Abstract
Purpose
To evaluate whether intraprocedural 3D quantification of Lipiodol deposition on cone-beam computed tomography (CBCT) can predict tumor response on follow-up contrast-enhanced magnetic resonance imaging (CE-MRI) in patients with hepatocellular carcinoma (HCC) treated with conventional transarterial chemoembolization (cTACE).
Materials and Methods
This IRB approved, retrospective analysis included 36 patients with 51 HCC target lesions, who underwent cTACE with CBCT. CE-MRI was acquired at baseline and 1 month after cTACE. Overall tumor volumes as well as intra-tumoral Lipiodol volumes on CBCT were measured and compared with the overall and necrotic (non-enhancing) tumor volumes on CE-MRI using the paired student’s t-test. Tumor response on CE-MRI was assessed using modified Response Evaluation Criteria in Solid Tumors (mRECIST). A linear regression model was used to correlate tumor volumes, Lipiodol volumes and the percentage of Lipiodol deposition on CBCT with the corresponding parameters on CE-MRI. Nonparametric spearman rank order correlation and trend test were used to correlate the percentage of Lipiodol deposition in the tumor with tumor response.
Result
A strong correlation between overall tumor volumes on CBCT and CE-MRI was observed (R2=0.986). In addition, a strong correlation was obtained between the volume of Lipiodol deposition on CBCT and tumor necrosis (in cm3) on CE-MRI (R2=0.960); and between the percentage of Lipiodol deposition and tumor necrosis (R2=0.979). Importantly, the extent of Lipiodol deposition (in percentage of total tumor volume) correlated strongly with tumor response on CE-MRI (Spearman rho=0.84, p<0.001).
Conclusions
Intraprocedural 3D quantification of Lipiodol deposition on CBCT can be used to predict tumor response on follow-up CE-MRI.
Keywords: Hepatocellular carcinoma, transcatheter arterial chemoembolization, cone-beam computed tomography, three-dimensional, quantitative, tumor response
Introduction
Two landmarks studies have demonstrated survival benefits for patients who were treated with conventional transarterial chemoembolization (cTACE) compared to symptomatic treatment [1, 2]. Although overall survival continues to be the ultimate end point in cTACE clinical trials, imaging biomarkers of tumors response are frequently used as surrogates for TACE efficacy [3-5]. In this context, identifying non-responders as early as possible is of high clinical relevance for the decision on whether or not to retreat the patient [6,7]. Some studies have identified intratumoral Lipiodol deposition as a strong biomarker of tumor necrosis and have confirmed it to be a predictor of tumor response and potentially survival outcomes after cTACE [3,5,8-12]. Many of these reports relied on post-procedural multi-detector CT (MDCT) in order to assess the Lipiodol distribution after cTACE, thus making the feedback available only upon completion of the procedure. In addition, most intraprocedural evaluation relied on non-quantitative and two dimension (2D) fluoroscopic assessment or manual measurements of the Lipiodol deposition. In this context, the emerging role of intraprocedural cone-beam computed tomography (CBCT) has not yet been fully explored [5,11]. The advent and growing availability of immediate, intraprocedural CBCT offers immediate cross-sectional analysis of the Lipiodol deposition [13]. In addition, the development of new 3D quantitative assessment tools provides the basis for objectifying the visual impression by volumetrically quantifying the distribution and deposition of Lipiodol immediately after injection [5, 4, 15]. Previous studies validated the ability of 3D image analysis to accurately quantify the deposition of Lipiodol on CBCT [16, 17]
The purpose of our study was to explore the use of intraprocedural CBCT for immediate 3D quantitative evaluation of Lipiodol deposition during cTACE as a predictor of tumor response on follow-up contrast-enhanced MRI (CE-MRI).
Materials and Methods
Patient Selection
This was a retrospective, single institution study, compliant with Health Insurance Portability and Accountability Act and approved by the Institutional Review Board. Informed consent was obtained before cTACE in all patients. Diagnosis of HCC was confirmed by liver biopsy or by typical imaging features on dynamic CE-CT or CE-MRI (hypervascularity in the arterial phase and washout in the venous phase) and an alpha-fetoprotein level of 200 ng/mL or higher. Patients with unresectable HCC were referred to cTACE after discussion at the multidisciplinary liver tumor conference. Eligibility criteria for cTACE were as follows: focal or multifocal unresectable HCC; Child-Pugh classification A or B; Eastern Cooperative Oncology Group performance status 0 or 1, and no contraindication to contrast medium. The patients with tumor burden greater than 70%, presence of extensive extrahepatic disease, or complete tumor occlusion of the portal vein were excluded. The inclusion of target lesion into image analysis was based on the following eligibility criteria: 1) dynamic CE-MRI acquired within 1 month before and after cTACE; 2) intraprocedural CBCT acquired immediately after cTACE; 3) therapy-naive target lesion visualized without artifacts on both modalities; 4) well-defined tumor borders. Patients and lesions that did not meet the aforementioned criteria were excluded from our study. Between June 2012 and February 2014, the liver tumor board discussed the care of 628 patients who had HCC. Of the 628 patients, 122 patients were treated with cTACE. Of those, 61 patients underwent CE-MRI within one month before and after the treatment, and intra-procedural CBCT immediately after TACE. 25 patients were excluded due to atypical lesion morphology, portal vein invasion and breathing artifact on CE-MRI. Thus, the final analysis included a total of 36 patients with a total of 51 HCC target lesions.
