Abstract
Cocaine dependence (CD) is associated with several cognitive deficits. Accumulating evidence, based on human and animal studies, has led to models for interpreting the neural basis of cognitive functions as interactions between functionally related brain regions. In this review, we focus on the magnetic resonance imaging (MRI) studies using brain connectivity techniques as related to CD. The majority of these brain connectivity studies indicated that cocaine use is associated with altered brain connectivity between different structures, including cortical-striatal regions and default mode network. In cocaine users, some of the altered brain connectivity measures are associated with behavioral performance, history of drug use, and treatment outcome. The implications of these brain connectivity findings to the treatment of CD and the pros and cons of the major brain connectivity techniques are discussed. Finally potential future directions in cocaine use disorder research using brain connectivity techniques are briefly described.
Keywords: cocaine use disorder, magnetic resonance imaging, functional connectivity, effective connectivity, brain connectivity
Introduction
Cocaine use disorder (CD) (See Table 1 for all abbreviations used in this article) is associated with several cognitive deficits [1–2]. Accumulating evidence from human and animal studies has led to models for interpreting the neural basis of cognitive functions as interactions between functionally related brain regions [3–6]. One of the theories is that CD affects three separate and interacting systems: the prefrontal cortex (PFC) dependent (reflective) system, the amygdala-striatum dependent (automatic, habitual or salient) system, and the insula system that translates interoceptive signals into conscious feelings of desire and decision-making processes related to uncertain risk and reward [7–8]. Imbalance among these systems has been theorized to contribute to compulsive substance use and loss of control in substance use disorders [7,9]. In addition, successful abstinence from drugs is associated with improvement in prefrontal structure and function [10], with likely improvement in the control of PFC over the striatal regions [10–11]. Furthermore, Robbins et al. [12] proposed ‘neurocognitive endophenotypes’, whereby changes in behavioral or cognitive processes are associated with deficits in neural systems. According to Robbins et al. [12], four ‘frontostriatal loops’ putatively associated with different aspects of impulsivity and compulsivity. Among these loops, two loops are relevant to impulsivity: i.e., the ventromedial PFC, subgenual cingulate cortex, ventral striatal loop associated with reward, and the ventrolateral PFC, ACC, pre-supplementary motor area, caudate, and putamen loop associated with stop-signal inhibition. Thus, brain connectivity analysis may be a potentially powerful tool to understand the neural correlates underlying impaired cognitive functions, and to test theories such as the aforementioned triple-system theory.
Table 1.
Abbreviations used in this manuscript.
| Abbreviations | Definition |
|---|---|
| AB | attentional bias |
| AcbC | nucleus accumbens core |
| AcbS | nucleus accumbens shell |
| ACC | anterior cingulate cortex |
| BLA | basolateral amygdala |
| CCA | cross correlation analysis |
| CD | cocaine dependence |
| CDs | Cocaine dependent subjects |
| CMA | corticomedial amygdala |
| coc | cocaine |
| CPu | caudate putamen |
| CTLs | controls |
| DBS | deep brain stimulation |
| DC | dorsal caudate |
| DCM | dynamic causal modeling |
| DCP | dorsal caudal putamen |
| DLPFC | dorsolateral prefrontal cortex |
| DMN | default mode network |
| DMT | delayed memory task |
| DRP | dorsal rostral putamen |
| ECN | executive control network |
| EEG | electroencephalography |
| FC | functional connectivity |
| FCD | functional connectivity density |
| fMRI | functional magnetic resonance imaging |
| ICA | independent component analysis |
| ICD | intrinsic connectivity distribution |
| ICN | intrinsic connectivity networks |
| IFC | inferior frontal cortex |
| IFG | inferior frontal gyrus |
| IFS | inferior frontal sulcus |
| IMaGES | Independent Multi-sample Greedy Equivalence Search |
| IMT | immediate memory task |
| L | left |
| LR | bilateral |
| M1 | primary motor cortex |
| MCC | middle cingulate cortex |
| MDN | mediodorsal nucleus |
| MFC | medial frontal cortex |
| MFG | middle frontal gyrus |
| MPH | methylphenidate |
| MRI | magnetic resonance imaging |
| MTG | middle temporal gyrus |
| MTL | medial temporal lobe |
| NAC | nucleus accumbens |
| NCOC | utero exposure to non-cocaine drugs |
| OFC | orbital frontal cortex |
| PAG | periaqueductal gray |
| pCASL | pseudo-continuous arterial spin labeling |
| PCC | posterior cingulate cortex |
| PCE | prenatal cocaine exposure |
| PDE | prenatal drug exposure |
| PFC | prefrontal cortex |
| PGs | individuals with pathological gambling |
| pHp | posterior hippocampus |
| PPC | posterior parietal cortex |
| PPI | psychophysiological interaction |
| PrL | prelimbic cortex |
| R | right |
| ROIs | regions of interest |
| rsFC | resting state functional connectivity |
| RSN | resting state network |
| rTMS | repeated trans-cranial magnetic stimulation |
| S1 | primary sensory cortex |
| S2 | secondary sensory cortex |
| SA | self-administration |
| SFG | superior frontal gyrus |
| SMA | supplementary motor area |
| SN | salience network |
| STR | striatum |
| VLPFC | ventrolateral prefrontal cortex |
| VRP | ventral rostral putamen |
| VSi | inferior ventral striatum |
| VSs | superior ventral striatum |
| VTA | ventral tegmental area |
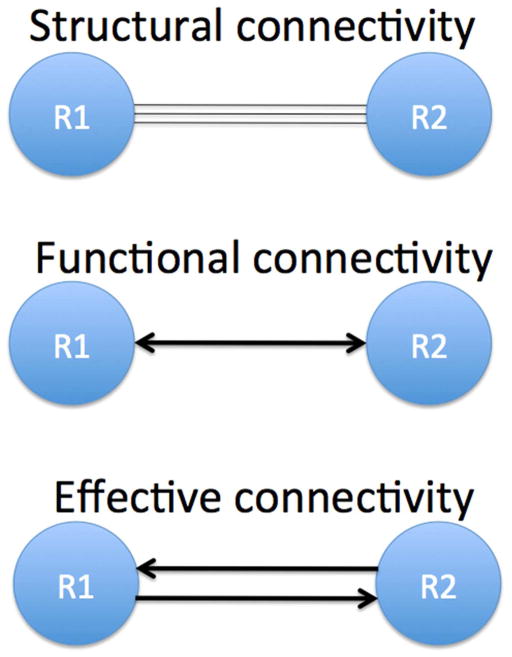
Magnetic resonance imaging (MRI) based brain connectivity analysis is generally classified into functional connectivity [13], effective connectivity [13], and structural connectivity [14]. Functional connectivity refers to the correlations between spatially remote neurophysiological events [13]. Unlike functional connectivity, effective connectivity models the causal effect that one region’s activity has on another region [13]. Thus the direction of connectivity is determined in effective connectivity. Structural connectivity can be measured using white matter tractography, which is used to visually represent fiber tracts using MRI diffusion tensor imaging [14]. Figure 1 shows a schematic diagram illustrating structural connectivity (fiber pathways, top), functional connectivity (correlations, middle), and effective connectivity (causal relationship, bottom) among between two brain regions (R1 and R2).
