Abstract
Objective
To characterize predictors of insulin-like growth factor (IGF)-1 and IGF binding protein (IGFBP)-3 in acute critical illness with the hypothesis that acute factors associated with critical illness will more strongly predict circulating IGF-1 and IGFBP-3 than chronic clinical or genetic factors.
Design
Observational study nested within a large prospective study using multivariable linear regression to model circulating IGF-1 and IGFBP-3 with acute and chronic clinical variables, and genotype from five polymorphisms in IGF pathway genes.
Setting and Patients
Five-hundred forty-three Caucasian patients with risk factors for acute respiratory distress syndrome (ARDS) and available plasma from early in critical illness, recruited from intensive care units (ICUs) of two large academic medical centers.
Interventions
None.
Measurements and Main Results
Total IGF-1 and IGFBP-3 were measured in plasma using IMMULITE assays. We examined age, gender, body mass index (BMI), cirrhosis, and diabetes, as well as APACHE III score, acute hepatic dysfunction, pneumonia and aspiration, sepsis/septic shock, ARDS, and receipt of corticosteroids. BMI, cirrhosis, and ARDS were strongly associated with IGF-1 and IGFBP-3 levels; APACHE III was strongly associated with IGF-1 levels; and age was strongly associated with IGFBP-3‥ Five polymorphisms (IGF1: rs1520220, rs35767, rs2946834; IGFBP1: rs4619; IGFBP3: rs2854746) were analyzed for associations with plasma levels. When genotypes were added to models, rs2854746 was significantly associated with plasma IGFBP-3. Genotype explained an additional 2% of variability with an overall adjusted R-square of 0.18.
Conclusions
Despite the acute derangements of critical illness, both acute and chronic health factors significantly influence circulating levels of IGF-1 and IGFBP-3 early in critical illness. Rs2854746 is also significantly associated with IGFBP-3 levels in this ICU cohort. Overall, phenotypic and genotypic factors explained only a modest amount of variability in IGF-1 and IGFBP-3. Further research is needed to understand how to apply these findings to patient care.
Keywords: Acute respiratory distress syndrome, single nucleotide polymorphisms, insulin-like growth factor-1, insulin-like growth factor binding protein-3, molecular epidemiology, critical care
INTRODUCTION
In acute critical illness, there are numerous derangements in endocrine pathways, many as direct markers of catabolism and stress. This has led to interest in various endocrine molecules as potential prognostic markers or even treatment options for acute critical illness (1, 2). Insulin-like growth factor (IGF)-1 and insulin-like growth factor binding protein (IGFBP)-3 have been of particular interest because of their relationship with growth hormone (GH) and mediation of its anabolic actions (3–5). IGF-1 and IGFBP-3 are both decreased in acute critical illness despite increasing GH due to peripheral GH resistance, decreased GH receptor expression, and down-regulation of hepatic synthesis and secretion of IGF-1 and IGFBP-3 (1, 2, 4, 6, 7). This cascade may be a key contributor to the catabolism of critical illness that could have major prognostic implications (2, 6, 8). In our previous work, we showed that lower levels of IGF-1 and IGFBP-3 were independently associated with ARDS in a cohort of at-risk patients, and lower levels were associated with mortality in ARDS cases (9).
IGF-1 is an anabolic peptide hormone with structural similarities to proinsulin (10). IGF-1 has also been linked to fibrogenesis, apoptosis, mitogenesis, and cell cycle regulation and differentiation (10, 11). In circulation, over 90% of IGF-1 is bound to IGFBP-3, the most abundant of the six known IGFBPs (5, 7). The anabolic actions of IGF-1 are controlled primarily by growth hormone, and IGF-1 itself indirectly mediates anabolic actions of GH (3).
IGF-1 and IGFBP-3 levels vary considerably between individuals, and various population-based cohorts have studied factors associated with these differences (12–15). There has been consistent evidence that IGF-1 declines with age, and that men have higher IGF-1 levels than women (12–14). IGFBP-3 tends also to decline with age and be lower in men, but evidence has been less striking than for IGF-1 (12, 13, 15). There has been less consistency in associations between circulating IGF-1 and IGFBP-3 and factors such as body mass index (BMI), smoking, and dietary factors (12–15).
Genetic factors have also been studied as determinants of circulating IGF-1 and IGFBP-3 levels. Twin studies have reported estimated heritability of IGF-1 to be 38%, and IGFBP-3 to be 60% (16). Numerous polymorphisms of IGF1 and IGFBP3 have been studied in association with IGF-1 and IGFBP-3 levels in several large cohorts (11, 17–20). Perhaps the most consistent finding has been the association of rs2854746 (IGFBP3) with IGFBP-3 levels, although there have been many polymorphisms associated with alterations in circulating IGF-1 and IGFBP-3 (11, 17–20). Some of these associations have varied by gender or race/ethnicity. Thus, there is evidence that circulating IGF-1 and IGFBP-3 are complex traits with both significant genetic and non-genetic influences (21).
Genetic and other chronic factors, many unmodifiable, contribute significantly to IGF-1 and IGFBP-3 levels in patients without acute illness. However, acute derangements in the growth hormone (GH)/IGF-1 axis during critical illness strongly affect circulating levels of IGF-1 and its binding proteins. Given the potential links to prognosis in acute and prolonged critical illness, we sought to further investigate the relative contributions of acute and chronic clinical factors, as well as genetic factors, to levels of IGF-1 and IGFBP-3 during acute medical critical illness. We hypothesized that acute factors associated with critical illness would more strongly predict circulating IGF-1 and IGFBP-3 than chronic clinical or genetic factors.
