Abstract
Adults with psychotic disorders have dysconnectivity in critical brain networks including the default mode (DM) and the cingulo-opercular (CO) networks. However, it is unknown whether such deficits are present in youths with less severe symptoms. We conducted a multivariate connectome-wide association study (CWAS) examining dysconnectivity with resting state functional MRI in a population-based cohort of 188 youths ages 8–22 with psychosis-spectrum (PS) symptoms and 204 typically developing (TD) comparators. We found evidence for multi-focal dysconnectivity in PS youths, implicating the bilateral anterior cingulate, frontal pole, medial temporal lobe, opercular cortex, and right orbitofrontal cortex. Follow-up seed-based and network-level analyses demonstrated that these results were driven by hyper-connectivity among DM regions and diminished connectivity among CO regions, as well as diminished coupling between frontal regions and DM regions. Collectively, these results provide novel evidence for functional dysconnectivity in PS youths, which show marked correspondence to abnormalities reported in adults with established psychotic disorders.
Keywords: psychosis, development, resting state fMRI, connectivity, connectome, adolescence
INTRODUCTION
Psychotic disorders are frequently devastating in adults (1–3), and it is increasingly recognized that sub-threshold psychotic-spectrum symptoms in youth also impact functioning (4–6). Sub-threshold symptoms are therefore important both dimensionally (2,7–9) and as potential harbingers of conversion to clinically diagnosed psychotic disorders (10). Convergent evidence from epidemiologic data of maternal infections, human neuroimaging data, and animal models indicates that psychotic symptoms may be the result of abnormal neurodevelopment (3,11). This conceptualization has led to the hope that a better understanding of the course of psychosis will allow early interventions to “bend the curve” of abnormal brain development and lead to improved patient outcomes (3,11). However, this approach requires large-scale studies in youth and selection of informative brain phenotypes.
Both structural (12,13) and functional (14–16) brain abnormalities are found in psychotic adults and in help-seeking youths at high risk for psychosis. Based on accumulating evidence, psychosis can be studied as a syndrome of dysconnectivity among multiple large-scale functional brain networks (17–21). In particular, evidence from both chronic adult clinical samples (18,22,23), first-episode psychosis (24–27), unaffected relatives (28,29), and youth at clinical risk (30,31) implicates deficits in the resting-state functional connectivity within the default mode (DM) network as well as the cingulo-opercular (CO) and fronto-parietal (FP) cognitive control networks. Consistently described abnormalities include hyper-connectivity within the DM network (23,28,32), diminished connectivity within the FP and CO cognitive control networks (18,23), and a change in connectivity between the task-negative DM and the task-positive FP and CO networks (23,31,33). As connectivity within these networks has been implicated in individual differences in cognition (34,35), it is possible that dysconnectivity may additionally relate to the cognitive deficits seen in adults with psychosis (36–38) and in youth with psychosis-spectrum (PS) symptoms (6).
Studies investigating the relationship between functional network abnormalities and developmental vulnerability to psychosis have been hampered in part by two factors. First, most studies have examined functional connectivity on a regional basis using traditional seed-based analyses or within a restricted set of brain networks. By definition, such an approach cannot reveal potentially important effects in brain regions that were not studied. In contrast, here we investigated functional connectivity using multivariate distance-based matrix regression (MDMR) as part of a Connectome-Wide Association Study (CWAS) (39). Previously applied in analysis of large-scale ecological and genetic datasets (40), MDMR allows one to interrogate how the overall multivariate pattern of connectivity differs at each voxel in association with PS symptoms.
Second, while some larger studies in adults with psychosis now include up to several hundred patients (18), most studies in youth remain small in size, diminishing statistical power and producing heterogeneous results. Notably, the ascertainment strategy we used differs substantially from studies of ultra-high risk help-seeking youth (1), applying instead a population-based approach that examines psychosis-spectrum symptoms in non-help seeking youth (4,41–43). While such a design will likely produce lower rates of transition to frank psychosis, early sub-clinical psychotic symptoms may nonetheless be valuable for elucidating the neurodevelopmental etiology of psychosis (42–45). To our knowledge there has been only one small study of functional network abnormalities in youth with psychosis-spectrum symptoms (46), and it remains relatively unknown whether psychosis-spectrum symptoms in this population demonstrate patterns of dysconnectivity similar to those found in clinical risk and schizophrenia samples. Similarities to adult clinical phenotypes would provide support for a dimensional view of psychosis symptomatology (42–45).
We hypothesized that youth with PS symptoms would demonstrate functional network abnormalities in the DM network as well as cognitive control networks such as the CO and FP networks. Our analytic approach was not biased by a priori network selection, but rather explored the entire complexity of the functional connectome using MDMR. This strategy was facilitated by a large sample of PS youth imaged as part of the Philadelphia Neurodevelopmental Cohort (PNC) (47), a population-based study of brain development. As described below, we offer novel evidence of functional network abnormalities in PS youths that in part mirror those seen in adults with psychotic disorders.
MATERIALS AND METHODS
Participants
Study participants included 188 PS youths and 204 typically developing (TD) comparators with no significant psychopathology, all of whom were imaged as part of the PNC (see Table 1 for demographics) (47). PS and TD criteria were identical to those used in prior reports (4,6); recruitment and assessment details are available in the Supplementary Methods. All study procedures were approved by the Institutional Review Boards of the University of Pennsylvania and Children’s Hospital of Philadelphia. Adult participants provided informed consent; minors provided assent and their parent or guardian provided informed consent.