Conventional TACE Protocol
cTACE was performed according to our standard institutional protocol, and all procedures were performed by an interventional radiologist (JFG) with 18 years of experience in hepatic interventions. With use of the Seldinger’s technique, a 5-F vascular sheath was placed in the right common femoral artery over a 0.035-inch guide wire (Bentson, Cook, Bloomington, IN). Under fluoroscopic guidance, a 5-F glide Simmons-1 catheter (Cordis, Miami, FL) was advanced and formed into the aortic arch and then used to select the celiac axis. A 3-F Renegade High-Flo catheter was coaxially advanced through the glide catheter over a 0.014-inch Transcend wire (Boston Scientific, Natick, MA), and manipulated to achieve lobar or segmental catheterization. An emulsion containing 50 mg doxorubicin (Adriamycin; Pharmacia &Upjohn, Peapack, NJ), and 10 mg mitomycin C in a 1:1 mixture with lipiodol (Lipiodol; Guerbet, Paris, France) was infused and followed by the infusion of gelatin-coated trisacryl microspheres (Embosphere Microspheres; Biosphere Medical, Rockland, MA) until arterial inflow was substantially reduced as seen on fluoroscopy.
Intraprocedural C-Arm Cone-Beam CT
All patients underwent CBCT immediately after TACE. Briefly, CBCT was performed using a commercially available angiographic system (Allura Xper FD20, Philips Healthcare, Best, The Netherlands) with the XperCT option, enabling CBCT acquisition and volumetric image reconstruction. Within 5 seconds, 312 projection images (60 frames per second) were acquired with the motorized C-arm covering a 240° clockwise arc under a fixed 120kVp setting. The two dimensional projection images were reconstructed using Feldkamp back projection into 3D volumetric images for a 250×250×194mm field of view (matrix size 384×384×296) with a voxel size of 0.6mm3. The patients were instructed to be at end-expiration apnea during the CBCT scanning.
CE-MRI Technique
All patients underwent baseline and post-TACE CE-MRI using a 1.5-T MR unit (CV/I, GE Medical Systems, Milwaukee, WI) and a phased-array torso coil within 4 weeks before cTACE. The imaging protocol included: 1) axial T2-weighted fast spin-echo images (repetition time [TR]/echo time [TE], 5000/100 msec; matrix size, 256 × 256; slice thickness, 8 mm; interslice gap, 2 mm; receiver bandwidth, 32 kHz); 2) axial single-shot breath-hold gradient-echo diffusion weighted echo-planar images (TR/TE, 5000–6500/110 msec; matrix size, 128×128; slice thickness, 8 mm; inter slice gap, 2 mm; b value, 500 second/mm2; receiver bandwidth, 64 kHz); and 3) axial breath-hold unenhanced and contrast-enhanced (0.1 mmol/kg intravenously of gadodiamide, Omniscan, General Electric, Princeton, NJ) T1-weighted three-dimensional fat-suppressed spoiled gradient-echo images (TR/TE, 5.1/1.2 m sec; field of view,320–400 mm2; matrix size, 192 x 160; slice thickness, 4–6 mm; receiver bandwidth, 64-kHz; flip angle, 15) in the arterial and portal venous phases (20 and 70 seconds after intravenous contrast administration, respectively). The arterial of the CE-MRI were used for the study.
Quantitative Measurement of Lipiodol Deposition in Tumor on CBCT
The volumetric measurement of the overall tumor and Lipiodol deposition on CBCT were performed by an experienced interventional radiologist who did not perform the TACE procedure (ZW, 10 years of experience). Overall tumor volumes as well as the Lipiodol volumes (in cm3) were measured using a semi-automated quantification software, which has been previously demonstrated as accurate and reproducible [16, 18-20]. Briefly, the quantification included three steps. First, the intraprocedural CBCT images were selected; second, a segmentation of the overall tumor volume including Lipiodol deposition was performed (Figure 1, 2: A/C) [18,19]. Third, a region-of-interest-based, semi-automatic quantification of the Lipiodol volume as well as percentage in relation to the overall lesion volume was performed (Figure 1, 2: E) [16,20].
