Abstract
Background
Plasma levels of cardiotonic steroids (CTS) are elevated in volume-expanded states such as chronic kidney disease, but the role of these natriuretic hormones in subjects with heart failure (HF) is unclear. We sought to determine the prognostic role of the CTS marinobufagenin (MBG) in HF, particularly in relation to long-term outcomes.
Methods and Results
We first measured plasma MBG levels and performed comprehensive clinical, laboratory, and echocardiographic assessment in 245 HF patients. All-cause mortality, cardiac transplantation, and HF hospitalization were tracked for 5 years. In our study cohort, median [interquartile range] MBG was 583 [383-812] pM. Higher MBG was associated with higher myeloperoxidase (MPO, r=0.42, p<0.0001), BNP (r=0.25, p=0.001), and asymmetric dimethylarginine (ADMA, r=0.32, p<0.001). Elevated levels of MBG were associated with measures of worse right ventricular function (RV s’: r= −0.39, p<0.0001) and predicted increased risk of adverse clinical outcomes (MBG ≥574 pM: HR 1.58 [1.10-2.31], p=0.014) even after adjustment for age, gender, diabetes mellitus, and ischemic etiology. In mice, a left anterior descending coronary artery ligation model of heart failure lead to increases in MBG, while infusion of MBG into mice for 4 weeks lead to significant increases in MPO, ADMA, and cardiac fibrosis.
Conclusions
In the setting of heart failure, elevated plasma levels of MBG are associated with right ventricular dysfunction and predict worse long-term clinical outcomes in multivariable models adjusting for established clinical and biochemical risk factors. Infusion of MBG appears to directly contribute to increased nitrative stress and cardiac fibrosis.
Keywords: heart failure, cardiotonic steroids, outcome, nitrative stress, cardiac fibrosis
Cardiotonic steroids (CTS) are a class of endogenous volume-sensitive hormones that bind to the Na/K-ATPase, and include cardenolides (such as digoxin and ouabain) as well as aglycone bufadienolides (such as telocinobufagin and marinobufagenin [MBG])1, 2. Increased circulating levels of CTS have been proposed as a compensatory mechanism for natriuresis and vascular tone in volume-expanded conditions including salt-sensitive hypertension, chronic kidney disease, and preeclampsia3-5. In these settings, CTS contribute to enhanced natriuresis by inducing endocytosis of proximal tubule cell Na/K-ATPase. This serves to remove the Na/K-ATPase from the basolateral membrane and thus reduces the transport of sodium from the tubular lumen to the blood compartment, thereby increasing sodium excretion6, 7. However, CTS may exert “off-target” signal transduction effects beyond their direct effects on the sodium pump2, 8. Hence, chronic stimulation of Na/K-ATPase signaling by CTS has important implications for not only for the natriuretic response to increased salt load9 but also has been implicated in pathological adaptation to volume expansion including hypertension, hypertrophy, and fibrosis10, 11. Accumulation of MBG and other CTS has been documented in a variety of cardiovascular disease states beyond conditions marked by plasma volume expansion and fluid retention4, 12-15. Furthermore, myocardial hypertrophy and growth effects of CTS have been proposed in the setting of hypertension and renal dysfunction16, and supported by both experimental and clinical data demonstrating the association between an endogenous “digoxin-like substance” and the development and severity of heart failure (HF)17, 18. Clinical and experimental evidence from our group and others has also demonstrated the pro-oxidant and pro-fibrotic effects of these steroid hormones in both cardiac and renal tissue19-23. As the relationship among MBG and myocardial structure and performance in the contemporary HF population has not been examined, we hypothesized that an increase in circulating MBG levels may track with disease severity and provide important prognostic information in the setting of HF. Further, as MBG in increased in volume overloaded states and associated with diastolic dysfunction in animal models, we hypothesized that circulating levels would be increased in HF patients with preserved ejection fraction and associate with echocardiographic indices of diastolic dysfunction. Herein, we examined the relationship among circulating levels of MBG with echocardiographic parameters, as well as long-term adverse clinical outcomes in patients with HF. We also used an animal infusion model to demonstrate the contribution of MBG to the clinical phenotype observed in our human study.
Methods
Study Design and Population
We prospectively enrolled 245 HF patients (≥18 years) seen at the Cleveland Clinic with a clinical diagnosis of HF and New York Heart Association (NYHA) functional class I-IV symptoms, who were free of significant renal, hepatic, and valvular diseases. Study participants were excluded if they experienced any of the following: (1) major cardiovascular event (myocardial infarction, unstable angina, stroke, transient ischemic attack, pulmonary embolism) within the preceding 30 days; (2) significant lung diseases, including chronic obstructive pulmonary disease, pulmonary fibrosis, pulmonary arterial hypertension, and asthma; or (3) major surgery or use of inotropic agents within the past month. We prospectively followed a composite endpoint including adverse clinical events (all-cause mortality, cardiac transplantation and HF hospitalization) and all-cause mortality through a process confirmed by chart review and the Social Security Death Index. Thirteen sex-matched apparently healthy volunteer participants without a history of heart failure served as non-heart failure controls. They were prospectively recruited outside of any healthcare institution setting, and did not report any active medical conditions at the time of blood draw. The study protocol was approved by the Cleveland Clinic Institutional Review Board and written informed consent was obtained from each of the study participants prior to their participation in the study.
Biochemical Assays
Blood samples were collected in ethylenediaminetetraacetic acid (EDTA)-plasma vacutainers at the time of clinical and echocardiographic and hemodynamic evaluation, were immediately aliquotted and stored at −80°C until analysis. Plasma samples were extracted for MBG measurements using C18 SepPak cartridges (Waters Inc., Cambridge, MA) and MBG levels were measured using a competitive fluoroimmunoassay [dissociation enhanced fluoroimmunoassay (DELFIA)] as previously described22, 24. The MBG DELFIA uses a murine monoclonal antibody (anti-MBG 4G4) and employs competition between immobilized antigen (MBG-glycoside-thyroglobulin) and MBG, other cross-reactants, or endogenous cardiotonic steroids within the sample for a limited number of binding sites on the 4G4 anti-MBG monoclonal antibodies. Secondary (goat anti-mouse) europium labeled antibody was obtained from Perkin-Elmer (Waltham, MA). Data on cross reactivity of the MBG antibody have been reported previously3, 23. For analysis of MBG tissue levels, adrenal glands were homogenized (TissueLyserII, Quiagen, 5 mm stainless steel beads) and homogenate was extracted with 10 fold excess of methyl tert-butyl ether before drying under nitrogen gas and resuspension in assay buffer (50 mM Tris-hydrochloride, 154 mM sodium chloride, 7.7 mM sodium azide, pH 7.8). Plasma myeloperoxidase (MPO) levels were determined by an enzyme-linked immunosorbent assay (CardioMPO II test, Cleveland Heart Labs, Cleveland OH). Arginine metabolomic profiles were quantified by liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) as previously described25, 26. All other assays including BNP, basic metabolic profile and lipid profile were measured on the Architect platform (Abbott Laboratories, Abbott Park IL). The 4-variable Modification of Diet in Renal Disease (MDRD) equation was used to calculate estimated glomerular filtration rate (eGFR)27.