MATERIALS AND METHODS
Parent Study Population and Design
The current observational study is part of the ongoing prospective Molecular Epidemiology of Acute Respiratory Distress Syndrome (ARDS) Study of which study design has been described in depth previously (22). The Human Subjects Committees at the Massachusetts General Hospital (MGH), Beth Israel Deaconess Medical Center (BIDMC), and the Harvard School of Public Health approved this study. Recruitment of adult intensive care unit (ICU) admissions at MGH (Boston, MA) began in January 1999, and at BIDMC (Boston, MA) in January 2007. Enrollment for the current cohort continued through March 2009. Admissions were screened daily for clinical risk factors for ARDS: pneumonia, sepsis or septic shock, aspiration, massive transfusions, pulmonary contusion or multiple fractures. Standard definitions of sepsis and septic shock were used (23). Eligible patients with at least one risk factor for ARDS were approached and enrolled after informed written consent was obtained from subjects or appropriate surrogates. Patients in the parent cohort were followed prospectively for development of ARDS, defined by meeting American-European Consensus Conference (AECC) criteria (24). Thus, the cohort as a whole represents patients with significant critical illness defined by major risk factors for ARDS.
IGF-1 and IGFBP-3 Measurements
Details of blood drawing protocols and laboratory methods have been published elsewhere (9). Briefly, per the parent study protocol, blood was drawn within the first 2 days of ICU admission, or within 2 days of onset of ARDS in patients with delayed onset of ARDS. In the parent cohort, onset of ARDS significantly delayed from ICU admission occurred in <10% of patients (9). Whole blood samples were centrifuged, and plasma was removed and stored at −80°C until analysis. The stability of IGF-1 and IGFBP-3 in frozen samples has been shown previously (25). Total IGF-1 and IGFBP-3 levels were measured using an automated IMMULITE assay on an IMMULITE 1000 instrument (Siemens, Malvern, PA). Lab personnel were blinded to clinical information.
Genotyping
DNA was extracted using PureGene DNA Isolation Kits (Gentra Systems, Research Triangle, NC). Five single nucleotide polymorphisms (SNPs) in the IGF pathway were previously chosen for analysis based on existing literature and information from the SNPseek Database (snp.wustl.edu/SNPseek/index.cgi last accessed November 17, 2009) which included >90,000 putatively functional human SNPs. SNPseek integrated data from the International HapMap project to include minor allele frequency distribution, population-specificity, and genomic annotations. SNPs were chosen for being functional, presumed functional, or tagging, and were also selected to be common with a minor allele frequency (MAF) > 0.05. The candidate SNPs ultimately included 3 polymorphisms on IGF1 (rs35767, rs1520220, rs2946834), and 1 each on IGFBP1 (rs4619) and IGFBP3 (rs2854746) (Table 1). SNPs were not selected specifically for Caucasians, but all have MAF >10% in all racial populations available in HapMap.
Table 1.
Single nucleotide polymorphisms genotyped
| Gene | Chr | SNP | Gene Region | MAF |
|---|---|---|---|---|
| IGF1 | 12 | rs35767 | Intergenic/Upstream | 16.2% |
| IGF1 | 12 | rs1520220 | Intron 3 | 19.4% |
| IGF1 | 12 | rs2946834 | Intergenic/Downstream | 35.2% |
| IGFBP1 | 7 | rs4619 | Exon | 33.5% |
| IGFBP3 | 7 | rs2854746 | Exon 1 | 44.6% |
Chr = chromosome; MAF = minor allele frequency, and is reported for the current cohort.
In 2008, these five SNPs were genotyped using 5’ nuclease allelic discrimination assays (Taqman®, Applied Biosystems, Foster City, CA) in over 1400 patients from the parent cohort. With this platform, allele detection is achieved by exonuclease cleavage of taq polymerase using custom primers and probes. In 2010, the high-density (50K) custom array designed by the Institute of Translational Medicine and Therapeutics, the Broad Institute, and the National Heart Lung and Blood Institute-supported Candidate gene Association Resource Consortium (ITMAT-Broad_CARe (IBC) array; Illumina®, San Diego, CA) was used to genotype over 2000 patients from the parent cohort. IBC samples were genotyped at the Center for Applied Genomics, Children’s Hospital of Philadelphia (Philadelphia, PA) using the gene-centric high-density chip (26). With the IBC chip, the SNP allele is distinguished by enzymatic single-base extension to incorporate a labeled nucleotide.
For the current study, results of both Taqman® and IBC genotyping platforms was combined to maximize patients with both biomarker and genotyping data available. Because of different technological designs which are associated with different allele-calling algorithms, a small number of discordant callings of genotypes among replicates is expected, typically <1%.In both methods, genotyping personnel were blinded to clinical information. For quality control, 10% of samples were randomly reanalyzed.
Statistical Analysis
All statistical analyses were performed using SAS Version 9.3 (SAS Inc., Cary, North Carolina).
Demographic and clinical characteristics between groups were compared using χ2 tests for categorical variables, and Student t-tests and/or nonparametric tests for continuous variables. Correlations between plasma IGF-1 and IGFBP-3 and clinical variables were estimated using Spearman correlation.
IGF-1 and IGFBP-3 were log-transformed to approximate normality, and log-transformed values were used in all models. A two-phase approach was used for model-building, first with phenotypic factors only, and then with genotypic factors included. First, linear regression models were used to examine the association between the acute and chronic health factors of interest and circulating IGF-1 and IGFBP-3 levels. Chronic factors included age, gender, BMI, diabetes, cirrhosis, and smoking status (current, past, never). The acute factors included Acute Physiology, Age and Chronic Health Evaluation (APACHE) III score as a marker of severity of illness; acute hepatic dysfunction; pneumonia including aspiration; presence of sepsis or septic shock; ARDS case status; and receipt of corticosteroids.
