Abstract
Background
We previously demonstrated that tissue plasminogen activator (tPA) reduces infarct size after mechanical middle cerebral artery occlusion (MCAO) in wild-type (WT) mice and transgenic mice expressing human leukocyte antigen DR2 (DR2-Tg). Clinically, tPA limits ischemic damage by dissolving the clot blocking blood flow through a cerebral artery. To mimic the clinical situation, we developed a new mouse model of thromboembolic stroke, and tested the efficacy of tPA in WT and DR2-Tg mice.
New Method
Autologous blood is withdrawn into a PE-8 catheter filled with 2 IU α-thrombin. After exposing the catheter briefly to air, the catheter is reintroduced into the external (ECA) and advanced into the internal carotid artery (ICA) to allow for intravascular injection of thrombin at the MCA bifurcation. To validate the model, we tested the effect of tPA on laser-Doppler perfusion (LDP) over the MCA territory and infarct size in WT and DR2-Tg mice.
Results
The procedure results in a consistent drop in LDP, and leads to a highly reproducible ischemic lesion. When administered at 15 min after thrombosis, tPA restored LDP and resulted in a significant reduction in infarct size at 24 hours after thrombosis in both WT and DR2-Tg.
Comparison with Existing Methods
Our model significantly reduces surgery time, requires a single anesthesia exposure, and produces a consistent and predictable infarction, with low variability and mortality.
Conclusion
We validated the efficacy of tPA in restoring blood flow and reducing infarct in a new model of endovascular thromboembolic stroke in the mouse.
Keywords: Cerebral Ischemia, Stroke, Thromboembolic, Mouse, tPA, Optical Microangiography
1. INTRODUCTION
Stroke is the fourth leading cause of death and a leading cause of serious, long-term disability in the United States (Towfighi and Saver, 2011). Approximately 87% of acute strokes are ischemic (Go et al., 2013), in which the middle cerebral artery (MCA) is most frequently affected (Gillum, 2002). Ischemic stroke occurs as a result of cerebral thrombosis or embolism. Cerebral thrombosis is the most common type of ischemic stroke (Zhang et al., 2013; Juliana B Casals et al., 2011), which refers to a thrombus that develops at the clogged part of cerebral vessel due to atherosclerosis of brain arteries, caused by a buildup of fatty deposits inside the blood vessels. An embolus is most often a piece of a thrombus that has broken free and travels to brain usually from an atherosclerotic plaque or heart.
Several stroke models have been developed in rodents to mimic ischemic stroke in humans, including the mechanical (intraluminal filament) occlusion model (Longa et al., 1989; Belayev et al., 1996; Kitagawa et al., 1998; Hata et al., 2000), the embolic (fibrin-rich clot) occlusion model (Overgaard et al., 1992; Busch et al., 1997; Zhang et al., 1997; Zhang et al., 1997; Ren et al., 2012; Zhang et al., 2005) and the photochemical in-situ thrombosis occlusion model (Yao et al., 1996; Cai et al., 1998; Watson et al., 2002; Yao et al., 2003). These models were useful in understanding the consequences of cerebral ischemia, although none reflected the natural course and pathophysiology of cerebral thrombosis (Hossmann, 2012). Zhang et al. described a rat thrombotic stroke model, whereby an autologous clot is formed as a result of direct injection of thrombin endovascularly (Zhang, et al., 1997). A mouse model of in situ thromboembolic stroke has also been described, in which an autologous thrombus is directly induced inside the MCA by local microinjection of purified thrombin, resulting in a stable and reproducible infarct volume with low mortality rate (Orset et al., 2007; 23. Ansar et al., 2014).However, this model requires craniotomy, penetration of dura mater and may alter intracranial pressure (ICP). Finally, and more recently, an atherothrombotic model of stroke in rats and mice was developed by collagen injection directly into the cerebral circulation (Schunkeet et al., 2015). Authors note, however, that in the mouse, the model produces multifocal strokes with variable size infarcts.
In the present study, we developed a reproducible thromboembolic mouse model with low mortality and variability, which requires short surgery time and a single anesthesia exposure. The model, which is based on the endovascular introduction of autologous blood and thrombin, was used in wild-type (WT) mice and mice expressing human leukocyte antigen DR2 transgene (DR2-Tg), to test the effect of thrombolysis by intravenous administration of recombinant tissue plasminogen activator (tPA) on infarct size and cerebral blood flow (CBF) recovery. We have previously demonstrated that Recombinant T Cell Receptor Ligands (RTL), partial MHC class II (pMHC) molecules covalently bound to myelin peptides, reverse brain tissue infiltration by leukocytes, which contributes to increased infarct size and worse outcome after middle cerebral artery occlusion (MCAO) (Subramanian et al., 2009). However, the potential application of RTL therapy to human stroke is limited by the need to match recipient MHC class II with the pMHC construct. In order to test the efficacy of human pMHC in experimental stroke, we used humanized transgenic mice, which express the human MHC class II region carrying the HLA-DR2 haplotype (DR2-Tg mice). For RTL therapy to be used in humans, it has to be combined with tPA, the standard stroke therapy. Therefore, in addition to using wild-type (WT) in the current study, we also validated tPA therapy in DR2-Tg mice, in anticipation of combining tPA with pMHC therapy in future studies.