Figure 1.
Schematic diagram illustrating structural connectivity (fiber pathways, top), functional connectivity (correlations, middle), and effective connectivity (causal relationship, bottom) between two brain regions (R1 and R2).
In this article, we review MRI-based brain connectivity studies that investigated the effects of cocaine use in humans and animals with a focus on the aforementioned triple-system theory. We summarize the relationship between the altered brain connectivity measures and the behavioral performance, drug use history, and treatment outcomes in the CD subjects. We discuss the implications of the brain connectivity findings on the treatment of CD and the pros and cons of the major brain connectivity techniques used in these studies. Finally we discuss potentially useful future directions in CD research using brain connectivity.
Included studies and major findings
We searched http://www.ncbi.nlm.nih.gov/pubmed/ using the key words “cocaine connectivity MRI” and found 47 articles. The search date was June 1st 2015. Out of these, 33 articles used MRI-based brain connectivity analysis in chronic cocaine use. Therefore these 33 articles were included in this review. Thirty of these included studies on human subjects except for three in which monkeys or rats were used as the subjects. Twenty of these 33 studies investigated resting state functional connectivity (rsFC) in humans. The study subjects, rsFC analysis methods, and the major findings of these rsFC human studies are summarized in Table 3. Ten of these 33 studies investigated functional connectivity or effective connectivity in humans during tasks. The study subjects, the task used, the brain connectivity analysis methods, and the major findings of these task-based brain connectivity studies are summarized in Table 4. Three of these 33 studies investigated rsFC in animals. The study subjects, rsFC analysis methods, and the major findings of these animal studies are summarized in Table 5. The detailed seed regions and/or regions of interest (ROIs) used are also provided for the brain connectivity analytical methods.
Table 3.
Resting state functional connectivity in humans. See Table 1 for the abbreviations used in this table.
| Study | Subjects | rsFC methods | Major findings |
|---|---|---|---|
| Adinoff et al. [30] | 22 relapsed CDs, 18 early-remission CDs and 20 CTLs | Cross correlation analysis (CCA) with L pHp as the seed |
|
| Camchong et al. [25] | 27 CDs and 24 CTLs | CCA with subgenua, caudal, dorsal, rostral, perigenual ACC as seeds |
|
| Cisler et al. [74] | 41 CDs and 19 CTLs | In dependent component analysis (ICA) & CCA with R ventral anterior insula, R mid-insula as seeds |
|
| Contreras-Rodriguez et al. [22] | 20 CDs, 19 PGs, and 21 CTLs | Global connectivity analysis & CCA with OFC, L and R caudate, amygdala, thalamus as the seeds |
|
| Gu et al. [26] | 39 cocaine users (34 current CDs, 4 current cocaine abusers, 1 current recreational user) and 39 CTLs | CCA with NAC, amygdala, hippocampus, medial dorsal thalamus, and rostral ACC as seeds |
|
| Hu et al. [23] | 56 cocaine users (53 CDs and 3 cocaine abusers) and 56 CTLs | CCA with VSi, VSs, DC, VRP, DRP, DCP as seeds |
|
| Kelly et al. [75] | 25 CDs and 24 CTLs | CCA with L and R IFS as seeds |
|
| Konova et al. [32] | 18 cocaine users (17 CDs and 1 cocaine abusers) and 16 CTLs | CCA with L and R VTA, NAC, amygdala, hippocampus, MDN of thalamus, rostral ACC as seeds |
|
| Konova et al. [27] | 19 cocaine users (18 CDs and 1 cocaine abuser) and 15 CTLs | Functional connectivity density (FCD) |
|
| Li et al. [76] | 33 PCE adolescents and 23 CTL adolescents | ICA & CCA with PCC as seed |
|
| Liang et al. [43] | 47 CDs and 47 CTLs | Modular analysis |
|
| McHugh et al. [77] | 45 CDs and 22 CTLs | CCA with L and R caudate, putamen, NAC as seeds |
|
| McHugh et al. [29] | 24 relapsed CDs, 21 non-relapse CDs, and 22 CTLs | CCA with L and R BLA, CMA as seeds |
|
| Ray et al. [28] | 20 CDs and 17 CTLs | ICA & CCA with 61 ROIs |
|
| Salzwedel et al. [78] | 33 PCE infants, 40 NCOC infants and 46 CTLs | CCA with insula, amygdala, L and R visual cortices as seeds |
|
| Schweitzer et al. [79] | 27 PDE adolescents (with intrauterine exposure to cocaine and/or heroin) and 20 CTLs | Graph theory |
|
| Verdejo-Garcia et al. [80] | 10 CDs and 14 CTLs | CCA with ACC, PAG, insula as seeds |
|
| Wang et al. [40] | 20 poly drug users (the primary diagnosis was cocaine dependence) and 19 CTLs | Graph theory using 90 ROIs |
|
| Wisner et al. [24] | 33 CDs and 32 CTLs | ICA |
|
Table 4.