Transthoracic Echocardiography
Comprehensive 2-dimensional echocardiography was performed in standard parasternal and apical views on all participants by an American Society of Echocardiography registered research sonographer using a Vivid 7 echocardiography machines (GE Healthcare, Waukesha WI) equipped with a phased-array transducer and following the American Society of Echocardiography recommendations28, 29. Further detail on echocardiographic analysis is described in the Supplemental Material.
Animal study
In order to assess MBG levels in a mouse model of post-myocardial infarction heart failure, left anterior descending (LAD) artery ligation was performed in C57BL6J mice and plasma MBG was assayed after 4 weeks. Briefly, mice were intubated and ventilated with 60% oxygen at 120 breaths per minute with an inspiratory pressure of 16 to 18 cm H2O using a rodent ventilator (Harvard Apparatus). After sternotomy was performed, the left atrium was retracted for visualization of the proximal LAD using a surgical microscope (Leica M500) and the LAD was ligated with 10-0 prolene suture. Blanching and dysfunction of the anterior wall verified LAD ligation. To directly test for a potential contribution of MBG to promotion of cardiac dysfunction and nitrative stress, osmotic minipumps (Alzet® model 1004) were placed intraperitoneally in order to deliver MBG (0.1 ug/g/day) or vehicle to mice for 4 weeks similar to what we have reported in the rat22. Quantitative real-time PCR was used to measure gene expression with 18S rRNA used as the internal control (TaqMan®, Life Technologies). These studies were approved by the Cleveland Clinic Institutional Animal Care and Use Committee and the procedures followed were in accordance with institutional guidelines.
Quantitative Histologic Techniques
Mason’s trichome and picosirius red staining was performed on deparafinized 5 μm serial heart sections. The sections were mounted under a Leica DM 2500 microscope and digitized with a QImaging MicroPublisher 5.0 RTV camera. Further detail on quantitative morphometric analysis is described in the Supplemental Material.
Statistical Analysis
Normally distributed continuous variables were summarized as mean ± standard deviation if normally distributed, or median (interquartile range [IQR]) if non-normally distributed. If more than two groups were compared, one-way analysis of variance (ANOVA) was performed prior to comparison of individual groups with the unpaired Student’s t-test with Bonferroni’s correction for multiple comparisons. If only two groups of normal data were compared, the Student’s t-test was used without correction. P values for non-parametric comparisons or those based on small sample size were performed using the Mann-Whitney test. Associations between changes in MBG and clinical, biochemical, and echocardiographic measures were performed using the Spearman’s rank correlation method. Clinical risk and time to clinical adverse events associated with increased MBG levels were assessed by Cox proportional hazard analysis and the units for hazard ratios represent the dichotomized values of MBG ≥574 pM and <574 pM. The optimal sensitivity and specificity cutoff of MBG (≥ 574 pM) levels in our cohort was determined using receiver operator characteristic (ROC) curve analyses in the context of the time to event and the Hazard Ratios reported represent the risk associated with MBG levels ≥ 574 pM. This value was chosen as the optimal ROC cut-point that maximized sensitivity plus specificity in ROC curve analysis of the nominal logistic regression fit between MBG modeled as a continuous variable and adverse clinical outcomes modeled as a nominal dichotomous variable. Survival curves (all-cause mortality, cardiac transplantation or HF hospitalization) were generated from Kaplan-Meier survival analysis. Continuous net reclassification improvement (NRI) was used as test of association and integrated discrimination improvement (IDI) was to measure improvement in model performance. The p values compare models with and without MBG. The model was adjusted for traditional risk factors, including age, sex, diabetes mellitus and ischemic etiology. Statistical analysis was performed using GraphPad Prism®, JMP 10.0 (SAS Institute, Cary, NC), and R 3.1.2 (Vienna, Austria). P values <0.05 were considered statistically significant.
Results
Subject Characteristics
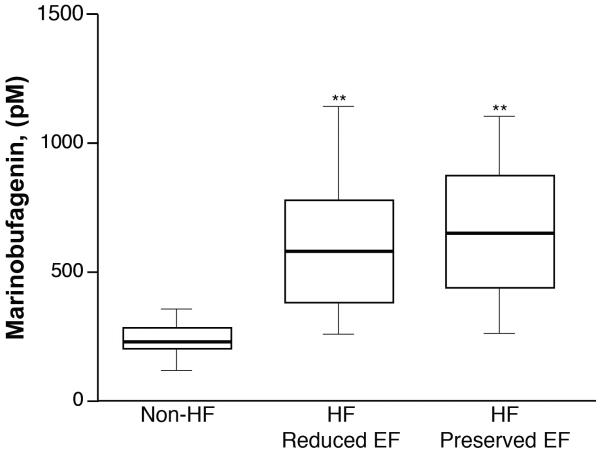
Baseline characteristics of our study cohort are presented in the Table. Median MBG level was 583 [IQR 383-812] pM in patients with HF compared to 241 [IQR 212, 281] pM in non-HF controls (n=13, mean age 43±12 years, 64% male, body mass index 27±3, 15% African American). Patients with HF have higher MBG levels when compared to that of non-HF controls, regardless of whether they have reduced or preserved LVEF (Figure 1). Furthermore, higher MBG was associated with higher indices of inflammation/oxidative stress (MPO: r=0.42, p<0.0001), myocardial stress (BNP: r=0.25, p=0.001), and nitrative stress [asymmetric dimethylarginine (ADMA): r=0.32, p<0.001; symmetric dimethylarginine (SDMA): r=0.34, p<0.001; and mono methyl arginine (MMA): r=0.40, p<0.0001]. In our study cohort, there was no significant association between MBG and cystatin C (p=0.485) and estimated GFR (p=0.345).