2. MATERIALS AND METHODS
2.1. Experimental Animals
All animal procedures were conducted in accordance with the National Institutes of Health guidelines for the use of animals in research, and protocols were approved by the Animal Care and Use Committees at Oregon Health & Science University and the Portland Veteran Affairs Medical Center. Male C57BL/6 mice (WT, 10–14 weeks of age, 23–28 g body weight) were purchased form Jackson Laboratory (Sacramento, CA, USA) and male HLA-DRB1*1502 transgenic mice (DR2-Tg, 10–14 weeks of age and weighing 23–28 g), which are also on a C57BL/6 background, were produced at the Portland VA Medical Center using foundation breeders provided by Dr. Chella David (Zhu et al., 2014). The mice were housed in temperature-controlled rooms on a 12-hour light and 12-hour dark cycle with water and food ad libitum. Mice were randomized to one of the following experimental groups: sham surgery, control (experimentally naïve) group and a treatment group (vehicle or tPA). Surgeons were blinded to treatment groups.
2.2. Pre-Surgery Catheter Preparation
The tip of a PE-8 tubing (inner diameter (I.D.): 0.20 mm, outer diameter (O.D.): 0.35mm, Strategic Applications Inc, Lake Villa, IL, USA) was stretched under a heating lamp to further reduce its O.D.to 0.23–0.25 mm. This will also smooth the catheter’s tip and make it more flexible, decreasing the risk of damaging vessel wall by the rigid tubing and sharp tip. A 31-gauge small hub removable needle (Hamilton Co., Reno, NV, USA) was inserted into a 15-cm long modified PE-8 catheter and attached to a 50 μL Hamilton syringe (Model 1705 RN SYR, Reno, NV, USA). Bovine α-thrombin (10μL, 0.2 NIHU/μL; T7513, Sigma-Aldrich, Co. LLC, St. Louis, MO, USA) was filled into catheter and syringe and subsequently stored at 4°C until needed for infusion.
2.3. Animal Model
Mice were anesthetized with 5% isoflurane for induction, and anesthesia was maintained with 1.0–1.5% isoflurane in O2-enrichedair using a face mask. Rectal temperature was maintained at 36.5 ± 0.5°C with warm water pads and heating lamp during surgery. During recovery, we keep a warm water pad set at 36.5°C under the cage. Under the operating microscope, the right common (CCA), external (ECA) and internal carotid arteries (ICA) were exposed through a 2-cm midline incision in the front of the neck. The ECA was cauterized and transected. The ICA was then carefully isolated to avoid damage to the Vagus nerve. A 6-0 suture (ETHICON, Inc., Somerville, NJ, USA) was tied loosely at the origin of the ECA. The right CCA and ICA were temporarily suspended using 6-0 suture. A modified PE-8 catheter, filled with 2 NIHU α-thrombin (10 μL of 0.2 NIHU/μL) was gently introduced into the ECA lumen through a small puncture. The suture around the origin of the ECA was tightened around the intraluminal catheter to prevent bleeding. A sample of 3 μL blood was withdrawn from ECA into this catheter, which was then exposed to air for 15 min to accelerate clot formation at the tip of the catheter (Figure 1C). The sutures on the CCA and ICA were temporarily tightened; 8-mm length of catheter was reintroduced into the ECA and gently advanced 7-8 mm into ICA (Figure 1B). At this point, the tip of the intraluminal catheter was 1–2 mm from the origin of the MCA. The blood clots and 50% of the thrombin were briskly injected to occlude the vessel, which was confirmed by the sudden drop in laser-Doppler perfusion (LDP; < 30% of baseline) over the MCA territory (Figure 1E). The rest of thrombin was then very gently injected over 1 min making sure LDP remained low. At 10 min after injection, the catheter was withdrawn and the right ECA was ligated. The suture on the right CCA was released at 60 min after onset of occlusion. For sham surgeries, the right CCA was ligated for 60 min, and the modified PE-8 catheter was placed in the ECA, but not advanced into ICA. No blood was withdrawn and no thrombin was injected in sham animals. Physiological variables (blood pressure, blood gases and rectal temperature) were measured in a separate group of mice under anesthesia at baseline and at 60 min after thrombin injection. Mice were then allowed to recover from anesthesia in cages (with warming pads) and briefly re-anesthetized to measure rectal temperature again at 3 hours after occlusion (thrombin injection) to measure body temperature during recovery.
Figure 1. Illustration of the Mouse Model of Thromboembolic Stroke.