Table 1.
| Typically Developing (TD) | Psychosis-Spectrum (PS) | p value | |
|---|---|---|---|
| n | 204 | 188 | NA |
| # Female | 103 | 108 | 0.2 |
| # Caucasian | 121 | 55 | <0.001 |
| Age (mean [S.D.] in years) | 15.46 (3.7) | 15.74 (2.9) | 0.41 |
| Maternal Education (mean [S.D.] in years) | 14.8 (2.6) | 13.6 (2.1) | <0.001 |
| Global Cognition (mean [S.D.] z-score) | 0.3 (0.7) | −0.1 (1.0) | <0.001 |
| In-Scanner Motion (mean [S.D.] in mm) | 0.66 (0.04) | 0.7 (0.04) | 0.25 |
| # Treated with Psychotropic Medication | 0 | 31 | <0.001 |
Image acquisition and processing
All data were acquired on the same scanner using the same imaging sequences, which have been previously described and are detailed in Supplementary Methods (47–50). Time series data were processed using a validated confound regression procedure (49) that has been optimized to reduce the influence of participant motion, which is of particular concern for studies of developmental psychopathology (48); see Supplementary Methods for details. After 6mm FWHM smoothing, processed participant-level BOLD images were distortion corrected with FUGUE (51), co-registered to the T1 image using boundary-based registration (52), registered to the MNI 1mm template using the top-performing diffeomorphic SyN registration included in ANTs (53–55), and down-sampled to 4mm isotropic voxels prior to CWAS for computational feasibility (39). All transformations were concatenated so that only one interpolation was performed in the entire process.
Connectome-wide association study (CWAS) using multivariate distance based matrix regression (MDMR)
As previously described (39), CWAS operates in three steps. First, the standard-space 4mm voxelwise participant time series data are used to conduct a seed-based connectivity analysis at each voxel within gray matter, by calculating the Pearson’s correlation coefficient between each voxel’s time series and the time series of every other gray matter voxel. Second, the overall multivariate pattern of connectivity for each voxel is compared among participants using a distance metric (Pearson’s correlation). Third, MDMR is used to test how well each phenotypic variable explains the distances between each participant’s connectivity patterns created in the second step. This provides a measure of how the overall pattern of connectivity is impacted by each group level variable. In contrast to other multivariate methods, this allowed us to examine group differences in connectivity while controlling for potentially confounding variables. Modeled variables included group (PS vs. TD), age, sex, race, and in-scanner motion (48,49,56). For each voxel’s connectivity pattern, MDMR yields a pseudo-F statistic, whose significance was assessed using 5,000 iterations of a permutation test. The ultimate product of this procedure is a voxelwise significance map showing how PS status impacts the overall pattern of connectivity at each voxel; as in Shehzad et al. (2014) type I error was controlled using cluster-correction with a voxel height of z> 1.64, and a corrected cluster probability of p<0.01 using 10,000 Monte-Carlo simulations (57). Results were displayed using cortical surface projections in Caret (58).
Follow-up seed-based analyses
While MDMR identifies clusters where a group difference in the overall multivariate pattern of connectivity is present, it does not describe what pattern of connectivity is driving the significant result. Accordingly, we conducted post-hoc seed-based analyses from each cluster returned by MDMR. Seed analyses were conducted in standard fashion, using Fischer’s z-transformed voxelwise Pearson’s correlations and group level covariates as above. It should be emphasized that, except for analyses examining relationships with cognition, all follow-up analyses subsequent to MDMR do not constitute a unique hypothesis test, as the seeds were selected based on the significance of the MDMR result.
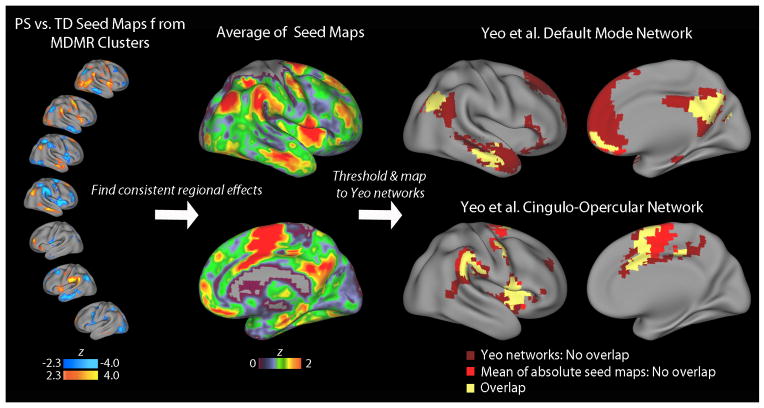
As described in Results below, examination of seed-based maps revealed that dysconnectivity was present in specific brain regions across multiple seeds. To assist in visualization of such regionally consistent patterns of dysconnectivity, we computed the average statistical effect of PS status at each voxel across all MDMR-based seed maps (see Figure 3). To do this, we calculated the absolute value of the group difference z-statistic map for each seed, and then averaged across all seeds. Clusters that exhibited consistent group effects in the averaged seed maps were identified using the same voxelwise cluster-corrected threshold as for MDMR (z>1.64, p<0.01). To assess correspondence to known large-scale functional networks, overlap maps were constructed using the commonly used 7-network parcellation of Yeo et al. (59).
Figure 3.
