Abstract
Merlin, the protein encoded by the NF2 gene, is a member of the band 4.1 familly of cytoskeleton-associated proteins and functions as a tumor suppressor for many types of cancer. However, the roles and mechanism of Merlin expression in pancreatic cancer have remained unclear. In this study, we sought to determine the impact of Merlin expression on pancreatic cancer development and progression using human tissue specimens, cell lines, and animal models. Decreased expression of Merlin was pronounced in human pancreatic tumors and cancer cell lines. Functional analysis revealed that restored expression of Merlin inhibited pancreatic tumor growth and metastasis in vitro and in vivo. Furthermore, Merlin suppressed the expression of Wnt/β-catenin signaling downstream genes and the nuclear expression of β-catenin protein, and overexpression of Forkhead box M1 (FOXM1) attenuated the suppressive effect of Merlin on Wnt/β-catenin signaling. Mechanistically, Merlin decreased the stability of FOXM1 protein, which plays critical roles in nuclear translocation of β-catenin. Collectively, these findings demonstrated that Merlin critically regulated pancreatic cancer pathogenesis by suppressing FOXM1/β-catenin signaling, suggesting that targeting novel Merlin/FOXM1/β-catenin signaling is an effective therapeutic strategy for pancreatic cancer.
Keywords: FOXM1, Merlin, metastasis, growth, pancreatic cancer
Introduction
Pancreatic ductal adenocarcinoma (PDA) has a dismal prognosis and is the seventh leading cause of cancer-related deaths (1). Its incidence is increasing annually, and in the United States, researchers estimated that 46,420 new PDA cases would be diagnosed and 39,590 patients would die of it in 2014 (2). Despite improvements in early diagnosis and treatment, more than 80% of PDA cases are diagnosed at late stages, and the 5-year survival rate for PDA is less than 5% (3). Thus, identification of the molecular mechanisms underlying PDA development and progression is urgently needed.
Merlin, the protein encoded by the NF2 gene, is a member of the band 4.1 families of cytoskeleton-associated proteins, which link the integral membrane proteins with the actin cytoskeleton (4,5). Investigators first identified Merlin as being associated with neurofibromatosis type 2 and that it functions as a tumor suppressor (6). A number of studies demonstrated that Merlin is a versatile tumor suppressor that can inhibit cancer cell proliferation and motility by modulating a wide range of signaling pathways (7). Furthermore, mutation of the NF2 gene and loss of Merlin protein occur in many different types of cancer, suggesting a general tumor-suppressive role for Merlin (8–13). Evidently, Merlin shuttles between the cell cortex and the nucleus and that both cortical Merlin and nuclear Merlin have been implicated in tumor suppression via interaction with angiomotin and CRL4-DCAF1, respectively (11,13). In a study of Ezrin, another member of the band 4.1 protein superfamily, overexpression of Merlin inhibited SW1990 PDA cell proliferation, migration, and adhesion (14). However, the roles and mechanism of Merlin expression in PDA development and progression have remained unclear.
Wnt/β-catenin is one of the most important signaling pathways in PDA development and progression (15). Briefly, this pathway is initiated by binding of Wnt ligands to receptors of the Frizzled family and co-receptors on the cell surface. With ligand binding, the cytoplasmic degradation complex, which consists of Axin, adenomatous polyposis coli, glycogen synthase kinase-3β, and casein kinase 1, is inhibited, leading to nuclear localization of β-catenin. In the nucleus, β-catenin binds to T-cell factor/lymphoid enhancer factor to activate Wnt downstream target genes, including cyclin D1, matrix metalloproteinase 2 (MMP2), MMP9, and vascular endothelial growth factor (VEGF). Although studies of mouse models demonstrated that activation of Wnt/β-catenin signaling was insufficient to initiate PDA, this pathway played key roles in PDA stem cell maintenance, progression, and drug resistance (15,16). Recent studies indicated that Merlin suppressed the Wnt/β-catenin signaling pathway (9,17,18); however, the molecular mechanism of this suppression and the relevance of the interaction between Merlin and catenin remains to be elucidated.
Forkhead box M1 (FOXM1) is a transcription factor in the FOX superfamily characterized by a conserved winged helix DNA-binding domain (19). FOXM1 is a key regulator of cell-cycle progression, and series of studies suggested that it plays critical roles in PDA growth, angiogenesis, invasion, and metastasis (20,21). Recently, we found that FOXM1 bound directly to β-catenin and had a key role in mediation of nuclear accumulation of β-catenin and downstream target gene expression (22,23).
In the present study, we sought to determine the roles and mechanism of Merlin expression in PDA growth and metastasis. We discovered that expression of Merlin was suppressed in PDA cells and that restored expression of Merlin inhibited PDA cell growth and metastasis in vitro and in vivo. Further studies demonstrated that re-expression of Merlin decreased the nuclear translocation of β-catenin by inducing instability of FOXM1 protein.
Materials and Methods
Cell culture and treatment
Human embryonic kidney 293 (HEK293) cells and the human PDA cell lines PANC-1, MiaPaCa-2, AsPC-1, BxPC-3, CaPan-1, CaPan-2, and PA-TU-8902 were purchased from the American Type Culture Collection. The PDA cell line MDA Panc-28 was a gift from Dr. Paul J. Chiao (The University of Texas MD Anderson Cancer Center, Houston, TX). The human PDA cell line FG was obtained from Michael P. Vezeridis (Brown Medical School, Providence, RI) (24). The human metastatic PDA cell line COLO357 and its fast-growing liver-metastatic variant in nude mice, L3.7, were described previously (20). All of these cell lines were maintained in plastic flasks as adherent monolayers in Eagle's minimal essential medium supplemented with 10% fetal bovine serum, sodium pyruvate, nonessential amino acids, L-glutamine, and a vitamin solution (Flow Laboratories). Immortalized human pancreatic ductal epithelial cells (provided by Dr. M.S. Tsao, Ontario Cancer Institute, Toronto, Ontario, Canada) were maintained in a keratinocyte serum-free medium supplemented with epidermal growth factor and bovine pituitary extract (Invitrogen). The cell lines obtained directly from the American Type Culture Collection were subjected to cell-line characterization or authentication via short tandem repeat profiling and passaged in our laboratory for fewer than 6 months after receipt. The following drugs were used in DNA methylation experiments: 5-aza-2'-deoxycytidine (5-aza), MG132, and cycloheximide (CHX) (Sigma-Aldrich). PDA cell lines were treated with 5-aza at 5 μM for 72 hours, and total RNAs were extracted from the cells and analyzed using quantitative real-time reverse transcription (RT)-PCR.
Human PDA specimens and immunohistochemical analysis
Tissue microarray (TMA) construction and immunohistochemical analysis were conducted with anti-Merlin and anti-FOXM1 antibodies (Santa Cruz Biotechnology) as described previously (25). The use of human specimens was approved by the relevant institutional review boards. The staining results were scored by two investigators blinded to the clinical data as described previously (26).