Task-based brain connectivity studies in humans. See Table 1 for the abbreviations used in this table.
| Study | Subjects | Task, and brain connectivity methods | Major findings |
|---|---|---|---|
| Albein-Urios et al. | 18 CDs with comorbid Cluster B personality disorders, 17 CDs without comorbidities and 21 CTLs | Re-appraisal task, Psychophysiologic al interaction (PPI) with subgenual ACC, OFC as ROIs |
|
| Albein-Urios et al. [82] | 17 CDs and 18 CTLs | Re-appraisal task, PPI with R DLPFC, R IFG as ROIs |
|
| Hanlon et al. [83] | 14 CDs and 15 CTLs | Finger-sequencing task, CCA with L M1, SMA, ACC, caudate, putamen, thalamus, R cerebellum as ROIs |
|
| Kilts et al. [84] | 42 CDs | Cocaine-Stroop task, ICA |
|
| Ma et al. [16] | 19 CDs and 14 CTLs | IMT/DMT verbal working memory task, DCM with L IFC, L MFG, L PPC, R PPC, LR SMA, L STR, R STR as notes |
|
| Ma et al. [17] | 13 CDs and 10 CTLs | Go/NoGo response inhibition task, dynamic causal modeling DCM with L DLPFC, R DLPFC, L ACC, R ACC, R VLPFC, L caudate, R hippocampus as nodes |
|
| Mitchell et al. [31] | 15 CDs and 15 CTLs | Cocaine-Stroop task, Intrinsic connectivity density (ICD) |
|
| Ray et al. [18] | 20 CDs and 17 CTLs | Cocaine-Stroop task, Independent Multisample Greedy Equivalence Search (IMaGES) S with LR amygdala, R hippocampus, LR dorsal striatum, LR insula, LR MFC, LR OFC, L DLPFC, ACC, R ventral striatum as nodes |
|
| Tomasi et al. [85] | 20 CDs and 20 CTLs | Cocaine-Stroop task, CCA with substantia nigra as seed |
|
| Worhunsky [86] | 20 CDs and 20 CTLs | Cocaine-Stroop task, ICA |
|
Table 5.
Resting state functional connectivity in animals. See Table 1 for the abbreviations used in this table.
| Study | Subjects | rsFC methods | Major findings |
|---|---|---|---|
| Chen et al. [19] | 7 cocaine SA rats, 5 saline SA rats, and 5 drug naïve rats. | CCA with CPu, M1, S1, S2, medial PFC, thalamus, insula as seeds |
|
| Lu et al. [20] | 13 cocaine SA rats, 13 sucrose SA rats, and 22 sedentary CTL rats | CCA with PrL, ACC, AcbC, AcbS, CPu as seeds |
|
| Murnane et al. [21] | 3 cocaine SA adult monkeys | CCA with NAC, amygdala, DLPFC, ACC, cerebellum as seeds |
|
Resting state functional connectivity studies in humans
During resting state, cocaine-dependent subjects (CDs), cocaine abusers, or subjects with prenatal cocaine exposure (PCE), had greater or lower FC between different regions compared to non-drug-using controls (CTLs). These results are summarized in Table 3. As can be seen from Table 3, the results across the studies are not always consistent. The conflicting findings could be due to different methods [15], small sample size, different cohorts [14] used in different studies and/or multiple other unknown factors [14].
Task-based brain connectivity studies in humans
As summarized in Table 4, compared to CTLs, the CD subjects had greater or lower FC between different brain regions.
Three effective connectivity studies were conducted to investigate neural correlates underlying working memory [16], response inhibition [17], and cue reactivity [18] in CDs. Ma et al. [16] used dynamic causal modeling (DCM) of a working memory task and found that during short memory delay condition, the inferior frontal gyrus to striatum effective connectivity was reduced in CDs but increased in CTLs. During the longer memory delay condition, the middle frontal gyrus to striatum effective connectivity was more reduced in CDs than in CTLs [14]. In another functional magnetic resonance imaging (fMRI) based DCM study, Ma et al. [17] used a Go/NoGo task to test response inhibition in CDs and matched CTLs. Results of this study showed differences between groups in effective connectivity during the Hard NoGo condition: the effective connectivity from right (R) dorsolateral prefrontal cortex (DLPFC) to left (L) caudate was increased in CTLs but remained the same in the CDs; the effective connectivity from R ventrolateral prefrontal cortex to L caudate was reduced in the CTLs but remained the same in the CDs; the effective connectivity from L anterior cingulate cortex (ACC) to L caudate remained the same in the CTLs but was reduced in the CDs. Ray et al. [18] found that during cocaine-cue exposure of a cocaine-Stroop task, CDs had a particular feed-forward effective connectivity among the nodes of the drug-cue processing network (amygdala→hippocampus→dorsalstriatum→insula→medial frontal cortex, DLPFC, ACC) that was not present in the CTLs. All these effective connectivities had positive strength except for the connectivity from insula to medial frontal cortex. Consistent with the triple-system theory [7–8], these effective connectivity studies indicated that CD is associated with an imbalance among the PFC regions, insula, and striatal regions: weakened control of the PFC regions over the striatal regions during working memory [16] and response inhibition [17] and strengthened control of the striatal and insular regions over the PFC regions during the cocaine-Stroop task [18].
Resting state functional connectivity studies in animals
Three resting state functional connectivity studies were conducted in rats [19–20] or monkeys [21]. The results are summarized in Table 5. Lu et al. [20] found that cocaine self-administration (SA) rats had lower rsFC between prelimbic cortex and entopeduncular nucleus and between nucleus accumbens core and dorsomedial PFC compared to both sucrose-SA and CTL rats. In addition, the rsFC between nucleus accumbens core and dorsomedial PFC was positively correlated with cocaine SA escalation in cocaine-SA rats. The other two studies investigated the acute effect of cocaine administration in cocaine-SA rats or cocaine-SA monkeys. Murnane et al. [21] found that acute cocaine administration selectively reduced the rsFC between ACC and nucleus accumbens, and between DLPFC and nucleus accumbens. In addition, the rsFC between DLPFC and nucleus accumbens during abstinence predicted cocaine intake when the monkeys were provided renewed access to cocaine [21]. Another animal study [19] also reported increased rsFC after acute cocaine administration. This was different from another study that reported decreased rsFC [21]. This difference may be related to the different species or seed regions used in the two studies.
