Abstract
Despite studies showing the relevance of different decision-making abilities, including response inhibition, to likelihood of using substances during adolescence, few have examined these neural processes among high-risk, substance-using youth. The current study explored associations between alcohol and marijuana use and functional activation differences during Stroop performance among a large sample (N = 80) of ethnically-diverse, high-risk youth in an fMRI-based task. In the absence of associations between substance use and task behavioral performance, adolescents with greater alcohol use showed less activation during the more cognitively difficult portion of the task across clusters in bilateral cuneus and precuneus, and right and left superior temporal gyrus. No associations were observed with marijuana use. The current results may suggest neural patterns of deactivation in regions important for cognitive control, such that alcohol use may confer additional risk for future decreased inhibition among these high-risk adolescents. The ability to inhibit prepotent responses has been shown to predict later response to treatment, and early interventions to encourage further development of cognitive control could represent promising options for treatment.
Keywords: adolescence, alcohol use, marijuana use, fMRI, Stroop
1. Introduction
Alcohol is the most widely used substance among teenagers, with two thirds of students reporting having consumed alcohol by the end of high school, while about one third of 10th and 12th grade students report having used marijuana within the last 12 months (Johnston et al., 2015). Even relatively modest levels of alcohol and marijuana use during adolescence may alter normal neural development (Tapert et al., 2007). These disruptions may have functional implications for the development and organization of critical neuronal networks in the brain, particularly complex frontolimbic connections related to self-control and decision-making that continue to develop throughout adolescence (Casey, 2015). Adolescents appear to be more sensitive to environmental cues than children or adults and less reactive to potentially threatening situations, which encourages healthy exploration (Casey, 2015). While much exploration is normative in adolescence, variability in executive function and decision-making processes (e.g., learning to balance consideration of both rewarding and negative consequences) is crucial in understanding the risk for negative consequences arising from exploratory behavior. Response inhibition is an important facet of the larger construct of executive functioning and may play a key role in determining an adolescent’s likelihood of engaging in risky substance-related behavior. For example, in a large longitudinal study, poor performance on behavioral measures of response inhibition during early and mid adolescence predicted later problematic drinking and illicit drug use, even after controlling for other common contributing factors such as parental substance abuse (Nigg et al., 2006). Further, greater appetite for reward and a diminished aversion to punishment has been shown to predict the frequency, quantity, and age of first substance use (Pardo et al., 2007), while impulsive sensation seeking is associated with increased consumption of both alcohol and marijuana in adolescence (Kong et al., 2013).
The Stroop paradigm is a useful measure of response inhibition, in addition to other cognitive demands (e.g., conflict monitoring; Silveri et al., 2011), and has been adapted for neuroimaging, although few have used the Stroop task with substance-using adolescents. In general, increasing age across childhood into young adulthood is associated with improved task performance and greater activation in prefrontal, anterior cingulate, and parietal cortices during color-word interference trials (Adleman et al., 2002). Among adolescent substance users, increased activation during the Stroop is generally observed in the absence of decreased performance. Regular marijuana users showed increased activation during a counting Stroop in several regions not typically observed during Stroop interference (e.g., rolandic operculum, supplementary motor area; Hatchard et al., 2014). Adolescents enrolled in an addiction treatment program showed similar behavioral performance as control participants, but the substance-using group activated additional parahippocampal, medial prefrontal, posterior, and subcortical regions during incongruent versus congruent trials (Banich et al., 2007). A similar pattern was observed among adolescents with a family history of alcoholism, such that family-history positive adolescents showed greater frontolimbic activation in the absence of differences in task performance (Silveri et al., 2011). In a study using a related paradigm, adolescents with a history of marijuana use performed comparably during a Go/No-Go task but had greater dorsolateral prefrontal and parietal activation during inhibition trials relative to controls (Tapert at al., 2007). Further, negative associations between past month substance use and Go/No-Go activation have been found in the left inferior frontal gyrus and right insula (Feldstein Ewing et al., 2015). Taken together, group comparisons between non-users and substance users have suggested compensatory mechanisms whereby substance users show increased activation in order to perform response inhibition tasks as well as non-users.
Similar findings have been observed among adults. A recent study among young adults found increased activation in low-level alcohol users compared to non-users during a counting Stroop in regions including the cerebellum, prefrontal cortex, and precuneus (Hatchard et al., 2015), again in the absence of task performance differences. Adults with alcohol use disorders (AUDs) showed greater cognitive control network activation during a multisensory Stroop task than healthy controls, with no difference in task accuracy but slower reaction times for those with AUDs (Wilcox et al., 2015). These results may suggest that behavioral differences in task performance indicate greater severity of substance use, including clinical symptoms of substance use disorders.
Neural activation during the Stroop has also been associated with behavior change following treatment. Interestingly, among adolescents participating in a smoking cessation treatment program, adolescents with greater activation during Stroop interference in inferior frontal gyrus, insula, thalamus, and anterior cingulate prior to beginning treatment were more successful in reducing smoking (Krishnan-Sarin et al., 2013). Similarly, among cannabis dependent adult patients, greater activation in regions associated with cognitive control predicted greater success in reducing use after a cognitive-behavioral treatment program (Kober et al., 2014). These results suggest that cognitive control processes are important for treatment response (i.e., reduced use).
The majority of the existing literature shows increased activation during Stroop among substance users in the absence of decreased task accuracy or slowed response times. These findings are consistent across adolescent and adult populations and across a wide range of substance use severity (e.g., limited use in the context of family history, regular low-level use, use disorder/dependence), and additional studies have suggested that neural activity during response inhibition may predict treatment outcome. Two studies have suggested a different pattern of baseline results. In the treatment study mentioned above, cannabis dependent adults showed relatively less Stroop-related activation in dorsolateral prefrontal cortex and ventral striatum compared to controls prior to beginning treatment (Kober et al., 2014). Among adolescents, decreased neural activation during a Go/No-Go task was observed in frontal, temporal, and parietal regions among adolescents who later transitioned into heavy alcohol use compared to those who did not, although no task performance differences were observed (Norman et al., 2011). The authors suggest that limited neural activity during response inhibition in early adolescence could indicate a trajectory of greater risk for future substance use (Norman et al., 2011).