A. The Circle of Willis and major cerebral vessels visualized on the mouse brain ventral surface after intravenous ink dye injection. ACA, MCA, ICA and PCA are the anterior, middle, interior and posterior cerebral arteries; SCA is the superior cerebellar artery; BA is the basilar artery, ACOM and PCOM are the anterior and posterior communicating arteries. B. Schematic illustration of the in-situ endovascular thrombin injection in mice. The catheter is introduced into the ICA, with final placement of its tip at 2 mm caudal to the origin of MCA. The catheter contains blood (red) and α-thrombin (gray). VA: vertebral artery; other abbreviations are the same as in “A”. C. A modified PE-8 catheter filled with blood (distal red segment) and α- thrombin (clear proximal end of the tubing). D. Soft, unformed red blood clots (arrow) immersed in saline as they appear prior to injection into the ICA. E. Laser-Doppler perfusion (LDP) drops by 20–40% of baseline after right common carotid artery occlusion (RCCAO). When blood clots and α-thrombin are injected, LDP abruptly drops further to ischemic level of ~10% of baseline. F. Thrombosis (yellow arrows) can be observed in the right middle (MCA) and internal (ICA) carotid arteries on the ventral surface of the brain at 60 min of thrombin injection. Mouse was perfused trans-cardially with 20 mL saline prior to brain extraction.
2.4. Monitoring Laser-Doppler Perfusion (LDP)
During the procedure, cerebrocortical perfusion was monitored by laser-Doppler (Model DRT4, Moor Instruments Inc., Wilmington, DE, USA) using a metal probe affixed to the surface of right side of the skull at the mid-point between the right ear and right eye (core of MCA territory). LDP was measured before CCA ligation (baseline), and measurements continued throughout procedure and 5 min after release of CCA ligature (Figure 1E). Animals were excluded if LDP did not drop below 30% of baseline after all thrombin was infused (Zhu et al., 2014).
2.5. Treatment with Recombinant Human Tissue Plasminogen Activator (tPA)
To test the efficacy of tPA in this thromboembolic model, human recombinant tPA (Genentech, Inc., San Francisco, CA, USA) was administered intravenously (I.V.) via the right jugular vein at a dose of 10 mg/kg body weight. Ten percent of tPA was given as a bolus and the remaining 90% was administered by continuous infusion over 30 min starting at 15 min of occlusion (thrombin injection) using a Syringe Pump (Model 1140-001; Harvard Apparatus, MA, USA) (Zhu et al., 2014). The control group received the vehicle in which tPA is dissolved, which consisted of 52.5 mg/mL L-arginine, 0.17 mg/mL polysorbate 80 and 15 mg/mL phosphoric acid in sterile water. Investigators were fully blinded to treatment code during both surgery and analysis of infarct size.
2.6. Measurement of Ischemic Lesion Volume
Mice were euthanized and brains collected at 24 hours after occlusion for measurement of infarct size using 2,3,5-triphenyltetrazolium chloride (TTC; Sigma, St. Louis, MO, USA) histology and digital image analysis, as previously described (Chen et al., 2014). Four slices of 2-mm thick coronal sections were incubated in 1.2 % TTC for 15 min at 37 °C, then fixed in 10% formalin overnight. Images were analyzed using Sigma Scan Pro 5.0 Software (Systat Software, Inc., Point Richmond, CA, USA). To control for edema, infarct size in each region (cortical, striatal and total hemispheric infarct) was determined by subtracting the ipsilateral non-infarcted tissue volume from the volume of the contralateral region, then dividing the difference by the contralateral region volume. The ratio is multiplied by 100 to yield regional infarct volume as a percent of the contralateral region. Investigators were fully blinded to treatment code during both surgery and analysis of infarct size.
2.7. Statistical Analysis
Data is presented as mean ± SD, and “n” refers to the number of mice. Differences among groups in infarct size were evaluated by one-way ANOVA with Student-Newman-Keuls post hoc test. Survival and success rates were compared using 2-sided Fisher’s exact test or Chi-Square test. Statistical significance was set at p <0.05. Physiological variables in Table 3 were evaluated using two-away ANOVA. Statistical analyses were performed using SigmaStat Statistical Software, Version 3.1 (SPSS, Inc., Chicago, IL, USA).
Table 3.
Physiological parameters before and after thrombin injection
| Groups | Before thrombin injection
|
1 hour after thrombin injection
|
||||||
|---|---|---|---|---|---|---|---|---|
| pH | PO2 (mm Hg) | PCO2 (mm Hg | MABP (mm Hg) | pH | PO2 (mm Hg) | PCO2 (mm Hg | MABP (mm Hg) | |
| Sham (n=4) | 7.42 ± 0.04 | 216 ± 21 | 20.6 ± | 82 ± 2 | 7.38 ± 0.07 | 219 ± | *30.5 ± | 77 ± 4 |
| 3.5 | 10 | 11.3 | ||||||
| Vehicle (n=4) | 7.39 ± 0.03 | 206 ± 12 | 21.5 ± | 86 ± 6 | # *7.29 ± | 205 ± | *39.1 ± | *74 ± 4 |
| 2.8 | 0.02 | 17 | 6.9 | |||||
| tPA treated (n=4) | 7.39 ± 0.02 | 200 ± 14 | 23.0 ± | 82 ± 1 | # *7.30 ± | 202 ± | *38.6 ± | 76 ± 4 |
| 1.5 | 0.04 | 17 | 5.3 | |||||
Values are mean ± SD. MABP, mean arterial blood pressure (right femoral artery).
indicates p<0.05 between baseline and 1 h after thrombin injection.
indicates p<0.05 compared with Sham group.