Relationship between brain connectivity and behavior, drug use history, and treatment outcome
Greater impulsivity was found to be associated with higher rsFC between orbital frontal cortex and subgenual ACC [22], and between striatum and DLPFC [23]. Greater impulsivity was found to be associated with lower resting state inter-network connectivity between an intrinsic connectivity network involving the anterior insula and ACC, and an intrinsic connectivity network involving the striatum [24]. In addition, the rsFC between perigenual ACC and DLPFC was significantly and positively correlated with reversal learning score [25]. In [22], the authors first computed the rsFC between right ventral striatum (superior part) and anterior prefrontal cortex/orbitofrontal cortex (Go circuit) and the rsFC between right ventral striatum (inferior part) and dorsal anterior cingulate cortex (STOP circuit). The GO circuit is hypothesized to promote compulsive behaviors while the STOP circuit may limit such behaviors [22]. They then computed rsFC (difference) = rsFC (GO circuit) - sFC (STOP circuit) and performed a correlation analysis and found that rsFC (difference) was positively correlated with compulsive like behaviors reflected in the DSM-IV-TR in cocaine users. The greater difference in rsFC between striatal-anterior prefrontal/orbital frontal cortex (GO) and striatal-dorsal ACC (STOP) circuits was associated with more loss of control over cocaine use [23]. These studies suggest that the impaired cortical-striatal connectivity may be an underlying factor in the impaired behavioral performance in CD.
The duration of cocaine use was associated with lower rsFC between ventral tegmental area and thalamus/lentiform nucleus/nucleus accumbens [26], greater short-range and long-range functional connectivity density in the regions of the default mode network (DMN) [27], lower intra-network connectivity strength of sensory motor cortex [28], and greater inter-network connectivity strength between occipital-limbic brain regions [28]. Greater peak cocaine level was associated with greater rsFC between caudate and thalamus and between amygdala and insula, and with lower rsFC between amygdala and cerebellum [22]. Greater recent cocaine use was associated with greater rsFC between striatum and DLPFC [23]. Greater cocaine use frequency and money spent on cocaine per week in abstinent CDs was associated with lower connectivity strength within occipital brain regions [28].
One study [29] compared rsFC across relapsed CDs, non-relapse CDs, and CTLs. The results of that study showed that relapsed CDs had lower rsFC between the L corticomedial amygdala and ventromedial PFC/rostral ACC than non-relapse CDs [29]. Adinoff et al. [30] found that rsFC between posterior hippocampus and posterior cingulate cortex (part of DMN) predicted relapse with 75% accuracy at 30, 60, and 90 days following treatment. During cocaine-Stroop tasks, greater Stroop-related intrinsic connectivity in bilateral thalamus, ventral striatum, and substantia nigra regions was associated with smaller number of self-reported days of consecutive abstinence during treatment [31]. In addition, greater Stroop-related intrinsic connectivity in bilateral thalamus, ventral striatum, and substantia nigra, R insula and L hippocampus was associated with more positive urine screens [31]. Greater effective connectivity from insula to DLPFC was associated with greater cocaine craving ratings [18]. In monkeys, impaired connectivity between PFC and striatal regions during abstinence predicted cocaine intake when the monkeys were able to access cocaine again [21]. These studies suggest that impaired cortical-striatal connectivity and DMN may be predictive of treatment outcomes.
Implication for the treatment of cocaine use disorder
The findings of the brain connectivity studies suggest that the cortico-striatal circuits could be therapeutic targets in CD. For example, Konova et al. [32] found that short-term methylphenidate (MPH) administration reduced an abnormally strong rsFC between ventral striatum and the dorsal striatum (putamen/globus pallidus), and lower rsFC between these regions with placebo administration robustly correlated with less severe addiction. In addition, short-term MPH strengthened several corticolimbic and corticocortical connections. Konova et al. [27] found that while the effects of MPH on functional connectivity density only partly overlapped with those of CD, MPH was associated with reduced density of short-range connections to the putamen/thalamus, a network of core relevance to habit formation and addiction [33]. These studies [27,32] did not report if the rsFC affected by the MPH is correlated with the clinical measures such as cocaine use or impulsivity. In addition to medications, deep brain stimulation (DBS) and repeated trans-cranial magnetic stimulation (rTMS) are two promising approaches which can be used for treating CD, with cortical-striatal circuits as the targets [11].
Expert Commentary
While activation measures or structural measures provide local information, the connectivity measures provide information about the relationship among distinct brain regions. Previous studies (reviewed by Rowe [34]) showed that the functional or effective connectivity methods could be sensitive to the presence or severity of disease and/or treatment, even where activation analysis is insensitive. However, as suggested by Rowe [34], the connectivity methods should be used as complementary to, not a substitute for, the activation or structural measures.
The majority of the existing brain connectivity studies indicate that CD is associated with altered brain networks including cortical-striatal regions and DMN. Within the CD subjects, some of the altered brain connectivity measures are associated with behavioral performance, drug use history, or treatment outcomes. Some studies, e.g., Ma et al. [16,35], have speculated that the change of connection could be due to the effect of change of neurotransmitters. The direct “toxic” effect of cocaine could be another causal factor. Future studies are needed to test these hypotheses. Given the alterations reviewed here, it is an interesting topic to describe which of these alterations in connectivity are specific of CD, and which others are shared by consumption of other drugs of abuse.
The cortical-striatal circuits could be promising therapeutic targets for CD. There is a pressing need to develop clinically useful biomarkers for treatment or prognosis of substance use disorders [36–37]. To date, however, it is still one of the major challenges to identify such useful biomarkers [38]. Rowe [34] has demonstrated that the connectivity approaches are relatively more sensitive to the presence or severity of disease and/or treatment than the approaches of regionally specific dysfunction. Thus, brain connectivity approaches could be clinically useful biomarkers for the treatment or prognosis of cocaine use disorder although there is still a paucity of such studies in the literature of cocaine use disorder.
Resting state functional connectivity and task-based effective connectivity are the two major brain connectivity techniques used in the studies described above. During the resting state fMRI scan, the subject is not required to perform any task. Thus resting state design is particularly attractive for animal studies or studies on patients incapable of task performance during the scan. This is an advantage of the resting state design over the task-based design. However, some neuronal processes essential in CD studies can only be measured with task-based fMRI [39]. In addition, resting state fMRI data has relatively low signal to noise ratio and requires extensive preprocessing steps to increase the signal to noise ratio. One should choose either task-based or resting state or both designs depending on the research question. Functional connectivity, which is based on correlation, cannot provide the direction of connectivity. However, functional connectivity is generally easy to compute although more complicated methods such as the graph theoretical analysis have been prposed [40]. Functional connectivity analysis could be hypothesis driven [41] or data driven (e.g., ICA) [42]. Although originally, functional connectivity needed ROIs to compute the correlation coefficients, whole brain functional connectivity methods such as the modular analysis [43] are now available and eliminates this requirement. Effective connectivity can provide the direction of connectivity, which may be important for understanding the neurobiology of CD. Effective connectivity analysis is generally complicated. In its original implementation, effective connectivity was hypothesis driven, but currently data driven effective connectivity techniques such as the DCM network discovery [44–45] are available. Effective connectivity needs ROIs and whole brain effective connectivity technique available are not yet available. DCM, one of the implementation of effective connectivity analysis, works on underlying neuronal level rather than at the hemodynamic level [46]. Therefore, confounders such as disease or medications may have less effect on the DCM analysis [46–49]. Originally, DCM was based on task-based fMRI data. However, currently novel DCM techniques have been developed for the analysis of resting state fMRI [50–52].