Table.
Baseline Characteristics (n=245).
| Variable | Value |
|---|---|
| Demographics: | |
| Age (years) | 58 ± 13 |
| Male gender, n (%) | 164 (67%) |
| Body mass index (kg/m2) | 30 ± 6 |
| African American, n (%) | 45 (18%) |
| Heart failure history: | |
| Ischemic etiology, n (%) | 92 (39%) |
| NYHA class III or IV, n (%) | 95 (66%) |
| Co-morbidities: | |
| Hypertension, n (%) | 130 (54%) |
| Diabetes mellitus, n (%) | 92 (38%) |
| Echocardiographic indices: | |
| LV mass index (g/m2) | 159 ± 57 |
| LV end-diastolic volume index (mL/m2) | 97 ± 42 |
| LV ejection fraction (%) | 31 ± 13 |
| Mitral E/e’ ratio | 22 ± 12 |
| Mitral E/A ratio | 1.9 ± 1.0 |
| Mitral DT (ms) | 160 ± 52 |
| Tricuspid E/e’ ratio | 7.1 ± 3.8 |
| LA volume index (mL/m2) | 41 ± 17 |
| RV end-diastolic area (cm2) | 26 ± 9 |
| RV s’ (cm/s) | 9 ± 3 |
| RV Fractional area change | 34 ± 12 |
| Tricuspid DT (ms) | 189 ± 61 |
| RA volume index (mL/m2) | 34 ± 18 |
| Medications: | |
| Angiotensin converting enzyme inhibitors or angiotensin receptor blockers, n (%) |
143 (61%) |
| Beta-blockers, n (%) | 171 (73%) |
| Spironolactone, n (%) | 87 (37%) |
| Loop diuretics, n (%) | 151 (64%) |
| Digoxin, n (%) | 70 (30%) |
| Laboratory data: | |
| MBG (pM) | 583 [383, 812] |
| Myeloperoxidase (pM) | 124 [77- 253] |
| BNP (pg/mL) | 431 [97, 1417] |
| eGFR (mL/min/1.73m2) | 71 ± 32 |
| Cystatin C (mg/L) | 1.85 [1.31 – 2.61] |
| ADMA (μM) | 1.05 [0.82 – 1.33] |
| SDMA (μM) | 1.70 [1.05 – 2.96] |
| MMA (nM) | 50 [34 – 92] |
Abbreviation: NYHA, New York Heart Association; LV, left ventricular; RV, right ventricular; MBG, marinobufagenin (MBG); eGFR, estimated glomerular filtration rate; BNP, B-type natriuretic peptide, ADMA, Asymmetric dimethylarginine; SDMA, symmetric dimethylarginine; MMA, mono methyl arginine.
Figure 1. Marinobufagenin levels in heart failure.
Comparison of plasma marinobufagenin levels between non-heart failure (non-HF) participants and HF participants with reduced (≤ 40) or preserved (>40) left ventricular ejection fraction (EF). ** p <0.001 vs control, by Student’s t-test with Bonferroni’s correction.
MBG Levels and Myocardial Structure and Performance
Supplemental Table 1 presents the relationships between MBG levels and echocardiographic parameters of cardiac structure and performance. In univariate analysis, higher MBG was associated with indices of LV diastolic function (Mitral deceleration time: r= −0.24, p=0.007) and RV diastolic function (Tricuspid E/e’: r=0.22, p=0.027; Tricuspid deceleration time: r= −0.38, p=0.002), as well as larger RV size (RV end-diastolic area: r=0.21, p=0.023). Elevated levels of MBG were associated with measures of worse RV systolic function (RV s’: r= −0.39, p<0.0001), but not left-sided systolic function (Supplemental Table 1).
MBG Levels and Prognosis
In our study cohort, 118 patients experienced an adverse event of death, cardiac transplantation, or HF hospitalization over the 5 year follow-up. When divided as dichotomous variable according to optimal cut-point (574 pM), elevated MBG was a predictor of increased risk of 5-year adverse outcomes, with higher MBG predicting increased risk of adverse clinical events (Hazard ratio 1.58 [95% confidence interval 1.10-2.31], p=0.014, Figure 2A). The predictive value of MBG remained statistically significant after adjustment for age, gender, diabetes mellitus, and ischemic etiology, but not eGFR (Supplemental Table 2). Moreover, the addition of MBG to traditional risk factors such as age, gender, ischemic etiology, and diabetes resulted in an 33.6% event-specific net reclassification (95% confidence interval 9.3%-57.8%, p=0.007) and a 3% integrated discrimination improvement (Supplemental Table 3). When defined by quartiles, this trend was confirmed using Cochran-Amitage test for the trend over quartiles (p=0.0297), although the trend was not monotonic (Supplemental Table 4). MBG did not predict events when modeled as a continuous variable (Hazard ratio 1.14 [0.95 – 1.37], p=0.15, per 1 standard deviation increment 0.62 with MBG modeled as a natural log transformed continuous variable).
Figure 2. Kaplan-Meier analysis of major adverse cardiac events (MACE) in participants with heart failure.
(A) Heart failure patients (n=245) stratified according to optimal cutoff for plasma marinobufagenin (MBG) as follows: “Low” MBG (<574 pM) or “High” MBG (≥ 574 pM). (B) Subgroup analysis of heart failure patients (n=115) with serial blood draws from admission to pre-discharge at 48-72 hours stratified according to optimal cutoff for change in plasma MBG as follows: “decreasing” MBG (<5%) or “increasing” MBG (≥ 5%).
We next selected a subgroup of the HF patients that had serial blood draws available at the time of their initial presentation in the hospital and 48-72 hours after admission (n=115 and included 35 events) and measured MBG. Rising MGB levels over the course of admission predicted increased risk of adverse outcomes [%ΔMBG modeled as a continuous variable per standard deviation increments where 1 standard deviation = 0.91%, Hazard ratio 1.30 (95% confidence interval 1.04-1.56), p=0.025, per 1 standard deviation increment, Figure 2B].
