcome Department of Imaging Neuro-Science, London, UK). Images were spatially realigned to correct for head motion, warped to standardized Montreal Neurological Institute (MNI) space using the participant’s T1 image, resampled to 2 mm3 voxels, and smoothed with an 8 mm3 kernel to minimize noise and residual differences in gyral anatomy. The general linear model was applied to the time series, convolved with the canonical hemodynamic response function and with a 128-s high-pass filter. Condition effects were modeled with box-car regressors representing the occurrence of each block type. Effects were estimated at each voxel and for each subject.
Individual contrast maps (statistical parametric maps) for R condition spins versus NI condition spins (R > NI) were generated for each participant. Contrast maps were then entered into a second-level independent samples t-test. To examine our primary aim, each individuals’ EEG asymmetry value during reward anticipation (i.e., EEG asymmetry difference score: R – NI spins) was entered as a regressor of interest. Age was entered as a covariate. Because of the exploratory nature of the current analyses, we considered activations that survived p < 0.001 (uncorrected), with a cluster extent threshold of greater than 20 contiguous voxels (volume > 160mm3), as significant to balance between Type I and Type II error (Lieberman et al., 2009); similar to prior studies (Banks et al., 2007; Labuschagne et al., 2010). Only significant activations in a priori regions of interest (i.e., reward-related neural circuitry) were interpreted. For visualization purposes only, we extracted the same 5mm (radius) spheres from the model in which EEG asymmetry values were not included as a regressor to accurately display the independent associations between EEG asymmetry values and BOLD signal response.
Results
Effects of EEG Reward Task
In the current sample, EEG asymmetry was significantly greater during R conditions compared with NI conditions (t(39) = 3.20, p < 0.05), suggesting that across subjects the task successfully elicited reward anticipation. Additional analyses indicated that there were no group differences in EEG asymmetry during the NI condition (F(2, 39) = 0.80, p = 0.46) or the R condition relative to the NI condition (i.e., R > NI; F(2, 39) = 0.17, p = 0.84). R > NI values were also not correlated with current depressive symptoms (r = 0.04, ns; assessed via the Hamilton Rating Scale for Depression [HAM-D; Hamilton, 1960]).
Effects of fMRI Reward Task
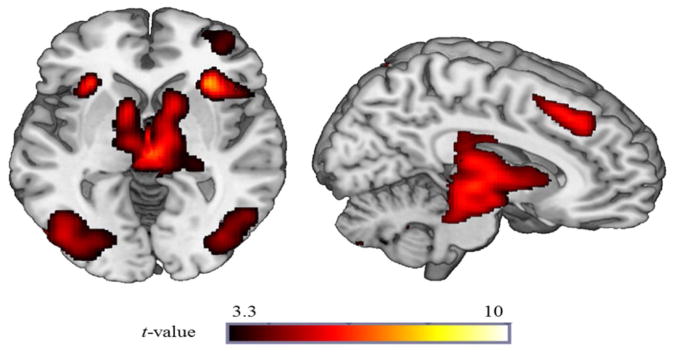
Across the entire sample, reward anticipation (R condition spins > NI condition spins) significantly activated a large contiguous cluster of mesolimbic reward regions including bilateral nucleus accumbens, caudate, lateral globus pallidum, and putamen (peak MNI [−12, 6, −4], k=130560mm3, Z = 7.04, p < 0.001). Thus, the fMRI task successfully probed reward-related regions (Figure 1). Additional regions including the insula, medial frontal gyrus, and ACC were also activated and are reported in Table 2.
Figure 1. Neural regions activated during R condition spins relative to NI condition spins.
Voxel-wise statistical t-map displayed on a canonical brain illustrating significant clusters at p < 0.001, uncorrected across the entire sample.
Table 2.