Five Year View
Almost all human studies reviewed here are cross sectional in nature. Thus, these studies cannot distinguish the preexisting altered brain connectivity from that caused by chronic cocaine use. Given the fact that both could be underlying factors, it is necessary to distinguish these two causes. Animal studies are particularly appropriate to address this question because the drug use can be well-controlled. Currently there are few brain connectivity studies in experimental CD. Resting state functional connectivity and structural connectivity analyses are particularly suitable for animal studies. Alternatively, longitudinal studies using non-treatment-seeking CD subjects can hopefully also address this question. However, we are not aware of any such publications.
Similarly, there exists the possibility that a remote region that has not been directly damaged shows a change of functional (or effective) connectivity. Such a phenomenon is called “connectional diaschisis” [53–54]. As suggested by Carrera and Tononi [54], the connectivity showing connectional diaschisis could be the target of therapeutic strategies. Brain lesion has been used to locate directly damaged brain region in [53]. For cocaine use disorder, brain regions showing altered structure or function could be used as directly damaged brain regions.
Functional connectivity and effective connectivity are related to white matter structural connectivity [55–56]. Previous MRI diffusion studies have reproducibly shown that CD is associated with significant white matter changes in both humans [57–63] and animals [64–65]. The impaired white matter integrity could be associated with impulsivity [57], poor decision-making [62], or worse treatment outcomes [66]. However, only one study [67] has used MRI diffusion-derived tractography to investigate the impaired structural connectivity in the PCE subjects.
Combined neuroimaging modalities can provide more information than a single imaging modality alone. Adinoff et al. [30] combined pseudo-continuous arterial spin labeling (pCASL) and resting state fMRI. These authors first analyzed the pCASL data to locate the region showing group difference in regional cerebral blood flow (rCBF). They then used this region as a seed for the resting state fMRI functional connectivity analysis. Coullaut-Valera et al. [68] used electroencephalography (EEG) to investigate impaired functional connectivity in polydrug users. EEG has low spatial resolution and high temporal resolution and so it is natural to combine it with fMRI which has a high spatial resolution, but relatively low temporal resolution, to gain more understanding of brain connectivity. A review [69] of 12 papers correlating EEG and fMRI-based resting state networks in adult human subjects suggests that spatially delimited theta and whole/local alpha waves could add important additional information to fMRI-based resting state networks (RSNs). Combined fMRI-based functional/effective connectivity and MRI diffusion-based structural connectivity may provide additional insight for the relationship between brain structure and function [55,70]. Prior information provided by MRI diffusion-based tractography (structural connectivity) can improve the results of DCM effective connectivity analysis [71].
DCM could be used to understand the consequences of pathophysiological changes. For example, DCM has been used to explain how acetylcholine enhances the precision of bottom-up synaptic transmission in cortical hierarchies [72]. In another study, Moran et al. [73] used DCM to infer the synaptic basis of ketamine-induced change in coordinated oscillations in the neural circuits of the rat happocampus and PFC. The information provided in these studies suggests that it is possible to use DCM to quantify the putative synaptic mechanisms underlying certain drug effects in terms of changes in effective (directional) connectivity between brain regions.
Table 2.
Connectivity techniques used in the studies reviewed in this manuscript.
| Techniques | Brief introduction |
|---|---|
| Cross correlation analysis (CCA) | CCA is a method in which functional connectivity is measured by evaluating the correlation between the time course of each voxel (or region of interest) and a reference function (often obtained from a seed). |
| Dynamic causal modeling (DCM) | DCM is a Bayesian procedure that measures effective connectivity through optimally predicting how fMRI data were generated. The effective connectivity, which is formulated in terms of stochastic or ordinary differential equations, is modeled at the hidden neuronal level rather than the observed fMRI level. |
| Functional connectivity density (FCD) | It estimates the number of global and local functional connections to a given region that exceed a specified correlation strength, based on correlations among all voxels, and the number of remote (long-range) connections, which is the difference between the former two numbers. |
| Global connectivity analysis | It is a data-driven FC method. It is a quantitative measure of the extent each voxel is connected to every other voxel in the brain, based on a correlation coefficient matrix obtained from the correlation analysis among all voxels. |
| Graph theory | A graph consists of a set of nodes (or vertices) and a set of connections (or edges). The adjacency matrix A contains the information about the connectivity structure of the graph. Ai,j = 1 when an edge exists between two vertices i and j, otherwise Ai,j = 0. The number of edges connecting to a vertex is called the degree k of this vertex. In graph theory, efficiency provides a physical meaning for topological characterization of the networks and measures the ability of information transfer of a network. Efficiency can be measured at the local or global level. Small-worldness refers to a phenomenon that most nodes are not neighbors of one another. However, every other node can reach these nodes through a small number of steps (or hops). |
| Intrinsic connectivity density (ICD) | ICD is based on the correlation among all voxels of interest. For each voxel, a histogram of correlations is constructed to estimate the distribution of connections to this voxel. The alpha parameter, which controls the spread of the distribution of connections, is a measure of number of high-correlation connections. Group level analysis is conducted based on the parametric image of the alpha parameter from all voxels for each subject. |
| In dependent component analysis (ICA) | ICA is data-driven method for functional connectivity analysis, which drives a set of measurement data into a number of independent components (or maps). It requires no reference function or predefined seed. |
| Independent Multisample Greedy Equivalence Search (IMaGES) | IMaGES measures effective connectivity. Given a set of ROIs and without a prescribed model, IMaGES uses a Bayesian algorithm to search for the best model. |
| Modular analysis | Based on the correlations among all voxels, the modular analysis aims to find an optimal partition of modules, which are groups of nodes that are strongly connected with each other in the same module. After the modules are determined, both inter-module connectivity and intra-module connectivity are then computed. |
| Psychophysiological interaction (PPI) | PPI measures functional connectivity between a brain region and the rest of the brain with relation to the performance of a particular psychological task. |
Acknowledgments
This work is supported by National Institute on Drug Abuse (NIDA) Grants # R01 DA034131 (LM), P50DA033935 (FGM/LM/JLS), U54 DA038999 (FGM/JLS/SDL), and P50 DA009262 (PAN) and NCRR Shared Instrumentation Grant # 1 S10 RR019186-01 (PAN).