Overall, existing data from Stroop and Go/No-Go paradigms suggest alterations in neural activity associated with alcohol and marijuana use even in the absence of behavioral differences in task performance between users and non-users. Such alterations in neural activity among adolescents who primarily use alcohol, marijuana, or both may provide insight into functional trajectories of substance use and other risky behaviors for early adulthood and later in life (e.g., Bava and Tapert, 2010; Norman et al., 2011). Despite studies showing the relevance of response inhibition to the likelihood of using substances, few have examined these neural processes among high-risk youth. The current study therefore explored associations between alcohol and marijuana use and functional activation differences during Stroop performance among a large sample of ethnically-diverse, high-risk youth (N = 80) with a range of substance use behavior. Past 3-month alcohol and marijuana use were entered as continuous variables in multiple regression analyses of activation during incongruent versus neutral and incongruent versus congruent trials. Based on evidence of impaired inhibition with alcohol use (e.g., among young adults; Pardo et al., 2007; Gan et al., 2014), it was hypothesized that alcohol and marijuana use would be negatively associated with neural activation during Stroop interference, particularly in regions subserving cognitive control processing such as prefrontal cortex and precuneus.
2. Methods
2.1. Sample and Procedures
Research assistants recruited adolescents from juvenile justice partner programs for a large, longitudinal intervention study in the southwest United States. All participants were required to be 14 to 18 years old and fluent in English, with no use of antipsychotic or anticonvulsant medications, no MRI contraindications (e.g., current pregnancy, irremovable metal implants or piercings, claustrophobia), and no traumatic brain injury (TBI) with loss of consciousness greater than 5 minutes in the last 6 months. Participants were also excluded from the parent study if they reported use of hard drugs (e.g., cocaine, heroin, methamphetamine) more than three times in the last month during the screening interview. All participants were assented and parental/legal guardian consent was obtained via audio recording prior to study participation. The participating institutional review board approved the study and a federal certificate of confidentiality was obtained. Participants completed behavioral measures and a single neuroimaging session. All neuroimaging data included herein were evaluated prior to random assignment to intervention conditions.
2.2. Measures
2.2.1. Substance use
Adolescents completed questions about alcohol and marijuana use frequency and quantity derived from a brief composite measure by White and Labouvie (1989), originally developed to assess alcohol use, and modified for this study to also evaluate marijuana use. In order to estimate overall consumption, an alcohol composite and a marijuana composite were calculated by multiplying two items for past 3-month quantity and frequency. Frequency of use in the last 3 months was rated on a 9-point ordinal scale ranging from 1=“never,” to 9=“every day.” Similarly, recent quantity of use was rated 1=“none” to 10=“more than 20 drinks” (or hits for marijuana use). Each composite variable was then log transformed due to non-normality for use in regression analyses.
2.2.2. Personality and behavior
On the self-report level, several questionnaires have been designed to assess factors closely related to response inhibition, among them the Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ; Torrubia et al., 2001) and the Impulsive Sensation Seeking Scale (IMPSS; Zuckerman, 2002). Both scales have been used in numerous studies of adolescent alcohol and marijuana consumption (e.g., Crawford et al., 2003; Pardo et al., 2007; Lopez-Vergara et al., 2012; Kong et al., 2013). The SPSRQ (Cronbach’s α = 0.66) is a 48-item measure divided into 2 subscales designed to measure behavioral inhibition (e.g., likely positively associated with Stroop performance; “Do you often refrain from doing something because of your fear of being embarrassed?”) and behavioral activation (e.g., likely negatively associated with Stroop performance; “Do you sometimes do things for quick rewards?”). The IMPSS (Cronbach’s α = 0.79) consists of 19 items that ask participants to agree or disagree with statements related to impulsivity and sensation seeking (e.g., “I like doing things just for the thrill of it”).
2.3. Stroop Task
Participants completed a shortened version of an empirically supported functional Stroop color-word interference task (Andrews-Hanna et al., 2011). As in a traditional Stroop task, participants select the color in which a word is printed across two main trial types: congruent (i.e., “red” shown in red text) and incongruent (i.e., “red” shown in green text). In addition, neutral trials were presented in which the stimulus is a color-neutral word (e.g., “jacket”) shown in colored text. Participants were instructed to select the printed color of each word (i.e., red, green, blue, or yellow) on a button box, and were not required to correct any errors before subsequent trials proceeded. Each trial displayed the word for 1500 ms, with 500 ms of fixation between trials.
Each participant completed two task runs during fMRI scanning for a total of 216 trials across congruent, incongruent, and neutral blocks. Briefly, in line with Andrews-Hanna et al. (2011), half of the trials in each block were comprised of stimuli that appeared only in that block [e.g., congruent (Cc), incongruent (Ic), and neutral (Nc)], and the other half of trials were neutral stimuli that were consistent across all blocks (see Figure 1). Inclusion of these neutral stimuli across all blocks reduces potential habituation effects and prevents participants from merely reading the words during the congruent trials. Contrasts of interest were incongruent - neutral trials (i.e., Ic - Nc; the Stroop interference effect), and incongruent - congruent trials (i.e., Ic - Cc as a measure of cognitive control; Andrews-Hanna et al., 2011).
Figure 1.