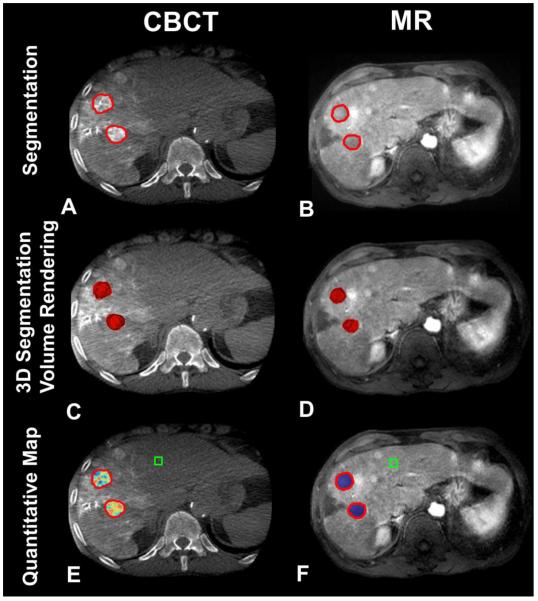
Figure 1.
3D volumetric semi-automatic evaluation of Lipiodol deposition (Complete Response according to mRECIST criteria) in HCC on a representative case. Segmentation of the tumor (red circle) on CBCT at corresponding slice level as on MR (A, B). 3D segmentation volume rendering on the same slice (C, D). Quantitative color map of Lipiodol deposition on CBCT (E) and tumor viability on follow-up MRI (F). The box represents the location of the background ROI. For the anterior target lesion: the tumor volume on CBCT and on MRI was 4.69cm3 and 4.60 cm3, respectively; the volume of Lipiodol on CBCT and non-enhancement on MRI was 4.66cm3 and 4.60 cm3, respectively. For the posterior target lesion: the tumor volume on CBCT and on MRI was 4.58cm3 and 4.51 cm3, respectively; the volume of Lipiodol on CBCT and non-enhancement on MRI was 4.55cm3 and 4.51 cm3, respectively.
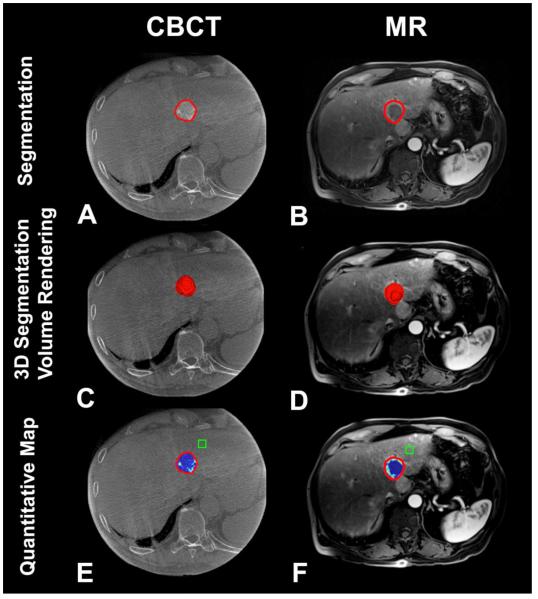
Figure 2.
3D volumetric semi-automatic evaluation of Lipiodol deposition (Partial Response according to mRECIST criteria) in HCC on a representative case. Segmentation of the tumor (red circle) on CBCT at corresponding slice level as on MR (A, B). 3D segmentation volume rendering on the same slice (C, D). Quantitative color map of Lipiodol deposition on CBCT (E) and tumor viability on follow-up MRI (E). The box represents the location of the background ROI. The tumor volume on CBCT and on MRI was 6.79cm3 and 6.80 cm3, respectively. The volume of Lipiodol deposition on CBCT and non-enhancement on MRI was 5.83cm3 and 6.12 cm3, respectively.
Quantitative Measurement of Tumor Necrosis on CE-MRI
Quantitative measurement of the overall as well as the non-enhancing necrotic tumor volume (as a surrogate for tumor necrosis) was performed using an analogous technique. Tumor enhancement was determined by using the 3D semi-automated method as described in the previous section [16,20] and is briefly described as follows: first, the specific CE-MRI scan was selected; second, a segmentation of the overall tumor volume was performed on the arterial-phase CE-MRI (Figure 1,2: B/D). Third, average enhancement values were obtained from a region of interest formed by a 10×10×10 mm cube placed within non-enhancing, extra-tumoral liver parenchyma. Fourth, enhancing / viable tumor tissue was defined as areas within the tumor where the enhancement exceeded that of the extra-tumoral parenchyma. The enhancement was then expressed in cm3 as well as a percentage of the total tumor volume (Figure 1, 2: F). The reciprocal values were used to quantify tumor necrosis [21]. A color-map was overlaid on the arterial-phase CE-MRI in order to demonstrate the regional tumor enhancement heterogeneity. The red color represented maximum enhancement (viable tumor), and blue represented minimum enhancement (nonviable tumor).