Acute hepatic dysfunction was defined as bilirubin ≥ 2.0 mg/dL within the first 24 hours of ICU admission. Because of the overlap between patients with cirrhosis and patients with elevated bilirubin at ICU admission, we considered models with separate variables for both cirrhosis and acute hepatic dysfunction (with cirrhotic patients excluded from the latter), as well as models with a single composite hepatic failure variable that included both cirrhosis and acute hepatic failure. ARDS case status was included based on our previous findings of an independent relationship between ARDS and circulating IGF-1 and IGFBP-3 (9). To avoid collinearity, score for age was removed from APACHE III in multivariable models also including age, and score for PaO2/FiO2 was removed in models including ARDS status. Receipt of corticosteroids was define as having received at least one dose of corticosteroid at any time during the first 3 days of critical illness. Multivariable models used a stepwise selection algorithm with conservative cutoffs of P ≤ 0.2 to enter and P ≤ 0.1 to stay. Models were refit with the surviving covariates.
In the second phase, linear regression models were used to examine the association between the SNPs of interest and circulating IGF-1 and IGFBP-3 levels. An additive genetic model was assumed for all SNPs. Univariate analyses examined relationships between IGF-1 and IGFBP-3 in association with genotype for each of the 5 SNPs of interest. Stepwise linear regression was then used to consider all relevant clinical covariates considered previously, along with genotypes of all five SNPs. Models were refit with the surviving covariates.
Final multivariable models thus considered the association of various factors to circulating IGF-1 and IGFBP-3 levels, with and without genetic contributions.
RESULTS
Patient population
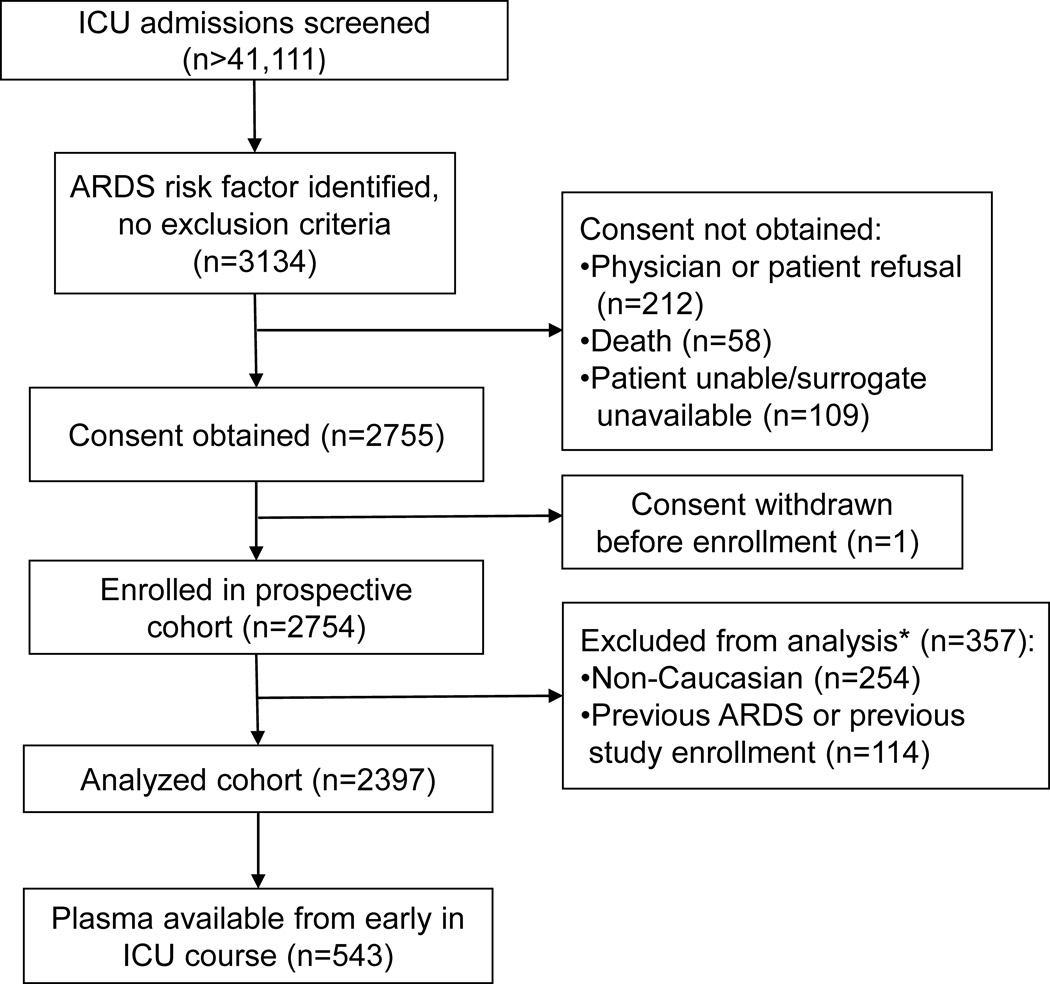
From January 1999 to March 2009, >44,111 consecutive ICU admissions were screened for possible inclusion (Figure 1). In total, 2397 patients were consented and enrolled in the parent study. Caucasians comprised >90% of the parent cohort, and given the lack of racial diversity of this cohort, the current nested study was limited to Caucasians to avoid population stratification.
Figure 1.
Study design and patient selection.
ICU = intensive care unit; ARDS = acute respiratory distress syndrome; *11 patients met both criteria for exclusion from current analysis.
There were 1854 patients who did not have available plasma for IGF-1 and IGFBP-3 measurements. There were no significant differences between those with and without plasma in terms of gender, diabetes, cirrhosis, sepsis or septic shock, pneumonia or aspiration, need for red cell transfusion, or trauma. There were, however, significant between-group differences in several variables: proportion of ARDS cases (33.9% with plasma vs. 25.6% without plasma; P = 0.0002); age (mean 63.8 ± 17.0 with plasma vs. 60.8 ± 17.6 without plasma; P = 0.0007); BMI (median 26.1, interquartile range (IQR) 7.5 with plasma vs. 26.6, IQR 8.0 without plasma; P = 0.039); and APACHE III (mean 73.3 ± 24.1 with plasma vs. 65.5 ± 23.0 without plasma; P < 0.0001).