Whole-brain results, across the entire sample, for R > NI
| Region | MNI Coordinates | Voxels (mm3) | Z-score | ||
|---|---|---|---|---|---|
|
| |||||
| X | Y | Z | |||
| L Inferior Parietal Lobe extending to Bilateral Caudate | −30 | −58 | 44 | 130560 | 7.04 |
| R Superior Parietal Lobe | 34 | −60 | 52 | 68112 | 6.56 |
| R Insula | 32 | 24 | −4 | 48904 | 6.17 |
| L Inferior Frontal Gyrus | −48 | 4 | 34 | 16688 | 5.61 |
| L Insula | −30 | 24 | −2 | 1808 | 5.23 |
| R Medial Frontal Gyrus | 6 | 34 | 44 | 5552 | 5.05 |
| L Medial Frontal Gyrus | −12 | −4 | 64 | 200 | 3.57 |
| Anterior Cingulate Gyrus | 10 | 44 | 18 | 224 | 3.32 |
Note. Reporting of all significant clusters at p < 0.001 (uncorrected) with a cluster extent threshold of k (number of contiguous voxels) >20. L =Left; R =Right; MNI = Montreal Neurologic Institute.
As is presented in Gorka et al. (2014), although all groups evidenced mesolimbic reward-related activation, the diagnostic groups differed in dACC activation during reward anticipation (MNI peak: [8, 8, 44], Z = 3.43, k = 116, p < 0.001). Specifically, the MDD-only group displayed greater dACC activation compared with controls (t(25) = 4.20, p < 0.001) and comorbid individuals (t(20) = 2.62, p < 0.05). The controls and comorbid individuals did not differ from each other. There were no other group differences in reward-related neural regions during the fMRI task.
Convergence of EEG and fMRI Reward Tasks
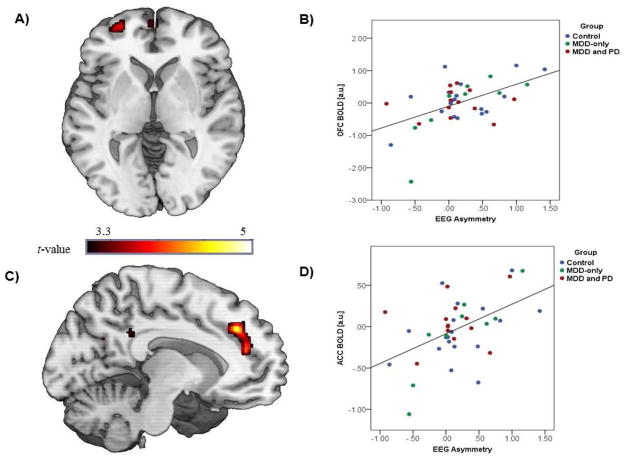
Results indicated that greater relative left frontal activity during reward anticipation was significantly associated with greater left ACC/medial PFC [MNI peak: [−10, 36, 34], k = 2480mm3, p < 0.001, uncorrected, r = 0.50) and left OFC [MNI peak [−28, 58, −2], k = 640mm3, p < 0.001, uncorrected, r = 0.52) activation during reward anticipation (see Figure 2). Post-hoc, we examined whether there were any group differences in the EEG asymmetry-ACC/mPFC and OFC correlations by conducting one-way analyses of variance (ANOVAs) on the extracted correlation parameter estimates. There were no significant group differences as the magnitude of the correlations were similar across groups (ACC/mPFC: F[2, 39] = 0.45, ns; Controls r = 0.47, MDD-only r = 0.62, MDD and PD r = 0.54; OFC: F[2, 39] = 0.31, ns; Controls r = 0.50, MDD-only r = 0.59, MDD and PD r = 0.55). The correlation magnitude was also not associated with current depressive symptoms (both p’s > 0.39). For completeness, all whole-brain results are presented in Table 3.
Figure 2. Correlation between EEG and fMRI reward anticipation measures.