Tumor Response Assessment of Target Lesions after cTACE
Tumor response of each target lesion was assessed by the same experienced radiologist according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [22]. Target tumor response was defined with the following four categories: Complete Response (CR), Partial Response (PR), Progressive Disease (PD) and Stable Disease (SD). CR: Disappearance of any intratumor arterial enhancement in target lesion. PR: at least a 30% decrease in the uni-dimensional diameter of viable (enhancement in the arterial phase) target lesion, taking as reference the baseline enhancing diameter of target lesion. PD: an increase of at least 20% in the diameter of viable (enhancing) target lesion, taking as reference the baseline enhancing diameter of target lesion. SD: changes that did not qualify for either PR or PD.
Statistics
Data analysis was performed using SPSS 15.0 (SPSS, Chicago, IL). The tumor volumes as well as the Lipiodol deposition volume and non-enhancing necrotic volume between CBCT and CE-MRI were compared using the two-tailed Student’s t-test for paired data. Linear regression was used to correlate both volumes (cm3) and percentage of Lipiodol deposition in the tumor and non-enhancement between CBCT and CE-MRI. Nonparametric Spearman rank order correlation and nonparametric trend test were used to correlate percentage of Lipiodol deposition in the tumor and short-term response assessed by using mRECIST. A P-value < 0.05 was considered statistically significant.
Results
Between June 2012 and February 2014, 36 patients were included in our study. There were twenty-nine men and seven women with a mean patient age of 57.6±8.1 years (range, 44-89 years). A total of 51 target lesions (mean lesions per patient, 1.4; range, 1-2; mean tumor diameter 2.25±1.02 cm) were included into the analysis (Table 1).
Table 1.
Baseline Patient Characteristics
| Parameter | Value |
|---|---|
| No. of patients | 36 |
| No. of lesions | 51 |
| Age(y)* | 57.6±8.1 |
| Sex | |
| Male | 27 |
| Female | 9 |
| Disease pattern | |
| Unifocal | 5 |
| Bifocal | 8 |
| Multifocal | 23 |
| Child-Pugh class | |
| A | 15 |
| B | 21 |
| Barcelona Clinic Liver Cancer stage | |
| A | 12 |
| B | 24 |
| ECOG | |
| 0 | 21 |
| 1 | 15 |
Data are expressed as means ± standard deviations
Volumes of Tumor, Lipiodol Deposition and Non-enhancement on CBCT and CE-MRI
The mean tumor volumes was 12.66±12.76cm3 (range, 0.79-50.68 cm3) on CBCT and 12.14±13.18 cm3 (range, 0.54-54.88 cm3) on CE-MRI, respectively, with no statistically significant difference between both modalities (P=0.898). The mean Lipiodol deposition volume on CBCT and unenhanced volume on CE-MRI were 8.22±8.49 cm3 (range, 0-30.95 cm3) and 8.06±9.19 cm3 (range, 0-34.54 cm3), respectively, with no statistically significant difference between both modalities (P=0.974).
Correlation of Tumor Volumes, Lipiodol Deposition on CBCT and non-enhancement on CE-MRI
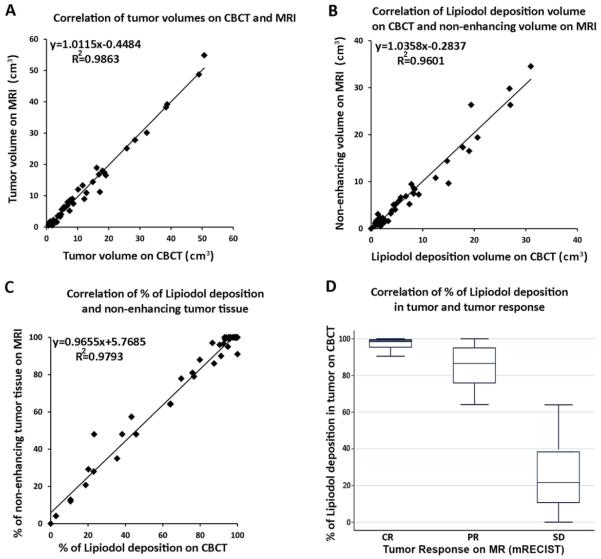
A strong correlation between tumor volumes was observed on CBCT and CE-MRI (Figure 3: A) (coefficient of determination: R2=0.986). A strong correlation between Lipiodol deposition volumes on CBCT and non-enhancing volumes on CE-MRI was observed (R2=0.960) (Figure 3: B). A strong correlation between % of Lipiodol deposition on CBCT and % of non-enhancement in tumor was also observed on CE-MRI (R2=0.979 respectively) (Figure 3: C).
Figure 3.
Correlation between CBCT and MR of tumor volume (A), and % of (B) and in cm3 (C) of Lipiodol deposition on CBCT with non-enhancing regions on MR (B). The linear regression model demonstrated a strong correlation between overall tumor volumes as well as the volume of Lipiodol and tumor necrosis on CBCT and CE-MRI, respectively (Figure 3: A/C). In addition, a strong correlation between the % of Lipiodol deposition on CBCT and % of non-enhancing tumor tissue on CE-MRI was observed (Figure 3: B). Importantly, the extent of Lipiodol deposition (in % of total tumor volume) correlated strongly with tumor response on CE-MRI (D).