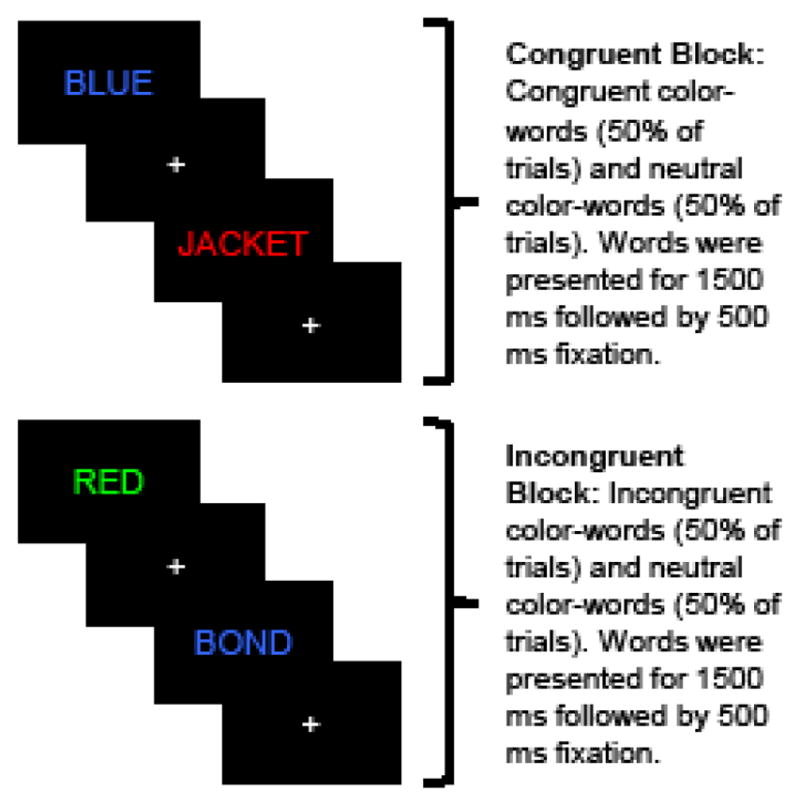
Schematic of fMRI Stroop paradigm.
2.4. Image Acquisition and Processing
MRI was performed on a 3T Siemens Trio (Erlangen, Germany) whole body scanner with a 12-channel phased array head coil. High resolution anatomical images were acquired with a multi-echo magnetization-prepared rapid gradient-echo (MP-RAGE) sequence with TE = 1.64, 3.50, 5.36, 7.22, and 9.08 ms, TR = 2.53 s, TI = 1.20 s, ip angle = 7°, NEX = 1, slice thickness = 1 mm, 33 slices, FOV (field of view) = 256 mm, and in-plane resolution = 256 × 256. During the Stroop task, functional images were acquired using a single-shot, gradient-echo echo-planar pulse sequence (TR = 2000 ms; TE = 29 ms; flip angle = 75°; FOV = 240 mm; matrix size = 64 × 64, voxel size = 3.75 × 3.75 × 4.55 mm). Structural images were acquired oblique to the anterior-posterior commissure line to diminish susceptibility artifacts.
The first image of each run along with two dummy scans was eliminated to account for T1 equilibrium effects, resulting in a total of 326 images for final analyses. Anomalous time-series values were first identified using a despiking algorithm in AFNI (Cox, 1996), and then replaced based on temporally neighbouring values. All time-series data were then spatially registered in two- and three-dimensional space to the second EPI image of the first run to reduce the effects of head motion, and were temporally interpolated to the first slice to account for differences in slice acquisition. Framewise displacement was calculated based on the first derivatives of the head motion data following transformation of rotations to a 100 mm diameter sphere (Power et al., 2012). Data were then spatially blurred using an 8 mm Gaussian full-width half-maximum filter and converted to standard stereotaxic coordinate space (Talairach and Tournoux, 1988).
A voxel-wise general linear model analysis was used to estimate data fit by convolving a double-gamma variate function with the study design matrix. A total of six regressors were used to model correct and incorrect trials during congruent, incongruent, and neutral blocks. Incorrect trials were modelled separately for each trial-type to eliminate error variance but were not analysed in the current study (Mayer et al., 2012). In addition, 12 nuisance motion regressors were included in the model (6 motion parameters and their derivatives). Percent signal change for correct trials was calculated by dividing the beta coefficients for each condition by the average model intercept (β0) across all runs and was used in all second level analyses.
2.5. Statistical Analyses
A whole-brain voxelwise multiple regression analysis was conducted for each contrast of interest to identify regions that were associated with task demands, and then to identify regions that showed a significant association between activation in cognitive control regions and alcohol or marijuana use. Age, gender, years of education, and mean framewise displacement were used as additional covariates in the analyses. Multiple comparisons were corrected using AFNI’s 3dClustSim through 10,000 Monte Carlo simulations of combined individual voxel probability (p < 0.005) and cluster size thresholding (2496 μl) for a whole brain correction of p < 0.05. Full width half maximum values along each axis were included as parameters in cluster thresholding.
3. Results
3.1. Sample Characteristics
Participants were omitted from the parent study pool because of missing substance use data (n = 9), framewise displacement > 0.5 (n = 16), and Stroop task accuracy of less than 66% correct responses across trials (n = 19). Participants excluded from imaging analyses did not differ from included participants in age, ethnicity, gender, years of education, IMPSS or SPSRQ total scores, or, for those with available substance use data, in frequency or quantity of alcohol or marijuana use in the past 3 months (all p > 0.15). Among participants who reported an age of first use, excluded participants were slightly older in age of first use for both alcohol [13.3 compared to 12.3 years of age; t(113) = 2.42, p < 0.02] and marijuana [12.6 compared to 11.4 years of age; t(113) = 2.80, p < 0.01]. Therefore, the evidence supports the comparable risk profile of our included versus excluded samples for this set of analyses.