A) Voxel-wise statistical t-map displayed on a canonical brain illustrating significant convergence between EEG asymmetry and left OFC BOLD response during reward anticipation; B) Scatter plot displaying the correlation between EEG asymmetry and OFC BOLD response; C) Voxel-wise statistical t-map displayed on a canonical brain illustrating significant convergence between EEG asymmetry and left ACC/medial PFC BOLD response during reward anticipation; D) Scatter plot displaying correlation between EEG asymmetry and ACC/medial PFC BOLD response; OFC = orbitofrontal cortex; ACC = anterior cingulate cortex; EEG = electroencephalogram; MDD = major depressive disorder; PD = panic disorder. Of note, estimates of ACC/mPFC and OFC activation were normally distributed across the sample (ACC/mPFC: M= −0.02±0.37, range: −1.06 – 0.68, skew: −0.37, kurtosis: 0.81; OFC: M= 0.02±0.66, range: −2.43 – 1.16, skew: −0.37, kurtosis: 2.01).
Table 3.
Whole-brain results for the correlation between frontal EEG asymmetry during reward anticipation and fMRI neural activation during reward anticipation.
| Region | MNI Coordinates | Voxels (mm3) | Pearson’s r value | ||
|---|---|---|---|---|---|
|
| |||||
| X | Y | Z | |||
| L Inferior Parietal Lobe | −30 | −40 | 36 | 1352 | 0.57 |
| L Anterior Cingulate Cortex/Medial Prefrontal Cortex | −10 | 36 | 34 | 2480 | 0.50 |
| R Inferior Frontal Lobe | 30 | 12 | 24 | 2152 | 0.54 |
| R Supp Motor Area | 20 | −28 | 54 | 664 | 0.45 |
| L Orbitofrontal Cortex | −28 | 58 | −2 | 640 | 0.52 |
| R Posterior Cingulate Gyrus | 10 | −38 | 28 | 360 | 0.49 |
| L Posterior Cingulate Gyrus | −12 | −40 | 32 | 232 | 0.44 |
| L Superior Frontal Gyrus | −2 | 60 | 2 | 200 | 0.45 |
Note. Reporting of all significant clusters at p < 0.001 (uncorrected) with a cluster extent threshold of k (number of contiguous voxels) >20.
Bold italics represent areas of interest. All correlation coefficients are positive as there were no significant negative correlations.
L =Left; R =Right; MNI = Montreal Neurologic Institute.
Discussion
A large body of evidence indicates that reduced reward anticipation is characterized by reduced relative left frontal activity and mesolimbic and PFC BOLD signal abnormalities during reward anticipation (Pizzagalli et al., 2009b; Smoksi et al., 2009; Shankman et al., 2007, 2013). Although it is assumed, on some level, that EEG asymmetry and fMRI capture similar mechanisms of dysfunction, no study to our knowledge has directly examined the convergence between these two measures during reward anticipation in the same sample. Therefore, the aim of the current study was to investigate if and where in the brain frontal EEG asymmetry and fMRI measures were correlated in a sample of adults with a range of internalizing psychopathology. Our results indicated that the two measures do indeed converge - specifically, individual differences in EEG asymmetry during reward anticipation were associated with individual differences in ACC/mPFC and OFC activation during reward anticipation. This suggests that these two measures may overlap in these neural regions.