PS youth demonstrate dysconnectivity with hubs of cingulo-opercular and default mode networks. Seed maps describing differences in connectivity between PS and TD youth (left column) suggested that connectivity with specific brain regions were consistently impacted across multiple CWAS-derived seeds. In order to visualize this, we computed the average absolute statistical effect (mean absolute z map) of group at each voxel across all CWAS-based seed maps (middle column). Clusters that exhibited consistent group effects across all seed maps were identified (average z>1.64, p<0.01; right column), and mapped to the large-scale functional networks defined by Yeo et al. (2011). This procedure revealed that regions identified by CWAS have prominent dysconnectivity with major hubs of the cingulo-opercular and default mode networks, with a very high degree of spatial overlap. Note that Yeo refers to the cingulo-opercular network as the ventral attention network.
Network construction and analysis
In order to summarize the observed pairwise interactions among implicated brain regions, we evaluated the data within a network framework. We constructed a graph of 18 nodes (see Supplementary Table 2) consisting of clusters identified by either MDMR (n=8) or overlap among seed maps (n=10). This graph was parsed into network modules using community detection techniques (see Supplementary Methods); network structure was displayed using Gephi (60). Group differences in connectivity among these modules were investigated using measures of within-network and between-network connectivity (61). Covariates were as above.
As a final step, to determine whether observed connectivity differences in the PS group had functional relevance, network measures were related to cognitive performance. As previously described (5,62), the Penn computerized neurocognitive battery (Penn CNB) was administered to all imaged PNC participants. A recent factor analysis (63) of the PNC CNB data determined that general cognitive efficiency (“g” cognition) can be effectively summarized using a single score from a bi-factor model of both accuracy and speed scores of CNB tests. The relationship between network measures that were abnormal in PS youth and cognitive performance within the PS group were investigated using linear regression with covariates as above. To determine if one cognitive domain was specifically impacted, we additionally evaluated three cognitive factors corresponding to the efficiency of executive function / complex reasoning, episodic memory, and social cognition (63). Although we anticipated the relationship between cognition and dysconnectivity would be focused primarily within PS youth, we additionally repeated these analyses in TD participants as well.
Supplementary Analyses
While the above analyses accounted for major known confounding variables including age, sex, in-scanner motion, and race through their inclusion as model covariates, we conducted additional analyses to further evaluate other potential confounds. Specifically, we re-evaluated network-level associations while including maternal education as a covariate. Additionally, while only a minority of PS youths were being treated with psychotropic medication, we re-ran network-level analyses with these participants excluded. Furthermore, we re-computed network-level analyses considering only participants over age 11. This was done for two reasons. First, participants under age 11 had higher levels of in-scanner motion, and we wanted to be certain that this did not systematically bias results. Second, participants under age 11 were only assessed using the collateral interview, and we wanted to exclude the possibility that the assessment strategy influenced observed results.
While all analyses included age and sex as covariates, as prior work has emphasized differences in developmental patterns across age and between sexes (50), we also examined age by group and group by sex interactions. Finally, relationships between network-level measures and both positive and negative/disorganized symptoms were examined as in Wolf et al. (64). Due to the computational expense of MDMR, all supplementary analyses were conducted on network-level summary data only.
RESULTS
MDMR reveals multifocal patterns of dysconnectivity in PS youth
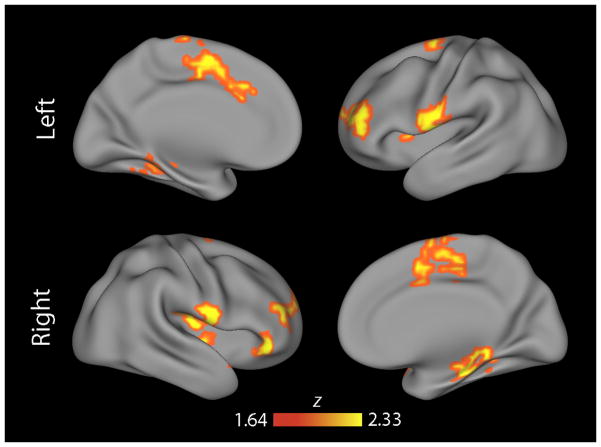
Comparison of TD and PS youths using MDMR revealed multiple regions where the multivariate pattern of connectivity differed between groups (Figure 1). These regions included the medial prefrontal and anterior cingulate cortex, bilateral frontal opercular cortex, bilateral frontal pole, right orbitofrontal cortex, and bilateral medial temporal lobe. However, as these MDMR results do not describe which specific connections form the basis for the observed multivariate results, each significant cluster from MDMR was next interrogated using a standard seed-based connectivity analysis.
Figure 1.
MDMR identified multiple foci of dysconnectivity in PS youth. Cortical projection displaying clusters identified by MDMR where the overall multivariate pattern of functional connectivity was significantly different between typically developing (TD) youth and those with psychosis-spectrum symptoms (PS). All clusters corrected for multiple comparisons at z>1.64, p<0.01.