Tumor Response at Follow-up MRI: Assessment with mRECIST
A CR was achieved in 26 HCC lesions, a PR in 11 lesions and a SD was noted in 14 lesions. The mean % of Lipiodol deposition in the tumor was 97.22 ± 2.84% in CR, 83.83 ±11.45% in PR and 24.00± 19.18% in SD. The nonparametric trend of percentage of Lipiodol deposition associated with ordinal levels of short-term response was highly statistical significant (P <0.001). There was a strong correlation between the % of Lipiodol deposition and tumor response (Spearman rho=0.84, P<0.001) (Figure 3: D).
Discussion
Our study demonstrates a strong correlation between the degree of Lipiodol deposition and necrotic non-enhancing tumor tissue on CE-MRI at 1 month follow-up. Furthermore, our study showed that 3D quantitative assessment of Lipiodol deposition on intraprocedural CBCT allowed monitoring and predicting short-term response at 1 month follow-up CE-MRI after cTACE in patients with HCC.
Tumor response is a predictive factor of survival [23,24]. Early determination of therapeutic response is helpful to guide patient treatment decisions for potential repeat TACE or additional treatments after chemoembolization. Tumor pathology is the gold standard to assess tumor response. However, it is not feasible in clinical practice and so imaging techniques such as contrast-enhanced MDCT and MRI are widely used to determine tumor response after TACE in order to make early clinical decisions. A strong correlation of imaging-based measurements of tumor response to TACE with tumor pathology provides critical foundation to link radiological findings with actual therapeutic effect. However, current tumor response methods, such as mRECIST and the European Association for the study of the Liver (EASL) guidelines have limitations in practice[25]. One of which is that they need upwards of 2 months after TACE to assess tumor response on follow-up imaging and so they can’t provide immediate feedback[26]. Recently, functional imaging techniques such as CT perfusion, diffusion-weighted MRI or PET-CT have been shown to exhibit changes hours to days after TACE to indicate treatment response, but the results of these approaches are variable and without standardized protocols [27,28].
The advent of new 3D methods of tumor assessment addresses the discordance between lesion diameter and non-spherical volume of the viable tumor tissue [29]. Based on workflow efficient and reproducible tumor segmentation software, these volumetric techniques include enhancement-based and diffusion-weighted MRI and are predictive of patient survival which address important concerns in regard to clinical practicability of 3D quantitative imaging [20,30-32]. A previous study has demonstrated the strong diagnostic accuracy and repeatability of 3D quantitative CE-MRI techniques with regard to pathologically measured tumor necrosis in HCC lesions treated with TACE [21]. Based on these findings, the present study demonstrated a strong correlation between the degree of Lipiodol deposition (volume and %) as seen on intra-procedural CBCT and non-enhancement necrosis as seen on follow-up MR. Importantly, the results demonstrated a strong correlation between the degree of Lipiodol deposition and short-term tumor response expand a new application of CBCT in monitoring and quantification of tumor response to TACE intraprocedurally.
Compared with other conventional imaging techniques to determine intraprocedural Lipiodol deposition, such as fluoroscopy and MDCT, volumetric measurement on intraprocedural CBCT has several clinical advantages in practice. When compared with fluoroscopy, it is a 3D quantitative method while fluoroscopy is a 2D qualitative method [33]. Compared with MDCT, it is workflow-efficient and time-saving to directly assess Lipiodol deposition after TACE without the need of transferring the patient to the MDCT room. Assessing Lipiodol deposition at time of TACE allows for intraprocedural feedback for modification of delivery endpoint [34]. CBCT has also lower radiation exposure than MDCT [35]. Although further technology development and intraprocedural workflow optimization are already on the horizon, CBCT with 3D segmentation software has already sufficient ability and repeatability to detect and volumetrically measure tumors as well as Lipiodol deposition [16-19,36]. Further, the new software used in our study has several unique advantages. First, it differentiates Lipiodol from tumor or liver parenchyma based on enhancement and calculates the volume of Lipiodol deposition. Second, it automatically measures the HU value of the Lipiodol deposition regions. Third, the degree of Lipiodol in tumor can be visualized clearly with different colors which can offer feedback for TACE [16]. Fourth, it requires only a single phase CBCT without the need of contrast media injection.
Our study had some limitations. First, the sample size was small. As the use of CBCT becomes more routine, we imagine the availability of imaging data to increase. Second, most of the lesions were small (mean diameter: 2.61±1.10 cm). The results of our study should be validated in future studies that have more patients and larger lesions. The scope of our work was for therapy response assessment and in future work, prediction of overall survival using analysis of intra-procedural imaging would have even more clinical value. Third, only HCC lesions that had clear borders (not diffuse/infiltrative disease) were included in our study. In future work, using whole liver 3D segmentation could solve this limitation. As the visibility of lesions improves through developments in imaging, and as staging systems improve to better identify patients for this kind of therapy, we anticipate the findings of our study to be more generalizable.