3. RESULTS
3.1. Success Rate and Physiological Monitoring
A total of 72 mice (half WT and half DR2-Tg) were used in the tPA treatment study. Mice within each strain were separated into three groups: untreated and tPA- and vehicle-treated groups (n=12 per group). A total of 12 (17%) mice were excluded from the study because of subarachnoid hemorrhage (SAH; 2 mice out of 12), failure to advance catheter or based on LDP exclusion criterion (failure to drop to less than 30% of baseline). Success rate and survival of animals up to 24 hours after surgery are shown in Table 1. Overall surgical success and 24-hour survival rates were not statistically different among the 3 treatment groups in either WT orDR2-Tg mouse. Rectal temperatures at end-ischemia (60 min MCAO) and at 3 hrs after thrombin injection were 36.6 ± 0.1 and 34.5 ± 0.3 in sham animals (n=4), 36.5 ± 0.2 and 31.2 ± 0.5 in untreated animals (n=4), and 36.6 ± 0.1 and 33.2 ± 0.7°C in tPA-treated animals (n=4), respectively. Arterial blood pressure, pH and pCO2 were maintained within their normal range prior to occlusion (pO2 was above 200 mmHg due to use of oxygen-enriched gas mixture; Table 3). At 60-min after thrombin injection, pCO2 increases above baseline in all groups, including sham animals. Blood pressure decreased slightly, but was statistically significant only in the untreated group. Finally, pH decreased in both groups subjected to ischemia relative to sham, regardless of tPA treatment.
Table 1.
Modeling success and animal survival at 24 hours after surgery
| Group | WT
|
DR2-Tg
|
||||||
|---|---|---|---|---|---|---|---|---|
| Untreated | Vehicle treated | tPA treated | Total | Untreated | Vehicle treated | tPA treated | Total | |
| Number of surgeries (n) | 12 | 12 | 12 | 36 | 12 | 12 | 12 | 36 |
| Success rate [n (%)] | 10 (83%) | 12 (100%) | 10 (83%) | 32 (89%) | 8 (67%) | 10 (83%) | 10 (83%) | 28 (78%) |
| 24-hours survival rate [n (%)] | 9 (90%) | 11 (92%) | 10 (100%) | 30 (94%) | 8 (100%) | 9 (90%) | 9 (90%) | 26 (93%) |
Surgeries on WT and DR2-Tg mice were performed by two different surgeons.
3.2. Effect of the tPA on LDP
Ligation of the right CCA decreased LDP in the right MCA territory by 20–40% of baseline (n=5 WT and n=3 DR2-Tg mice), and when ligature was release after 60 minutes, LDP quickly returned to normal, resulting in no apparent infarcts by TTC staining at 24 hours later (Data not shown). After introduction of autologous blood and thrombin, LDP in the ipsilateral MCA territory dropped precipitously to 10% of baseline in all groups(Figure 1E) and was restored close to baseline after tPA treatment(Figure 2). No significant differences in LDP were observed between the untreated and vehicle-treated groups at any time point during occlusion. Treatment with tPA restores perfusion approximately 15 minutes after start of tPA infusion. There was a significant increase in LDP response to tPA at all time points compared to vehicle-treated and untreated groups in both WT and DR2-Tg mice.
Figure 2. Effect of recombinant tissue plasminogen activator (tPA) on cerebral blood flow (CBF) recovery after thromboembolic stroke in WT (A) and DR2 mice (B).

Mice were administered tPA (10 mg/kg) through the jugular vein at 15 min after MCAO. CBF was measured by laser-Doppler and was monitored for 60 min after MCAO. Treatment with tPA restored CBF starting at 15 min after onset of treatment. Values are expressed as mean ± SD. Stars denote statistically significant differences compared to untreated and vehicle-treated groups (*p<0.05, **p<0.01).
3.3. Effect of tPA on infarct size
Injection of the clot and thrombin resulted in a significant infarct in the untreated and vehicle-treated WT and DR2-Tg mice at 24 hours after MCAO. No differences were observed in cortical, striatal, hippocampal or total hemispheric infarcts between untreated and vehicle-treated groups (Figure 3 and 4). In both strains of mice, treatment with tPA starting at 15 minutes after MCAO significantly reduced the size of cortical, striatal, hippocampal and total hemispheric infarcts compared to the untreated or vehicle-treated groups (Figure 3 and 4). Infarct size and its distribution were highly reproducible, as detected by TTC staining (Figure 4, with hippocampal infarct highlighted). No hemorrhage was observed within the infarction zone in either of the groups. The coefficient of variation (CV) for infarct size in untreated mice was 0.2 and 0.5 with tPA treatment.
Figure 3. Effect of tissue plasminogen activator (tPA) on infarct size after thromboembolic MCAO in WT and DR2-Tg mice.

Treatment with tPA significantly reduced cortical, striatal and total hemispheric infarcts at 24 after MCAO compared to untreated and vehicle-treated groups in both WT (A) and DR2-Tg mice (B). Values are expressed as mean ± SD. *P<0.05, **P<0.01.
Figure 4. Hippocampal Infarct after thromboembolic stroke in mice.