MBG Promotes Cardiac Fibrosis and Nitrative Stress in Animal Models
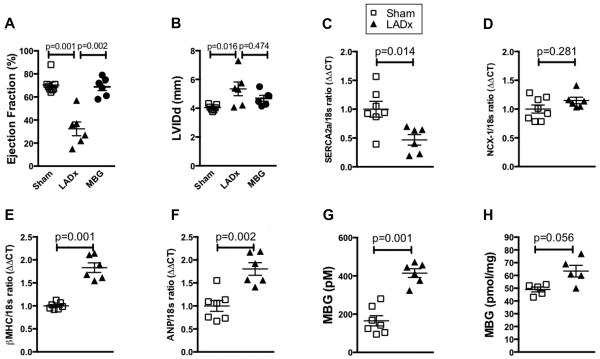
In order to assess MBG levels in a mouse model of post-myocardial infarction heart failure, left anterior descending artery ligation (LADx) was performed in C57BL6J mice. After 4 weeks of LADx we observed a significant decrease in left ventricular ejection fraction and increases left ventricular size (Figure 3A and B). We also observed changes in left ventricular homogenate of molecular markers of cardiac calcium handling [sarcoplasmic reticulum calcium ATPase 2a (SERCA2a) and sodium calcium exchanger (NCX-1)] and cardiac hypertrophy [beta myosin heavy chain (βMHC) and atrial natriuretic peptide (ANP)] consistent with a heart failure phenotype in this model (Figure 3C-F). Further, we noted increases in MBG levels in the post-MI heart failure mice (Figure 3G), with MBG levels observed within the range of values detected among HF subjects studied (50th percentile 585 pM). We also performed the LAD ligation procedure on a separate group of mice (n=5) and, after 1 week, isolated and extracted the adrenal glands and performed MBG measurement. Here we found that adrenal MBG levels were elevated vs control mice, but this did not reach statistical significance (Mann-Whitney p value = 0.056, Figure 3H).
Figure 3. Elevated MBG levels contribute to nitrative stress.
Echocardiographic measures of cardiac ejection fraction (A) and Diastolic Left Ventricular Internal Dimension (LVIDd) (B) after 4 weeks of either LAD ligation (LADx) or MBG infusion. Gene expression of calcium handling proteins sarcoplasmic reticulum calcium ATPase (SERCA2a) (C) and sodium calcium exchanger (NCX-1) (D), and hypertrophic markers beta myosin heavy chain (βMHC) (E) and atrial natriuretic peptide (ANP) (F) after 4 weeks of LADx. Plasma MBG (G) levels are increased 4 weeks after LAD ligation in a post myocardial infarction heart failure model. (H) Adrenal tissue MBG levels 1 week after LAD ligation. P values were calculated using the Mann-Whitney U test.
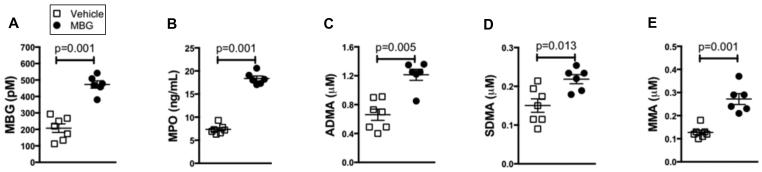
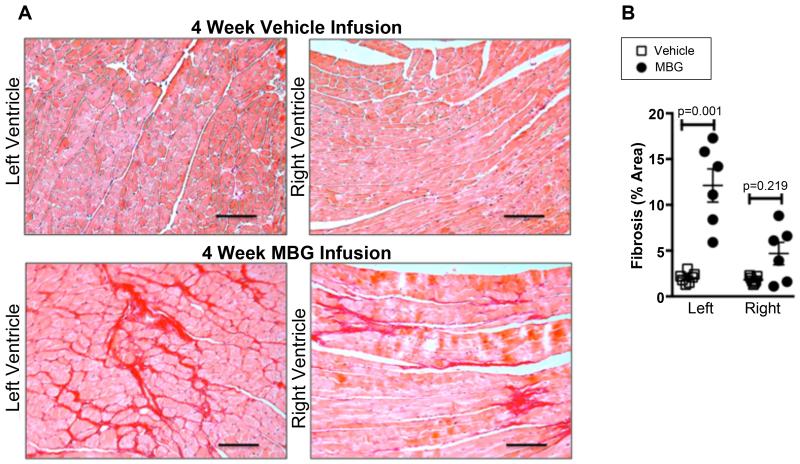
To directly test for a potential contribution of MBG to promotion of cardiac dysfunction and nitrative stress, mice were infused intraperitoneally with MBG (0.1 ug/g/day) which results in comparable levels to the post-MI heart failure mice (Figure 4A). MBG infusion did not result in decreased ejection fraction, but was accompanied by a significant increase in left ventricular size (Figure 3A and B). Importantly, mice infused with MBG demonstrated corresponding increases in MPO (Figure 4B) as well as increases in several methylated arginine markers of nitrative stress including ADMA, SDMA, and MMA (Figure 4 C-E). Furthermore, while MBG infused mice experienced significant left ventricular cardiac fibrosis vs vehicle treated controls, right ventricular cardiac fibrosis appeared to be increased vs vehicle treated controls but this did not reach statistical significance (Mann-Whitney p value = 0.219, Figure 5).
Figure 4. Elevated MBG levels contribute to nitrative stress.
After 4 week infusion of MBG, plasma levels of MBG (A) and MPO (B), as well as methylated arginine metabolites asymmetric dimethylarginine (ADMA, C), symmetric dimethylarginine (SDMA, D), and mono methyl arginine (MMA, E) are increased vs vehicle treated mice. P values were calculated using the Mann-Whitney U test.
Figure 5. Elevated MBG levels contribute to fibrosis.
Representative picosirius red histology (A) and quantitative morphometry (B) from mouse hearts after 4 week MBG infusion. Scale bar represents 100 um. P values were calculated using the Mann-Whitney U test.
Discussion
While elevated CTS have been associated with cardiac hypertrophy and dysfunction in subjects with hypertension30, 31, hypertrophic cardiomyopathy32, decompensated HF33, 34, and cardiomyopathy in chronic kidney disease15, 23, this is the first study to our knowledge that examines the relationship between plasma MBG and cardiac parameters as well as adverse clinical events in a broad and contemporary cohort of patients with HF. The relationships between elevated MBG and indices of inflammation/oxidative stress, myocardial stress, and nitrative stress and the predominantly diastolic dysfunction is consistent with the physiologic effects of MBG on the myocardium in volume-expanded states. These findings support future investigations on the potential role of modulation of MBG levels or activity as a novel targeted therapy in the population of HF patients burdened with significant cardiovascular disease and death.