The ACC/mPFC and OFC as the major sites of convergence between these two measures is interesting for numerous reasons. First and foremost, as was previously noted, source localization studies have suggested that frontal EEG asymmetry at rest is mediated by left OFC activation (Pizzagalli et al., 2005) and thus, there is some consistency with prior source localization findings and the current results. Second, both the ACC/mPFC and OFC are known to play major roles in emotion processing and are considered key components of the brain’s reward circuitry (Bush et al., 2000; McClure et al., 2004; Nestler & Carlezon, 2006). Converging evidence indicates that the ACC/mPFC is critically involved in the regulation of reward responses (Etkin, Egner, & Kalish, 2011; Phillips, Drevets, Rauch, & Lane, 2003), including evaluating anticipated reward magnitude and probability, tracking reward prediction errors, and forming associations between reward and appropriate action (Alexander & Brown, 2010; Haber & Knutson, 2010; Knutson & Cooper, 2005). The ACC/mPFC also has dense projections to the ventral striatum and functionally interacts with this region (and others) to modulate reward processing and reward-related positive affect (Haber & Knutson, 2010). The OFC similarly projects to the ventral striatum and is another core node in the mesolimbic dopaminergic pathway (Haber et al., 1995; Schultz et al., 2000). Numerous studies have shown that the OFC is involved in sensory integration and assessing the value of rewarding stimuli (Gottfried et al., 2003; O’Doherty et al., 2001), as well as the subjective feeling of hedonia (Kringelbach, 2005; Kringelbach et al., 2003). The ACC/mPFC and OFC are therefore fundamental regions of the brain associated with reward processes, and the current findings suggest that EEG asymmetry and fMRI BOLD laboratory paradigms of reward anticipation may converge in these important areas.
It is also important to note that the ACC/mPFC and OFC are implicated in reward-related dysfunction across several disorders such as depression, bipolar disorder, and schizophrenia (Barch & Dowd, 2010; Gorka et al., 2014; Nusslock et al., 2012). For example, relative to healthy controls, individuals with depression have been shown to exhibit increased ACC/mPFC activation during reward anticipation (Knutson et al., 2008), and blunted OFC activation during rewarding outcomes (Dichter et al., 2012). Bipolar patients have meanwhile been found to have heightened OFC activation during reward anticipation (Nusslock et al., 2012). In addition to studies with patient populations, it has been demonstrated that lesions of the ACC/mPFC produce depressive-like symptoms including abnormal reward cue estimation, apathy, loss of motivation, and social deficits (Damasio & Van Hoesen, 1983; Drevets et al., 1997). Collectively, these findings suggest that these PFC regions may be related to the pathophysiology of the reward anticipation deficits across several disorders. The fact that frontal EEG asymmetry and fMRI converge in these regions may suggest that these two literatures capture shared underlying mechanisms. Along these same lines, it is worth mentioning that in the current study, greater relative left frontal activity was associated with greater left ACC/mPFC and left OFC activation. The lateralization of these findings underscores the potential overlap of these measures in the left PFC.
In addition to the relevance of the location of convergence it is worth noting that the simple fact that these two separate neural measures correlate is meaningful. As was previously mentioned, one of the major hopes of NIMH’s RDoC initiative is that measures across units of analysis (e.g., neural circuits and physiology) will converge to ultimately reflect core mechanisms of psychopathology (Cuthbert & Kozak, 2013). This study is one of the first in a series of investigations that are necessary to map out the unique and shared components of discrete “units of analysis” (or measures) of the same construct. In order to truly hone in on mechanisms of dysfunction, there is a need to integrate and synthesize parallel literatures. Moreover, knowing areas of overlap may eventually aid in future study design. If ACC/mPFC and OFC functioning during reward anticipation corresponds with frontal EEG asymmetry during reward anticipation, a study seeking to capture functioning in these neural regions may decide to use EEG rather than fMRI as it is cheaper and less invasive. Before these conclusions can be drawn, however, additional studies utilizing simultaneous EEG and fMRI data collection are needed to confirm that these two measures correspond within the aforementioned regions. This represents an important future direction of this line of work.
The current study did not find diagnostic group differences in EEG asymmetry, ACC/mPFC and OFC BOLD response, or the convergence of the two measures. Given the small sample size and uneven group distribution (i.e., 18 controls, 9 MDD-only, and 13 MDD and PD), it is possible that the current study was underpowered to detect group differences and thus, the role of diagnosis on method convergence remains an important, yet relatively untested, question. It is also possible that these null findings reflect the fact that DSM diagnoses are heterogeneous constructs that do not neatly map on to underlying neurobiological functioning and that convergence is less impacted (or moderated) by categorical diagnoses than may be anticipated. Importantly, regardless of whether diagnosis does or does not impact convergence, data on this topic is useful and could help further refine mechanisms of dysfunction within specific diagnostic groups or point to potential transdiagnostic (or agnostic) processes.