Seed-based connectivity analyses reveal abnormalities within DM and CO networks
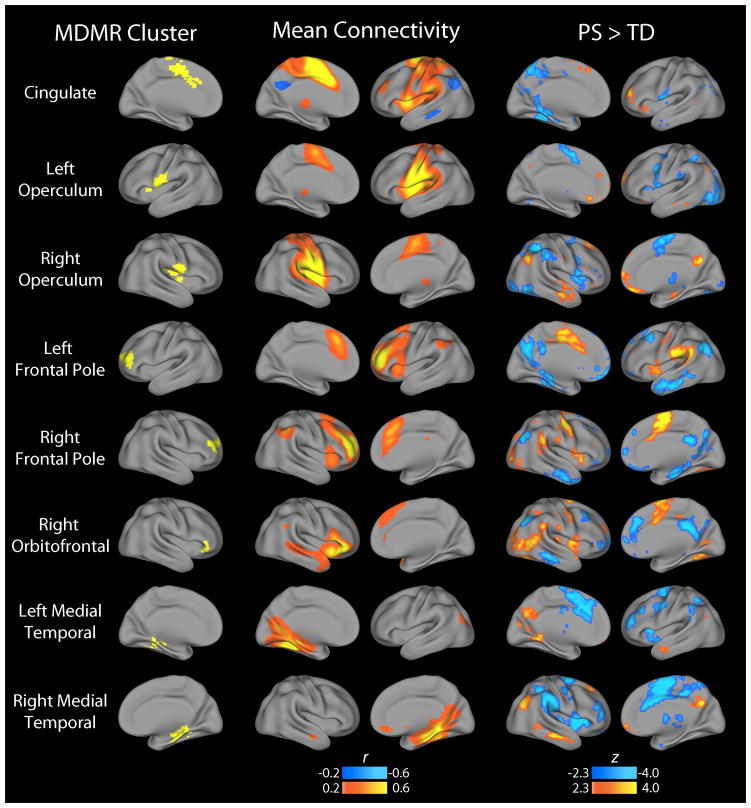
Follow-up analyses used the clusters identified by MDMR as the basis for seed-based connectivity analyses, which examined the connectivity from that cluster with the rest of the brain on a voxelwise basis. These analyses demonstrated that the multivariate results from MDMR were driven by dysconnectivity within specific functional networks (Figure 2). For example, opercular clusters demonstrated diminished connectivity with other elements of the CO network, including the anterior cingulate and insula. In contrast, medial temporal regions showed increased connectivity with hubs of the DM network including the posterior cingulate, inferior parietal cortex, and lateral temporal cortex. Finally, frontopolar and orbitofrontal regions showed a pattern of increased connectivity with CO regions but diminished connectivity with DM hubs.
Figure 2.
Follow-up seed-based connectivity analyses explain patterns of dysconnectivity that drive MDMR results. The multivariate results of CWAS identify regions where there is a significant difference in the overall pattern of connectivity between PS and TD youth, but do not describe what that pattern is. Accordingly, each cluster identified by CWAS (Figure 1) was used as a seed in order to understand what changes in connectivity led to the significant finding. The middle column in the figure displays the mean connectivity across all subjects from each seed; the right hand column displays the group difference between PS and TD youth. As results were relatively symmetric bilaterally, only the ipsilateral hemisphere is displayed.
Considered collectively, seed-based analyses using CWAS clusters consistently implicated the CO and DM networks. In order to aid visualization of this effect, we averaged the PS vs. TD difference across all seed maps, and compared the average difference to the widely used functional parcellation defined by Yeo et al. (Figure 3) (59). This procedure identified regions where PS participants had abnormalities in pairwise connectivity with the clusters identified by MDMR, revealing a very high degree of overlap with hubs of the DM and CO networks; very little overlap was seen with other functional networks.
Network analysis delineates dissociable changes within and between large-scale networks
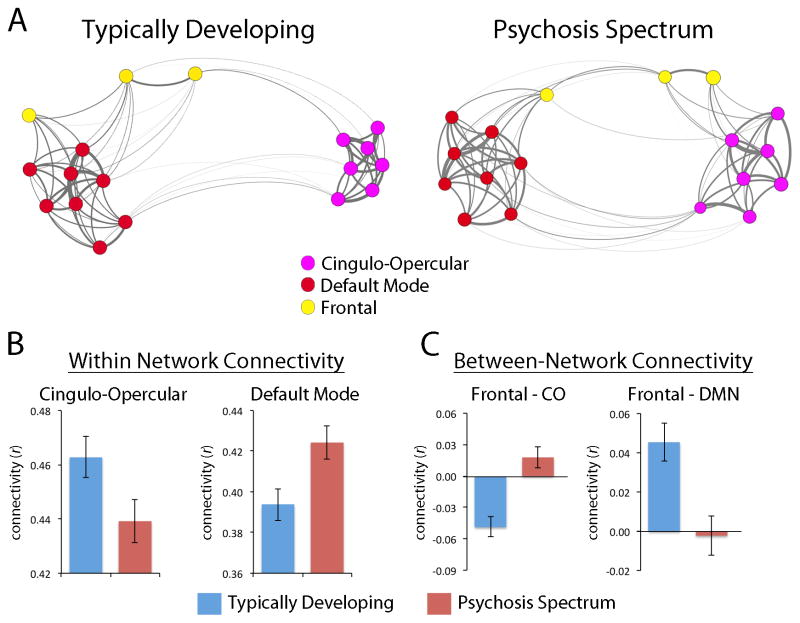
These results suggest that while the strongest differences in connectivity between PS and TD youth are present in the clusters identified by MDMR, these results are driven by abnormalities in the relationship with known hubs of the CO and DM networks. To concisely summarize how relationships among these regions are altered in PS youth, we conducted network analyses in a system where nodes included MDMR regions (Figure 1) and those regions consistently implicated in seed analyses (Figure 3). Application of community detection procedures identified three network modules: a DM module (red in Figure 4A), a CO module (purple), and a module composed of the three frontal regions identified by the MDMR (bilateral frontopolar and right orbitofrontal cortex; yellow).
Figure 4.
Dissociable aberrations of network connectivity in PS youth. A. Kamada-Kawai layout of mean connectivity within a network of nodes defined by CWAS and overlap of seed maps. Network is composed of nodes identified by CWAS (Figure 1) or those consistently identified by overlap of seed connectivity maps (Figure 3); network communities were detected within this system using a consensus-based assignment of participant-level partitions. B. PS youth have diminished connectivity within the cingulo-opercular network, but enhanced connectivity within the default mode network. C. PS youth have enhanced connectivity between frontal regions and the cingulo-opercular network, but diminished connectivity between default mode and frontal regions.