Contribution of CTS in Heart Failure
While previous human studies have demonstrated the detection of elevated CTS in the setting of acute myocardial infarction13, 35, 36 and HF37, the novel findings in the current study was the relationship between elevated MBG and worsened right ventricular function (a condition often associated with venous congestion) as assessed by standard echocardiographic indices as well as adverse clinical outcomes and nitrative stress. Further, the data from the subgroup of patients who had serial MBG measurements suggests that MBG levels are dynamic and supports the view that rising levels of MGB during admission predict worse long-term clinical outcomes.
The relationship between cardiac structure, hemodynamics and CTS has also been observed in several other cohort studies. Endogenous plasma ouabain levels are elevated in patients with severely impaired left ventricular function (EF< 30%)34 and demonstrated significant positive correlation with hemodynamics such as blood pressure as well as cardiac indices such as left ventricular mass index, left ventricular end diastolic volume, as well as eccentric remodeling in hypertensive patients30, 31. Both circulating and myocardial tissue CTS immunoreactivity was positively correlated with left ventricular end-diastolic pressures and inversely correlated with cardiac index in patients with hypertrophic cardiomyopathy32. The shift from compensated left ventricular hypertrophy to CHF is not only marked by a three-fold increase in endogenous plasma ouabain levels in decompensated CHF, but also an increase in the sensitivity of cardiac Na/K-ATPase to ouabain33, 34. Levels of circulating endogenous ouabain also predicted HF progression in idiopathic dilated cardiomyopathy patients38 and left ventricular hypertrophy in the setting of end-stage renal disease39. Increased plasma MBG levels parallel the progression of HF37 and are associated with a “uremic cardiomyopathy” in chronic kidney disease15, 22, 23. Experimentally, Dahl salt-sensitive rats fed a high-salt diet demonstrated compensated left ventricular hypertrophy progressing to dilated cardiomyopathy in parallel with increasing plasma MBG level as well as increased expression and sensitivity of the Na/K-ATPase α-1 to MBG33. Interestingly, in our study, MBG was not significantly associated with cystatin C or estimated GFR, suggesting factors beyond renal insufficiency that influence MBG production. As we have previously demonstrated that MBG is synthetized by adrenocortical cells and that Angiotensin II can be its secretagogue 40, 41 we isolated and extracted the adrenal glands and performed MBG measurement in a group of mice one week after LADx and noted it was significantly elevated. This supports the adrenal tissue as a significant contributor to the pool of circulating MBG in a post-MI heart failure model. Taken together with our findings, these observations suggest that the elevated CTS levels which accompany edematous states like HF may promote downstream adverse cardiovascular consequences.
Mechanisms Linking CTS to Cardiovascular Pathology in Heart Failure
In addition to their well known effects on the ion transporting functions of the Na/K-ATPase, CTS also bind to and initiated signaling through a non-pumping pool of the Na/K-ATPase which reside in caveolae6. CTS confer a conformational change to the Na/K-ATPase that releases the Src kinase domain, thus activating Src kinase and multiple downstream targets2, 8, 11. This novel Na/K-ATPase-mediated signaling is responsible for a variety of key cellular roles involving cell growth/hypertrophy, reactive oxygen species production, and collagen synthesis6.
In the present study we demonstrate for the first time that increased circulating levels of MBG in human heart failure associate with elevations in markers of inflammation and nitrative stress including MPO and the methylated arginine metabolites ADMA, SDMA, and MMA. Using an animal model of post-MI heart failure, we also demonstrate a significant increase in plasma MBG 4 weeks after ligation of the left anterior descending artery in mice. Further, infusion of mice with MBG, which results in similar circulating levels as those seen in both human and experimental heart failure, recapitulated the increases in MPO as well as ADMA, SDMA, and MMA seen in our human heart failure study.
The mechanism whereby MBG may increase methylated arginine metabolites is unclear.We and others have shown that cardiotonic steroids such as MBG and ouabain increase ROS and inflammatory cytokines in cardiac42 and renal43 cell types, and also decrease NO bioavailability without changes in eNOS expression44. Inflammation and increased oxidant stress can significantly impact methyltransferases necessary for arginine methylation, the proteases involved in release of free methylargnine metabolites, and the catabolic dimethylarginine dimethylaminohydrolases responsible for metabolism of ADMA45. Thus, it is possible that some of the observed associations between methylated arginine metabolites and MBG may occur in part via ROS mediated perturbations in these enzymatic pathways.
The association of CTS with markers of inflammation and nitrative stress is not however without precedent. We have previously reported in the rat that treatment of both cardiac myocytes and isolated perfused hearts with the CTS ouabain yielded increased nitrative modification and decreased activity of cardiac calcium handling proteins as well as diastolic dysfunction46. We have also shown that ouabain induces increases in inflammatory cytokine expression from both macrophage and renal proximal tubular cell types43. Our findings are also in parallel with animal models such as partial (5/6th) nephrectomy showing elevations in circulating MBG levels that stimulate systemic oxidant stress, oxidative modification and fibrosis of cardiac tissue and cardiac dysfunction in the rat19, 22, 47. This cardiac phenotype can also be recapitulated by infusion of MBG. In contrast, both active and passive immunization against MBG as well as lowering circulating MBG levels via adrenalectomy significantly reduce the oxidant stress and cardiac dysfunction independent of changes in blood pressure.
In our study, mice infused with MBG also demonstrated significant increases in cardiac fibrosis. MBG and ouabain have both been shown to increase [3H]proline incorporation in addition to collagen expression (both mRNA and protein) in cardiac and renal fibroblast cell types20 and these effects were blocked by pharmacological antagonism of the TGF beta pathway19. We have also noted that decreases in Fli-1 (a negative regulator of collagen synthesis) expression appear to be necessary for MBG to induce increases in collagen in several types of fibroblasts (cardiac, renal, and dermal). Additionally, MBG induces translocation of PKCdelta from the cytosol to the nucleus in a PLC dependent manner, and this translocation of PKCdelta causes the phosphorylation and subsequent degradation of Fli-120. In several fibroblast cell types, CTS also stimulate Na/K-ATPase and oxidant signaling which induce collagen production19, 22, 43,. These signaling pathways are not only significantly attenuated by oxidant scavenging and inhibition of Src kinase, but also through competitive inhibitory mechanisms induced by spironolactone and canrenone binding to the Na/K-ATPase19, 22, 48, 49. Thus, the pro-inflammatory and pro-fibrotic CTS-Na/K-ATPase signaling axis may provide a novel therapeutic target in settings such as HF where elevated CTS induce inflammation and cardiac fibrosis.