References
- 1.Spronk DB, van Wel JH, Ramaekers JG, Verkes RJ. Characterizing the cognitive effects of cocaine: a comprehensive review. Neurosci Biobehav Rev. 2013;37(8):1838–1859. doi: 10.1016/j.neubiorev.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Potvin S, Stavro K, Rizkallah E, Pelletier J. Cocaine and cognition: a systematic quantitative review. Journal of addiction medicine. 2014;8(5):368–376. doi: 10.1097/ADM.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 3.Collette F, Van der Linden M. Brain imaging of the central executive component of working memory. Neurosci Biobehav Rev. 2002;26(2):105–125. doi: 10.1016/s0149-7634(01)00063-x. [DOI] [PubMed] [Google Scholar]
- 4.D’Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci. 2007;362(1481):761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funahashi S. Prefrontal cortex and working memory processes. Neuroscience. 2006;139(1):251–261. doi: 10.1016/j.neuroscience.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Vuilleumier P, Driver J. Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Philos Trans R Soc Lond B Biol Sci. 2007;362(1481):837–855. doi: 10.1098/rstb.2007.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 8.Noel X, Brevers D, Bechara A. A neurocognitive approach to understanding the neurobiology of addiction. Curr Opin Neurobiol. 2013;23(4):632–638. doi: 10.1016/j.conb.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garavan H, Brennan KL, Hester R, Whelan R. The neurobiology of successful abstinence. Curr Opin Neurobiol. 2013;23(4):668–674. doi: 10.1016/j.conb.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kravitz AV, Tomasi D, LeBlanc KH, et al. Cortico-striatal circuits: Novel therapeutic targets for substance use disorders. Brain Res. 2015 doi: 10.1016/j.brainres.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn Sci. 2012;16(1):81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Friston KJ. Functional and fffective connectivity: A review. Brain Connectivity. 2011;1(1):13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- 14.Bastiani M, Roebroeck A. Unraveling the multiscale structural organization and connectivity of the human brain: the role of diffusion MRI. Frontiers in neuroanatomy. 2015;9:77. doi: 10.3389/fnana.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma L, Wang B, Chen X, Xiong J. Detecting functional connectivity in the resting brain: a comparison between ICA and CCA. Magn Reson Imaging. 2007;25(1):47–56. doi: 10.1016/j.mri.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 16.Ma L, Steinberg JL, Hasan KM, Narayana PA, Kramer LA, Moeller FG. Stochastic dynamic causal modeling of working memory connections in cocaine dependence. Hum Brain Mapp. 2014;35(3):760–778. doi: 10.1002/hbm.22212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma L, Steinberg JL, Cunningham KA, et al. Inhibitory behavioral control: A stochastic dynamic causal modeling study comparing cocaine dependent subjects and controls. NeuroImage. Clinical. 2015;7:837–847. doi: 10.1016/j.nicl.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray S, Haney M, Hanson C, Biswal B, Hanson SJ. Modeling Causal Relationship Between Brain Regions Within the Drug-Cue Processing Network in Chronic Cocaine Smokers. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen YI, Famous K, Xu H, et al. Cocaine self-administration leads to alterations in temporal responses to cocaine challenge in limbic and motor circuitry. Eur J Neurosci. 2011;34(5):800–815. doi: 10.1111/j.1460-9568.2011.07806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu H, Zou Q, Chefer S, et al. Abstinence from cocaine and sucrose self-administration reveals altered mesocorticolimbic circuit connectivity by resting state MRI. Brain Connect. 2014;4(7):499–510. doi: 10.1089/brain.2014.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murnane KS, Gopinath KS, Maltbie E, Daunais JB, Telesford QK, Howell LL. Functional connectivity in frontal-striatal brain networks and cocaine self-administration in female rhesus monkeys. Psychopharmacology (Berl) 2015;232(4):745–754. doi: 10.1007/s00213-014-3709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Contreras-Rodriguez O, Albein-Urios N, Vilar-Lopez R, et al. Increased corticolimbic connectivity in cocaine dependence versus pathological gambling is associated with drug severity and emotion-related impulsivity. Addict Biol. 2015 doi: 10.1111/adb.12242. [DOI] [PubMed] [Google Scholar]
- 23.Hu Y, Salmeron BJ, Gu H, Stein EA, Yang Y. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA psychiatry. 2015;72(6):584–592. doi: 10.1001/jamapsychiatry.2015.1. [DOI] [PubMed] [Google Scholar]
- 24.Wisner KM, Patzelt EH, Lim KO, MacDonald AW., 3rd An intrinsic connectivity network approach to insula-derived dysfunctions among cocaine users. Am J Drug Alcohol Abuse. 2013;39(6):403–413. doi: 10.3109/00952990.2013.848211. [DOI] [PubMed] [Google Scholar]
- 25.Camchong J, MacDonald AW, 3rd, Nelson B, et al. Frontal hyperconnectivity related to discounting and reversal learning in cocaine subjects. Biol Psychiatry. 2011;69(11):1117–1123. doi: 10.1016/j.biopsych.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu H, Salmeron BJ, Ross TJ, et al. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53(2):593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konova AB, Moeller SJ, Tomasi D, Goldstein RZ. Effects of chronic and acute stimulants on brain functional connectivity hubs. Brain Res. 2015 doi: 10.1016/j.brainres.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ray S, Gohel SR, Biswal BB. Altered Functional Connectivity Strength in Abstinent Chronic Cocaine Smokers Compared to Healthy Controls. Brain Connect. 2015 doi: 10.1089/brain.2014.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McHugh MJ, Demers CH, Salmeron BJ, Devous MD, Sr, Stein EA, Adinoff B. Cortico-amygdala coupling as a marker of early relapse risk in cocaine-addicted individuals. Front Psychiatry. 2014;5:16. doi: 10.3389/fpsyt.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adinoff B, Gu H, Merrick C, et al. Basal Hippocampal Activity and Its Functional Connectivity Predicts Cocaine Relapse. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell MR, Balodis IM, Devito EE, et al. A preliminary investigation of Stroop-related intrinsic connectivity in cocaine dependence: associations with treatment outcomes. Am J Drug Alcohol Abuse. 