Five-hundred forty-three patients had plasma measurements of IGF-1 and IGFBP-3 completed plus available genotype information for the SNPs of interest. Although the aim of the study was to analyze IGF-1 and IGFBP-3 at the earliest possible point in critical illness, 27 (<5%) patients had analysis of IGF-1 and IGFBP-3 from blood samples collected after Day 3 of ICU admission. These were all patients who developed ARDS delayed from ICU admission.
Baseline patient characteristics of the current cohort are shown in Table 2.Three-hundred twenty-nine (60.6%) patients received at least one dose of corticosteroid within the first 3 days of ICU admission; of these, 328 had a diagnosis of sepsis, septic shock, or ARDS. Of the patients with septic shock, 250 (88.3%) received at least one dose of corticosteroids within the first 3 days of ICU admission. All 184 patients with ARDS received at least one dose of corticosteroid within the first 3 days of ICU admission.
Table 2.
Baseline cohort characteristics.
| Characteristic | (n = 543) |
|---|---|
| Age, mean ± SD | 63.8 ± 17.0 |
| Female gender, n (%) | 210 (38.7) |
| BMI, median (IQR) | 26.1 (7.5) |
| Smoking status, n (%)a | |
| Never smoker | 155 (28.9) |
| Past smoker | 171 (31.9) |
| Current smoker | 110 (20.6) |
| Diabetes, n (%) | 141 (26.1) |
| Cirrhosis, n (%) | 25 (4.6) |
| Acute hepatic dysfunction, n (%)b | 65 (12.0) |
| APACHE III score, mean ± SD | 73.3 ± 24.0 |
| Sepsis syndrome, n (%)c | 179 (33.0) |
| Pulmonary source | 109 (60.9) |
| Septic shock, n (%)c | 283 (52.1) |
| Pulmonary source (%) | 143 (50.5) |
| Pneumonia, n (%) | 282 (51.9) |
| Aspiration, n (%) | 42 (7.7) |
| Trauma, n (%) | 39 (7.2) |
| ARDS cases, n (%) | 184 (33.9) |
| Total IGF-1 (ng/mL), median (IQR) | 67.3 (59.9) |
| IGFBP-3 (ng/mL), median (IQR) | 1989.7 (1626.1) |
SD = standard deviation; IQR = interquartile range; BMI = body mass index; APACHE = Acute Physiology, Age and Chronic Health Evaluation; IGFMR = molar ratio of IGF-1 and IGFBP-3.
Data missing for 107 (19.6%) patients.
Defined by bilirubin ≥ 2.0 mg/dL within 24 hours of ICU admission, and not including patients with chronic cirrhosis.
Sepsis syndrome does not include patients with septic shock; the total number of patients in the cohort with sepsis and/or septic shock is 462 (85.1%) with 252 (54.6%) from a pulmonary source
Phenotypic predictors of circulating IGF-1 and IGFBP-3
In univariate analyses of log-transformed biomarkers, significant (P < 0.05) predictors of plasma IGF-1 were age (β −0.0049, P = 0.007), BMI (β 0.0089, P = 0.031), smoking status (β −0.090, P = 0.042), diabetes (β −0.19, P = 0.008), cirrhosis (β −0.78, P < 0.0001), composite variable for acute and/or chronic hepatic dysfunction (β −0.39, P < 0.0001), sepsis/septic shock (β −0.28, P = 0.001), pneumonia/aspiration (β −0.13, P = 0.036), ARDS (β −0.28, P < 0.0001), APACHE III (β −0.0096, P <0.0001), and receipt of corticosteroids (β −0.38, P <0.0001).
Significant predictors of IGFBP-3 were similar: age (β −0.0072, P <0.0001), BMI (β 0.013, P 0.0002), female gender (β 0.11, P = 0.049), cirrhosis (β −0.78, P <0.0001), acute hepatic dysfunction β −0.062, P = 0.46), sepsis/septic shock (β −0.28, P = 0.0002), pneumonia/aspiration (β −0.14, P = 0.009), ARDS (β −0.19, P = 0.001), APACHE III (β −0.0058, P <0.0001), and receipt of corticosteroids (β −0.28, P <0.0001).
The final models of phenotypic variables in association with IGF-1 and IGFBP-3 are shown in Table 3. BMI, cirrhosis, and ARDS were strongly associated with both IGF-1 and IGFBP-3; APACHE III was strongly associated with IGF-1; and age was strongly associated with IGFBP-3.
Table 3.
Parameter estimates for multivariable models of plasma IGF-1 and IGFBP-3.
| logIGF-1 | P | logIGFBP-3 | P | |
|---|---|---|---|---|
| Age | NS | -- | −0.0068 | <0.0001 |
| Female | NS | -- | NS | -- |
| Smoking | NS | -- | NS | -- |
| BMI | 0.010 | 0.010 | 0.010 | 0.003 |
| Diabetes | −0.17 | 0.013 | NS | -- |
| Cirrhosis | −0.61 | <0.0001 | −0.76 | <0.0001 |
| Sepsis/septic shock | NS | -- | −0.19 | 0.009 |
| Pneumonia/aspiration | NS | -- | NS | -- |
| APACHE III | −0.0064 | <0.0001 | NS | -- |
| ARDS | −0.22 | 0.0005 | −0.19 | 0.0004 |
| Adjusted R-square | 0.145 | -- | 0.160 | -- |
Cells show parameter estimates (β) except as noted. IGF-1 is measured in ng/mL; IGFBP-3 is measured in ug/mL. NS = not significant (variables that did not survive the selection algorithm prior to model refit); BMI = body mass index; ARDS = acute respiratory distress syndrome; APACHE = Acute Physiology, Age and Chronic Health Evaluation.