Study limitations
Despite being the largest study to our knowledge of reporting the relationship between clinical outcomes and MBG in HF, the current study is still limited in the relatively small number of patients as well as the selection bias that potentially confounds interpretation of such cohort studies. We did not have sufficient data to analyze central venous pressure or other indices of right ventricular function, thus the measurements indicating worsened RV function are based solely on echocardiographic indices. Our Cox proportional hazard analyses with multiple covariables was limited by lack of power due to missing data. Similarly, when defined by quartiles, while the trend between MBG and worse clinical outcomes was similar to that obtained by the optimal cutpoint analysis, it was not statistically significant. Nevertheless, our study examines for the first time the contribution of MBG to incident cardiovascular outcomes in a cohort of patients with HF and demonstrates a novel association between CTS and markers of inflammation and nitrative stress. We would emphasize that the purpose of this study was to demonstrate underlying physiology rather than to propose MBG as a biomarker as it tracks with worsening renal function. The increased inflammation, nitrative stress and cardiac fibrosis seen with elevations in MBG may be an important mechanism of cardiovascular dysfunction in patients with heart failure and requires further investigation.
Conclusion
Elevated levels of MBG are associated with indices of worse cardiac dysfunction as well as increased risk for development of adverse clinical outcomes in patients with HF even after multivariable models adjustment for established clinical risk factors. Similarly, changes in MBG levels over time appear to be of prognostic benefit in HF patients, and infusion of MBG in an animal model appears to directly contribute to increased nitrative stress, cardiac fibrosis and dysfunction. Thus, MBG may serve as an important therapeutic target in patients with heart failure.
Supplementary Material
Acknowledgments
Sources of Funding
This research was supported by grants from the National Institutes of Health (NIH, R01HL103931) and the NIH/Office of Dietary Supplements (P20HL113452), and the Cleveland Clinic Clinical Research Unit of the Case Western Reserve University CTSA (UL1TR 000439). Dr. Kennedy was supported by an American Heart Association Scientist Development Grant 14SDG18650010. Drs. Fedorova and Bagrov are supported by Intramural Research Program, National Institute on Aging, National Institutes of Health. Mass spectrometry studies were performed on instruments housed in a facility supported in part by a Center of Innovations Award by AB SCIEX.
Footnotes
Disclosures
None.
References
- 1.Bagrov AY, Shapiro JI. Endogenous digitalis: pathophysiologic roles and therapeutic applications. Nat Clin Pract Nephrol. 2008;4:378–92. doi: 10.1038/ncpneph0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev. 2009;61:9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fedorova OV, Simbirtsev AS, Kolodkin NI, Kotov AY, Agalakova NI, Kashkin VA, Tapilskaya NI, Bzhelyansky A, Reznik VA, Frolova EV, Nikitina ER, Budny GV, Longo DL, Lakatta EG, Bagrov AY. Monoclonal antibody to an endogenous bufadienolide, marinobufagenin, reverses preeclampsia-induced Na/K-ATPase inhibition and lowers blood pressure in NaCl-sensitive hypertension. J Hypertens. 2008;26:2414–25. doi: 10.1097/HJH.0b013e328312c86a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komiyama Y, Dong XH, Nishimura N, Masaki H, Yoshika M, Masuda M, Takahashi H. A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clinical Biochemistry. 2005;38:36–45. doi: 10.1016/j.clinbiochem.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Lopatin DA, Ailamazian EK, Dmitrieva RI, Shpen VM, Fedorova OV, Doris PA, Bagrov AY. Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J Hypertens. 1999;17:1179–87. doi: 10.1097/00004872-199917080-00018. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Xie ZJ. The sodium pump and cardiotonic steroids-induced signal transduction protein kinases and calcium-signaling microdomain in regulation of transporter trafficking. Biochim Biophys Acta. 2010;1802:1237–45. doi: 10.1016/j.bbadis.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Kennedy DJ, Yan Y, Shapiro JI. Reactive Oxygen Species Modulation of Na/K ATPase Regulates Fibrosis and Renal Proximal Tubular Sodium Handling. International Journal of Nephrology. 2012;2012:1–14. doi: 10.1155/2012/381320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie JX, Shapiro AP, Shapiro JI. The Trade-Off between Dietary Salt and Cardiovascular Disease; A Role for Na/K-ATPase Signaling? Front Endocrinol (Lausanne) 2014;5:97. doi: 10.3389/fendo.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loreaux EL, Kaul B, Lorenz JN, Lingrel JB. Ouabain-Sensitive alpha1 Na,K-ATPase enhances natriuretic response to saline load. J Am Soc Nephrol. 2008;19:1947–54. doi: 10.1681/ASN.2008020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaustein MP, Zhang J, Chen L, Song H, Raina H, Kinsey SP, Izuka M, Iwamoto T, Kotlikoff MI, Lingrel JB, Philipson KD, Wier WG, Hamlyn JM. The pump, the exchanger, and endogenous ouabain: signaling mechanisms that link salt retention to hypertension. Hypertension. 2009;53:291–8. doi: 10.1161/HYPERTENSIONAHA.108.119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lingrel JB. The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na,K-ATPase. Annu Rev Physiol. 2010;72:395–412. doi: 10.1146/annurev-physiol-021909-135725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagrov AY, Fedorova OV, Austin-Lane JL, Dmitrieva RI, Anderson DE. Endogenous marinobufagenin-like immunoreactive factor and Na+, K+ ATPase inhibition during voluntary hypoventilation. Hypertension. 1995;26:781–8. doi: 10.1161/01.hyp.26.5.781. [DOI] [PubMed] [Google Scholar]