Plasmids and small interfering RNAs
The plasmids pcDNA3.1-FOXM1B (pFOXM1), Flag-tagged FOXM1B (Flag-FOXM1), and a control vector were described previously (22,27). The plasmid pcDNA3.0-Flag-tagged Merlin was the full-length Merlin isoform I and was obtained from Addgene. Full-length Merlin was cloned into the pcDNA3.0 (pMerlin) and pcDNA3.0-hemagglutinin (HA; HA-Merlin) vectors as a BamHI-EcoRI fragment. Mutated Merlin-S518A was generated via overlapping polymerase chain reaction (PCR) and inserted into pcDNA3.0 (pM-S518A) and pcDNA3.0-HA (HA-M-S518A) vectors (amplified via two-step PCR using the following primers: 5'-atgcggatccatggccggggccatcgcttcccg-3' and 5'-ctttttctttctctatctccatggcaagccgcttcatgtcagtatctttg-3' plus 5'-caaagatactgacatgaagcggcttgccatggagatagagaaagaaaaag-3' and 5'-atgcgatatcctagagctcttcaaagaaggccact-3' for the first reaction and 5'-atgcggatccatggccggggccatcgcttcccg-3' and 5'-atgcgatatcctagagctcttcaaagaaggccact-3' for the second reaction). Mutated Merlin-S518D was obtained using the same method (primers: 5'-atgcggatccatggccggggccatcgcttcccg-3' and 5'-ctttttctttctctatctccatgtcaagccgcttcatgtcagtatctttg-3' plus 5'-caaagatactgacatgaagcggcttgacatggagatagagaaagaaaaag-3' and 5'-atgcgatatcctagagctcttcaaagaaggccact-3' for the first reaction and 5'-atgcggatccatggccggggccatcgcttcccg-3' and 5'-atgcgatatcctagagctcttcaaagaaggccact-3' for the second reaction). An HA-ubiquitin vector described previously was used as a control (28). Full-length Merlin was cloned into the retroviral expression vector pBABEpuro as a BamHI-EcoRI fragment (pBABE-Merlin). Retroviruses were produced by transfecting packaging cells (HEK293) with a three-plasmid system consisting of the empty pBABEpuro vector or pBABE-Merlin, the packaging plasmid pUMVC, and the envelope plasmid pCMV-VSV-G. pUMVC and pCMV-VSVG were obtained from Addgene. Retroviruses were frozen at −20°C or −80 for long-term storage. Each amplified DNA fragment was verified by sequencing the inserts and flanking regions of the plasmids. Small interfering RNA #1 (siRNA#1) targeting Merlin (siMerlin), which was obtained from Cell Signaling Technology, was a pool of siRNAs for the NF2 gene (sense strand: 5'-ccuguaaauuuguucuuua-3'; antisense strand: 5'-uaaagaacaaauuuacagg-3'). SiRNA#2 targeting Merlin was designed based on the sequences specific to human NF2 gene cDNA (sense strand: 5'-ggacaagaagguacuggaucaugau-3'; antisense strand: 5'-aucaugauccaguaccuucuugucc-3'), which was reported previously (29). SiRNA targeting p21-activated kinase 1 (PAK1) was designed based on the sequences specific to human PAK1 gene cDNA (sense strand: 5'-cucacacccgcuaggauucuucuuu-3'; antisense strand: 5'-aaagaagaauccuagcgggugugag-3'), which was reported previously (30). SiRNA targeting FOXM1 (siFOXM1) was described previously (25).
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR analysis of the expression of the Merlin, FOXM1, and β-catenin genes was performed using total RNA and the SYBR Green reagent with an ABI Prism 7000HT sequence detection system (Applied Biosystems) (28). The sequences of the PCR primers were as follows: Merlin, 5'-cggtgtccttgatcgtgtactg-3' (forward) and 5'-tcaattgcgagatgaagtggaa-3' (reverse); FOXM1, 5'-acgtccccaagccaggctc-3' (forward) and 5'-ctactgtagctcaggaataa-3' (reverse); β-catenin, 5'-tgttcgtgcacatcaggatacc-3' (forward) and 5'-acatcccgagctaggatgtgaag-3' (reverse); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5'-acagtccatgccatcactgcc-3' (forward) and 5'-gcctgcttcaccaccttcttg-3' (reverse).
Methylation-specific PCR
Methylation-specific PCR was performed using genomic DNA from the tumor cells, which was modified with bisulfate using an EZ DNA Methylation Kit according to the manufacturer's instructions. For detection of unmethylated DNA, the forward primer was 5'-agttattttaaaggaggtgggatgg-3', and the reverse primer was 5'-aaacaaaacccctaaacaacaa-3'. For detection of methylated DNA, the forward primer was 5'-gagttattttaaaggaggcgggac-3', and the reverse primer was 5'-gaaacccctaaacgacaacgac-3'. The primers were designed using the MethPrimer software program as described previously (31). The negative control was water. The positive control was genomic DNA that was methylated using the CpG methylase M.Sss I (New England Biolabs). The PCR conditions consisted of an initial 5 minutes at 95°C; 40 cycles of 94°C for 25 seconds, 58°C for 25 seconds, and 72°C for 30 seconds; and a final extension step at 72°C for 10 minutes. The PCR products were confirmed using 2% agarose gel electrophoresis and ethidium bromide staining.
Gene transfection
For retroviral transduction, PDA cells were plated into six-well plate 24 hours prior to viral infection (approximately 50% confluent) and were infected with a mixture of retroviruses and hexadimethrine bromide (Polybrene; 5 μg/mL), and stable populations of the cells were obtained via selection with 2 μg/mL puromycin. For transient transfection, cells were transfected with plasmids or siRNA for 48 hours before functional assays using Lipofectamine LTX and Lipofectamine 2000 CD (Invitrogen), respectively. PDA cells treated with the transfection reagents alone were included as mock controls.
Western blot analysis
Total cell lysates, cytoplasmic and nuclear protein fractions were extracted as described previously (20). Standard Western blotting was carried out using primary anti-Merlin, anti-FOXM1, -β-catenin, -cyclin D1, -c-Myc, -μPAR, -MMP9, and -VEGF (rabbit; Santa Cruz Biotechnology), and anti-MMP2 (rabbit; Cell Signaling Technology) antibodies. Equal protein-sample loading was monitored using an anti-GAPDH antibody for total cell protein lysates (rabbit; Santa Cruz Biotechnology), an anti-α-tubulin antibody for cytoplasmic fractions (mouse; Oncogene), or an anti-histone H1 antibody for nuclear fractions (mouse; Santa Cruz Biotechnology). Secondary antibodies were anti-mouse IgG or anti-rabbit IgG (Santa Cruz Biotechnology). The bands were quantified using the Quantity One analysis software program (version 4.6; Bio-Rad).
Pulse-chase analysis
Pulse-chase experiments were performed as reported previously (32). Briefly, BxPC-3 cells were co-transfected with pFOXM1B and a control vector or pMerlin, and PANC-1 cells were co-transfected with pFOXM1 and siMerlin or a control vector 24 hours before being subjected to treatment with CHX at a final concentration of 100 μg/mL for indicated times. Protein extracts were prepared and analyzed via Western blotting for FOXM1 expression.
Immunoprecipitation and ubiquitination assays
HEK293 cells were co-transfected with HA-ubiquitin, Flag-FOXM1, HA-Merlin, or HA-M-S518A/D as indicated. Thirty hours after transfection, the cells were treated with 10μM MG132, a proteasome inhibitor for 6 hours and then harvested in IP lysis buffer (Thermo Scientific). Cell lysates were incubated with prewashed Flag affinity beads (Sigma) overnight at 4°C. Immunoprecipitates of the lysates were washed four times with the lysis buffer, boiled for 5 minutes in a protein loading buffer, and analyzed using Western blotting. Ubiquitinated FOXM1 was detected using an HA-tagged antibody (rabbit; Cell Signaling Technology).
Colony-formation assay
Two hundred cells from each group as indicated were plated in 24-well plates and allowed to grow for 14 days in culture medium; the medium was changed twice a week. Cells were then fixed with 4% paraformaldehyde and stained with 0.1% crystal violet solution for 10 minutes. Colonies (>20 cells) were counted using an inverted microscope at 40× magnification. The results were calculated as the percentage of proper control. All experiments were performed in triplicate and repeated twice.