Acute hepatic dysfunction (exclusive of cirrhosis) was not significant in multivariable models. The composite variable for acute/chronic hepatic dysfunction was significant, but this appeared to be driven by cirrhosis with the parameter estimate for cirrhosis alone being much larger with a lower P value. The inclusion of cirrhosis vs. composite hepatic dysfunction did not significantly affect parameter estimates of other variables. Thus, cirrhosis alone was included in the final models.
Receipt of steroids appeared to act as a confounder for sepsis/septic shock and/or ARDS. Receipt of steroids was indeed highly correlated with the presence of septic shock and/or ARDS (ρ = 0.83, P < 0.0001).
IGF pathway genotyping
Of the 543 patients with plasma IGF-1 and IGFBP-3 measurements, 479 had genotyping using the IBC platform, and 445 had genotyping using the Taqman platform. There were 381 patients with genotypes from both platforms. Genotype discrepancies between platforms for the 5 SNPs of interest were found in <0.5% of patients, and these were excluded. Overall genotyping success rate was 99%.
IGF pathway genotypes and circulating IGF-1 and IGFBP-3
There were no significant deviations from Hardy-Weinberg equilibrium for any polymorphisms tested. In univariate analyses, there was no significant association between log-transformed IGF-1 and any of the 5 candidate SNPs (rs35767, rs1520220, rs2946834, rs4619, rs2854746). For IGFBP-3, however, there was a significant association with rs2854746 genotype (β 0.12, P = 0.0025).
In multivariable models that included both genotype and clinical variables, rs2854746 genotype remained a significant predictor of IGFBP-3 (Table 4). Because of the strong correlation between IGF-1 and IGFBP-3, we took a conservative approach and forced rs2854746 genotype in to the final multivariable model of IGF-1, although it had been rejected in the selection algorithm (Table 4). Models were very stable with no changes in significant clinical variables, and with minimal change in parameter estimates for those variables when adding rs2854746 to the models. This suggests that genotype added additional information to the model of IGFBP-3 and is not a confounder. This is further supported by an increase in adjusted R-square with rs2854746 genotype explaining and additional 2% of variation in IGFBP-3 levels than clinical variables alone.
Table 4.
Parameter estimates for multivariable models of plasma IGF-1 and IGFBP-3, including genotype at rs2854746 (IGFBP3).
| logIGF-1 | P | logIGFBP-3 | P | |
|---|---|---|---|---|
| Age | NS | -- | −0.0067 | <0.0001 |
| Female | NS | -- | NS | -- |
| Smoking | NS | -- | NS | -- |
| BMI | 0.011 | 0.008 | 0.011 | 0.002 |
| Diabetes | −0.18 | 0.012 | NS | -- |
| Cirrhosis | −0.61 | <0.001 | −0.77 | <0.0001 |
| Sepsis/septic shock | NS | -- | −0.18 | 0.014 |
| Pneumonia/aspiration | NS | -- | NS | -- |
| APACHE III | −0.0063 | <0.0001 | NS | -- |
| ARDS | −0.23 | 0.0003 | −0.21 | 0.0001 |
| rs2854746a | 0.075 | 0.081 | 0.13 | 0.0006 |
| Adjusted R-square | 0.148 | 0.179 |
Genotype forced in to model for logIGF-1. Cells show parameter estimates (β) except as noted. IGF-1 is measured in ng/mL; IGFBP-3 is measured in ug/mL. NS = not significant (variables that did not survive the selection algorithm prior to model refit); BMI = body mass index; ARDS = acute respiratory distress syndrome; APACHE = Acute Physiology, Age and Chronic Health Evaluation.
DISCUSSION
The somatotropic axis including GH, IGF-1 and IGFBP-3 has been of great interest in critical care because of its relationships to metabolism, catabolism, and nutritional status, factors that are important during critical illness. These factors may also have significant prognostic implications for recovery from critical illness. Thus, we sought to further elucidate derangements in the somatotropic axis during critical illness by better understanding contributors to IGF-1 and IGFBP-3 levels in acute critical illness.
In this ICU cohort, acute and chronic health factors as well as genetic factors, were associated with circulating IGF-1 and IGFBP-3. While there were numerous strong associations, the clinical and genetic variables explained only a modest amount of variability in IGF-1 and IGFBP-3 which is consistent with prior studies (13, 19). The most variability was explained for IGFBP-3, also consistent with prior studies (13, 19). However, perhaps most interesting is that age and gender, factors that have been strongly and consistently associated with IGF-1 (11–14, 16, 18, 19), were insignificant in the face of critical illness. This is consistent with a prior study of 88 patients with a range of illness severity showing no association between age or gender and IGF-1 measured early in critical illness (27). Gender was also not significantly associated with IGFBP-3 in our cohort. BMI has had very inconsistent and largely null associations with IGF-1 and IGFBP-3 outside of critical illness (12–14). Yet there was a strong association between BMI and both IGF-1 and IGFBP-3 levels in our study. Given that IGF-1 and IGFBP-3 are hepatically synthesized, it is not surprising that there was a strong negative relationship between hepatic dysfunction and circulating levels. As above, this effect appeared to be driven by cirrhosis and not acute hepatic dysfunction.
We also saw a strong relationship with severity of illness. For IGF-1, this manifested as a negative association with APACHE III; for IGFBP-3 there was a negative association with both pneumonia and sepsis. This was not seen in at least two prior studies in critically ill patients (2, 28). Consistent with our prior work in this cohort, ARDS was also significantly associated with lower IGF-1 and IGFBP-3 levels (9).