These network modules were used to derive summary measures of within-network and between-network connectivity. This approach demonstrated that PS youth have enhanced connectivity within the DM network (t=2.7, p= 8.0 × 10−3), but diminished connectivity within the CO network (t= 2.1, p=0.03; Figure 4B). Furthermore, between-network connectivity was systematically altered as well (Figure 4C), with frontal regions becoming more connected with CO regions (t=4.7, p=2.7×10−6) and less coupled with the DM regions (t=3.4, p=7.0×10−4) in PS youth. There was no group difference between DMN and CO coupling, but as in our prior work females showed diminished CO connectivity with the DMN (50). Inclusion of maternal education as a covariate did not impact results. Exclusion of PS participants taking psychoactive medication or subjects under age 11 reduced the finding of increased connectivity in CO regions to a non-significant trend; all other results were not impacted and remained equally significant. Age by group and sex by group interactions were not significant and thus not retained in the model.
Default mode network hyper-connectivity is related to cognitive impairment in youth with psychosis-spectrum symptoms
As a last step, we sought to determine the functional relevance of connectivity aberrations in PS youth by relating changes in network connectivity to cognitive performance. This analysis revealed that elevated connectivity in the DMN is associated with impaired cognitive performance in PS youth (t=2.7, p=6.7×10−3). This association was present across all three components of cognition identified by a prior factor analysis, including executive function & complex reasoning (t=−2.7, p=7.1×10−3), episodic memory (t=−2.6, p=9.3×10−3), and social cognition (t=−2.3, p=0.023). The relationship remained significant when medicated participants or participants under the age of 11 were excluded and when maternal education was included as a covariate. Other alterations in network connectivity in PS youth were not associated with variation in cognitive performance; no associations with the reported severity of psychosis-spectrum symptoms were found. Finally, there were no significant associations between cognitive performance and network-level measures in TD participants.
DISCUSSION
In the largest study to date of psychosis-spectrum symptoms in youth, we used MDMR to identify multifocal patterns of dysconnectivity. While PS youth demonstrated diminished connectivity among CO network regions, they also displayed hyper-connectivity among DM regions, which was significantly related to cognitive impairment. Furthermore, frontal regions were de-coupled from DM regions in PS youth, and showed increased connectivity with CO regions. Taken together, these findings delineate for the first time a pattern of functionally relevant large-scale network dysconnectivity in PS youth that has marked parallels to observed abnormalities seen in adults with frank psychosis.
Hyper-connectivity in default mode regions scales with cognitive deficits in PS youth
Abnormally enhanced connectivity within the DM network in adults with schizophrenia is one of the most commonly reported and oft-cited findings in psychiatric neuroimaging. Since the original reports in adults (22,28), this result has been replicated in other adult samples (23), early onset schizophrenia (32,65), and youth at clinical risk of psychosis (30). In our sample, this effect was most prominent in the medial temporal lobe cluster identified by MDMR, which had enhanced connectivity with multiple hubs of the DM including the posterior cingulate, lateral temporal cortex, and inferior parietal cortex. Task-based fMRI studies in adults with psychosis have also described diminished DM de-activation during challenging cognitive tasks (66,67), which has been interpreted as cognitive interference. Such an interpretation is consistent with the observed relationship between hyper-connectivity among DM regions and diminished cognitive performance in our data. As discussed below, DM hyper-connectivity may be related to diminished top-down regulation by frontal cognitive control regions, which showed decreased coupling with DM regions.
PS youth demonstrate abnormalities of cognitive control regions
In addition to the elevated connectivity seen among DM regions, PS youth had abnormalities affecting regions within the CO network and frontal regions that fall within the FP network. Specifically, PS youth had diminished connectivity among CO regions, and shift in connectivity among frontal regions away from DM regions towards CO regions. These results accord well with prior reports suggesting diminished connectivity among cognitive control networks in both adults with psychosis and youth at clinical risk for psychosis (18,23,25,31,33,68). In contrast, the relationship between cognitive control regions and the DM is more variable across studies. Unlike the effects observed here, several studies in adults have found evidence of elevated connectivity between cognitive control and DM regions (23,30,31,33). While speculative, it is possible that this divergence between our findings and those seen in adults reflects differential alterations of connectivity related to disease chronicity and symptom severity. Indeed, such an account was offered by a recent study that found evidence for early hyper-connectivity in PFC regions (65). Early de-coupling of frontal regions from DM regions might reverse over time as internally directed thoughts related to psychotic symptoms become more advanced.
MDMR allows full exploration of the connectome in PS youth
The majority of studies investigating abnormalities of functional connectivity in psychosis or psychosis risk have utilized either seed-based or network-based approaches with regions of interest (nodes) defined a priori. While data-driven network analyses (using independent component analyses or similar techniques) include fewer assumptions than hypothesis-driven seed-based analyses, they are nonetheless not fully exploratory, as they require substantial data reduction. In contrast, we approached the current study with a fully data-driven analysis using MDMR. Remarkably, this exploratory multivariate analysis identified abnormalities in regions most commonly implicated in studies of psychosis in adults.
Notably, while the clusters of maximal group difference identified by MDMR associate with known functional networks, they lie at the edge of network partition boundaries; this was most prominent for the opercular regions identified by MDMR but was true to a lesser extent for frontal and medial temporal regions as well. In contrast, the regions consistently identified by follow-up seed analyses clearly align with known hubs of the DM and CO networks (59). This pattern suggests that dysconnectivity in PS youth may impact network partition boundaries defined by such edge-hub connectivity patterns; such a pattern of results was also observed in a study of autism-spectrum traits in adults (69). While the present results do not evaluate this possibility explicitly, they motivate future studies that examine alterations in functional parcellation boundaries in detail (59,70–73).