- 13.Bagrov AY, Fedorova OV, Dmitrieva RI, Howald WN, Hunter AP, Kuznetsova EA, Shpen VM. Characterization of a urinary bufodienolide Na+,K+-ATPase inhibitor in patients after acute myocardial infarction. Hypertension. 1998;31:1097–103. doi: 10.1161/01.hyp.31.5.1097. [DOI] [PubMed] [Google Scholar]
- 14.Fedorova OV, Doris PA, Bagrov AY. Endogenous marinobufagenin-like factor in acute plasma volume expansion. Clin Exp Hypertens. 1998;20:581–91. doi: 10.3109/10641969809053236. [DOI] [PubMed] [Google Scholar]
- 15.Gonick HC, Ding Y, Vaziri ND, Bagrov AY, Fedorova OV. Simultaneous measurement of marinobufagenin, ouabain, and hypertension-associated protein in various disease states. Clin Exp Hypertens. 1998;20:617–27. doi: 10.3109/10641969809053240. [DOI] [PubMed] [Google Scholar]
- 16.Schreiber V, Kolbel F, Stepan J, Gregorova I, Pribyl T. Digoxin-like immunoreactivity in the serum of rats with cardiac overload. J Mol Cell Cardiol. 1981;13:107–10. doi: 10.1016/0022-2828(81)90232-7. [DOI] [PubMed] [Google Scholar]
- 17.Liu ZQ, Ma AQ, Zhang L, Yang DY. Intra-cellular electrolyte changes and levels of endogenous digoxin-like substance within the plasma in patients with congestive heart failure. Int J Cardiol. 1990;27:47–53. doi: 10.1016/0167-5273(90)90190-g. [DOI] [PubMed] [Google Scholar]
- 18.Morise T, Okamoto S, Takasaki H, Ikeda M, Takeda R, Kiuti F, Tuda Y. Biological activity of partially purified digitalis-like substance and Na-K-ATPase inhibitor in rats. Jpn Circ J. 1988;52:1309–16. doi: 10.1253/jcj.52.1309. [DOI] [PubMed] [Google Scholar]
- 19.Elkareh J, Kennedy DJ, Yashaswi B, Vetteth S, Shidyak A, Kim EG, Smaili S, Periyasamy SM, Hariri IM, Fedorova L, Liu J, Wu L, Kahaleh MB, Xie Z, Malhotra D, Fedorova OV, Kashkin VA, Bagrov AY, Shapiro JI. Marinobufagenin stimulates fibroblast collagen production and causes fibrosis in experimental uremic cardiomyopathy. Hypertension. 2007;49:215–24. doi: 10.1161/01.HYP.0000252409.36927.05. [DOI] [PubMed] [Google Scholar]
- 20.Elkareh J, Periyasamy SM, Shidyak A, Vetteth S, Schroeder J, Raju V, Hariri IM, El-Okdi N, Gupta S, Fedorova L, Liu J, Fedorova OV, Kahaleh MB, Xie Z, Malhotra D, Watson DK, Bagrov AY, Shapiro JI. Marinobufagenin induces increases in procollagen expression in a process involving protein kinase C and Fli-1: implications for uremic cardiomyopathy. Am J Physiol Renal Physiol. 2009;296:F1219–26. doi: 10.1152/ajprenal.90710.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedorova LV, Raju V, El-Okdi N, Shidyak A, Kennedy DJ, Vetteth S, Giovannucci DR, Bagrov AY, Fedorova OV, Shapiro JI, Malhotra D. The cardiotonic steroid hormone marinobufagenin induces renal fibrosis: implication of epithelial-to-mesenchymal transition. Am J Physiol Renal Physiol. 2009;296:F922–34. doi: 10.1152/ajprenal.90605.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy DJ, Vetteth S, Periyasamy SM, Kanj M, Fedorova L, Khouri S, Kahaleh MB, Xie Z, Malhotra D, Kolodkin NI, Lakatta EG, Fedorova OV, Bagrov AY, Shapiro JI. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension. 2006;47:488–95. doi: 10.1161/01.HYP.0000202594.82271.92. [DOI] [PubMed] [Google Scholar]
- 23.Kolmakova EV, Haller ST, Kennedy DJ, Isachkina AN, Budny GV, Frolova EV, Piecha G, Nikitina ER, Malhotra D, Fedorova OV, Shapiro JI, Bagrov AY. Endogenous cardiotonic steroids in chronic renal failure. Nephrol Dial Transplant. 2011;26:2912–9. doi: 10.1093/ndt/gfq772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fedorova OV, Talan MI, Agalakova NI, Lakatta EG, Bagrov AY. Endogenous ligand of alpha(1) sodium pump, marinobufagenin, is a novel mediator of sodium chloride--dependent hypertension. Circulation. 2002;105:1122–7. doi: 10.1161/hc0902.104710. [DOI] [PubMed] [Google Scholar]
- 25.Shao Z, Wang Z, Shrestha K, Thakur A, Borowski AG, Sweet W, Thomas JD, Moravec CS, Hazen SL, Tang WH. Pulmonary hypertension associated with advanced systolic heart failure: dysregulated arginine metabolism and importance of compensatory dimethylarginine dimethylaminohydrolase-1. J Am Coll Cardiol. 2012;59:1150–8. doi: 10.1016/j.jacc.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson Tang WH, Tong W, Shrestha K, Wang Z, Levison BS, Delfraino B, Hu B, Troughton RW, Klein AL, Hazen SL. Differential effects of arginine methylation on diastolic dysfunction and disease progression in patients with chronic systolic heart failure. Eur Heart J. 2008;29:2506–13. doi: 10.1093/eurheartj/ehn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 29.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 30.Manunta P, Stella P, Rivera R, Ciurlino D, Cusi D, Ferrandi M, Hamlyn JM, Bianchi G. Left ventricular mass, stroke volume, and ouabain-like factor in essential hypertension. Hypertension. 1999;34:450–6. doi: 10.1161/01.hyp.34.3.450. [DOI] [PubMed] [Google Scholar]
- 31.Pierdomenico SD, Bucci A, Manunta P, Rivera R, Ferrandi M, Hamlyn JM, Lapenna D, Cuccurullo F, Mezzetti A. Endogenous ouabain and hemodynamic and left ventricular geometric patterns in essential hypertension. Am J Hypertens. 2001;14:44–50. doi: 10.1016/s0895-7061(00)01225-5. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi T, Ijiri Y, Toko H, Shimomura H, Okabe M, Terasaki F, Kitaura Y, Kawamura K. Increased digitalis-like immunoreactive substances in patients with hypertrophic cardiomyopathy. Eur Heart J. 2000;21:296–305. doi: 10.1053/euhj.1999.1744. [DOI] [PubMed] [Google Scholar]