Although our cohort is made up exclusively of patients with risk factors for ARDS, it is generally representative of the medically critically ill patient population with high severity of illness and 88% of patients meeting criteria for sepsis and/or septic shock, pneumonia, or aspiration. Although our cohort does include 39 patients with major trauma as a risk factor for ARDS, 7 of those patients also had pneumonia, aspiration, sepsis, or septic shock, or a combination of these. Thus, only 5.9% of our cohort had trauma without other medical critical illness.
With the large number patients with sepsis and ARDS, we found that the majority of our patients received corticosteroids within the first 3 days of ICU admission, consistent with standard of care for septic shock and ARDS during most of the enrollment period represented. There is evidence that glucocorticoid treatment can down-regulate expression of IGF-1 and its receptor (IGF-1R). However, it has been shown that glucocorticoid treatment does not affect circulating free or total IGF-1, nor circulating IGFBP-3 (29). Thus, we do not believe our results are significantly altered by corticosteroid administration, and any small effect that might be present would be well-distributed across the cohort.
Most studies measuring IGF-1 in critically ill patients have not measured IGFBP-3 (1, 27, 28, 30). The few existing ICU studies measuring both IGF-1 and IGFBP-3 are all small (2, 3, 8). In these studies, IGFBP-3 has shown less consistent decreases than IGF-1, perhaps because IGFBP-3 has a long half-life, and although critically ill patients are acutely catabolic, the effects of protein catabolism may not be reflected in lower IGFBP-3 levels right away (6). We did see low levels of both IGF-1 and IGFBP-3 in this study, however, which may speak to the high level of illness severity in our patient cohort.
In our study, the minor allele of rs2854746 (Ala32Gly) in IGFBP3 was associated with increasing circulating IGFBP-3. Rs2854746 is a non-synonymous missense polymorphism that is a putative exon splicing silencer and enhancer. Rs2854746 is in the same haplotype block as rs2854744, the most extensively studied IGFBP3 SNP, and these two SNPs are in strong linkage disequilibrium (LD). Phenotypically, rs2854746 has been associated with increases in circulating IGFBP-3 in several studies (11, 17, 18, 20). Cheng, et al. hypothesized that higher levels of IGFBP-3 due to genetic variation may decrease IGF-1 bioavailability ultimately affecting its bioactivity in circulation and tissues (18). This may be particularly relevant in critical illness when there is global depression of IGF-1 and IGFBP-3. We did not see any significant relationships, either in univariate or multivariate analyses, between IGF-1 or IGFBP-3 and any other polymorphisms tested. In studies of non-critically ill patients, rs35767 has been associated with higher IGF-1 in at least one study (17), with no significant association in other studies (11, 20). Results for rs1520220 and rs2946834 have been similarly mixed with higher IGF-1 seen in some studies (11, 17), particularly among women (19), but no relationship seen elsewhere (20). Rs4619 has been less well-studied, but it has been of interest because it is a nonsynonymous missense polymorphism in a coding region. No consistent relationship has been found between rs4619 and circulating IGF-1 or IGFBP-3 (17, 20).
While caution must be taken in interpreting the significance of limited SNP data in a single study, our findings are bolstered by the associations between rs2854746 and increased circulating IGFBP-3 observed in non-critically ill cohorts (11, 17, 18, 20). But just as with IGF-1 levels, it is likely that there are many genetic (and non-genetic) determinants of IGFBP-3 that remain undefined (31). It is clear that circulating IGF-1 and IGFBP-3 are complex traits, and it may be that the small effects of individual polymorphisms are overshadowed by the influences of acute critical illness.
This study has several strengths including that, to our knowledge, this is the largest study examining both IGF-1 and IGFBP-3 in critically ill patients, and the first study to examine genetic polymorphisms in the IGF pathway in critically ill patients. This is also the first study to combine both phenotypic and genotypic data to model the IGF pathway in an ICU cohort. The cohort is a prospectively enrolled, thoroughly characterized cohort with minimal misclassification of clinical variables. Biomarker measurements were done using state-of-the-art methodology by personnel blinded to clinical information. Assay variability was minimal, and although assay errors cannot be excluded, such errors would result in random misclassification, biasing toward the null. Genotyping was high-quality with minimal failures or discrepancies between the two platforms used.
There are several limitations to this study. This is an observational study with measurements of IGF-1 and IGFBP-3 measured at one time point only, although the earliest point in critical illness was chosen because the majority of ICU patients demonstrate acute and often severe derangements at this early point, whereas derangements at later time points would be expected to vary substantially based on the trajectory of illness toward recovery, death, or chronic critical illness. We have not examined variables that may be more reflective of muscle weakness as a consequence of protein catabolism, and IGF-1 and IGFBP-3 levels at later time points may be relevant to such variables, particularly in patients with prolonged critical illness and those who develop significant muscle weakness.
Although our patient cohort is very well-characterized, we do not have information about significant non-diabetic endocrine comorbidities such as thyroid or adrenal disease, both of which could affect the IGF pathway. We also do not have detailed information on hormone supplements such as oral contraceptives or post-menopausal hormone replacement therapy. We did not measure GH levels, and thus we cannot comment on the true relationship between GH and the biomarkers measured. Despite this, GH has been well-characterized as consistently elevated in acute critical illness, absent any primary disorders of GH. We do not have detailed data on insulin administration around the time of blood sampling, although when IGF-1 is measured in outpatient clinical settings, patients are not generally instructed to avoid insulin. In uncontrolled Type 1 diabetes, IGF-1 levels are known to be low and subsequently rise with adequate insulin treatment, but the story in Type 2 diabetes is much less clear with no consistent changes in IGF-1 levels compared to non-diabetic controls (31). It seems the more relevant factor may be nutritional status, specifically malnutrition or recent starvation. We did not have any dietary information from the time around ICU admission, and we know that nutritional status is an important regulator of circulating IGF-1 in particular with low levels seen in acute starvation and chronic malnutrition (31). We would expect relative starvation with poor nutritional intake as part of the immediate prodrome of acute critical illness extending into the early phase of ICU admission (corresponding with timing of blood draws). However, it is an important limitation that we were unable to include nutritional variables in our analyses.