The final dataset contained 80 adolescents (see Table 1). Participants were predominantly male (74%) with a mean age of 15.94 (SD = 1.17), and were ethnically diverse (51.2% Hispanic, 22.5% Multiracial, 11.3% Caucasian, 7.5% African American, 5.0% Native American, and 2.5% not specified). This sample reported a past 3-month average of 2 to 3 drinks per month, and 7–9 hits of marijuana on 4–5 occasions per month. Important for the purposes of comparison, this sample also included adolescents who had not used any alcohol (n = 20; 25%) or marijuana (n = 26; 32.5%) within the last 3 months. For substance using adolescents, use of alcohol and marijuana were marginally correlated [r(72) = 0.20, p = 0.09].
Table 1.
Sample characteristics.
| Measures | Females (n = 21) Mean (SD) |
Males (n = 59) Mean (SD) |
|---|---|---|
| Demographics | ||
| Age | 15.81 (1.17) | 15.98 (1.18) |
| Ethnicity (% Hispanic) | 61.9 | 47.4 |
| Years of Education (Last Grade Completed) | 9.14 (1.39) | 9.12 (1.02) |
| Total Scale Scores | ||
| Impulsive Sensation Seeking (IMPSS) | 12.71 (2.51) | 11.49 (3.59)a |
| Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) | 24.24 (6.38) | 22.00 (6.94) |
| Substance Use | ||
| Age of first alcohol use | 12.55 (1.50)b | 12.19 (2.34)c |
| Age of first marijuana use | 12.05 (2.42) | 11.24 (2.28) |
| Past 3 months alcohol use frequency† | 3.10 (2.05) | 3.19 (2.14) |
| Past 3 months alcohol use quantity (drinks)‡ | 3.52 (1.97) | 3.36 (2.02) |
| Past 3 months marijuana use frequency† | 5.76 (3.69) | 4.86 (3.54) |
| Past 3 months marijuana use quantity (hits)‡ | 5.10 (3.51) | 5.51 (3.50) |
n = 53;
n = 20;
n = 58
1=“Never,” 2=“Occasionally,” 3=“Once a month,” 4=“2–3 times a month,” 5=“4–5 times a month,” 6=“Once a week,” 7=“2–3 times a week,” 8=“4–5 times a week,” 9=“Every day.”
1=“None,” 2=“1,” 3=“2–3,” 4=“4–6,” 5=“7–9,” 6=“10–12,” 7=“13–15,” 8=“16–18,” 9=“19–20,” 10=“More than 20.”
3.2. Behavioral Correlations
Associations between alcohol consumption and scores on the SPSRQ and IMPSS were not significant, nor were associations between marijuana consumption and scores on the SPSRQ or IMPSS. Given this lack of significant associations, SPSRQ and IMPSS variables were not examined further as covariates.
3.3. Task Performance
Overall, participants responded correctly to congruent trials at a rate of approximately 91% (correct trials out of 72; M = 65.61, SD = 5.16), with 88% correct responses to neutral (correct trials out of 72; M = 63.40, SD = 5.68) and incongruent (correct trials out of 72; M = 63.09, SD = 4.88) trials. As expected, participants had greater accuracy during congruent than incongruent trials, with more correct [t(79) = 6.75, p < 0.001] and fewer incorrect [t(79) = −5.41, p < 0.001] responses. Reaction times were calculated for correct trials only, and were shortest for congruent trials (M = 708.84 ms, SD = 91.30 ms), followed by neutral (M = 723.56 ms, SD = 88.62 ms) and incongruent (M = 762.71 ms, SD = 97.82 ms) trials. Further, reaction times were significantly shorter for congruent than incongruent trials [t(79) = −11.71, p < 0.001]. Neither alcohol nor marijuana consumption was significantly correlated with accuracy measures or reaction time for congruent or incongruent trials.
3.4. Task Activation
3.4.1. Incongruent-Neutral
Alcohol consumption was negatively associated with incongruent-neutral task activation in 2 clusters (see Figure 2 and Table 2). The largest cluster (4593 μl) extended across right precentral gyrus, postcentral gyrus, and central opercular cortex. The second cluster (4121 μl) extended across right superior lateral occipital cortex, superior parietal lobule, angular gyrus, and precuneus. Marijuana consumption was not significantly associated with activation in any region.
Figure 2.