This study significantly adds to the current literature; although, there are several limitations worth nothing. First, as is noted above, the current sample size included individuals with specific DSM diagnoses and it is unclear whether the findings would generalize to other samples. For instance, although individuals with schizophrenia also display reward anticipation deficits, it is unknown whether the ACC/mPFC and OFC would also be the sites of convergence within this clinical population. Second, although our EEG and fMRI reward tasks were analogous, they were not identical and minor visual or timing differences could have impacted the results. Related to this point, the order of EEG and fMRI reward tasks was not counterbalanced, due to the aims of the larger study (i.e., Shankman et al., 2013), and it is possible there was an impact of task order. Future studies are therefore needed to replicate the current findings.
Despite these limitations, there are several important inferences that can be drawn from the present study. Most notably, two commonly used measures of reward anticipation – frontal alpha EEG asymmetry and fMRI BOLD signal-were found to correlate. Specifically, EEG asymmetry reflecting greater relative left frontal activity during reward anticipation was associated with greater ACC/mPFC and OFC activation during reward anticipation. Given that these regions are key nodes in the neural reward circuit and are implicated in the pathophysiology of depression and schizophrenia, the convergence of these two measures in these regions is noteworthy and highlights potential areas of overlap between the seemingly parallel EEG and fMRI literatures.
Highlights.
Correlation between EEG asymmetry and fMRI measures of reward anticipation examined
The two reward anticipation measures were indeed correlated
EEG asymmetry was associated with ACC/mPFC and OFC activation
Acknowledgments
This study was supported by a grant from Brain and Behavior Research Foundation and an R21 from the National Institute of Mental Health (R21 MH080689; PI: Shankman). The funding sources had no role in the present manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander WH, Brown JW. Computational models of performance monitoring and cognitive control. Topics in Cognitive Science. 2010;2(4):658–677. doi: 10.1111/j.1756-8765.2010.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JJB, Coan JA, Nazarian M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology. 2004;67:183–218. doi: 10.1016/j.biopsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala–frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2(4):303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal–striatal interactions. Schizophrenia Bulletin. 2010;36(5):919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Epstein N, Brown G, Steer R. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wüstenberg T, Hein J, Kienast T, Kahnt T, Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biological Psychiatry. 2009;66(8):734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Becker MP, Nitsch AM, Miltner WH, Straube T. A single-trial estimation of the feedback-related negativity and its relation to BOLD responses in a time-estimation task. The Journal of Neuroscience. 2014;34(8):3005–3012. doi: 10.1523/JNEUROSCI.3684-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum H, Oltmanns TF. Emotional experience and expression in schizophrenia and depression. Journal of Abnormal Psychology. 1992;101:37–44. doi: 10.1037//0021-843x.101.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neuroscience. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JE, Quitkin FM. Regional brain asymmetries in major depression with or without an anxiety disorder: a quantitative electroencephalographic study. Biological Psychiatry. 1997;41(9):939–948. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review. 2008;28(4):676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G. Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. Neuroimage. 2011;57(4):1608–1616. doi: 10.1016/j.neuroimage.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Choi SH, Lee H, Ku J, Yoon KJ, Kim JJ. Neural basis of anhedonia as a failure to predict pleasantness in schizophrenia. The World Journal of Biological Psychiatry. 2013;15:525–533. doi: 10.3109/15622975.2013.819121. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Kozak MJ. Constructing constructs for psychopathology: The NIMH research domain criteria. Journal of Abnormal Psychology. 2013;122:928–937. doi: 10.1037/a0034028. [DOI] [PubMed] [Google Scholar]