Although we have data on smoking status, the categorical smoking variable is imprecise, and there is missing data for almost 20% of the cohort.
We only tested associations with a limited number of candidate SNPs, and there may be additional information genotypes or haplotypes not tested here.
Finally, we acknowledge the possibility of selection bias given some significant differences between patients with available plasma and genotyping data, and those without this information. This stems primarily from difficulty in rapidly enrolling and obtaining blood samples after ICU admission, a challenge commonly encountered in critical care research. We did include those variables that differed between groups (age, BMI, APACHE III, and ARDS case status) in our multivariable models. It is still possible that there are additional unmeasured variables that differ between these groups. Such differences, however, would be more likely to affect generalizability than internal validity.
CONCLUSION
Both acute and chronic health factors significantly influence circulating levels of IGF-1 and IGFBP-3 early in critical illness. Rs2854746 is also significantly associated with IGFBP-3 levels in this ICU cohort, even after adjustment for other relevant covariates. Overall, these combined phenotypic and genotypic factors explain only a modest amount of variability in IGF-1 and IGFBP-3. Further research is needed to understand how these findings may be directly applied to patient care.
ACKNOWLEDGEMENTS
We thank Yael Tarshish, Hanae Fujii-Rios, Kezia Ellison, and Ian Taggart for patient recruitment; Elizabeth Baker, Lauren Searl, and Konstantinos Aronis for specimen processing; Marcia Chertok, Julie DelPrato, and Janna Frelich for data management; and Andrea Shafer for research support.
Conflicts of Interest and Source of Funding: AMA is currently receiving a grant (10FTF3440007) from the American Heart Association. BTT has received a one-time honorarium from GlaxoSmithKline and is currently receiving funding (1U01HL123009-1) from the National Institutes of Health/National Heart, Lung & Blood Institute (NHLBI). DCC is currently receiving funding from the National Institutes of Health (NHLBI: R01HL060710 and T32HL116275; National Cancer Institute: P30CA006516 and R01CA092824; National Institute of Environmental Health Sciences: R01 ES009860 and T32 ES007069); and from the Centers for Disease Control (National Institute for Occupational Safety & Health: R01OH002421 and T42OH008416). For the remaining authors, none were declared. The current work was supported by the National Institutes of Health (R01HL60710) and the American Heart Association (10FTF3440007).
Copyright form disclosures:
Dr. Ahasic received support for article research from the National Institutes of Health (NIH) and the American Heart Association. Her institution received grant support from the American Heart Association (Dr. Ahasic has a career development grant funding this research, disclosed in the manuscript) and received support for travel from the American Heart Association (The career development grant allows up to $3000/yr for travel to academic conferences relevant to the current research. No travel money has been received outside of this grant). Dr. Mantzoros disclosed government work. Dr. Thompson consulted for GlaxoSmithKline (One time ARDS advisory board meeting) and received support for article research form the NIH. His institution received grant support from the NHLBI (Conduct sepsis and ARDS clinical research). Dr. Christiani received support for article research from the NIH. Dr. Christiani and his institution received grant support from the NIH (NHLBI).
Footnotes
The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Takala J, Ruokonen E, Webster NR, et al. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341(11):785–792. doi: 10.1056/NEJM199909093411102. [DOI] [PubMed] [Google Scholar]
- 2.Schuetz P, Müller B, Nusbaumer C, et al. Circulating levels of GH predict mortality and complement prognostic scores in critically ill medical patients. Eur J Endocrinol. 2009;160(2):157–163. doi: 10.1530/EJE-08-0786. [DOI] [PubMed] [Google Scholar]
- 3.Timmins AC, Cotterill AM, Hughes SC, et al. Critical illness is associated with low circulating concentrations of insulin-like growth factors-I and -II, alterations in insulin-like growth factor binding proteins, and induction of an insulin-like growth factor binding protein 3 protease. Crit Care Med. 1996;24(9):1460–1466. doi: 10.1097/00003246-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Van den Berghe G. Growth hormone secretagogues in critical illness. Horm Res. 1999;51(Suppl 3):21–28. doi: 10.1159/000053158. [DOI] [PubMed] [Google Scholar]
- 5.Elijah IE, Branski LK, Finnerty CC, et al. The GH/IGF-1 system in critical illness. Best Pract Res Clin Endocrinol Metab. 2011;25(5):759–767. doi: 10.1016/j.beem.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang CH, Frost RA. Role of growth hormone, insulin-like growth factor-I, and insulin-like growth factor binding proteins in the catabolic response to injury and infection. Curr Opin Clin Nutr Metab Care. 2002;5(3):271–279. doi: 10.1097/00075197-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Mesotten D, Van den Berghe G. Changes within the GH/IGF-I/IGFBP axis in critical illness. Crit Care Clin. 2006;22(1):17–28. doi: 10.1016/j.ccc.2005.09.002. v. [DOI] [PubMed] [Google Scholar]