Conclusion
Intraprocedural 3D quantification of Lipiodol deposition on CBCT can be used to predict tumor response on follow-up MRI.
Footnotes
Conflict of Interest
ML and AR are Philips employees. JFG is a grant recipient of Philips. The control of the data and the information submitted for publication were maintained by the remaining authors (ZW, RC, RD, YZ, GY, JC, RS), who had no conflicts of interest.
References
- 1.Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–9. doi: 10.1016/S0140-6736(02)08649-X. doi: 10.1016/S0140-6736(02)08649-X. PMID: 12049862. [DOI] [PubMed] [Google Scholar]
- 2.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–71. doi: 10.1053/jhep.2002.33156. doi: 10.1053/jhep.2002.33156. PMID: 11981766. [DOI] [PubMed] [Google Scholar]
- 3.Herber S, Biesterfeld S, Franz U, Schneider J, Thies J, Schuchmann M, et al. Correlation of multislice CT and histomorphology in HCC following TACE: predictors of outcome. Cardiovascular and interventional radiology. 2008;31(4):768–77. doi: 10.1007/s00270-007-9270-8. doi: 10.1007/s00270-007-9270-8. PMID: 18196335. [DOI] [PubMed] [Google Scholar]
- 4.Takayasu K, Muramatsu Y, Maeda T, Iwata R, Furukawa H, Muramatsu Y, et al. Targeted transarterial oily chemoembolization for small foci of hepatocellular carcinoma using a unified helical CT and angiography system: analysis of factors affecting local recurrence and survival rates. AJR American journal of roentgenology. 2001;176(3):681–8. doi: 10.2214/ajr.176.3.1760681. doi: 10.2214/ajr.176.3.1760681. PMID: 11222205. [DOI] [PubMed] [Google Scholar]
- 5.Loffroy R, Lin M, Yenokyan G, Rao PP, Bhagat N, Noordhoek N, et al. Intraprocedural C-arm dual-phase cone-beam CT: can it be used to predict short-term response to TACE with drug-eluting beads in patients with hepatocellular carcinoma? Radiology. 2013;266(2):636–48. doi: 10.1148/radiol.12112316. doi: 10.1148/radiol.12112316. PMID: 23143027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamanaka K, Hatano E, Kitamura K, Iida T, Ishii T, Machimito T, et al. Early evaluation of transcatheter arterial chemoembolization-refractory hepatocellular carcinoma. Journal of gastroenterology. 2012;47(3):343–6. doi: 10.1007/s00535-011-0511-x. doi: 10.1007/s00535-011-0511-x. PMID: 22183859. [DOI] [PubMed] [Google Scholar]
- 7.Kim HY, Park JW, Joo J, Jung SJ, An S, Woo SM, et al. Severity and timing of progression predict refractoriness to transarterial chemoembolization in hepatocellular carcinoma. Journal of gastroenterology and hepatology. 2012;27(6):1051–6. doi: 10.1111/j.1440-1746.2011.06963.x. doi: 10.1111/j.1440-1746.2011.06963.x. PMID: 22098152. [DOI] [PubMed] [Google Scholar]
- 8.Monsky WL, Kim I, Loh S, Li CS, Greasby TA, Deutsch LS, et al. Semiautomated segmentation for volumetric analysis of intratumoral ethiodol uptake and subsequent tumor necrosis after chemoembolization. AJR American journal of roentgenology. 2010;195(5):1220–30. doi: 10.2214/AJR.09.3964. doi: 10.2214/AJR.09.3964. PMID: 20966331. [DOI] [PubMed] [Google Scholar]
- 9.Lee HS, Kim KM, Yoon JH, Lee TR, Suh KS, Lee KU, et al. Therapeutic efficacy of transcatheter arterial chemoembolization as compared with hepatic resection in hepatocellular carcinoma patients with compensated liver function in a hepatitis B virus-endemic area: a prospective cohort study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(22):4459–65. doi: 10.1200/JCO.2002.02.013. PMID: 12431969. [DOI] [PubMed] [Google Scholar]
- 10.El Khaddari S, Gaudin JL, Abidi H, Picaud G, Rode A, Souquet JC. [Chemoembolization in hepatocellular carcinoma: multivariate analysis of survival prognostic factors after the first session] Gastroenterologie clinique et biologique. 2002;26(8-9):728–34. PMID: 12434077. [PubMed] [Google Scholar]
- 11.Suk Oh J, Jong Chun H, Gil Choi B, Giu Lee H. Transarterial chemoembolization with drug-eluting beads in hepatocellular carcinoma: usefulness of contrast saturation features on cone-beam computed tomography imaging for predicting short-term tumor response. Journal of vascular and interventional radiology : JVIR. 2013;24(4):483–9. doi: 10.1016/j.jvir.2013.01.001. doi: 10.1016/j.jvir.2013.01.001. PMID: 23452553. [DOI] [PubMed] [Google Scholar]