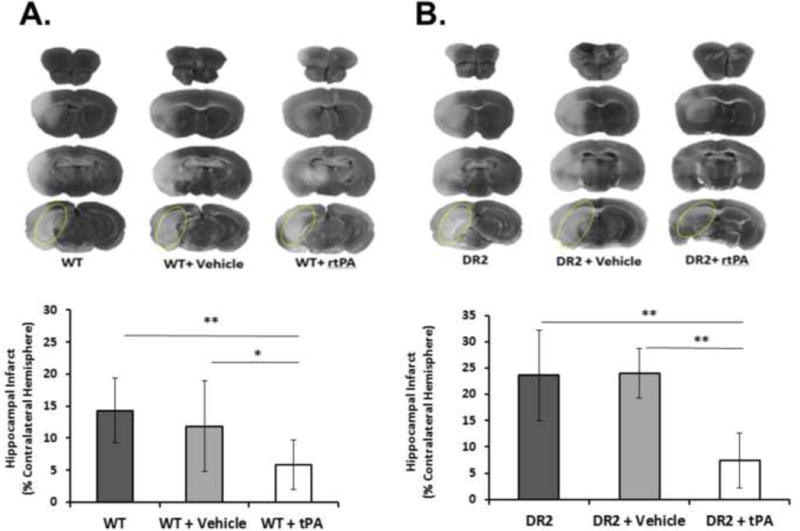
Representative TTC-stained coronal sections showing infarcts at 24 hrs after MCAO in WT (A) and DR2 mice (B). Hippocampal infarct is highlighted by dotted yellow ovals in caudalmost brain section, and quantified in the graphs in the lower panels. Treatment with tissue plasminogen activator (tPA) significantly reduced hippocampal infarct size at 24 after MCAO compared to untreated and vehicle-treated groups in both WT (A) and DR2-Tg mice (B).Values are expressed as mean ± SD. *P<0.05, **P<0.01.
4. DISCUSSION
Remarkable success has recently been achieved in treating acute ischemic stroke and decreasing associated mortality. Nevertheless, the only approved therapy for acute thromboembolic stroke remains thrombolysis using tPA given to selected patients within the first 4.5 hours after onset of symptoms (Hacke et al., 2008; Wahlgren et al., 2008). Furthermore, recanalization efficacy is far from optimal, as only 20–40% of patients treated with tPA achieve partial or full recanalization (Saver, 2011; del Zoppoet al., 1992). Moreover, potential side effects of tPA, such as hemorrhage, may aggravate stroke injury and offset the benefits provided by reperfusing an occluded artery. Development of new therapies requires the availability of reproducible animal models that faithfully model human stroke as essential tools of preclinical research.
Over the past three decades, several different stroke models have been developed in rodents and used to investigate the pathophysiology of ischemia and to discover novel therapeutic targets for ischemic stroke (Table 2). Mechanical occlusion of cerebral vessels, such as the intraluminal suture MCAO model, was developed to study downstream ischemic changes subsequent to vascular occlusion, but is not suitable to study thrombolysis. Embolic stroke models, on the other hand, mimic the pathophysiology of human stroke more closely, and are appropriate to study therapies targeting the occluding thrombus. Current thromboembolic stroke models in rodents introduce well-formed blood clots, which require 1–2 days to mature ex vivo. Other models require invasive surgeries, injection of laser-activated dyes, invasive surgery or collagen injection. More importantly, these models lack reproducibility and uniformity in infarct size and location (Ren et al., 2012). Thrombosis is a dynamic process, which in living tissue results from various combinations of stasis, hyper-coagulation and endothelial injury, collectively known as the Virchow’s triad (Bagot and Arya, 2008). In our model, which uses young healthy mice, endothelial injury is minimal, and may result from intravascular catheter introduction. The more important triggers of thrombosis in our model are local hypercoagulation induced by thrombin and the reduction in blood flow (stasis) due to CCA ligation.
Table 2.