- 8.Weber-Carstens S, Deja M, Koch S, et al. Risk factors in critical illness myopathy during the early course of critical illness: a prospective observational study. Crit Care. 2010;14(3):R119. doi: 10.1186/cc9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahasic AM, Zhai R, Su L, et al. IGF1 and IGFBP3 in acute respiratory distress syndrome. Eur J Endocrinol. 2012;166(1):121–129. doi: 10.1530/EJE-11-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemmons DR. Involvement of insulin-like growth factor-I in the control of glucose homeostasis. Curr Opin Pharmacol. 2006;6(6):620–625. doi: 10.1016/j.coph.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 11.D'Aloisio AA, Schroeder JC, North KE, et al. IGF-I and IGFBP-3 polymorphisms in relation to circulating levels among African American and Caucasian women. Cancer Epidemiol Biomarkers Prev. 2009;18(3):954–966. doi: 10.1158/1055-9965.EPI-08-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morimoto LM, Newcomb PA, White E, et al. Variation in plasma insulin-like growth factor-1 and insulin-like growth factor binding protein-3: personal and lifestyle factors (United States) Cancer Causes Control. 2005;16(8):917–927. doi: 10.1007/s10552-005-2702-3. [DOI] [PubMed] [Google Scholar]
- 13.DeLellis K, Rinaldi S, Kaaks RJ, et al. Dietary and lifestyle correlates of plasma insulin-like growth factor-I (IGF-I) and IGF binding protein-3 (IGFBP-3): the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2004;13(9):1444–1451. [PubMed] [Google Scholar]
- 14.Kaklamani VG, Linos A, Kaklamani E, et al. Age, sex, and smoking are predictors of circulating insulin-like growth factor 1 and insulin-like growth factor-binding protein 3. J Clin Oncol. 1999;17(3):813–817. doi: 10.1200/JCO.1999.17.3.813. [DOI] [PubMed] [Google Scholar]
- 15.Kaklamani VG, Linos A, Kaklamani E, et al. Dietary fat and carbohydrates are independently associated with circulating insulin-like growth factor 1 and insulin-like growth factor-binding protein 3 concentrations in healthy adults. J Clin Oncol. 1999;17(10):3291–3298. doi: 10.1200/JCO.1999.17.10.3291. [DOI] [PubMed] [Google Scholar]
- 16.Harrela M, Koistinen H, Kaprio J, et al. Genetic and environmental components of interindividual variation in circulating levels of IGF-I, IGF-II, IGFBP-1, and IGFBP-3. J Clin Invest. 1996;98(11):2612–2615. doi: 10.1172/JCI119081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel AV, Cheng I, Canzian F, et al. IGF-1, IGFBP-1, and IGFBP-3 polymorphisms predict circulating IGF levels but not breast cancer risk: findings from the Breast and Prostate Cancer Cohort Consortium (BPC3) PLoS One. 2008;3(7):e2578. doi: 10.1371/journal.pone.0002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng I, DeLellis Henderson K, Haiman CA, et al. Genetic determinants of circulating insulin-like growth factor (IGF)-I, IGF binding protein (BP)-1, and IGFBP-3 levels in a multiethnic population. J Clin Endocrinol Metab. 2007;92(9):3660–3666. doi: 10.1210/jc.2007-0790. [DOI] [PubMed] [Google Scholar]
- 19.Al-Zahrani A, Sandhu MS, Luben RN, et al. IGF1 and IGFBP3 tagging polymorphisms are associated with circulating levels of IGF1, IGFBP3 and risk of breast cancer. Hum Mol Genet. 2006;15(1):1–10. doi: 10.1093/hmg/ddi398. [DOI] [PubMed] [Google Scholar]
- 20.Su X, Colditz GA, Willett WC, et al. Genetic variation and circulating levels of IGF-I and IGFBP-3 in relation to risk of proliferative benign breast disease. Int J Cancer. 2010;126(1):180–190. doi: 10.1002/ijc.24674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jernström H, Deal C, Wilkin F, et al. Genetic and nongenetic factors associated with variation of plasma levels of insulin-like growth factor-I and insulin-like growth factor-binding protein-3 in healthy premenopausal women. Cancer Epidemiol Biomarkers Prev. 2001;10(4):377–384. [PubMed] [Google Scholar]
- 22.Gong MN, Thompson BT, Williams P, et al. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33(6):1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 23.Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101(6):1481–1483. doi: 10.1378/chest.101.6.1481. [DOI] [PubMed] [Google Scholar]
- 24.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 25.Ito Y, Nakachi K, Imai K, et al. Stability of frozen serum levels of insulin-like growth factor-I, insulin-like growth factor-II, insulin-like growth factor binding protein-3, transforming growth factor beta, soluble, Fas, and superoxide dismutase activity for the JACC study. J Epidemiol. 2005;15(Suppl 1):S67–S73. doi: 10.2188/jea.15.S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Y, Lanktree MB, Taylor KC, et al. Gene-centric meta-analyses of 108 912 individuals confirm known body mass index loci and reveal three novel signals. Hum Mol Genet. 2013;22(1):184–201. doi: 10.1093/hmg/dds396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyle UG, Jolliet P, Genton L, et al. Clinical evaluation of hormonal stress state in medical ICU patients: a prospective blinded observational study. Intensive Care Med. 2005;31(12):1669–1675. doi: 10.1007/s00134-005-2832-9. [DOI] [PubMed] [Google Scholar]
- 28.Hajsadeghi S, Khamseh ME, Gholami S, et al. IGF-I concentration and changes in critically ill patients. J Res Med Sci. 2011;16(2):170–178. [PMC free article] [PubMed] [Google Scholar]
- 29.Frystyk J, Schou AJ, Heuck C, et al. Prednisolone reduces the ability of serum to activate the IGF1 receptor in vitro without affecting circulating total or free IGF1. Eur J Endocrinol. 2013;168(1):1–8. doi: 10.1530/EJE-12-0518. [DOI] [PubMed] [Google Scholar]
- 30.Kythreotis P, Kokkini A, Avgeropoulou S, et al. Plasma leptin and insulin-like growth factor I levels during acute exacerbations of chronic obstructive pulmonary disease. BMC Pulm Med. 2009;9:11. doi: 10.1186/1471-2466-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clemmons DR. Clinical utility of measurements of insulin-like growth factor 1. Nat Clin Pract Endocrinol Metab. 2006;2(8):436–446. doi: 10.1038/ncpendmet0244. [DOI] [PubMed] [Google Scholar]