Tumor-cell migration/ invasion assay
Both cell scratch-wound assay and modified Boyden chambers assay were performed to determine the migrating and invading ability of PDA cells with altered Merlin expression as described previously (33).
Animal experiments
Female pathogen-free athymic nude mice were purchased from the National Cancer Institute. The animals were maintained in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International in accordance with the current regulations and standards of the U.S. Department of Agriculture and Department of Health and Human Services. To analyze tumorigenicity, pancreatic cancer cells (1×106) in 0.1 mL of Hank's balanced salt solution were injected subcutaneously into the right scapular region of nude mice. The tumor-bearing mice were killed when they became moribund or on day 35 after inoculation and their tumors were removed and weighed.
Statistical analysis
The significance of the patient specimen data was determined using the Pearson correlation coefficient. The two-tailed χ2 test or Fisher exact test was used to determine the significance of the differences among the covariates. All the in vitro and in vivo experiments were repeated 2 to 4 times or as indicated, and the data from independent experiments were shown as mean ± SEM or as indicated otherwise, and the significance of the data was determined using the Student t-test (two-tailed) or one-way analysis of variance. In all of the tests, P values less than 0.05 were considered statistically significant. The SPSS software program (version 13.0; IBM Corporation) was used for statistical analysis.
Results
Reduced expression of Merlin directly associated with pathologic features in PDA
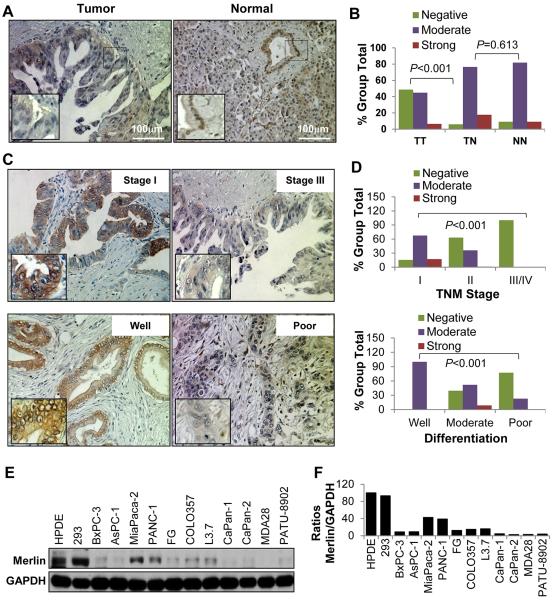
To determine the role of Merlin expression in PDA pathogenesis, we first investigated Merlin protein expression in the 154 primary PDA specimens, 34 tumor-adjacent tissue specimens, and 22 normal pancreatic tissue specimens in the TMA described previously (25). The clinicopathologic characteristics of the TMA specimens are listed in Supplementary Table S1. We observed Merlin-positive staining in the cytoplasm and nuclei (Supplementary Fig. S1) and that the expression of Merlin in the tumor-adjacent and normal pancreatic tissue specimens was much higher than that in the tumors (Fig. 1A and 1B). Merlin expression was negatively correlated with disease pT classification (P<0.001), regional lymph node metastasis (P=0.021), TNM stage (P<0.001), and tumor differentiation (P<0.001); and cytosolic Merlin predicted advanced grade as well as loss of Merlin did (Supplementary Table S2 and S3; Fig. 1C and 1D). Consistently, Merlin protein expression was markedly lower in most of the pancreatic cancer cell lines than that in human pancreatic ductal epithelial and HEK293 cells (Fig. 1E and 1F). These data indicated that Merlin may be a tumor suppressor for PDA and that decreased expression of Merlin may promote PDA development and progression.
Figure 1.
Expression of Merlin in and its association with clinicopathologic features of PDA. TMA PDA specimens were immunostained with a specific anti-Merlin antibody. A, representative images of Merlin expression in PDA specimens. Adjacent normal pancreatic tissue specimens are also shown. B, shown was markedly lower Merlin expression in tumors (TT) than in adjacent normal tissue (TN) and normal tissue (NN) and no difference in Merlin expression between adjacent normal and normal tissue specimens. C and D, negative association of the expression of Merlin with TNM stage (upper panels) and tumor differentiation (lower panels). E, Western blot analysis of the expression of Merlin in PDA cell lines. F, quantitative results of the Western blot analysis obtained via densitometric analysis, standardized according to GAPDH expression, and expressed as the percentage of HPDE.
The frequently reduced expression of Merlin in PDA cells suggested a higher rate of NF2 gene inactivation than predicted from mutation analysis, because only about 10% of the PDA cases had heterozygous losses or inactivating mutations at the NF2 locus in The Cancer Genome Atlas data set. Previous studies demonstrated that hypermethylation of the CpG islands in the promoter region of the NF2 gene played critical roles in decreased expression of Merlin (34,35). Indeed, the promoter region of NF2 contains typical CpG islands (Supplementary Fig. S2A). Methylation-specific PCR revealed that AsPC-1, CaPan-1, and PA-TU-8902 cells exhibited hypermethylation in the promoter region of NF2 (Supplementary Fig. S2B). Furthermore, we incubated 8 PDA cell lines, which had relatively low expression levels of Merlin, in a medium alone or containing the demethylating agent 5-azacytidine for 72 hours and analyzed the NF2 mRNA expression in them using quantitative PCR. As shown in Supplementary Fig. S2C, exposure to 5-azacytidine produced higher NF2 mRNA expression in AsPC-1 and PA-TU-8902 cells than in their control cells. Therefore, promoter hypermethylation may contribute to reduced Merlin expression in a subset of PDA cases.
Merlin suppressed PDA cell growth, migration, and invasion in vitro, and tumorigenicity and metastasis in vivo
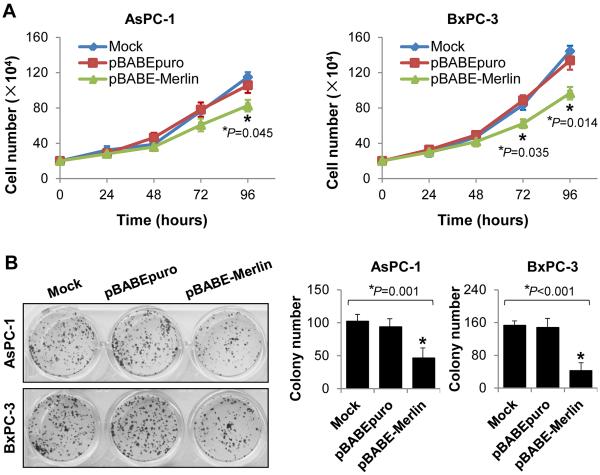
To assess the impact of Merlin expression on PDA biology, we infected AsPC-1, BxPC-3, and FG cells, which have very low or intermediate levels of endogenous Merlin expression, with retroviruses containing Merlin (AsPC-1/BxPC-3/FG-pBABE-Merlin) and empty retroviral expression vectors used as controls (AsPC-1/BxPC-3/FG-pBABEpuro). After selection of the infected cells with puromycin, we found the pooled drug-resistant cells to have high levels of Merlin expression and that the restored Merlin expression was still a little lower than the endogenous level of Merlin expression in HEK293 cells (Supplementary Fig. S3A). As shown in Fig. 2A and 2B, restored expression of Merlin significantly suppressed the growth of and colony formation by PDA cells (P<0.05).
Figure 2.