- 33.Fedorova OV, Talan MI, Agalakova NI, Lakatta EG, Bagrov AY. Coordinated shifts in Na/K-ATPase isoforms and their endogenous ligands during cardiac hypertrophy and failure in NaCl-sensitive hypertension. Journal of Hypertension. 2004;22:389–97. doi: 10.1097/00004872-200402000-00025. [DOI] [PubMed] [Google Scholar]
- 34.Gottlieb SS, Rogowski AC, Weinberg M, Krichten CM, Hamilton BP, Hamlyn JM. Elevated concentrations of endogenous ouabain in patients with congestive heart failure. Circulation. 1992;86:420–5. doi: 10.1161/01.cir.86.2.420. [DOI] [PubMed] [Google Scholar]
- 35.Bagrov A, Fedorova OV, Maslova MN, Roukoyatkina NI, Ukhanova MV, Zhabko EP. Endogenous plasma Na,K-ATPase inhibitory activity and digoxin like immunoreactivity after acute myocardial infarction. Cardiovasc Res. 1991;25:371–7. doi: 10.1093/cvr/25.5.371. [DOI] [PubMed] [Google Scholar]
- 36.Bagrov AY, Kuznetsova EA, Fedorova OV. Endogenous digoxin-like factor in acute myocardial infarction. J Intern Med. 1994;235:63–7. doi: 10.1111/j.1365-2796.1994.tb01033.x. [DOI] [PubMed] [Google Scholar]
- 37.Fridman AI, Matveev SA, Agalakova NI, Fedorova OV, Lakatta EG, Bagrov AY. Marinobufagenin, an endogenous ligand of alpha-1 sodium pump, is a marker of congestive heart failure severity. J Hypertens. 2002;20:1189–94. doi: 10.1097/00004872-200206000-00032. [DOI] [PubMed] [Google Scholar]
- 38.Pitzalis MV, Hamlyn JM, Messaggio E, Iacoviello M, Forleo C, Romito R, de Tommasi E, Rizzon P, Bianchi G, Manunta P. Independent and incremental prognostic value of endogenous ouabain in idiopathic dilated cardiomyopathy. Eur J Heart Fail. 2006;8:179–86. doi: 10.1016/j.ejheart.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Stella P, Manunta P, Mallamaci F, Melandri M, Spotti D, Tripepi G, Hamlyn JM, Malatino LS, Bianchi G, Zoccali C. Endogenous ouabain and cardiomyopathy in dialysis patients. J Intern Med. 2008;263:274–80. doi: 10.1111/j.1365-2796.2007.01883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fedorova OV, Agalakova NI, Talan MI, Lakatta EG, Bagrov AY. Brain ouabain stimulates peripheral marinobufagenin via angiotensin II signalling in NaCl-loaded Dahl-S rats. Journal of Hypertension. 2005;23:1515–23. doi: 10.1097/01.hjh.0000174969.79836.8b. [DOI] [PubMed] [Google Scholar]
- 41.Dmitrieva RI, Bagrov AY, Lalli E, Sassone-Corsi P, Stocco DM, Doris PA. Mammalian bufadienolide is synthesized from cholesterol in the adrenal cortex by a pathway that Is independent of cholesterol side-chain cleavage. Hypertension. 2000;36:442–8. doi: 10.1161/01.hyp.36.3.442. [DOI] [PubMed] [Google Scholar]
- 42.Priyadarshi S, Valentine B, Han C, Fedorova OV, Bagrov AY, Liu J, Periyasamy SM, Kennedy D, Malhotra D, Xie Z, Shapiro JI. Effect of green tea extract on cardiac hypertrophy following 5/6 nephrectomy in the rat. Kidney Int. 2003;63:1785–90. doi: 10.1046/j.1523-1755.2003.00914.x. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy DJ, Chen Y, Huang W, Viterna J, Liu J, Westfall K, Tian J, Bartlett DJ, Tang WH, Xie Z, Shapiro JI, Silverstein RL. CD36 and Na/K-ATPase-alpha1 form a proinflammatory signaling loop in kidney. Hypertension. 2013;61:216–24. doi: 10.1161/HYPERTENSIONAHA.112.198770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wenceslau CF, Davel AP, Xavier FE, Rossoni LV. Long-term ouabain treatment impairs vascular function in resistance arteries. J Vasc Res. 2011;48:316–26. doi: 10.1159/000322576. [DOI] [PubMed] [Google Scholar]
- 45.Pope AJ, Druhan L, Guzman JE, Forbes SP, Murugesan V, Lu D, Xia Y, Chicoine LG, Parinandi NL, Cardounel AJ. Role of DDAH-1 in lipid peroxidation product-mediated inhibition of endothelial NO generation. Am J Physiol Cell Physiol. 2007;293:C1679–86. doi: 10.1152/ajpcell.00224.2007. [DOI] [PubMed] [Google Scholar]
- 46.Kennedy DJ, Vetteth S, Xie M, Periyasamy SM, Xie Z, Han C, Basrur V, Mutgi K, Fedorov V, Malhotra D, Shapiro JI. Ouabain decreases sarco(endo)plasmic reticulum calcium ATPase activity in rat hearts by a process involving protein oxidation. Am J Physiol Heart Circ Physiol. 2006;291:H3003–11. doi: 10.1152/ajpheart.00603.2006. [DOI] [PubMed] [Google Scholar]
- 47.Kennedy DJ, Elkareh J, Shidyak A, Shapiro AP, Smaili S, Mutgi K, Gupta S, Tian J, Morgan E, Khouri S, Cooper CJ, Periyasamy SM, Xie Z, Malhotra D, Fedorova OV, Bagrov AY, Shapiro JI. Partial nephrectomy as a model for uremic cardiomyopathy in the mouse. Am J Physiol Renal Physiol. 2008;294:F450–4. doi: 10.1152/ajprenal.00472.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Okdi N, Smaili S, Raju V, Shidyak A, Gupta S, Fedorova L, Elkareh J, Periyasamy S, Shapiro AP, Kahaleh MB, Malhotra D, Xie Z, Chin KV, Shapiro JI. Effects of cardiotonic steroids on dermal collagen synthesis and wound healing. J Appl Physiol. 2008;105:30–6. doi: 10.1152/japplphysiol.00119.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian J, Shidyak A, Periyasamy SM, Haller S, Taleb M, El-Okdi N, Elkareh J, Gupta S, Gohara S, Fedorova OV, Cooper CJ, Xie Z, Malhotra D, Bagrov AY, Shapiro JI. Spironolactone attenuates experimental uremic cardiomyopathy by antagonizing marinobufagenin. Hypertension. 2009;54:1313–20. doi: 10.1161/HYPERTENSIONAHA.109.140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.