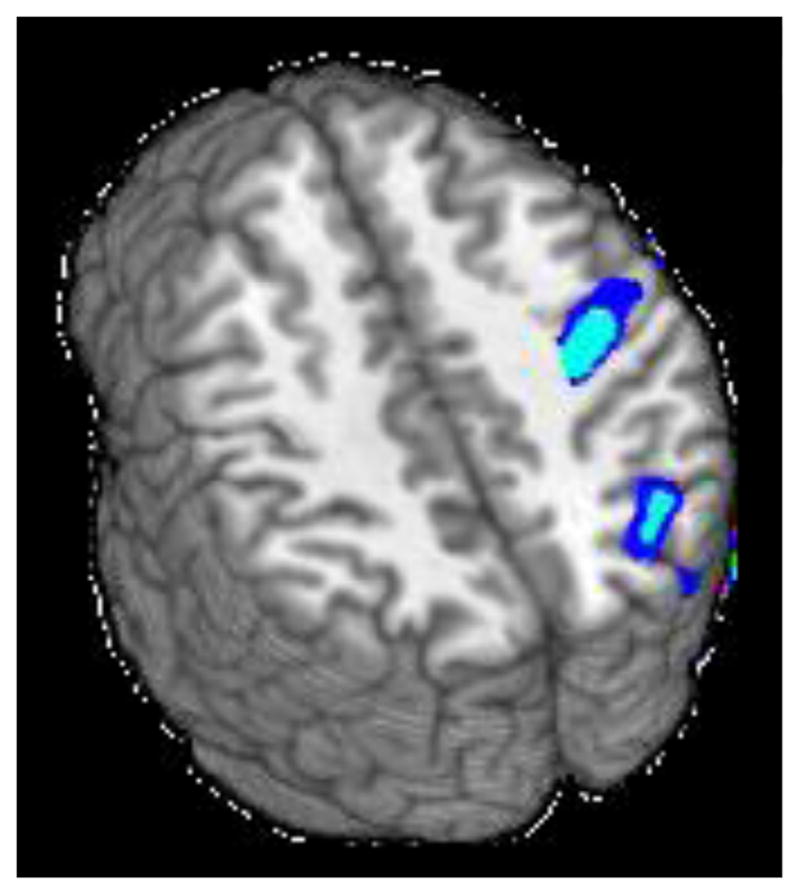
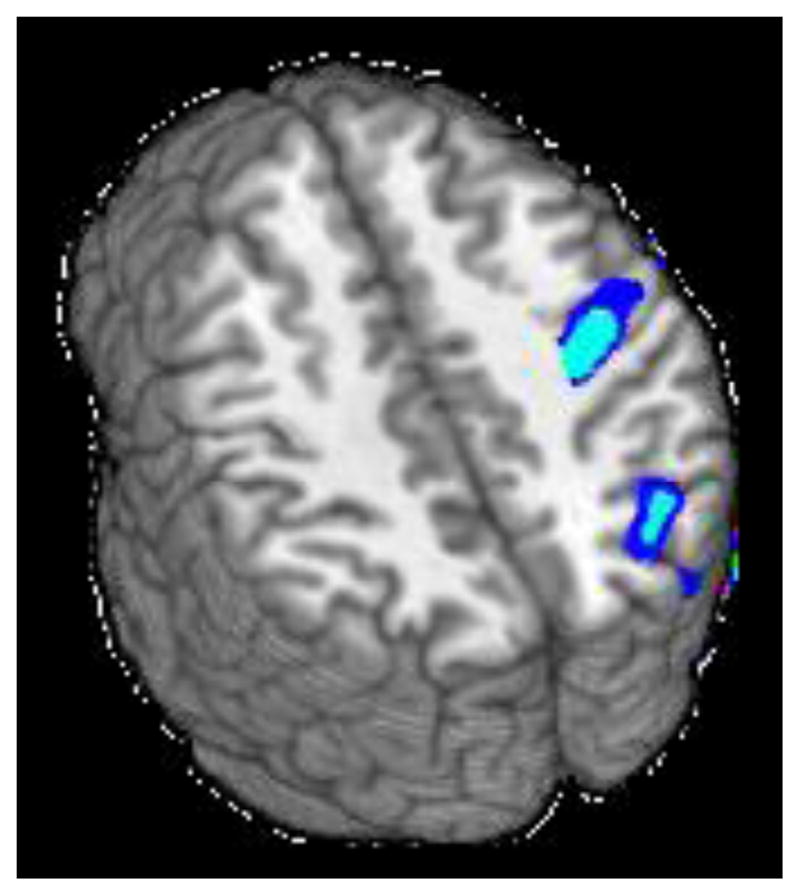
Clusters of significant negative association between alcohol use and Stroop activation for the incongruent-neutral contrast (z = 35 and 42).
Table 2.
Coordinates of task activations during incongruent-neutral and incongruent-congruent contrasts.
| Annotation† | Cluster Size (voxels) | MNI Coordinates‡ | ||
|---|---|---|---|---|
| X | Y | Z | ||
| Incongruent-Neutral | ||||
| R precentral gyrus; R postcentral gyrus; central opercular cortex | 4593 | 48 | 111 | 110 |
| R superior lateral occipital cortex; R superior parietal lobule; R angular gyrus; R precuneus | 4121 | 57 | 63 | 110 |
| Incongruent-Congruent | ||||
| Bilateral cuneus and precuneus; R angular gyrus; R middle occipital gyrus; R superior occipital gyrus; R superior parietal lobule | 9256 | 82 | 54 | 108 |
| R superior temporal gyrus; R postcentral gyrus; R insula; R precentral gyrus | 7234 | 37 | 119 | 94 |
| L superior temporal gyrus; L postcentral gyrus | 4049 | 145 | 115 | 92 |
L=Left; R=Right
Harvard-Oxford Cortical Structural Atlas
Center of mass
3.4.2. Incongruent-Congruent
Alcohol consumption was negatively associated with incongruent-congruent task activation in 3 clusters (see Figure 3 and Table 2). The largest cluster (9256 μl) extended across bilateral cuneus and precuneus, into right angular gyrus, right middle occipital gyrus, right superior occipital gyrus, and right superior parietal lobule. The second cluster (7234 μl) extended from right superior temporal gyrus into right postcentral gyrus, insula, and right precentral gyrus. The third cluster (4049 μl) extended from left superior temporal gyrus to left postcentral gyrus. Marijuana consumption was not significantly associated with incongruent-congruent task activation in any region.
Figure 3.