Merlin suppressed PDA cell growth in vitro. AsPC-1 and BxPC-3 cells were infected with retroviruses and stably overexpressed Merlin. Cell growth was assessed via cell counting at the indicated time points (A), colony formation assay was performed in 24-well plates and numbers of colonies were counted 14 days after retroviral infection (B). Data represented mean ± SEM of three independent experiments; *P values were indicated, as compared between “pBABE-Merlin” and “Mock” or “pBABEpuro” control.
Conversely, we decreased Merlin expression using specific siRNA against Merlin, and found that both siRNA#1 and siRNA#2 knocked down Merlin expression effectively (Supplementary Fig. S3B). In scratch-wound assay, incased Merlin expression decreased the flattening and spreading of BxPC-3 cells, whereas knockdown of Merlin expression promoted the flattening and spreading of PANC-1 cells (P<0.05). Similarly, increased Merlin expression suppressed the migration and invasion of BxPC-3 cells, whereas knockdown of Merlin expression attenuated the migration and invasion of PANC-1 cells (P<0.05) (Supplementary Fig. S4A and S4B).
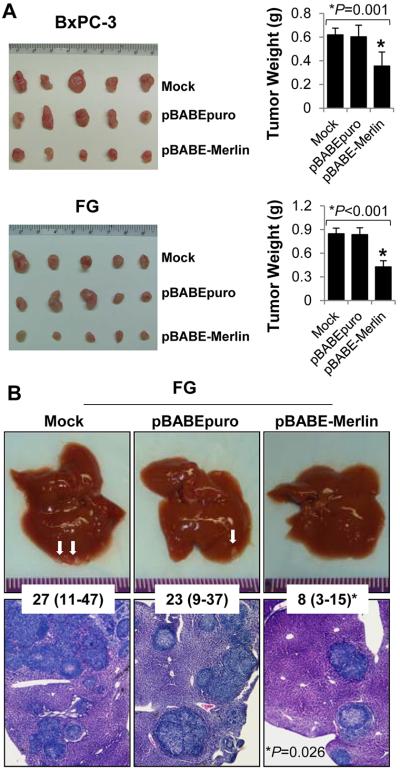
Next, we determined the effect of altered Merlin expression on PDA growth and metastasis in vivo. We found that increased expression of Merlin markedly suppressed tumor growth and experimental liver metastasis (P<0.05) (Fig. 3A and 3B). Collectively, these data clearly demonstrated that Merlin suppressed the growth, migration, and invasion of PDA cells in vitro, and PDA tumorigenicy and metastasis in vivo, supporting that Merlin might function as a tumor suppressor for PDA.
Figure 3.
Merlin Inhibited PDA growth and metastasis in vivo. A, BxPC-3 and FG cells with stable Merlin overexpression were injected subcutaneously into the right scapular region in nude mice (n=5). The resulting tumors were removed from the mice and weighted. Data represented mean ± SD of 5 mice per group. This experiment was repeated once with similar results. B, FG cells with stable Merlin overexpression were injected intravenously into the ileocolic vein in nude mice (n=5). Representative gross livers with metastasis and hematoxylin and eosin-stained liver metastasis sections obtained from the mice are shown. The numbers in the insert represent the median (range) numbers of liver metastases in each group. This experiment was repeated once with similar results. *P values were indicated, as compared between “pBABE-Merlin” and “Mock” control or “pBABEpuro” control.
Merlin regulated Wnt/β-catenin pathway in PDA cells
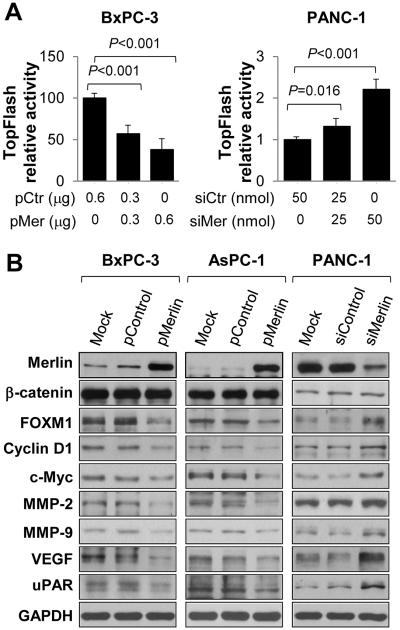
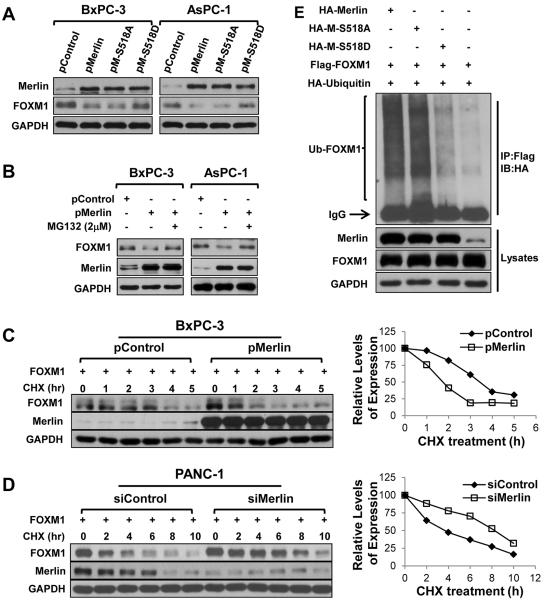
Wnt/β-catenin pathway is one of the most important signaling pathways in PDA development and progression (9,15,17,18). To further identify the molecular mechanisms underlying the tumor-suppressive function of Merlin in PDA cases, we initially focused on the impact of Merlin on Wnt/β-catenin signaling. We found that increased expression of Merlin in BxPC-3 cells drastically suppressed the activity of the TOP Flash reporter, a tool used to evaluate the transcriptional activity of β-catenin, and that knockdown of expression of Merlin in PANC-1 cells increased its activity (Fig. 4A). Previous studies demonstrated the existence of Merlin in both phosphorylation and de-phosphorylation, and that the requirement of de-phosphorylation for the tumor-suppressive activity of Merlin (10,36,37). Interconversion between the phosphorylation and de-phosphorylation forms of Merlin is regulated by PAK, which phosphorylates Merlin at Ser518 and maintains the protein in the inactive form (10,38). To investigate the effect of Merlin phosphorylation on β-catenin activity, we generated a Merlin-S518A expression vector, which cannot be phosphorylated by PAK, and a Merlin-S518D expression vector, which mimics the phosphorylated form of Merlin. As shown in Supplementary Fig.S6A, Merlin-S518A decreased TOP Flash reporter activity similar to that of wild-type Merlin, whereas Merlin-S518D did not significantly decrease the TOP Flash reporter activity (4,10).
Figure 4.
Merlin suppressed Wnt/β-catenin signaling in PDA cells. A, TOPFlash reporter activity assay. BxPC-3 cells were co-transfected with 0.2 μg of TOPFlash reporter and 0, 0.3, or 0.6μg of pMerlin (“pMer”) or a control pBABEpuro vector (“pCtr”). PANC-1 cells were co-transfected with 0.2μg of TOPFlash reporter and 0, 25, or 50nmol/L siMerlin (“siMer”) or control siRNA (“siCtr”). Promoter activity (Mean±SD) was examined using a dual luciferase assay kit. Data represented mean ± SEM of three independent experiments. *P values were indicated, as compared between “pBABE-Merlin” and “pBABEpuro” control, or between “siMerlin” and “control siRNA”. B, BxPC-3 and AsPC-1 cells were transfected with pMerlin or a control vector for 48 hours, and PANC-1 cells were transfected with siMerlin or a control siRNA for 48 hours. Total cell proteins were extracted and expression levels of Merlin and others were measured using Western blotting. This experiment was repeated once with similar results.