Strengths and limitations of a population-based sampling strategy
The population-based approach used here allowed us to study younger participants than typically included in studies of help seeking clinical risk individuals, and also to accrue a substantially larger sample size at a single site and scanner. Establishing the presence of network abnormalities in younger individuals is an advantage for potential interventions that are designed to alter disease trajectory (2,3,7,74). However, the cross-sectional analysis presented here does not allow us to determine how much the observed dysconnectivity is driven by a sub-sample of youths who will later become frankly psychotic or attenuated by individuals who will show no further symptoms (75). Preliminary data from ongoing follow-up studies suggests that approximately 10% of PS youth will develop frank symptoms of psychosis within 2–3 years; we expect this figure will increase as longitudinal follow-up is extended. Additionally, participants with psychosis-spectrum symptoms also have elevated levels of co-morbid psychopathology (e.g., Supplementary Table 1) (4). Future studies will be necessary to further parse such heterogeneity and determine whether the observed results were due to psychosis per se or other dimensions of psychopathology. These effects underline the need for multivariate tools that allow data-driven identification of more homogenous sub-populations. When combined with longitudinal follow-up, such tools may allow for effective prediction of future risk.
Conclusions
These results establish that psychosis-spectrum symptoms in youth are associated with a pattern of functional dysconnectivity previously seen in adults with psychosis. Considered jointly with our recent report using task-based fMRI from the same sample (64), this finding suggests that abnormalities within specific vulnerable brain networks emerge at a young age, and are not due to disease chronicity or the confounding influence of psychotropic medication. Network dysconnectivity may evolve to become a biomarker that can be used in both drug discovery and clinical trials of novel treatments. Especially when integrated with an array of cognitive and genomic data, such imaging phenotypes may help stage risk and target interventions for specific sub-populations that cannot be distinguished on clinical symptoms alone.
Supplementary Material
Acknowledgments
Thanks to the acquisition and recruitment team: Karthik Prabhakaran, Ryan Hopson, Jeff Valdez, Raphael Gerraty, Marisa Riley, Jack Keefe, Elliott Yodh, and Rosetta Chiavacci. Thanks to Mark Elliott for image acquisition support. Thanks to Frank Mentch for data management. Supported by RC2 grants from the National Institute of Mental Health MH089983 and MH089924 and P50MH096891. Support for developing statistical analyses (SNV, RTS, TDS) was provided by a seed grant by the Center for Biomedical Computing and Image Analysis (CBICA) at Penn. Support for network analytics was provided by the Institute for Translational Medicine and Therapeutics (ITMAT) at Penn to DSB and TDS. Additional support was provided by K23MH098130 to TDS, R01MH101111 to DHW, K01MH102609 to DRR, K08MH079364 to MEC, R01NS085211 to RTS, T32MH065218-11 to SNV, and the Dowshen Program for Neuroscience.
Footnotes
CONFLICT OF INTERESTS: The authors declare no competing financial interests.
References
- 1.Addington J, Cadenhead KS, Cannon TD, Cornblatt B, McGlashan TH, Perkins DO, et al. North american prodrome longitudinal study: A collaborative multisite approach to prodromal schizophrenia research. Schizophr Bull. 2007 May;33(3):665–72. doi: 10.1093/schbul/sbl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Insel TR. Translating scientific opportunity into public health impact: A strategic plan for research on mental illness. Arch Gen Psychiatry. 2009 Feb;66(2):128–33. doi: 10.1001/archgenpsychiatry.2008.540. [DOI] [PubMed] [Google Scholar]
- 3.Insel TR. Rethinking schizophrenia. Nature. 2010 Nov 11;468(7321):187–93. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 4.Calkins ME, Moore TM, Merikangas KR, Burstein M, Satterthwaite TD, Bilker WB, et al. The psychosis spectrum in a young U.S. Community sample: Findings from the philadelphia neurodevelopmental cohort. World Psychiatry. 2014 Oct;13(3):296–305. doi: 10.1002/wps.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012 Mar;26(2):251–65. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gur RC, Calkins ME, Satterthwaite TD, Ruparel K, Bilker WB, Moore TM, et al. Neurocognitive growth charting in psychosis spectrum youths. JAMA Psychiatry. 2014 Feb 5; doi: 10.1001/jamapsychiatry.2013.4190. [DOI] [PubMed] [Google Scholar]
- 7.Casey BJ, Oliveri ME, Insel T. A neurodevelopmental perspective on the research domain criteria (rdoc) framework. Biol Psychiatry. 2014 Sep 1;76(5):350–3. doi: 10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: The NIMH research domain criteria project. Schizophr Bull. 2010 Nov;36(6):1061–2. doi: 10.1093/schbul/sbq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (rdoc): Toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010 Jul;167(7):748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 10.McGlashan TH, Miller TJ, Woods SW. Pre-onset detection and intervention research in schizophrenia psychoses: Current estimates of benefit and risk. Schizophr Bull. 2001;27(4):563–70. doi: 10.1093/oxfordjournals.schbul.a006896. [DOI] [PubMed] [Google Scholar]