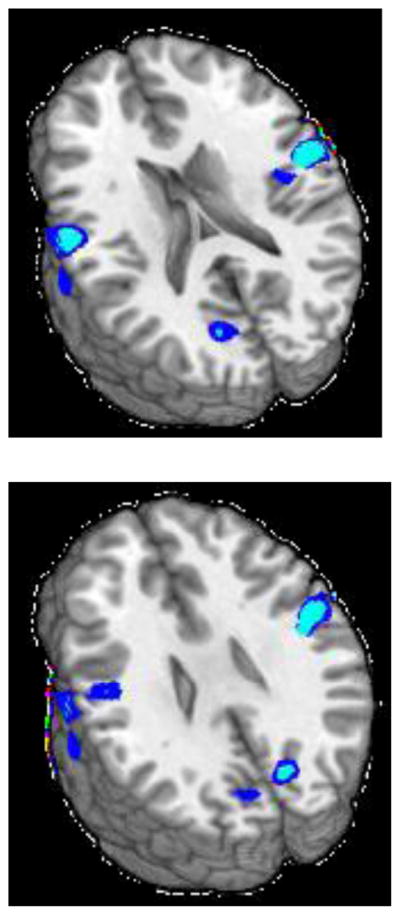
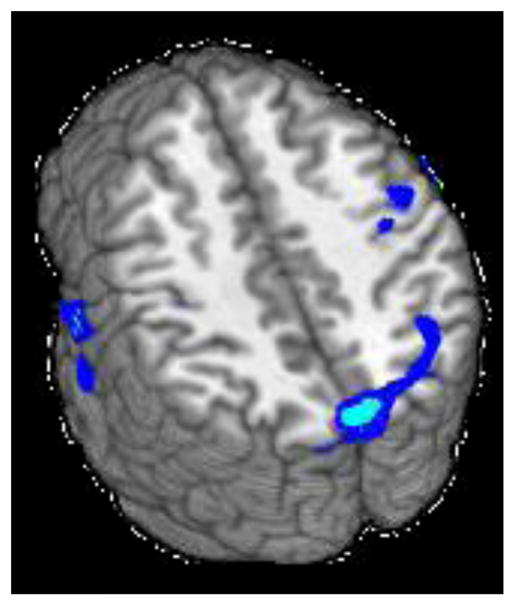
Clusters of significant negative association between alcohol use and Stroop activation for the incongruent-congruent contrast (z = 18, 26, and 42).
3.5. Post Hoc Analyses
Post hoc analyses examined possible alcohol use by marijuana use interactions within clusters significantly associated with alcohol use for both contrasts. Task activation was extracted for each cluster and used as the dependent variable in regressions that added an alcohol by marijuana interaction term with alcohol use, marijuana use, age, gender, years of education, and framewise displacement. No significant interaction effects were observed.
4. Discussion
4.1. Current Findings
The current study examined associations between functional activation during a response inhibition task and recent alcohol and marijuana use within an understudied subset of high-risk, ethnically diverse youth. Few studies have used the Stroop paradigm to examine response inhibition processes associated with risk behavior for youth. Further, those few studies have been limited to small sample sizes. A strength of this study is the strong variability of substance use within this sample, which facilitated including alcohol and marijuana use as continuous factors in regression analyses rather than using dichotomous grouping variables as has typically been done in prior studies (e.g., Banich et al., 2007). Moreover, each model included covariates for age, gender, years of education, and mean framewise displacement. This allowed for a more focused comparison of the potential associations of recent alcohol and marijuana use and variability in activation during a Stroop task.
We observed no associations among alcohol or marijuana use, task behavioral performance (i.e., accuracy or reaction time), and questionnaires assessing domains of personality typically associated with substance use (i.e., sensitivity to reward and punishment, impulsivity). This is somewhat surprising given studies among adolescents showing associations of impulsivity with increased alcohol and marijuana consumption (e.g., Kong et al., 2013) as well as reward response with frequency, quantity, and age of first substance use (Pardo et al., 2007). Interestingly, this lack of associations is in line with with other studies of marijuana use among high-risk youth (e.g., Robbins and Bryan, 2004; Weiland et al., 2015), which may indicate that these relationships are generally small within high-risk samples and are thus inconsistently observed and less predictive of substance use.
In terms of functional activation, alcohol use was negatively associated with activation in incongruent versus neutral trials and incongruent versus congruent trials during the Stroop paradigm, despite the absence of associations that might otherwise predict task activation (e.g., variation in task accuracy). For the incongruent versus neutral contrast, these results may suggest decreased reactive control such as evaluation of responses (Andrews-Hanna et al., 2011), such that adolescents with higher alcohol use are attending less to their own task performance. In terms of the Stroop effect, adolescents with greater alcohol use showed less activation during the interference condition across clusters in bilateral cuneus and precuneus, and right and left superior temporal gyrus. These results contrast with prior Stroop studies, in which behavioral performance did not differ between substance-using and control participants, but additional widespread brain regions were activated during incongruent versus congruent trials for substance users (e.g., Banich et al., 2007; Hatchard et al., 2015). One possibility for these differing patterns is that the adolescents in other studies were recruited to participate in treatment programs to reduce substance use, which might account for increased general engagement or increasing cognitive control with treatment (Banich et al., 2007; Krishnan-Sarin et al., 2013). Decreased activation in frontal, temporal, and parietal regions during inhibition on a Go/No-Go task has been observed among adolescents who later transitioned to heavier alcohol use (Norman et al., 2011), and the current results may suggest similar neural patterns.
The current analyses were designed to test the specific contributions of alcohol and marijuana use to neural functioning within a high-risk group of adolescents, above and beyond basic demographic factors (i.e., to the extent that these could be controlled by covariates for age, gender, and years of education). In that context, patterns of increased activation suggestive of compensatory processing or increased effort to complete the task were not observed, but rather we observed potential disengagement or decreased neural efficiency, such that alcohol use may confer additional risk for poorer behavioral performance or lack of inhibition among these high-risk adolescents (e.g., Feldstein Ewing et al., 2014). In other words, for high-risk youth, it is important to note that alcohol use during adolescence may carry a specific neural risk even in the context of short-term use. This is an area that clearly needs further examination to prospectively determine the relationship between timing of substance use onset, volume of substance use exposure, and related potential brain and cognitive impact.