Further studies demonstrated that increased expression of Merlin downregulated the expression of the Wnt/β-catenin target genes cyclin D1, c-Myc, MMP2, MMP9, VEGF, and μPAR, which are the key genes related to cell proliferation, invasion, and metastasis, whereas knockdown of expression of Merlin led to elevated expression of these genes (Fig. 4B). Consistent with published studies, increased Merlin expression did not significantly reduce β-catenin protein or mRNA expression (Fig. 4B and Supplementary Fig. S5) (15,18). Interestingly, we observed that increased Merlin expression markedly downregulated FOXM1 protein expression and that knockdown of Merlin expression upregulated FOXM1 expression but did not affect FOXM1 mRNA expression (Fig. 4B and Supplementary Fig. S3B and S5). Given that the interaction between FOXM1 and β-catenin and its critical roles in PDA growth, angiogenesis, invasion, and metastasis (18–21), our data suggest that the suppressive role of Merlin involves in FOXM1/Wnt/β-catenin signaling axis.
Merlin Negatively regulated FOXM1 expression in PDA cells
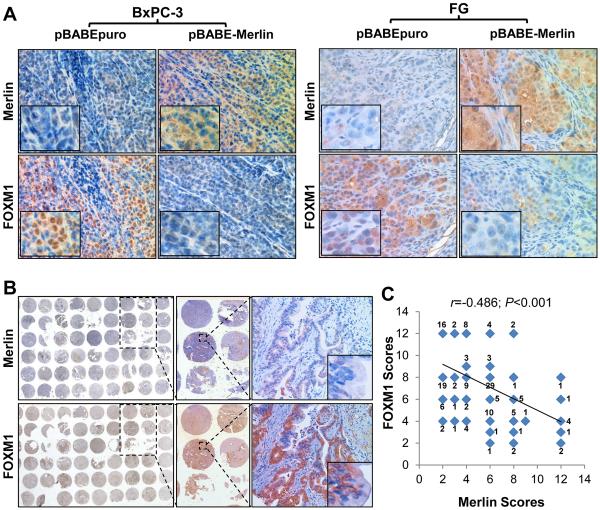
To confirm that Merlin negatively regulate FOXM1 expression in PDA cells, we further examined the effects of altered expression of Merlin on FOXM1 expression in various PDA cell lines. Clearly, increased Merlin expression reduced FOXM1 protein expression, whereas knockdown of Merlin expression led to upregulation of FOXM1 expression (Supplementary Fig. S6B). Merlin-S518A decreased FOXM1 expression similar to that of wild-type Merlin, whereas Merlin-S518D did not (Fig. 6A), confirming the role of Merlin phosphorylation in repressing the expression of FOXM1. Consistently increased expression of Merlin markedly decreased the expression of FOXM1 in xenograft tumors of BxPC-3 and FG cells (Fig. 5A). Furthermore, expression of FOXM1 was negatively associated with expression of Merlin at a statistically significant level (r=−0.486, P<0.001) (Fig. 5B and 5C) in the same cohort of human PDA TMA specimens (Fig. 1). Collectively, these experimental and clinical data strongly supported the notion that Merlin negatively regulated the expression of FOXM1 and that phosphorylation of Merlin by PAK abolished the suppressive effect of Merlin on FOXM1.
Figure 6.
Merlin promoted ubiquitination and degradation of FOXM1 in PDA cells. A, BxPC-3 and AsPC-1 cells were transfected with pMerlin, pMerlin-S518A, pMerlin-S518D, or a control vector. Total protein was harvested 48 hours after transfection to measure Merlin and FOXM1 expression using Western blotting. B, BxPC-3 and AsPC-1 cells transfected with pMerlin or a control vector were treated with or without MG132. Merlin and FOXM1 protein expression was determined using Western blotting. C, BxPC-3 cells co-transfected with pFOXM1 and pMerlin or pControl were treated with or without CHX for the indicated times. Total cell lysates were used to measure FOXM1 using Western blotting. Semiquantification was performed using GAPDH as a loading control and relative FOXM1 expression levels at time 0 was set as 100%. D, PANC-1 cells co-transfected with pFOXM1 and siMerlin or siControl were treated with or without CHX for the indicated times. The FOXM1 was determined as described above. E, HEK293 cells co-transfected with Flag-FOXM1, HA-ubiquitin, and HA-Merlin/HA-Merlin-S518A/HA-Merlin-S518D/Control were lysed in IP lysis buffer. Overexpressed Flag-FOXM1 protein was captured using Flag affinity beads and immunoblot analysis with an HA-tag antibody. All experiments were performed two times with similar results.
Figure 5.
Merlin suppressed FOXM1 expression in vivo. A, subcutaneous tumor specimens were stained immunohistochemically with specific anti-Merlin and anti-FOXM1 antibodies. Shown are representatively photographs of the expression of Merlin and FOXM1 in BxPC-3 and FG xenograft tumros. B, Merlin and FOXM1 protein expression TMA tissue sections were represented from the cohort described in Figure 1. C, Negative correlation of Merlin expression with FOXM1 expression was assessed using Pearson correlation coefficient analysis (n=154, r=−0.486, P<0.001). Some of the dots on the graph represent more than one specimen and the numbers of overlapping scores were then labeled.
Merlin promoted ubiquitination and degradation of FOXM1
Because Merlin did not change FOXM1 mRNA expression, we determined whether Merlin affected FOXM1 protein stability. We observed that MG132, a typical proteasome inhibitor, attenuated the downregulating effect of Merlin on FOXM1 protein expression (Fig. 6B). To further confirm that Merlin decreased the stability of FOXM1 protein, we investigated the effect of Merlin expression on the FOXM1 protein half-lives using pulse-chase analyses. As shown in Fig. 6C and 6D, increased Merlin expression shortened the half-life of FOXM1 from 3.5 to 1.5 hours in BxPC-3 cells, whereas knockdown of Merlin expression extended the half-life of FOXM1 from 4 to 8 hours in PANC-1 cells. Next, in protein ubiquitination experiment, we found that ubiquitination of FOXM1 was increased in the cells with increased Merlin or Merlin-S518A expression, but not in that with Merlin-S518D (Fig. 6E). Therefore, Merlin is a negative regulator of FOXM1 expression, by enhancing FOXM1 ubiquitination and subsequent degradation.
FOXM1 mediated the negative regulatory effect of Merlin on the Wnt/β-catenin signaling
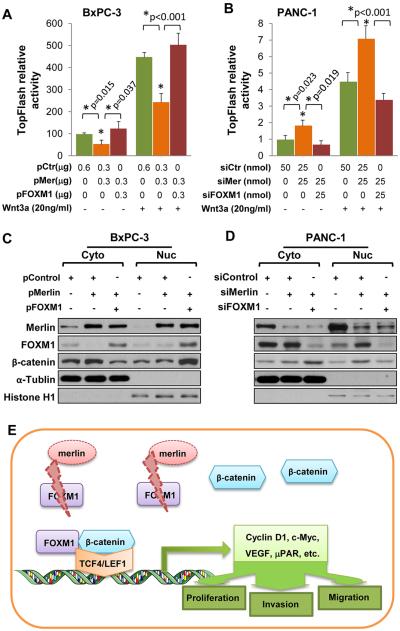
To determine the role of FOXM1 in mediation of the suppressive effect of Merlin on Wnt/β-catenin signaling, we reanalyzed the effect of Merlin on TOPFlash reporter activity with FOXM1 as a rescue. In BxPC-3 cells, restored expression of Merlin suppressed the activity of the TOPFlash reporter, whereas overexpression of FOXM1 attenuated this suppressive effect (Fig. 7A). Consistently, in PANC-1 cells, knockdown of FOXM1 and Merlin expression markedly suppressed the TOPFlash reporter activity, which was elevated by knockdown of Merlin expression (Fig. 7B). Furthermore, Wnt3a ligand treatment increased the regulatory effect of Merlin and Merlin plus FOXM1 on TOPFlash reporter activity (Fig. 7A and B).