Overview of common ischemic stroke models in mouse
| Model | Advantages | Limitations | References |
|---|---|---|---|
| Intraluminal filament model (Mechanical MCAO model) | Controlled timing of occlusion and reperfusion; transient and permanent occlusion; does not require craniotomy; minimal effects on coagulation and fibrinolytic systems;mimicsendovascular thrombectomy due to rapid recanalization after filament withdrawn; established and well recognized in the stroke research community | Lesion volume/variability highly dependent on collateralization and circle of Willis circle; reproducibility depends on filament size and shape; potential damage to endothelium; risk of subarachnoid hemorrhagesecondary to sutureinducedarterial rupture; not suitable for thrombolysis studies | Kitagawa et al., 1998; Hata et al., 2000; |
| Photothrombosis model | Reproducible, localized and controlled as it is triggered by light/laser beam; low mortality;intact skull; no mechanicaldamage of endothelium | Earlyvasogenic edema and bloodbrain barrier breakdown occur within minutes; small ischemic penumbra; injection of photosensitivedye; unsuitable for preclinical drug studies | Lee et al., 2006; Labatgest et al., 2013 |
| Embolic model | Mimics cerebral embolism in human; can be used to study thrombolysis; uses fibrin-rich (white) clots with predetermined size (length and diameter);does not require craniotomy | Location and duration of ischemia difficult to control; lacks reproducibility and uniformity relative to infarct volume; requires24 to 48 h for clots to mature; high mortality in long term recovery; spontaneous recanalization | Zhang Z et al., 1997; Zhang ZG et al., 2005; Ren et al., 2012 |
| Thromboembolicmodel | Reproducible infarcts; suitable for thrombolysis studies; resemblescerebral thrombosis in human | Small craniotomy and excision of dura to expose the MCA; small infarct size | Orset et al., 2007; Ansar et al., 2014 |
Our model has unique features, which gives it an advantage over existing thromboembolic rodent models of ischemic stroke. Most importantly, the model produces a predictable and reproducible focal infarction with low variability and low mortality, mostly attributed to the precise localization of thrombosis. The coefficient of variation (CV) for infarct in untreated mice in our study was 0.2. Not surprisingly, CV increases to 0.5 with tPA treatment. The CV of infarct volume in rodent experimental stroke models has a wide range between 0.05 to 2, depending on the model, choice of anesthetic and how well physiological and hemodynamic variables are controlled, among other factors (Liu et al., 2009). Similarly, Zhang F et al. found that infarct location and size were highly variable in mouse stroke models, with the thromboembolic model showing highest variability in infarct size of 0.44 and lowest 7-day survival (Zhang et al., 2012).
Exposure of 3 μL of fresh blood to air for only 15 min results in a fragile and not fully formed clot because the blood and thrombin do not mix well within the thin catheter (Figure 1 C and D). Injecting the unformed clot near the origin of MCA, where blood flow is already low due to CCA ligation, results in thrombus formation and prevents blood flow through ACA and MCA (Figure 1 B and F). Thrombin has a very short half-life time (1–2 minutes) (Siller-Matula et al., 2011), resulting in clot formation at the MCA / ACA bifurcation, without downstream expansion or migration of the clot. In support of this, no apparent infarction was observed outside the MCA territory, in contralateral hemisphere or in any other organ outside the brain. As seen in Figure 1F, precise lodging of thrombus at the MCA / ACA bifurcation was confirmed by visual inspection of the Circle of Willis. Finally, survival rate in our model exceeded 90%.
Our model produces stroke in a relatively short period of time. The time from skin incision to catheter withdrawn after MCA occlusion was kept at less than one hour by two trained surgeons. The model requires only a single anesthesia exposure. Zhang et al. reported a mouse focal cerebral ischemia model with a fibrin-rich embolus, but their model requires donor mice and over 24 h for clot preparation (Zhang et al., 1997; Zhang et al., 2005). Our endovascular approach for clot delivery allows precise placement of the clot, avoiding craniotomy and penetration of the dura mater.
Relevance of an animal model needs to be validated against standard of care for clinical stroke therapy by demonstrating a beneficial response tPA using relevant doses, administration routes and timing. Although tPA has previously been shown to afford benefit in thromboembolic stroke models, these studies were performed in the rat, with no reports in the mouse. With widespread use of genetically modified transgenic and gene-deleted mice in stroke research (Hossmann, 1998), it is import to have a validated mouse model of stroke and tPA therapy. Our study is first to show benefit of tPA in a mouse model of thromboembolic stroke.
In measuring temperature during recovery, we observed low body temperature in mice at hours after thromboembolic stroke, which may be related to reduced activity (Barber et al., 2004). This response is in contrast to what is observed in the rat, which shows hyperthermia after stroke (Reglodi et al., 2000). In agreement with our study, Barber PA et al. (Barber et al., 2004) found that after 45 min of MCAO, rectal temperature dropped to 33.1°C ± 2.3°C for the first 4 hours after the end of surgical temperature regulation, and despite warming efforts, temperature only reached 34.94°C ± 0.8°C. Similarly, in a recent study by Wu et al (Wu et al., 2014), rectal temperature decreased after MCAO over the first 10 hrs after MCAO compared to baseline, which reached lowest value (33.23 ± 0.57 C) at 1 hr after MCAO and increased thereafter. At 12 hr after MCAO, the temperature of control group eventually returned to normal without significant difference compared to baseline (37.11 ± 0.30 C). In the “temperature controlled” group, where mice were recovered in a 37 °C incubator, rectal temperature, nevertheless, was lower than baseline over the first 7 hrs after MCAO, with lowest value of around 35°C recorded at 1 hr after MCAO (Wu et al., 2014).
In summary, we have developed a novel thromboembolic stroke model in the mouse, which uses an endovascular approach, and we have validated the model using tPA. Our model significantly reduces surgery time, requires a single anesthesia exposure, and produces consistent and predictable infarction, with low variability and mortality. The model will contribute to studies aimed at understanding stroke pathophysiology and the development of stroke therapies, especially studies performed in genetically altered mice.
Highlights.