Interestingly, in light of ongoing questions surrounding the impact of marijuana on the developing brain (Filbey et al., 2014; Gilman et al., 2014; Weiland et al., 2015), marijuana use was not associated with activation during the Stroop task in the current study. Previous studies have found increased activation during reward anticipation among adolescent heavy marijuana users (Jager et al., 2013), as well as differences in functional activation patterns between marijuana users and controls during working memory tasks (Schweinsburg et al., 2005, 2010). Neural activity of response inhibition during Stroop and Go/No-Go paradigms has been associated with longer-term heavy marijuana use (e.g., Tapert et al., 2007; Hatchard et al., 2014; Kober et al., 2014), and the current study utilized self-reported marijuana use over the past 3 months. It is therefore possible that recent marijuana exposure does not have the same degree of neural impact as longer-term marijuana use or alcohol use in functional response or developing brain areas (e.g., Weiland et al., 2015). Alternately, it might be the case that the adolescent brain is quite plastic, and thus, is able to recover and/or compensate following exposure to marijuana use in particular (Lisdahl et al., 2013). It will continue to be important to explore this issue given the prevalence of marijuana use among adolescents.
4.2. Limitations
A number of potential limitations should be considered when interpreting these results. The reported data are cross-sectional, which prevents consideration of whether any observed neural activation differences are indicative of pre-existing risk factors for heavier substance use or results of substance use. These adolescents have already come into contact with the juvenile justice system and represent increased general behavioral risk, and it is therefore difficult to parse the specific contributions of substance use to neural activation beyond inclusion of covariates. In particular, participants were not specifically excluded on the basis of any psychiatric disorders including Attention Deficit Hyperactivity Disorder (ADHD), but rather only if they indicated psychotropic medication use suggestive of greater severity of psychopathology. Similarly, limited information was available for recent or lifetime drug use other than alcohol or marijuana, and participants reporting recent hard drug use were excluded so that other drug use could not be covaried in the models. It is important to note that these inclusion/exclusion criteria are less stringent than many of the cited studies on adolescent substance use, although we believe that this improves generalizability of these results across high-risk youth. Finally, substance use data relied on self-report as opposed to an interview format. Previous studies have documented the validity of self-reporting substance use among adolescents (Marlatt et al., 1998; Clark and Winters, 2002), but greater detail could likely be achieved via interview.
4.3. Conclusions
At this time, there are over 31 million adolescents under the jurisdiction of the juvenile court system (Hockenberry and Puzzanchera, 2014), and these young people have an established higher rate of substance use and related consequences (e.g., Sarver et al., 2014). Thus, it is exactly this type of adolescent for whom there is greater need to understand patterns of use to guide prevention and intervention program development. The ability to inhibit prepotent responses has been shown to predict later response to treatment in terms of successful reduction of substance use (Krishnan-Sarin et al., 2013; Kober et al., 2014), and early interventions to encourage further development of cognitive control (i.e., or provide cognitive rehabilitation for those with greater severity of substance use) could represent promising options for treatment.
Highlights.
We examined fMRI activation during a Stroop task among high-risk, diverse youth.
Recent alcohol use was negatively associated with activation but not performance.
Recent marijuana use was not associated with activation or performance.
Even short-term alcohol use during adolescence may carry specific neural risk.
Acknowledgments
This research was supported by 1R01NR013332-01 to SWFE and ADB. Portions of this paper were presented as a poster at the 2014 Flux Congress in Hollywood, CA. SWFE and ADB designed research; RET, ABD, ARM, and JML designed and performed analyses; RET, ABD, and NSH wrote the paper and revisions; SWFE and ADB edited the paper and revisions.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL. A developmental fMRI study of the Stroop color-word task. NeuroImage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Mackiewicz Seghete KL, Claus ED, Burgess GC, Ruzic L, Banich MT. Cognitive control in adolescence: Neural underpinnings and relation to self-report behaviors. PLoS One. 2011;6:e21598. doi: 10.1371/journal.pone.0021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT, Crowley TJ, Thompson LL, Jacobson BL, Liu X, Raymond KM, Claus ED. Brain activation during the Stroop task in adolescents with severe substance and conduct problems: A pilot study. Drug and Alcohol Dependence. 2007;90:175–182. doi: 10.1016/j.drugalcdep.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Tapert SF. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychology Review. 2010;20:398–413. doi: 10.1007/s11065-010-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology. 2015;66:295–319. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Clark DB, Winters KC. Measuring risks and outcomes in substance use disorders prevention research. Journal of Consulting and Clinical Psychology. 2002;70:1207–1223. doi: 10.1037//0022-006x.70.6.1207. [DOI] [PubMed] [Google Scholar]