- 12.Kim DY, Ryu HJ, Choi JY, Park JY, Lee DY, Kim BK, et al. Radiological response predicts survival following transarterial chemoembolization in patients with unresectable hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35(11):1343–50. doi: 10.1111/j.1365-2036.2012.05089.x. [DOI] [PubMed] [Google Scholar]
- 13.Tacher V, Radaelli A, Lin M, Geschwind J-F. How I do it: Cone Beam Computed Tomography during Transarterial Chemoembolization for Liver Cancer. Radiology. 2014 doi: 10.1148/radiol.14131925. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tognolini A, Louie JD, Hwang GL, Hofmann LV, Sze DY, Kothary N. Utility of C-arm CT in patients with hepatocellular carcinoma undergoing transhepatic arterial chemoembolization. Journal of vascular and interventional radiology : JVIR. 2010;21(3):339–47. doi: 10.1016/j.jvir.2009.11.007. doi: 10.1016/j.jvir.2009.11.007. PMID: 20133156. [DOI] [PubMed] [Google Scholar]
- 15.Loffroy R, Lin M, Rao P, Bhagat N, Noordhoek N, Radaelli A, et al. Comparing the detectability of hepatocellular carcinoma by C-arm dual-phase cone-beam computed tomography during hepatic arteriography with conventional contrast-enhanced magnetic resonance imaging. Cardiovascular and interventional radiology. 2012;35(1):97–104. doi: 10.1007/s00270-011-0118-x. doi: 10.1007/s00270-011-0118-x. PMID: 21328023. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Lin M, Lesage D, Chen R, Chapiro J, Gu T, et al. Three-dimensional Evaluation of Lipiodol Retention in HCC after Chemoembolization: A Quantitative Comparison between CBCT and MDCT. Academic radiology. 2014;21(3):393–9. doi: 10.1016/j.acra.2013.11.006. doi: 10.1016/j.acra.2013.11.006. PMID: 24507426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen R, Geschwind JF, Wang Z, Tacher V, Lin M. Quantitative assessment of lipiodol deposition after chemoembolization: comparison between cone-beam CT and multidetector CT. Journal of vascular and interventional radiology : JVIR. 2013;24(12):1837–44. doi: 10.1016/j.jvir.2013.08.017. doi: 10.1016/j.jvir.2013.08.017. PMID: 24094672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tacher V, Lin M, Chao M, Gjesteby L, Bhagat N, Mahammedi A, et al. Semiautomatic volumetric tumor segmentation for hepatocellular carcinoma: comparison between C-arm cone beam computed tomography and MRI. Academic radiology. 2013;20(4):446–52. doi: 10.1016/j.acra.2012.11.009. doi: 10.1016/j.acra.2012.11.009. PMID: 23498985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pellerin O, Lin M, Bhagat N, Ardon R, Mory B, Geschwind JF. Comparison of semi-automatic volumetric VX2 hepatic tumor segmentation from cone beam CT and multi-detector CT with histology in rabbit models. Academic radiology. 2013;20(1):115–21. doi: 10.1016/j.acra.2012.07.011. doi: 10.1016/j.acra.2012.07.011. PMID: 22947274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin M, Pellerin O, Bhagat N, Rao PP, Loffroy R, Ardon R, et al. Quantitative and volumetric European Association for the Study of the Liver and Response Evaluation Criteria in Solid Tumors measurements: feasibility of a semiautomated software method to assess tumor response after transcatheter arterial chemoembolization. Journal of vascular and interventional radiology : JVIR. 2012;23(12):1629–37. doi: 10.1016/j.jvir.2012.08.028. doi: 10.1016/j.jvir.2012.08.028. PMID: 23177109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin M, Cornish T, Wang Z, Geschwind J-F. 3D Evaluation of Tumor Necrosis in HCC Patients after TACE – A Radiologic-Pathologic Correlation. Radiology. 2014 [Google Scholar]
- 22.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132. Epub 2010/02/23. doi: 10.1055/s-0030-1247132. PMID: 20175033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Georgiades C, Geschwind JF, Harrison N, Hines-Peralta A, Liapi E, Hong K, et al. Lack of response after initial chemoembolization for hepatocellular carcinoma: does it predict failure of subsequent treatment? Radiology. 2012;265(1):115–23. doi: 10.1148/radiol.12112264. doi: 10.1148/radiol.12112264. PMID: 22891361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Memon K, Kulik L, Lewandowski RJ, Wang E, Riaz A, Ryu RK, et al. Radiographic response to locoregional therapy in hepatocellular carcinoma predicts patient survival times. Gastroenterology. 2011;141(2):526–35. doi: 10.1053/j.gastro.2011.04.054. 