We developed a new mouse model of thromboembolic stroke
Stroke induction does not require craniotomy
The model requires short surgery time and a single anesthesia exposure
Model produces consistent infarction, with low variability and mortality
We validated the model using tissue plasminogen activator in two mouse strains
Acknowledgments
This project was supported by NIH grants R42NS065515 (Offner & Alkayed) and R01NS070837 (Alkayed).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ansar S, Chatzikonstantinou E, Wistuba-Schier A, Mirau-Weber S, Fatar M, Hennerici MG, Meairs S. Characterization of a new model of thromboembolic stroke in C57 black/6J mice. Translational Stroke Research. 2014;5(4):526–533. doi: 10.1007/s12975-013-0315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot CN, Arya R. Virchow and his triad: a question of attribution. British Journal of Haematology. 2008;143(2):180–190. doi: 10.1111/j.1365-2141.2008.07323.x. [DOI] [PubMed] [Google Scholar]
- Barber PA, Hoyte L, Colbourne F, Buchan AM. Temperature-regulated model of focal ischemia in the mouse: a study with histopathological and behavioral outcomes. Stroke. 2004;35(7):1720–5. doi: 10.1161/01.STR.0000129653.22241.d7. [DOI] [PubMed] [Google Scholar]
- Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture: neurological and pathological evaluation of an improved model. Stroke. 1996;27(9):1616–1623. doi: 10.1161/01.str.27.9.1616. [DOI] [PubMed] [Google Scholar]
- Busch E, Kruger K, Hossmann KA. Improved model of thromboembolic stroke and rt-PA induced reperfusion in the rat. Brain research. 1997;778(1):16–24. doi: 10.1016/s0006-8993(97)01008-1. [DOI] [PubMed] [Google Scholar]
- Cai H, Yao H, Ibayashi S, Uchimura H, Fujishima M. Photothrombotic middle cerebral artery occlusion in spontaneously hypertensive rats: influence of substrain, gender, and distal middle cerebral artery patterns on infarct size. Stroke. 1998;29(9):1982–1987. doi: 10.1161/01.str.29.9.1982. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bodhankar S, Murphy SJ, Vandenbark AA, Alkayed NJ, Offner H. Intrastriatal B-cell administration limits infarct size after stroke in B-cell deficient mice. Metab Brain Dis. 2012;27(4):487–493. doi: 10.1007/s11011-012-9317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ, Poeck K, Pessin MS, Wolpert SM, Furlan AJ, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992;32(1):78–86. doi: 10.1002/ana.410320113. [DOI] [PubMed] [Google Scholar]
- Gillum RF. New considerations in analyzing stroke and heart disease mortality trends: the Year 2000 Age Standard and the International Statistical Classification of Diseases and Related Health Problems, 10th Revision. Stroke; a journal of cerebral circulation. 2002;33(6):1717–1721. doi: 10.1161/01.str.0000016925.58848.ea. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics–2013 update: a report from the American Heart Association [published corrections appear in Circulation. 2013; 127: e841 and Circulation. 2013;127:doi: 10.1161/CIR.0b013e31828124ad] Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D, ECASS Investigators Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- Hata R, Maeda K, Hermann D, Mies G, Hossmann K-A. Evolution of brain infarction after transient focal ischemia in mice. J Cereb Blood Flow Metab. 2000;20(6):937–946. doi: 10.1097/00004647-200006000-00006. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Experimental models for the investigation of brain ischemia. Cardiovascular Research. 1998;39(1):106–120. doi: 10.1016/s0008-6363(98)00075-3. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. The two pathophysiologies of focal brain ischemia: implications for translational stroke research. J Cereb Blood Flow Metab. 2012;32(7):1310–1316. doi: 10.1038/jcbfm.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals Juliana B, Pieri Naira CG, Feitosa Matheus LT, Ercolin Anna CM, Roballo Kelly CS, Barreto Rodrigo SN, Bressan Fabiana F, Martins Daniele S, Miglino Maria A, Ambrósio Carlos E. The Use of Animal Models for Stroke Research: A Review. Comp Med. 2011 Aug;61(4):305–313. [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Yang G, Mabuchi T, Yagita Y, Hori M, Yanagihara T. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: evaluation of the patency of the posterior communicating artery. J Cereb Blood Flow Metab. 1998;18(5):570–579. doi: 10.1097/00004647-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Liu S, Zhen G, Meloni BP, Campbell K, Winn HR. Rodent stroke omdel guidelines for preclinical stroke trials (1st edition) Journal of experimental stroke & translational medicine. 2009;2(2):2–27. doi: 10.6030/1939-067x-2.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Orset C, Macrez R, Young AR, Panthou D, Angles-Cano E, Maubert E, et al. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke. 2007;38(10):2771–2778. doi: 10.1161/STROKEAHA.107.487520. [DOI] [PubMed] [Google Scholar]