Figure 7.
Merlin/FOXM1 signaling regulates Wnt/β-catenin pathway. A, the TOPFlash reporter was cotransfected into BxPC-3 cells with a control vector, pMerlin, or pMerlin plus pFOXM1 with or without Wnt3a treatment. The resulting promoter activity (Mean±SD of triplicates; *Student t test) was analyzed using a dual luciferase assay. Data represented mean ± SEM of three independent experiments. *P values were indicated, as compared between groups indicated. B, the TOPFlash reporter was cotransfected into PANC-1 cells with control siRNA, siMerlin, or siMerlin plus siFOXM1 with or without Wnt3a treatment, and the resulting promoter activity was analyzed. C and D, a control vector, pMerlin, or pMerlin plus pFOXM1 were transfected into BxPC-3 cells (C) and control siRNA, siMerlin, or siMerlin plus siFOXM1 were transfected into PANC-1 cells (D) for 48 hours. Cytosolic and nuclear proteins were extracted, and the levels of Merlin, FOXM1, and β-catenin protein expression were measured using Western blotting. These experiments were repeated once with similar results. E, Model of Merlin inactivating FOXM1/β-catenin signaling in PDA cells. Loss of Merlin stabilizes FOXM1 protein and subsequently enhances Wnt/β-catenin pathway, resulting in increased expression of Wnt downstream target genes and promoting PDA development and progression.
Moreover, subcellular protein fractionation study confirmed the presence of Merlin in the nucleus in positive lines; increased Merlin expression reduced both cytoplasmic and nuclear FOXM1 protein expression, and drastically reduced nuclear β-catenin expression (Fig. 7C), whereas knockdown of Merlin did the opposite (Fig. 7D). Consistent with the results of TOPFlash reporter activity assay, FOXM1 overexpression attenuated the suppressive effect of Merlin on nuclear translocation of β-catenin, and knockdown of FOXM1 reduced the nuclear expression of β-catenin, which was partially reversed by downregulation of Merlin expression (Fig. 7C and 7D). These data demonstrated that FOXM1 plays a critical role in mediation of the suppressive effect of Merlin expression on the Wnt/β-catenin pathway.
In addition to being phosphorylated by PAK, Merlin is an inhibitor of PAK activity, and knockdown of Merlin expression leads to increased PAK activation because of the presence of a negative feedback loop (38). Previous studies also demonstrated that Merlin activated Wnt/β-catenin signaling via the Rac/PAK pathway (18). We therefore examined the role of PAK1 in mediation of the suppressive function of Merlin on Wnt/β-catenin signaling. The results demonstrated that knockdown of Merlin expression in PANC-1 cells resulted in increased β-catenin transcriptional activity, whereas knockdown of PAK1 and Merlin expression rescued increased TOPFlash activity (Supplementary Fig. S6C). Collectively, these data demonstrated that both FOXM1 and PAK1 played important roles in mediation of the suppressive effect of Merlin expression on Wnt/β-catenin signaling in PDA cells.
Discussion
In the present study, we obtained five lines of experimental and clinical evidence supporting the tumor-suppressive role of Merlin by aberrant activation of FOXM1/β-catenin signaling in PDA development and progression. First, the expression of Merlin was markedly downregulated in PDA and negatively associated with disease pT classification, regional lymph node metastasis, TNM stage, and tumor differentiation; Merlin in the nucleus in vivo and that loss of nuclear staining predicted higher grade as well as loss of total staining. Second, restored expression of Merlin inhibited PDA cell growth, invasion, migration, and metastasis in vitro and in vivo. Third, expression of Merlin was markedly downregulated in PDA cells, and Merlin negatively regulated the expression of FOXM1 by promoting ubiquitination and degradation of FOXM1. Additionally, the expression of Merlin was inversely correlated with that of FOXM1 in PDA specimens. Fourth, increased Merlin expression reduced TOPFlash reporter activity and nuclear β-catenin and Wnt/β-catenin downstream target gene expression. Fifth, FOXM1 attenuated the suppressive effect of Merlin on the activity of the TOPFlash reporter and nuclear translocation of β-catenin. Collectively, these findings demonstrated novel Merlin/FOXM1/β-catenin signaling in PDA cells, which contributed to pancreatic tumorigenesis.
NF2 gene is first identified in 1993 and classified as a tumor suppressor for two reasons. First, an inactivating mutation of NF2 led to loss of the Merlin protein and may occur in schwannoma, meningioma, hamartoma, and ependymoma cases sporadically or as part of the autosomal dominant syndrome neurofibromatosis type 2 (7). Second, expression of Merlin was lost in many types of cancer, and modulated several signaling pathways that played critical roles in cancer cell proliferation, motility, and apoptosis (13). In PDA cells, Merlin expression was elevated during cell-cycle progression and mitotic arrest; furthermore, overexpression of Merlin inhibited SW1990 cell proliferation, migration, and adhesion in vitro (14,39). These findings indicated a tumor-suppressive role for Merlin expression in PDA. In the present study, we systematically analyzed the Merlin expression patterns in PDA cell lines and tumor specimens. The results revealed that expression of Merlin was downregulated in most of the cell lines. Additionally, statistical analysis of the relationships among Merlin expression and clinicohistopathologic PDA parameters demonstrated drastically decreased Merlin expression in PDA and that the relationships were closely correlated with the malignant features of PDA. In functional studies, our in vitro and in vivo experimental results revealed that Merlin overexpression suppressed PDA cell growth and metastasis, which was consistent with the role of Merlin in other types of cancer (9,11,40,41). Taken together, these clinical and experimental findings strongly and clearly supported Merlin as a tumor suppressor in PDA. Moreover, reduced expression of Merlin in nervous system tumors predominantly resulted from mutations, loss of heterozygosity, and hypermethylation of CpG islands in the promoter region of the NF2 gene (35). Also, in breast cancer, osteopontin-initiated, Akt-mediated phosphorylation of Merlin contributed to loss expression of Merlin (12,40,42–44). Because only 10% of PDA harbored heterozygous losses or mutations of the NF2 gene, we analyzed the role of hypermethylation in Merlin expression in PDA cell lines. We observed that hypermethylation contributed to reduced expression of Merlin in a subset of PDA cases. However, histone methylation may contribute little to the reduced expression of Merlin in pancreatic cancer (Supplementary Figure S7). Our results are consistent with the findings from prior reports (45,46).