35 e1-2. doi: 10.1053/j.gastro.2011.04.054. PMID: 21664356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapiro J, Lin M, Duran R, Schernthaner RE, Geschwind JF. Assessing tumor response after loco-regional liver cancer therapies: the role of 3D MRI. Expert review of anticancer therapy. 2014:1–7. doi: 10.1586/14737140.2015.978861. Epub 2014/11/06. doi: 10.1586/14737140.2015.978861. PMID: 25371052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillmore R, Stuart S, Kirkwood A, Hameeduddin A, Woodward N, Burroughs AK, et al. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. Journal of hepatology. 2011;55(6):1309–16. doi: 10.1016/j.jhep.2011.03.007. Epub 2011/06/28. doi: 10.1016/j.jhep.2011.03.007. PMID: 21703196. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y, Yaghmai V, Salem R, Lewandowski RJ, Nikolaidis P, Larson AC, et al. Imaging tumor response following liver-directed intra-arterial therapy. Abdom Imaging. 2013;38(6):1286–99. doi: 10.1007/s00261-013-0017-5. doi: DOI 10.1007/s00261-013-0017-5. PMID: WOS:000328076000014. [DOI] [PubMed] [Google Scholar]
- 28.Chung JC, Naik NK, Lewandowski R, Deng J, Mulcahy MF, Kulik LM, et al. Diffusion-weighted magnetic resonance imaging to predict response of hepatocellular carcinoma to chemoembolization. World J Gastroentero. 2010;16(25):3161–7. doi: 10.3748/wjg.v16.i25.3161. doi: DOI 10.3748/wjg.v16.i25.3161. PMID: WOS:000279765300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantatzis M, Kakolyris S, Amarantidis K, Karayiannakis A, Prassopoulos P. Treatment response classification of liver metastatic disease evaluated on imaging. Are RECIST unidimensional measurements accurate? European radiology. 2009;19(7):1809–16. doi: 10.1007/s00330-009-1327-4. doi: 10.1007/s00330-009-1327-4. PMID: 19238395. [DOI] [PubMed] [Google Scholar]
- 30.Bonekamp S, Halappa VG, Geschwind JF, Li Z, Corona-Villalobos CP, Reyes D, et al. Unresectable hepatocellular carcinoma: MR imaging after intraarterial therapy. Part II. Response stratification using volumetric functional criteria after intraarterial therapy. Radiology. 2013;268(2):431–9. doi: 10.1148/radiol.13121637. doi: 10.1148/radiol.13121637. PMID: 23616632. [DOI] [PubMed] [Google Scholar]
- 31.Prasad SR, Jhaveri KS, Saini S, Hahn PF, Halpern EF, Sumner JE. CT tumor measurement for therapeutic response assessment: comparison of unidimensional, bidimensional, and volumetric techniques initial observations. Radiology. 2002;225(2):416–9. doi: 10.1148/radiol.2252011604. doi: 10.1148/radiol.2252011604. PMID: 12409574. [DOI] [PubMed] [Google Scholar]
- 32.Duran R, Chapiro J, Frangakis C, Lin M, Schlachter TR, Schernthaner RE, et al. Uveal Melanoma Metastatic to the Liver: The Role of Quantitative Volumetric Contrast-Enhanced MR Imaging in the Assessment of Early Tumor Response after Transarterial Chemoembolization. Translational oncology. 2014;7(4):447–55. doi: 10.1016/j.tranon.2014.05.004. doi: 10.1016/j.tranon.2014.05.004. PMID: 24953419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeon UB, Lee JW, Choo KS, Kim CW, Kim S, Lee TH, et al. Iodized oil uptake assessment with cone-beam CT in chemoembolization of small hepatocellular carcinomas. World journal of gastroenterology : WJG. 2009;15(46):5833–7. doi: 10.3748/wjg.15.5833. PMID: 19998505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwazawa J, Ohue S, Hashimoto N, Muramoto O, Mitani T. Survival after C-arm CT-assisted chemoembolization of unresectable hepatocellular carcinoma. European journal of radiology. 2012;81(12):3985–92. doi: 10.1016/j.ejrad.2012.08.012. doi: 10.1016/j.ejrad.2012.08.012. PMID: 22959287. [DOI] [PubMed] [Google Scholar]
- 35.Kim S, Yoshizumi TT, Toncheva G, Yoo S, Yin FF. Comparison of radiation doses between cone beam CT and multi detector CT: TLD measurements. Radiation protection dosimetry. 2008;132(3):339–45. doi: 10.1093/rpd/ncn305. doi: 10.1093/rpd/ncn305. PMID: 19074786. [DOI] [PubMed] [Google Scholar]
- 36.Taguchi K, Funama Y, Zhang M, Fishman EK, Geschwind JF. Quantitative measurement of iodine concentration in the liver using abdominal C-arm computed tomography. Academic radiology. 2009;16(2):200–8. doi: 10.1016/j.acra.2008.08.002. doi: 10.1016/j.acra.2008.08.002. PMID: 19124106. [DOI] [PubMed] [Google Scholar]