- 11.Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: Update 2012. Mol Psychiatry. 2012 Apr 10; doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haijma SV, Van Haren N, Cahn W, Koolschijn PCMP, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: A meta-analysis in over 18 000 subjects. Schizophr Bull. 2013 Sep;39(5):1129–38. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vita A, De Peri L, Deste G, Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: A meta-analysis and meta-regression of longitudinal MRI studies. Transl Psychiatry. 2012;2:e190. doi: 10.1038/tp.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000 Nov;10(11):1078–92. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 15.Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Valdez JN, Siegel SJ, et al. Association of enhanced limbic response to threat with decreased cortical facial recognition memory response in schizophrenia. Am J Psychiatry. 2010 Apr;167(4):418–26. doi: 10.1176/appi.ajp.2009.09060808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper D, Barker V, Radua J, Fusar-Poli P, Lawrie SM. Multimodal voxel-based meta-analysis of structural and functional magnetic resonance imaging studies in those at elevated genetic risk of developing schizophrenia. Psychiatry Res. 2014 Jan 30;221(1):69–77. doi: 10.1016/j.pscychresns.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Nekovarova T, Fajnerova I, Horacek J, Spaniel F. Bridging disparate symptoms of schizophrenia: A triple network dysfunction theory. Front Behav Neurosci. 2014;8:171. doi: 10.3389/fnbeh.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker JT, Holmes AJ, Masters GA, Yeo BTT, Krienen F, Buckner RL, Ongur D. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014 Feb;71(2):109–18. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calhoun VD, Sui J, Kiehl K, Turner J, Allen E, Pearlson G. Exploring the psychosis functional connectome: Aberrant intrinsic networks in schizophrenia and bipolar disorder. Front Psychiatry. 2011;2:75. doi: 10.3389/fpsyt.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubinov M, Bullmore E. Fledgling pathoconnectomics of psychiatric disorders. Trends Cogn Sci. 2013 Nov 15; doi: 10.1016/j.tics.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. Neuroimage. 2012 Oct 1;62(4):2296–314. doi: 10.1016/j.neuroimage.2011.12.090. [DOI] [PubMed] [Google Scholar]
- 22.Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007 Mar;164(3):450–7. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 23.Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011 Aug;130(1–3):86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren W, Lui S, Deng W, Li F, Li M, Huang X, et al. Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. Am J Psychiatry. 2013 Nov;170(11):1308–16. doi: 10.1176/appi.ajp.2013.12091148. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, et al. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fmri. Neurosci Lett. 2007 May 7;417(3):297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]
- 26.Alonso-Solis A, Corripio I, de Castro-Manglano P, Duran-Sindreu S, Garcia-Garcia M, Proal E, et al. Altered default network resting state functional connectivity in patients with a first episode of psychosis. Schizophr Res. 2012 Aug;139(1–3):13–8. doi: 10.1016/j.schres.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol Psychiatry. 2011 Jul 1;70(1):64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009 Jan 27;106(4):1279–84. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Buuren M, Vink M, Kahn RS. Default-mode network dysfunction and self-referential processing in healthy siblings of schizophrenia patients. Schizophr Res. 2012 Dec;142(1–3):237–43. doi: 10.1016/j.schres.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 30.Shim G, Oh JS, Jung WH, Jang JH, Choi C-H, Kim E, et al. Altered resting-state connectivity in subjects at ultra-high risk for psychosis: An fmri study. Behav Brain Funct. 2010;6:58. doi: 10.1186/1744-9081-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wotruba D, Michels L, Buechler R, Metzler S, Theodoridou A, Gerstenberg M, et al. Aberrant coupling within and across the default mode, task-positive, and salience network in subjects at risk for psychosis. Schizophr Bull. 2013 Nov 16; doi: 10.1093/schbul/sbt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang J, Liao Y, Song M, Gao J-H, Zhou B, Tan C, et al. Aberrant default mode functional connectivity in early onset schizophrenia. PLoS One. 2013;8(7):e71061. doi: 10.1371/journal.pone.0071061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tu P-C, Lee Y-C, Chen Y-S, Li C-T, Su T-P. Schizophrenia and the brain’s control network: Aberrant within- and between-network connectivity of the frontoparietal network in schizophrenia. Schizophr Res. 2013 Jul;147(2–3):339–47. doi: 10.1016/j.schres.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Cole MW, Yarkoni T, Repovs G, Anticevic A, Braver TS. Global connectivity of prefrontal cortex predicts cognitive control and intelligence. J Neurosci. 2012 Jun 27;32(26):8988–99. doi: 10.1523/JNEUROSCI.0536-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006 Dec 20;26(51):13338–43. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, et al. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry. 1991 Jul;48(7):618–24. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- 37.Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994 Feb;51(2):124–31. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 38.Greenwood TA, Lazzeroni LC, Murray SS, Cadenhead KS, Calkins ME, Dobie DJ, et al. Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the consortium on the genetics of schizophrenia. Am J Psychiatry. 2011 Sep;168(9):930–46. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shehzad Z, Kelly C, Reiss PT, Cameron Craddock R, Emerson JW, McMahon K, et al. A multivariate distance-based analytic framework for connectome-wide association studies. Neuroimage. 2014 Jun;93(Pt 1):74–94. doi: 10.1016/j.neuroimage.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zapala MA, Schork NJ. Statistical properties of multivariate distance matrix regression for high-dimensional data analysis. Front Genet. 2012;3:190. doi: 10.3389/fgene.2012.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: Evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009 Feb;39(2):179–95. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- 42.Kelleher I, Connor D, Clarke MC, Devlin N, Harley M, Cannon M. Prevalence of psychotic symptoms in childhood and adolescence: A systematic review and meta-analysis of population-based studies. Psychol Med. 2012 Sep;42(9):1857–63. doi: 10.1017/S0033291711002960. [DOI] [PubMed] [Google Scholar]