- Crawford AM, Pentz MA, Chou CP, Li C, Dwyer JH. Parallel developmental trajectories of sensation seeking and regular substance use in adolescents. Psychology of Addictive Behaviors. 2003;17:179–192. doi: 10.1037/0893-164X.17.3.179. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Houck JM, Bryan AD. Neural activation during response inhibition is associated with adolescents’ frequency of risky sex and substance use. Addictive Behaviors. 2015;44:80–87. doi: 10.1016/j.addbeh.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Sakhardande A, Blakemore SJ. The effect of alcohol consumption on the adolescent brain: A systematic review of MRI and fMRI studies of alcohol-using youth. Neuroimage: Clinical. 2014;5:420–437. doi: 10.1016/j.nicl.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Venner KL, Mead HK, Bryan AD. Exploring racial/ethnic differences in substance use: A preliminary theory-based investigation with juvenile justice-involved youth. BioMed Central Pediatrics. 2011;11:71. doi: 10.1186/1471-2431-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, Segall J. Long-term effects of marijuana use on the brain. Proceedings of the National Academy of Sciences USA. 2014;111:16913–16918. doi: 10.1073/pnas.1415297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan G, Guevara A, Marxen M, Neumann M, Junger E, Kobiella A, Mennigen E, Pilhatsch M, Schwarz D, Zimmermann US, Smolka MN. Alcohol-induced impairment of inhibitory control is linked to attenuated brain responses in right fronto-temporal cortex. Biological Psychiatry. 2014;76:698–707. doi: 10.1016/j.biopsych.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Kuster JK, Lee S, Lee MJ, Kim BW, Makris N, van der Kouwe A, Blood AJ, Breiter HC. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. Journal of Neuroscience. 2014;34:5529–5538. doi: 10.1523/JNEUROSCI.4745-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchard T, Fried PA, Hogan MJ, Cameron I, Smith AM. Regular marijuana use impacts cognitive interference: An fMRI investigation in young adults using the Counting Stroop task. Journal of Addiction Research and Therapy. 2014;5:197–203. [Google Scholar]
- Hatchard T, Smith AM, Halchuk RE, Longo CA, Fried PA, Hogan MJ, Cameron I. Effects of low-level alcohol use on cognitive interference: An fMRI study in young adults. Alcohol. 2015;49:7–13. doi: 10.1016/j.alcohol.2014.07.020. [DOI] [PubMed] [Google Scholar]
- Hockenberry S, Puzzanchera C. Juvenile Court Statistics 2011. Pittsburgh: National Center for Juvenile Justice; 2014. [Google Scholar]
- Jager G, Block RI, Luijten M, Ramsey NF. Tentative evidence for striatal hyperactivity in adolescent cannabis-using boys: A cross-sectional multicenter fMRI study. Journal of Psychoactive Drugs. 2013;45:156–167. doi: 10.1080/02791072.2013.785837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use: 1975–2014: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan; 2015. [Google Scholar]
- Kober H, DeVito EE, DeLeone CM, Carroll KM, Potenza MN. Cannabis abstinence during treatment and one-year follow-up: Relationship to neural activity in men. Neuropsychopharmacology. 2014;39:2288–2298. doi: 10.1038/npp.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G, Smith AE, McMahon TJ, Cavallo DA, Schepis TS, Desai RA, Potenza MN, Krishnan-Sarin S. Pubertal status, sensation-seeking, impulsivity, and substance use in high-school-aged boys and girls. Journal of Addiction Medicine. 2013;7:116–121. doi: 10.1097/ADM.0b013e31828230ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Balodis IM, Kober H, Worhunsky PD, Liss T, Xu J, Potenza MN. An exploratory pilot study of the relationship between neural correlates of cognitive control and reduction in cigarette use among treatment-seeking adolescent smokers. Psychology of Addictive Behaviors. 2013;27:526–532. doi: 10.1037/a0032479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Frontiers in Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Vergara HI, Colder CR, Hawk LW, Jr, Wieczorek WF, Eiden RD, Lengua LJ, Read JP. Reinforcement sensitivity theory and alcohol outcome expectancies in early adolescence. The American Journal of Drug and Alcohol Abuse. 2012;38:130–134. doi: 10.3109/00952990.2011.643973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Baer JS, Kivlahan DR, Dimeff LA, Larimer ME, Quigley LA, Somers JM, Williams E. Screening and brief intervention for high-risk college student drinkers: Results from a 2-year follow-up assessment. Journal of Consulting and Clinical Psychology. 1998;66:604–615. doi: 10.1037//0022-006x.66.4.604. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Teshiba TM, Franco AR, Ling J, Shane MS, Stephen JM, Jung RE. Modeling conflict and error in the medial frontal cortex. Human Brain Mapping. 2012;33:2843–2855. doi: 10.1002/hbm.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug and Alcohol Dependence. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo Y, Aguilar R, Molinuevo B, Torrubia R. Alcohol use as a behavioural sign of disinhibition: Evidence from JA Gray’s model of personality. Addictive Behaviors. 2007;32:2398–2403. doi: 10.1016/j.addbeh.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins R, Bryan A. Relationships between future orientation, impulsive sensation seeking, and the risk behavior of adjudicated adolescents. Journal of Adolescent Research. 2004;19:428–445. doi: 10.1177/0743558403258860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver DE, McCart MR, Sheidow AJ, Letourneau EJ. ADHD and risky sexual behavior in adolescents: conduct problems and substance use as mediators of risk. Journal of Child Psychology and Psychiatry. 2014;55:1345–1353. doi: 10.1111/jcpp.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana. Drug and Alcohol Dependence. 2005;79:201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Medina KL, McQueeny T, Brown SA, Tapert SF. The influence of recency of use on fMRI response during spatial working memory in adolescent marijuana users. Journal of Psychoactive Drugs. 2010;42:401–412. doi: 10.1080/02791072.2010.10400703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Rogowska J, McCaffrey A, Yurgelun-Todd DA. Adolescents at risk for alcohol abuse demonstrate altered frontal lobe activation during Stroop performance. Alcoholism: Clinical and Experimental Research. 2011;35:218–228. doi: 10.1111/j.1530-0277.2010.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical; New York: 1988. [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrubia R, Avila C, Moltó J, Caseras X. The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Personality and Individual Differences. 2001;31:837–862. [Google Scholar]
- Weiland BJ, Thayer RE, Depue BE, Sabbineni A, Bryan AD, Hutchison KE. Daily marijuana use is not associated with brain morphometric measures in adolescents or adults. Journal of Neuroscience. 2015;35:1505–1512. doi: 10.1523/JNEUROSCI.2946-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HR, Labouvie EW. Towards the assessment of adolescent problem drinking. Journal of Studies on Alcohol. 1989;50:30–37. doi: 10.15288/jsa.1989.50.30. [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Mayer AR, Bogenschutz MP, Ling J, Dekonenko C, Cumbo H. Cognitive control network function in alcohol use disorder before and during treatment with Lorazepam. Substance Use and Misuse. 2015;50:40–52. doi: 10.3109/10826084.2014.957771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Zuckerman-Kuhlman Personality Questionnaire (ZKPQ): An alternative five-factorial model. In: De Raad B, Perugini M, editors. Big Five Assessment. Hogrefe and Huber; Cambridge, Maryland: 2002. pp. 377–397. [Google Scholar]