- Dalili MN, Penton-Voak IS, Harmer CJ, Munafò MR. Meta-analysis of emotion recognition deficits in major depressive disorder. Psychological Medicine. 2014:1–10. doi: 10.1017/S0033291714002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Van Hoesen GW. Emotional disturbances associated with focal lesions of the limbic frontal lobe. In: Heilman KM, Satz P, editors. Neuropsychology of Human Emotion. New York: Guilford Press; 1983. pp. 85–110. [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3(10):1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Asymmetric brain function, affective style, and psychopathology: The role of early experience and plasticity. Development and Psychopathology. 1994;6(04):741–758. [Google Scholar]
- Davidson RJ. Affective style and affective disorders: perspectives from affective neuroscience. Cognition & Emotion. 1998;12:307–330. [Google Scholar]
- Davidson RJ. What does the prefrontal cortex “do” in affect: perspectives on frontal EEG asymmetry research. Biological Psychology. 2004;67(1):219–234. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- de Leeuw M, Kahn RS, Vink M. Fronto-striatal dysfunction during reward processing in unaffected siblings of schizophrenia patients. Schizophrenia Bulletin. 2015;41(1):94–103. doi: 10.1093/schbul/sbu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Kozink RV, McClernon FJ, Smoski MJ. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. Journal of Affective Disorders. 2012;136(3):1126–1134. doi: 10.1016/j.jad.2011.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannler M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Engel M, Fritzsche A, Lincoln TM. Anticipatory pleasure and approach motivation in schizophrenia-like negative symptoms. Psychiatry Research. 2013;210(2):422–426. doi: 10.1016/j.psychres.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figee M, Vink M, de Geus F, Vulink N, Veltman DJ, Westenberg H, Denys D. Dysfunctional reward circuitry in obsessive-compulsive disorder. Biological Psychiatry. 2011;69(9):867–874. doi: 10.1016/j.biopsych.2010.12.003. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version. Washington, D.C: American Psychiatric Press; 1996. [Google Scholar]
- Forbes EE, Christopher May J, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, Dahl RE. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. Journal of Child Psychology and Psychiatry. 2006;47(10):1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: a scale development study. Journal of Research in Personality. 2006;40:1086–1102. [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophrenia Research. 2007;93(1):253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Huggins AA, Fitzgerald DA, Nelson BD, Phan KL, Shankman SA. Neural response to reward anticipation in those with depression with and without panic disorder. Journal of Affective Disorders. 2014;164:50–56. doi: 10.1016/j.jad.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301(5636):1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Grimm O, Heinz A, Walter H, Kirsch P, Erk S, Haddad L, Meyer-Lindenberg A. Striatal response to reward anticipation: Evidence for a systems level intermediate phenotype for schizophrenia. JAMA Psychiatry. 2014 doi: 10.1001/jamapsychiatry.2014.9. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. The Journal of Neuroscience. 2006;26(32):8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. Journal of Neuroscience. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann D, Hewig J, Seifert J, Naumann E, Bartussek D. The latent state-trait structure of resting EEG asymmetry: Replication and extension. Psychophysiology. 2005;42:740–752. doi: 10.1111/j.1469-8986.2005.00367.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser TU, Iannaccone R, Stämpfli P, Drechsler R, Brandeis D, Walitza S, Brem S. The feedback-related negativity (FRN) revisited: new insights into the localization, meaning and network organization. Neuroimage. 2014;84:159–168. doi: 10.1016/j.neuroimage.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Helzer JE, Kraemer HC, Krueger RF. The feasibility and need for dimensional psychiatric diagnoses. Psychological Medicine. 2006;36:1671–1680. doi: 10.1017/S003329170600821X. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. Journal of Abnormal Psychology. 1991;100:535–545. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- Horan WP, Wynn JK, Mathis I, Miller GA, Green MF. Approach and Withdrawal Motivation in Schizophrenia: An Examination of Frontal Brain Asymmetric Activity. PloS One. 2014;9(10):e110007. doi: 10.1371/journal.pone.0110007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research Domain Criteria (RDoC): Developing a valid diagnostic framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. Journal of Abnormal Psychology. 2007;116:80–85. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biological Psychiatry. 2008;63:686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Current opinion in neurology. 