Mechanisms underlying Merlin's suppressive function are unclear. The Wnt/β-catenin pathway is widely dysregulated in cancer, and it is one of the pivotal drivers of pancreatic tumorigenesis (15,47). Stabilization and nuclear translocation of β-catenin are the core events in persistent activation of this pathway. In colorectal cancer cases, high levels of nuclear β-catenin result from mutations in Wnt/β-catenin–associated signaling cascades (48). However, in PDA, mutations in the Wnt/β-catenin pathway are seldom detected, and β-catenin does not contain a nuclear localization sequence. Thus, cross-talk with other factors and epigenetic modulations play key roles in activation of Wnt/β-catenin signaling (15). In previous studies, we demonstrated that FOXM1 bound directly to β-catenin and facilitated nuclear translocation of it (3,22). Authors also recently reported that Merlin inhibited the Wnt/β-catenin pathway and suppressed downstream target gene expression (9,17,18,49). Mechanistically, Merlin regulated the formation of adherence junctions by directly binding with β-catenin and the E/N-cadherin complex at the plasma membrane. Additionally, Merlin decreased the number of Frizzled-1 receptors and reduced Wnt signaling. In the present study, we observed for the first time that Merlin suppressed TOPFlash reporter activity and Wnt downstream target gene expression in PDA cells. Furthermore, Merlin inhibited the nuclear translocation of β-catenin and activation of Wnt/β-catenin signaling via downregulation of FOXM1 protein expression in PDA cells, as overexpression of FOXM1 attenuated the suppressive effect of Merlin on Wnt/β-catenin signaling. This novel finding regarding Merlin/FOXM1/β-catenin signaling is critical to identifying the molecular mechanisms underlying PDA pathogenesis given that the Wnt/β-catenin pathway is one of the key drivers of PDA initiation and progression and that expression of Merlin is generally downregulated in PDA cells.
FOXM1 is a key regulator of tumor growth, angiogenesis, metastasis, and metabolism via transduction of upstream signals to downstream effectors (21,25). FOXM1 is overexpressed in PDA cells. However, the upstream regulators of FOXM1 expression are still unknown. A study of malignant mesothelioma demonstrated that the Hippo pathway transcriptional co-activator Yes-associated protein induced cell proliferation by transcriptionally upregulating the expression of FOXM1 (50). Furthermore, previous studies demonstrated that Merlin was an upstream regulator of the Hippo pathway and inhibited the activity of Yes-associated protein (13). However, in the present study, we found that Merlin did not regulate the expression of FOXM1 at the transcriptional level via Hippo signaling given that altered expression of Merlin did not change the FOXM1 mRNA expression. Instead, we found that Merlin regulated the expression of FOXM1 by promoting ubiquitination and degradation of FOXM1. Recently, authors reported that Merlin translocated to the nucleus and bound directly to the E3 ubiquitin ligase CRL4DCAF1. This binding inhibited the activity of CRL4DCAF1 and resulted in suppression of oncogenic gene expression and, finally, inhibition of tumorigenesis (11,13). Also, Merlin may indirectly regulate modification of FOXM1 protein, which decreases the stability of FOXM1. However, the detailed mechanisms underlying Merlin's regulation of FOXM1 protein stability warrant further elucidation.
In summary, the present study confirmed that Merlin is an important tumor suppressor in PDA and identified novel Merlin/FOXM1/β-catenin signaling in PDA pathogenesis. Given the importance of FOXM1 expression and the Wnt/β-catenin signaling pathway in PDA development and progression, our findings not only provide further understanding of the molecular mechanisms underlying FOXM1/β-catenin signaling activation but also identify aberrant Merlin/FOXM1/β-catenin signaling as a promising new therapeutic target for PDA.
Supplementary Material
Acknowledgments
We thank Don Norwood for editorial assistance and Xuemei Wang, Associate Director of Quantitative Research, for assistance in statistical analyses.
Financial Support: Supported by grants R01-CA129956, R01-CA148954, R01-CA152309, and R01-CA172233 from the National Cancer Institute, National Institutes of Health (to K. Xie); grant 81460461 (to S. Zheng); and grant 81120108017 from the National Natural Science Foundation of China (to Q. Huang).
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–17. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 4.Cooper J. Giancotti FG. Molecular insights into NF2/Merlin tumor suppressor function. FEBS Lett. 2014;588:2743–52. doi: 10.1016/j.febslet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W, Cooper J, Karajannis MA, Giancotti FG. Merlin: a tumour suppressor with functions at the cell cortex and in the nucleus. EMBO Rep. 2012;13:204–15. doi: 10.1038/embor.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;72:791–800. doi: 10.1016/0092-8674(93)90406-g. [DOI] [PubMed] [Google Scholar]
- 7.Pecina-Slaus N. Merlin, the NF2 gene product. Pathol Oncol Res. 2013;19:365–73. doi: 10.1007/s12253-013-9644-y. [DOI] [PubMed] [Google Scholar]
- 8.Morris ZS, McClatchey AI. Aberrant epithelial morphology and persistent epidermal growth factor receptor signaling in a mouse model of renal carcinoma. Proc Natl Acad Sci U S A. 2009;106:9767–72. doi: 10.1073/pnas.0902031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau YK, Murray LB, Houshmandi SS, Xu Y, Gutmann DH, Yu Q. Merlin is a potent inhibitor of glioma growth. Cancer Res. 2008;68:5733–42. doi: 10.1158/0008-5472.CAN-08-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sher I, Hanemann CO, Karplus PA, Bretscher A. The tumor suppressor merlin controls growth in its open state, and phosphorylation converts it to a less-active more-closed state. Dev Cell. 2012;22:703–5. doi: 10.1016/j.devcel.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, You L, Cooper J, Schiavon G, Pepe-Caprio A, Zhou L, et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 2010;140:477–90. doi: 10.1016/j.cell.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrow KA, Das S, Metge BJ, Ye K, Mulekar MS, Tucker JA, et al. Loss of tumor suppressor Merlin in advanced breast cancer is due to post-translational regulation. J Biol Chem. 2011;286:40376–85. doi: 10.1074/jbc.M111.250035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper J, Li W, You L, Schiavon G, Pepe-Caprio A, Zhou L, et al. Merlin/NF2 functions upstream of the nuclear E3 ubiquitin ligase CRL4DCAF1 to suppress oncogenic gene expression. Sci Signal. 2011;4:pt6. doi: 10.1126/scisignal.2002314. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Feng Y, Tao K, Su Z, Yu X, Zheng J, et al. The expression and phosphorylation of ezrin and merlin in human pancreatic cancer. Int J Oncol. 2014;44:2059–67. doi: 10.3892/ijo.2014.2381. [DOI] [PubMed] [Google Scholar]
- 15.Morris JPt, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010;10:683–95. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui J, Jiang W, Wang S, Wang L, Xie K. Role of Wnt/beta-catenin signaling in drug resistance of pancreatic cancer. Curr Pharm Des. 2012;18:2464–71. doi: 10.2174/13816128112092464. [DOI] [PubMed] [Google Scholar]