- Overgaard K, Sereghy T, Boysen G, Pedersen H, Høyer S, Diemer NH. A rat model of reproducible cerebral infarction using thrombotic blood clot emboli. J Cereb Blood Flow Metab. 1992;12(3):484–490. doi: 10.1038/jcbfm.1992.66. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Somogyvari-Vigh A, Maderdrut JL, Vigh S, Arimura A. Postischemic spontaneous hyperthermia and its effects in middle cerebral artery occlusion in the rat. Exp Neurol. 2000;163:399–407. doi: 10.1006/exnr.2000.7367. [DOI] [PubMed] [Google Scholar]
- Ren M, Lin ZJ, Qian H, Choudhury GR, Liu R, Liu H, Yang SH. Embolic middle cerebral artery occlusion model using thrombin and fibrinogen composed clots in rat. Journal of Neuroscience Methods. 2012;211(2):296–304. doi: 10.1016/j.jneumeth.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saver JL. Improving reperfusion therapy for acute ischaemic stroke. J ThrombHaemost. 2011 Jul 9;(Suppl 1):333–343. doi: 10.1111/j.1538-7836.2011.04371.x. [DOI] [PubMed] [Google Scholar]
- Schunke KJ, Toung TK, Zhang J, Pathak AP, Xu J, Zhang J, Koehler RC, Faraday N. A novel atherothrombotic model of ischemic stroke induced by injection of collagen into the cerebral vasculature. J Neurosci Methods. 2015;239:65–74. doi: 10.1016/j.jneumeth.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller-Matula JM, Schwameis M, Blann A, Mannhalter C, Jilma B. Thrombin as a multifunctional enzyme. Focus on in vitro and in vivo effects. ThrombHaemost. 2011;106(6):1020–1033. doi: 10.1160/TH10-11-0711. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Zhang B, Kosaka Y, Burrows GG, Grafe MR, Vandenbark AA, Hurn PD, Offner H. Recombinant T cell receptor ligand treats experimental stroke. Stroke. 2009;40(7):2539–45. doi: 10.1161/STROKEAHA.108.543991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towfighi A, Saver JL. Stroke Declines From Third to Fourth Leading Cause of Death in the United States. Stroke. 2011;42(8):2351–2355. doi: 10.1161/STROKEAHA.111.621904. [DOI] [PubMed] [Google Scholar]
- Wahlgren N, Ahmed N, Dávalos A, Hacke W, Millán M, Muir K, Roine RO, Toni D, Lees KR, SITS investigators Thrombolysis with alteplase 3–4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet. 2008;372(11):1303–1309. doi: 10.1016/S0140-6736(08)61339-2. [DOI] [PubMed] [Google Scholar]
- Watson BD, Prado R, Veloso A, Brunschwig J-P, Dietrich WD. Cerebral blood flow restoration and reperfusion injury after ultraviolet laserfacilitated middle cerebral artery recanalization in rat thrombotic stroke. Stroke. 2002;33(2):428–434. doi: 10.1161/hs0202.102730. [DOI] [PubMed] [Google Scholar]
- Wu L, Xu L, Xu X, Fan X, Xie Y, Yang L, Lan W, Zhu J, Xu G, Dai J, Jiang Y, Liu X. Keep warm and get success: the role of postischemictemperature in the mouse middle cerebral artery occlusion model. Brain Res Bull. 2014;101:12–7. doi: 10.1016/j.brainresbull.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Yao H, Ibayashi S, Sugimori H, Fujii K, Fujishima M. Simplified model of krypton laser-induced thrombotic distal middle cerebral artery occlusion in spontaneously hypertensive rats. Stroke. 1996;27(2):333–336. doi: 10.1161/01.str.27.2.333. [DOI] [PubMed] [Google Scholar]
- Yao H, Sugimori H, Fukuda K, Takada J, Ooboshi H, Kitazono T, Ibayashi S, Iida M. Photothrombotic middle cerebral artery occlusion and reperfusion laser system in spontaneously hypertensive rats. Stroke. 2003;34(11):2716–2721. doi: 10.1161/01.STR.0000094730.38343.73. [DOI] [PubMed] [Google Scholar]
- Zhang F, Guo RM, Yang M, Wen XH, Shen J. A stable focal cerebral ischemia injury model in adult mice: assessment using 7T MR imaging. AJNR Am J Neuroradiol. 2012;33(5):935–939. doi: 10.3174/ajnr.A2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain research. 1997;766(1–2):83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chopp M, Zhang RL, Goussev A. A mouse model of embolic focal cerebral ischemia. J Cereb Blood Flow Metab. 1997;17(10):1081–1088. doi: 10.1097/00004647-199710000-00010. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang RL, Jiang Q, Raman SB, Cantwell L, Chopp M. A new rat model of thrombotic focal cerebral ischemia. J Cereb Blood Flow Metab. 1997;17(2):123–135. doi: 10.1097/00004647-199702000-00001. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Ding G, Jiang Q, Zhang RL, Zhang X, Gan WB, Chopp M. A model of mini-embolic stroke offers measurements of the neurovascular unit response in the living mouse. Stroke. 2005;36(12):2701–2704. doi: 10.1161/01.STR.0000190007.18897.e3. [DOI] [PubMed] [Google Scholar]
- Zhu W, Libal NL, Casper A, Bodhankar S, Offner H, Alkayed NJ. Recombinant T cell receptor ligand treatment improves neurological outcome in the presence of tissue plasminogen activator in experimental ischemic stroke. Translational Stroke Research. 2014;5(5):612–617. doi: 10.1007/s12975-014-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