- 43.Kelleher I, Murtagh A, Molloy C, Roddy S, Clarke MC, Harley M, Cannon M. Identification and characterization of prodromal risk syndromes in young adolescents in the community: A population-based clinical interview study. Schizophr Bull. 2012 Mar;38(2):239–46. doi: 10.1093/schbul/sbr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelleher I, Cannon M, Cannon M. Psychotic-like experiences in the general population: Characterizing a high-risk group for psychosis. Psychol Med. 2011 Jan;41(01):1–6. doi: 10.1017/S0033291710001005. [DOI] [PubMed] [Google Scholar]
- 45.Kelleher I, Corcoran P, Keeley H, Wigman JT, Devlin N, Ramsay H, et al. Psychotic symptoms and population risk for suicide attempt: A prospective cohort study. JAMA Psychiatry. 2013 Sep;70(9):940–8. doi: 10.1001/jamapsychiatry.2013.140. [DOI] [PubMed] [Google Scholar]
- 46.Jacobson McEwen SC, Connolly CG, Kelly AMC, Kelleher I, O’Hanlon E, Clarke M, et al. Resting-state connectivity deficits associated with impaired inhibitory control in non-treatment-seeking adolescents with psychotic symptoms. Acta Psychiatr Scand. 2014 Feb;129(2):134–42. doi: 10.1111/acps.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, et al. Neuroimaging of the philadelphia neurodevelopmental cohort. Neuroimage. 2014 Feb 1;86:544–53. doi: 10.1016/j.neuroimage.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. Neuroimage. 2012 Jan 2;60(1):623–32. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013 Jan 1;64(0):240–56. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Satterthwaite TD, Wolf DH, Roalf DR, Ruparel K, Erus G, Vandekar S, et al. Linked sex differences in cognition and functional connectivity in youth. Cereb Cortex. 2014 Apr 25; doi: 10.1093/cercor/bhu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012 Aug 8;62(2):782–90. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 52.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009 Oct 10;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008 Feb;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ants similarity metric performance in brain image registration. Neuroimage. 2011 Feb 2;54(3):2033–44. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang M-C, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009 Jul 7;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Satterthwaite TD, Wolf DH, Ruparel K, Erus G, Elliott MA, Eickhoff SB, et al. Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. Neuroimage. 2013 Dec;83(0):45–57. doi: 10.1016/j.neuroimage.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996 Jun;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 58.Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;8(5):443–59. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011 Sep;106(3):1125–65. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bastian M, Heymann S, Jacomy M. Gephi: An open source software for exploring and manipulating networks. ICWSM. 2009;8:361–2. [Google Scholar]
- 61.Bassett DS, Porter MA, Wymbs NF, Grafton ST, Carlson JM, Mucha PJ. Robust detection of dynamic community structure in networks. Chaos. 2013 Mar;23(1):013142. doi: 10.1063/1.4790830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, et al. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: Standardization and initial construct validation. J Neurosci Methods. 2010 Mar 3;187(2):254–62. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moore TM, Reise SP, Gur RE, Hakonarson H, Gur RC. Psychometric properties of the penn computerized neurocognitive battery. Neuropsychology. 2014 Sep 1; doi: 10.1037/neu0000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolf DH, Satterthwaite TD, Calkins ME, Ruparel K, Elliott MA, Hopson RD, Jackson C, Prabhakaran K, Bilker WB, Hakonarson H, Gur RC, Gur RE. Functional neuroimaging abnormalities in psychosis spectrum youth. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2014.3169. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anticevic A, Hu X, Xiao Y, Hu J, Li F, Bi F, et al. Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change. J Neurosci. 2015 Jan 7;35(1):267–86. doi: 10.1523/JNEUROSCI.2310-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anticevic A, Repovs G, Barch DM. Working memory encoding and maintenance deficits in schizophrenia: Neural evidence for activation and deactivation abnormalities. Schizophr Bull. 2013 Jan;39(1):168–78. doi: 10.1093/schbul/sbr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seidman LJ, Rosso IM, Thermenos HW, Makris N, Juelich R, Gabrieli JDE, et al. Medial temporal lobe default mode functioning and hippocampal structure as vulnerability indicators for schizophrenia: A MRI study of non-psychotic adolescent first-degree relatives. Schizophr Res. 2014 Nov;159(2–3):426–34. doi: 10.1016/j.schres.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 68.Orliac F, Naveau M, Joliot M, Delcroix N, Razafimandimby A, Brazo P, et al. Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr Res. 2013 Aug;148(1–3):74–80. doi: 10.1016/j.schres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 69.Di Martino A, Shehzad Z, Kelly C, Roy AK, Gee DG, Uddin LQ, et al. Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. Am J Psychiatry. 2009 Aug;166(8):891–9. doi: 10.1176/appi.ajp.2009.08121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex. 2014 Oct 14; doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Craddock RC, James G, Holtzheimer PE, Hu XP, Mayberg HS. A whole brain fmri atlas generated via spatially constrained spectral clustering. Hum Brain Mapp. 2012 Aug;33(8):1914–28. doi: 10.1002/hbm.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011 Nov 17;72(4):665–78. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Honnorat N, Eavani H, Satterthwaite TD, Gur RE, Gur RC, Davatzikos C. GraSP: Geodesic graph-based segmentation with shape priors for the functional parcellation of the cortex. Neuroimage. 2014 Nov 11; doi: 10.1016/j.neuroimage.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008 Dec;9(12):947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TGM, et al. Progressive reduction in cortical thickness as psychosis develops: A multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015 Jan 15;77(2):147–57. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.