2013;39(6):392–402. doi: 10.3109/00952990.2013.841711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konova AB, Moeller SJ, Tomasi D, Volkow ND, Goldstein RZ. Effects of methylphenidate on resting-state functional connectivity of the mesocorticolimbic dopamine pathways in cocaine addiction. JAMA psychiatry. 2013;70(8):857–868. doi: 10.1001/jamapsychiatry.2013.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57(3):432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 34.Rowe JB. Connectivity Analysis is Essential to Understand Neurological Disorders. Front Syst Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma L, Steinberg JL, Cunningham KA, et al. Inhibitory behavioral control: a stochastic dynamic causal modeling study using network discovery analysis. Brain Connect. 2015;5(3):177–186. doi: 10.1089/brain.2014.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elkashef A, Vocci F. Biological markers of cocaine addiction: implications for medications development. Addict Biol. 2003;8(2):123–139. doi: 10.1080/1355621031000117356. [DOI] [PubMed] [Google Scholar]
- 37.Frank R, Hargreaves R. Clinical biomarkers in drug discovery and development. Nat Rev Drug Discov. 2003;2(7):566–580. doi: 10.1038/nrd1130. [DOI] [PubMed] [Google Scholar]
- 38.Guengerich FP. Mechanisms of drug toxicity and relevance to pharmaceutical development. Drug Metab Pharmacokinet. 2011;26(1):3–14. doi: 10.2133/dmpk.dmpk-10-rv-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daliri M, Behroozi M. Advantages and disadvantages of resting state functional connectivity magnetic resonance imaging for clinical applications. OMICS J Radiology. 2013;3:e123. [Google Scholar]
- 40.Wang Z, Suh J, Li Z, et al. A hyper-connected but less efficient small-world network in the substance-dependent brain. Drug Alcohol Depend. 2015;152:102–108. doi: 10.1016/j.drugalcdep.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 42.McKeown MJ, Makeig S, Brown GG, et al. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp. 1998;6(3):160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang X, He Y, Salmeron BJ, Gu H, Stein EA, Yang Y. Interactions between the Salience and Default-Mode Networks Are Disrupted in Cocaine Addiction. J Neurosci. 2015;35(21):8081–8090. doi: 10.1523/JNEUROSCI.3188-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friston K, Penny W. Post hoc Bayesian model selection. Neuroimage. 2011;56(4):2089–2099. doi: 10.1016/j.neuroimage.2011.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friston KJ, Li B, Daunizeau J, Stephan KE. Network discovery with DCM. Neuroimage. 2011;56(3):1202–1221. doi: 10.1016/j.neuroimage.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19(4):1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- 47.Stephan KE, Weiskopf N, Drysdale PM, Robinson PA, Friston KJ. Comparing hemodynamic models with DCM. Neuroimage. 2007;38(3):387–401. doi: 10.1016/j.neuroimage.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.David O, Guillemain I, Saillet S, et al. Identifying neural drivers with functional MRI: an electrophysiological validation. PLoS Biol. 2008;6(12):2683–2697. doi: 10.1371/journal.pbio.0060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li B, Daunizeau J, Stephan KE, Penny W, Hu D, Friston K. Generalised filtering and stochastic DCM for fMRI. Neuroimage. 2011;58(2):442–457. doi: 10.1016/j.neuroimage.2011.01.085. [DOI] [PubMed] [Google Scholar]
- 50.Di X, Biswal BB. Identifying the default mode network structure using dynamic causal modeling on resting-state functional magnetic resonance imaging. Neuroimage. 2014;86:53–59. doi: 10.1016/j.neuroimage.2013.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friston KJ, Kahan J, Biswal B, Razi A. A DCM for resting state fMRI. Neuroimage. 2014;94:396–407. doi: 10.1016/j.neuroimage.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Razi A, Kahan J, Rees G, Friston KJ. Construct validation of a DCM for resting state fMRI. Neuroimage. 2015;106:1–14. doi: 10.1016/j.neuroimage.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campo P, Garrido MI, Moran RJ, et al. Remote effects of hippocampal sclerosis on effective connectivity during working memory encoding: a case of connectional diaschisis? Cereb Cortex. 2012;22(6):1225–1236. doi: 10.1093/cercor/bhr201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carrera E, Tononi G. Diaschisis: past, present, future. Brain. 2014;137(Pt 9):2408–2422. doi: 10.1093/brain/awu101. [DOI] [PubMed] [Google Scholar]
- 55.Guye M, Bartolomei F, Ranjeva JP. Imaging structural and functional connectivity: towards a unified definition of human brain organization? Curr Opin Neurol. 2008;21(4):393–403. doi: 10.1097/WCO.0b013e3283065cfb. [DOI] [PubMed] [Google Scholar]
- 56.Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct. 2009 doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- 57.Moeller FG, Hasan KM, Steinberg JL, et al. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30(3):610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- 58.Moeller FG, Hasan KM, Steinberg JL, et al. Diffusion tensor imaging eigenvalues: preliminary evidence for altered myelin in cocaine dependence. Psychiatry Res. 2007;154(3):253–258. doi: 10.1016/j.pscychresns.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 59.Lim KO, Wozniak JR, Mueller BA, et al. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92(1–3):164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma L, Hasan KM, Steinberg JL, et al. Diffusion tensor imaging in cocaine dependence: regional effects of cocaine on corpus callosum and effect of cocaine administration route. Drug Alcohol Depend. 2009;104(3):262–267. doi: 10.1016/j.drugalcdep.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma L, Steinberg JL, Keyser-Marcus L, et al. Altered white matter in cocaine-dependent subjects with traumatic brain injury: A diffusion tensor imaging study. Drug Alcohol Depend. 2015 doi: 10.1016/j.drugalcdep.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lane SD, Steinberg JL, Ma L, et al. Diffusion tensor imaging and decision making in cocaine dependence. PLoS ONE. 2010;5(7):e11591. doi: 10.1371/journal.pone.0011591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bell RP, Foxe JJ, Nierenberg J, Hoptman MJ, Garavan H. Assessing white matter integrity as a function of abstinence duration in former cocaine-dependent individuals. Drug Alcohol Depend. 