- 17.Bosco EE, Nakai Y, Hennigan RF, Ratner N, Zheng Y. NF2-deficient cells depend on the Rac1-canonical Wnt signaling pathway to promote the loss of contact inhibition of proliferation. Oncogene. 2010;29:2540–9. doi: 10.1038/onc.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L, Ercolano E, Ammoun S, Schmid MC, Barczyk MA, Hanemann CO. Merlin-deficient human tumors show loss of contact inhibition and activation of Wnt/beta-catenin signaling linked to the PDGFR/Src and Rac/PAK pathways. Neoplasia. 2011;13:1101–12. doi: 10.1593/neo.111060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark KL, Halay ED, Lai E, Burley SK. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364:412–20. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 20.Huang C, Qiu Z, Wang L, Peng Z, Jia Z, Logsdon CD, et al. A novel FoxM1-caveolin signaling pathway promotes pancreatic cancer invasion and metastasis. Cancer Res. 2012;72:655–65. doi: 10.1158/0008-5472.CAN-11-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C, Du J, Xie K. FOXM1 and its oncogenic signaling in pancreatic cancer pathogenesis. Biochim Biophys Acta. 2014;1845:104–16. doi: 10.1016/j.bbcan.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang N, Wei P, Gong A, Chiu WT, Lee HT, Colman H, et al. FoxM1 promotes beta-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell. 2011;20:427–42. doi: 10.1016/j.ccr.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Li Z, Kong X, Xie D, Jia Z, Jiang W, et al. Downregulation of MicroRNA 494 via Loss of SMAD4 Increases FOXM1 and betacatenin Signaling in Pancreatic Ductal Adenocarcinoma Cells. Gastroenterology. 2014;147:485–97. doi: 10.1053/j.gastro.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 24.Vezeridis MP, Tzanakakis GN, Meitner PA, Doremus CM, Tibbetts LM, Calabresi P. In vivo selection of a highly metastatic cell line from a human pancreatic carcinoma in the nude mouse. Cancer. 1992;69:2060–3. doi: 10.1002/1097-0142(19920415)69:8<2060::aid-cncr2820690810>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25.Cui J, Shi M, Xie D, Wei D, Jia Z, Zheng S, et al. FOXM1 Promotes the Warburg Effect and Pancreatic Cancer Progression via Transactivation of LDHA Expression. Clin Cancer Res. 2014;20:2595–606. doi: 10.1158/1078-0432.CCR-13-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Wei D, Huang S, Peng Z, Le X, Wu TT, et al. Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res. 2003;9:6371–80. [PubMed] [Google Scholar]
- 27.Liu M, Dai B, Kang SH, Ban K, Huang FJ, Lang FF, et al. FoxM1B is overexpressed in human glioblastomas and critically regulates the tumorigenicity of glioma cells. Cancer Res. 2006;66:3593–602. doi: 10.1158/0008-5472.CAN-05-2912. [DOI] [PubMed] [Google Scholar]
- 28.Xue J, Lin X, Chiu WT, Chen YH, Yu G, Liu M, et al. Sustained activation of SMAD3/SMAD4 by FOXM1 promotes TGF-beta-dependent cancer metastasis. J Clin Invest. 2014;124:564–79. doi: 10.1172/JCI71104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JY, Moon HJ, Lee WK, Chun HJ, Han CW, Jeon YW, et al. Merlin facilitates ubiquitination and degradation of transactivation-responsive RNA-binding protein. Oncogene. 2006;25:1143–52. doi: 10.1038/sj.onc.1209150. [DOI] [PubMed] [Google Scholar]
- 30.Zhu G, Wang Y, Huang B, Liang J, Ding Y, Xu A, et al. A Rac1/PAK1 cascade controls beta-catenin activation in colon cancer cells. Oncogene. 2012;31:1001–12. doi: 10.1038/onc.2011.294. [DOI] [PubMed] [Google Scholar]
- 31.Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–31. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- 32.Hwang YC, Yang CH, Lin CH, Ch'ang HJ, Chang VH, Yu WC. Destabilization of KLF10, a tumor suppressor, relies on thr93 phosphorylation and isomerase association. Biochim Biophys Acta. 2013;1833:3035–45. doi: 10.1016/j.bbamcr.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Khoo CP, Micklem K, Watt SM. A comparison of methods for quantifying angiogenesis in the Matrigel assay in vitro. Tissue Eng Part C Methods. 2011;17:895–906. doi: 10.1089/ten.tec.2011.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kino T, Takeshima H, Nakao M, Nishi T, Yamamoto K, Kimura T, et al. Identification of the cis-acting region in the NF2 gene promoter as a potential target for mutation and methylation-dependent silencing in schwannoma. Genes Cells. 2001;6:441–54. doi: 10.1046/j.1365-2443.2001.00432.x. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Gomez P, Bello MJ, Alonso ME, Lomas J, Arjona D, Campos JM, et al. CpG island methylation in sporadic and neurofibromatis type 2-associated schwannomas. Clin Cancer Res. 2003;9:5601–6. [PubMed] [Google Scholar]
- 36.Sherman L, Xu HM, Geist RT, Saporito-Irwin S, Howells N, Ponta H, et al. Interdomain binding mediates tumor growth suppression by the NF2 gene product. Oncogene. 1997;15:2505–9. doi: 10.1038/sj.onc.1201418. [DOI] [PubMed] [Google Scholar]
- 37.Sher I, Hanemann CO, Karplus PA, Bretscher A. The tumor suppressor merlin controls growth in its open state, and phosphorylation converts it to a less-active more-closed state. Dev Cell. 2012;22:703–5. doi: 10.1016/j.devcel.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T. Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell. 2003;12:841–9. doi: 10.1016/s1097-2765(03)00382-4. [DOI] [PubMed] [Google Scholar]
- 39.Kokkinakis DM, Liu X, Neuner RD. Modulation of cell cycle and gene expression in pancreatic tumor cell lines by methionine deprivation (methionine stress): implications to the therapy of pancreatic adenocarcinoma. Mol Cancer Ther. 2005;4:1338–48. doi: 10.1158/1535-7163.MCT-05-0141. [DOI] [PubMed] [Google Scholar]
- 40.James MF, Han S, Polizzano C, Plotkin SR, Manning BD, Stemmer-Rachamimov AO, et al. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009;29:4250–61. doi: 10.1128/MCB.01581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhan Y, Modi N, Stewart AM, Hieronimus RI, Liu J, Gutmann DH, et al. Regulation of mixed lineage kinase 3 is required for Neurofibromatosis-2-mediated growth suppression in human cancer. Oncogene. 2011;30:781–9. doi: 10.1038/onc.2010.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nunes F, Shen Y, Niida Y, Beauchamp R, Stemmer-Rachamimov AO, Ramesh V, et al. Inactivation patterns of NF2 and DAL-1/4.1B (EPB41L3) in sporadic meningioma. Cancer Genet Cytogenet. 2005;162:135–9. doi: 10.1016/j.cancergencyto.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Wallace AJ, Watson CJ, Oward E, Evans DG, Elles RG. Mutation scanning of the NF2 gene: an improved service based on meta-PCR/sequencing, dosage analysis, and loss of heterozygosity analysis. Genet Test. 2004;8:368–80. doi: 10.1089/gte.2004.8.368. [DOI] [PubMed] [Google Scholar]
- 44.Lasota J, Wasag B, Dansonka-Mieszkowska A, Karcz D, Millward CL, Rys J, et al. Evaluation of NF2 and NF1 tumor suppressor genes in distinctive gastrointestinal nerve sheath tumors traditionally diagnosed as benign schwannomas: s study of 20 cases. Lab Invest. 2003;83:1361–71. doi: 10.1097/01.lab.0000087591.29639.e3. [DOI] [PubMed] [Google Scholar]
- 45.Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 2006;20:1123–36. doi: 10.1101/gad.381706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tiwari N, Tiwari VK, Waldmeier L, Balwierz PJ, Arnold P, Pachkov M, et al. Sox4 is a master regulator of epithelial-mesenchymal transition by controlling Ezh2 expression and epigenetic reprogramming. Cancer Cell. 2013;23:768–83. doi: 10.1016/j.ccr.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 47.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 48.White BD, Chien AJ, Dawson DW. Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142:219–32. doi: 10.1053/j.gastro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lallemand D, Curto M, Saotome I, Giovannini M, McClatchey AI. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev. 2003;17:1090–100. doi: 10.1101/gad.1054603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mizuno T, Murakami H, Fujii M, Ishiguro F, Tanaka I, Kondo Y, et al. YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle-promoting genes. Oncogene. 2012;31:5117–22. doi: 10.1038/onc.2012.5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.