2005;18(4):411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. NeuroReport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Kring AM, Barch DM. The motivation and pleasure dimension of negative symptoms: Neural substrates and behavioral outputs. European Neuropsychopharmacology. 2014;24(5):725–736. doi: 10.1016/j.euroneuro.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex. 2003;13(10):1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Nathan PJ. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35(12):2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social cognitive and Affective Neuroscience. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, York MK, Montague PR. The neural substrates of reward processing in humans: the modern role of FMRI. The Neuroscientist. 2004;10(3):260–268. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- Meehl PE. Hedonic capacity: some conjectures. Bulletin of the Menninger Clinic. 1975;39:295–307. [PubMed] [Google Scholar]
- Nusslock R, Almeida JR, Forbes EE, Versace A, Frank E, LaBarbara EJ, Phillips ML. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disorders. 2012;14(3):249–260. doi: 10.1111/j.1399-5618.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biological Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Pause BM, Raack N, Sojka B, Goder R, Aldenhoff JB, Ferstl R. Convergent and divergent effects of odors and emotions in depression. Psychophysiology. 2003;40:209–225. doi: 10.1111/1469-8986.00023. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry. 2003;54(5):504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2010;36(1):183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. American Journal Psychiatry. 2009a;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. Journal of Psychiatric Research. 2009b;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness a source-localization study. Psychological Science. 2005;16(10):805–813. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Plichta MM, Wolf I, Hohmann S, Baumeister S, Boecker R, Schwarz AJ, Brandeis D. Simultaneous EEG and fMRI reveals a causally connected subcortical-cortical network during reward anticipation. The Journal of Neuroscience. 2013;33(36):14526–14533. doi: 10.1523/JNEUROSCI.0631-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar P, Fannon D, Kuipers E, Simmons A, Frangou S, Kumari V. Emotional decision-making and its dissociable components in schizophrenia and schizoaffective disorder: a behavioural and MRI investigation. Neuropsychologia. 2008;46(7):2002–2012. doi: 10.1016/j.neuropsychologia.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61(5):720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Sherdell L, Waugh CE, Gotlib IH. Anticipatory pleasure predicts motivation for reward in major depression. Journal of Abnormal Psychology. 2012;121:51–60. doi: 10.1037/a0024945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Klein DN. The relation between depression and anxiety: an evaluation of the tripartite, approach-withdrawal and valence-arousal models. Clinical Psychology Review. 2003;23(4):605–637. doi: 10.1016/s0272-7358(03)00038-2. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Klein DN, Tenke CE, Bruder GE. Reward sensitivity in depression: A biobehavioral study. Journal of Abnormal Psychology. 2007;116(1):95–104. doi: 10.1037/0021-843X.116.1.95. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, Gorka SM. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology. 2013;122(2):322–338. doi: 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, Dichter GS. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. Journal of Affective Disorders. 2009;118:69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJB. Resting frontal EEG asymmetry as an endophenotype for depression risk: Sex-specific patterns of frontal brain asymmetry. Journal of Abnormal Psychology. 2010;119:502–512. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Coan JA, Towers DN, Allen JJ. Frontal EEG asymmetry during emotional challenge differentiates individuals with and without lifetime major depressive disorder. Journal of Affective Disorders. 2011;129(1):167–174. doi: 10.1016/j.jad.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomarken AJ, Dichter GS, Garber J, Simien C. Resting frontal brain activity: Linkages to maternal depression and socioeconomic status among adolescents. Biological Psychology. 2004;67:77–102. doi: 10.1016/j.biopsycho.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Tsurugizawa T, Uematsu A, Uneyama H, Torii K. Functional brain mapping of conscious rats during reward anticipation. Journal of Neuroscience Methods. 2012;206:132–137. doi: 10.1016/j.jneumeth.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 2009;45:327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]