2011;114(2–3):159–168. doi: 10.1016/j.drugalcdep.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Narayana PA, Ahobila-Vajjula P, Ramu J, Herrera J, Steinberg JL, Moeller FG. Diffusion tensor imaging of cocaine-treated rodents. Psychiatry Res. 2009;171(3):242–251. doi: 10.1016/j.pscychresns.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Narayana PA, Herrera JJ, Bockhorst KH, et al. Chronic cocaine administration causes extensive white matter damage in brain: diffusion tensor imaging and immunohistochemistry studies. Psychiatry Res. 2014;221(3):220–230. doi: 10.1016/j.pscychresns.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu J, DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. White matter integrity is associated with treatment outcome measures in cocaine dependence. Neuropsychopharmacology. 2010;35(7):1541–1549. doi: 10.1038/npp.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lebel C, Warner T, Colby J, et al. White matter microstructure abnormalities and executive function in adolescents with prenatal cocaine exposure. Psychiatry Res. 2013;213(2):161–168. doi: 10.1016/j.pscychresns.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coullaut-Valera R, Arbaiza I, Bajo R, et al. Drug polyconsumption is associated with increased synchronization of brain electrical-activity at rest and in a counting task. International journal of neural systems. 2014;24(1):1450005. doi: 10.1142/S0129065714500051. [DOI] [PubMed] [Google Scholar]
- 69.Nishida K, Razavi N, Jann K, et al. Integrating Different Aspects of Resting Brain Activity: A Review of Electroencephalographic Signatures in Resting State Networks Derived from Functional Magnetic Resonance Imaging. Neuropsychobiology. 2015;71(1):6–16. doi: 10.1159/000363342. [DOI] [PubMed] [Google Scholar]
- 70.Rykhlevskaia E, Gratton G, Fabiani M. Combining structural and functional neuroimaging data for studying brain connectivity: a review. Psychophysiology. 2008;45(2):173–187. doi: 10.1111/j.1469-8986.2007.00621.x. [DOI] [PubMed] [Google Scholar]
- 71.Stephan KE, Tittgemeyer M, Knosche TR, Moran RJ, Friston KJ. Tractography-based priors for dynamic causal models. Neuroimage. 2009;47(4):1628–1638. doi: 10.1016/j.neuroimage.2009.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moran RJ, Campo P, Symmonds M, Stephan KE, Dolan RJ, Friston KJ. Free energy, precision and learning: the role of cholinergic neuromodulation. J Neurosci. 2013;33(19):8227–8236. doi: 10.1523/JNEUROSCI.4255-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moran RJ, Jones MW, Blockeel AJ, Adams RA, Stephan KE, Friston KJ. Losing control under ketamine: suppressed cortico-hippocampal drive following acute ketamine in rats. Neuropsychopharmacology. 2015;40(2):268–277. doi: 10.1038/npp.2014.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cisler JM, Elton A, Kennedy AP, et al. Altered functional connectivity of the insular cortex across prefrontal networks in cocaine addiction. Psychiatry Res. 2013;213(1):39–46. doi: 10.1016/j.pscychresns.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kelly C, Zuo XN, Gotimer K, et al. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol Psychiatry. 2011;69(7):684–692. doi: 10.1016/j.biopsych.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Z, Santhanam P, Coles CD, et al. Increased “default mode” activity in adolescents prenatally exposed to cocaine. Hum Brain Mapp. 2011;32(5):759–770. doi: 10.1002/hbm.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McHugh MJ, Demers CH, Braud J, Briggs R, Adinoff B, Stein EA. Striatal-insula circuits in cocaine addiction: implications for impulsivity and relapse risk. Am J Drug Alcohol Abuse. 2013;39(6):424–432. doi: 10.3109/00952990.2013.847446. [DOI] [PubMed] [Google Scholar]
- 78.Salzwedel AP, Grewen KM, Vachet C, Gerig G, Lin W, Gao W. Prenatal drug exposure affects neonatal brain functional connectivity. J Neurosci. 2015;35(14):5860–5869. doi: 10.1523/JNEUROSCI.4333-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schweitzer JB, Riggins T, Liang X, et al. Prenatal drug exposure to illicit drugs alters working memory-related brain activity and underlying network properties in adolescence. Neurotoxicology and teratology. 2015;48:69–77. doi: 10.1016/j.ntt.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Verdejo-Garcia A, Contreras-Rodriguez O, Fonseca F, et al. Functional alteration in frontolimbic systems relevant to moral judgment in cocaine-dependent subjects. Addict Biol. 2014;19(2):272–281. doi: 10.1111/j.1369-1600.2012.00472.x. [DOI] [PubMed] [Google Scholar]
- 81.Albein-Urios N, Verdejo-Roman J, Soriano-Mas C, Asensio S, Martinez-Gonzalez JM, Verdejo-Garcia A. Cocaine users with comorbid Cluster B personality disorders show dysfunctional brain activation and connectivity in the emotional regulation networks during negative emotion maintenance and reappraisal. Eur Neuropsychopharmacol. 2013;23(12):1698–1707. doi: 10.1016/j.euroneuro.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 82.Albein-Urios N, Verdejo-Roman J, Asensio S, Soriano-Mas C, Martinez-Gonzalez JM, Verdejo-Garcia A. Re-appraisal of negative emotions in cocaine dependence: dysfunctional corticolimbic activation and connectivity. Addict Biol. 2014;19(3):415–426. doi: 10.1111/j.1369-1600.2012.00497.x. [DOI] [PubMed] [Google Scholar]
- 83.Hanlon CA, Wesley MJ, Stapleton JR, Laurienti PJ, Porrino LJ. The association between frontal-striatal connectivity and sensorimotor control in cocaine users. Drug Alcohol Depend. 2011;115(3):240–243. doi: 10.1016/j.drugalcdep.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kilts CD, Kennedy A, Elton AL, et al. Individual differences in attentional bias associated with cocaine dependence are related to varying engagement of neural processing networks. Neuropsychopharmacology. 2014;39(5):1135–1147. doi: 10.1038/npp.2013.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tomasi D, Volkow ND, Wang R, et al. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One. 2010;5(5):e10815. doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Worhunsky PD, Stevens MC, Carroll KM, et al. Functional brain networks associated with cognitive control, cocaine dependence, and treatment outcome. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 2013;27(2):477–488. doi: 10.1037/a0029092. [DOI] [PMC free article] [PubMed] [Google Scholar]