Abstract
The tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is a potent human carcinogen. Metabolic activation of NNK generates a number of DNA adducts including O2-methylthymidine (O2-Me-dT) and O2-[4-(3-pyridyl)-4-oxobut-1-yl]thymidine (O2-POB-dT). To investigate the biological effects of these O2-alkylthymidines in humans, we have replicated plasmids containing a site-specifically incorporated O2-Me-dT or O2-POB-dT in human embryonic kidney 293T (HEK293T) cells. The bulkier O2-POB-dT exhibited high genotoxicity and only 26% translesion synthesis (TLS) occurred, while O2-Me-dT was less genotoxic and allowed 55% TLS. However, O2-Me-dT was 20% more mutagenic (mutation frequency (MF) 64%) compared to O2-POB-dT (MF 53%) in HEK293T cells. The major type of mutations in each case was targeted T→A transversions (56% and 47%, respectively, for O2-Me-dT and O2-POB-dT). Both lesions induced a much lower frequency of T→G, the dominant mutation in bacteria. siRNA knockdown of the TLS polymerases (pols) indicated that pol η, pol ζ, and Rev1 are involved in the lesion bypass of O2-Me-dT and O2-POB-dT as the TLS efficiency decreased with knockdown of each pol. In contrast, MF of O2-Me-dT was decreased in pol ζ and Rev1 knockdown cells by 24% and 25%, respectively, while for O2-POB-dT, it was decreased by 44% in pol ζ knockdown cells, indicating that these TLS pols are critical for mutagenesis. Additional decrease in both TLS efficiency and MF was observed in cells deficient in pol ζ plus other Y-family pols. This study provided important mechanistic details on how these lesions are bypassed in human cells in both error-free and error-prone manner.
Keywords: O2-methylthymidine, O2-POB-thymidine, mutagenesis, TLS polymerase, HEK293T, NNK, tobacco-specific nitrosamine, siRNA knockdown
Graphical Abstract
1. Introduction
Tobacco use is the single largest preventable cause of disease and premature death in the US, yet more than 45 million Americans still smoke cigarettes. Tobacco products contain over 5000 compounds and over 70 carcinogens [1]. Amongst the plethora of carcinogens present in tobacco and tobacco smoke, likely candidates for initiating cancers are the tobacco-specific nitrosamines, including (4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)) and N'-nitrosonornicotine (NNN) [2–5]. Intermediates formed during metabolic activation of these compounds by P450 have been shown to covalently bind to DNA forming DNA adducts [6]. NNK thus far is the only carcinogen found in tobacco products that causes lung tumors in animal models, irrespective of the route of administration [7, 8].
NNK can be metabolically activated to generate methylating agents or to generate pyridyloxobutyalting agents [6, 7]. These agents react with DNA forming methyl (Me) and 4-(3-pyridyl)-4-oxobutyl (POB) adducts, respectively. The most common methylated DNA adducts identified in NNK-treated rodents formed in the methylation pathway are 7-Me-dG and O6-Me-dG [9–11]; however, other methylation products such as O2-Me-dC and O2-Me-dT are also most likely formed [9]. While mutagenicity and carcinogenicity of the methylation pathway of NNK-derived O6-Me-dG and its link to carcinogenesis is well documented [8, 10–14], the relative importance of the POB-pathway is still under investigation.
The pyridyloxobutylating pathway generates four POB adducts including O2-POB-dT, O2-POB-dC, O6-POB-dG and 7-POB-dG, which were detected in NNK treated rats and mice [15–17]. A/J mice treated with a pyridyloxobutylating agent, 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone (NNKOAc), showed that three POB DNA adducts, O2-POB-dT, O6-POB-dG and 7-POB-dG, form and persist in lung DNA at significant levels, suggesting their likely involvement in lung carcinogenesis [18]. However, O2-POB-dT was identified as the most abundant POB adduct in lung tissues of A/J female mice [19] as well as the most persistent adduct in lung and liver of male F344 rats treated with NNK [20]. NNKOAc-treated CHO cells produced point mutations mainly at the AT base pairs, implicating O2-POB-dT as a candidate lesion responsible for these mutation [21].
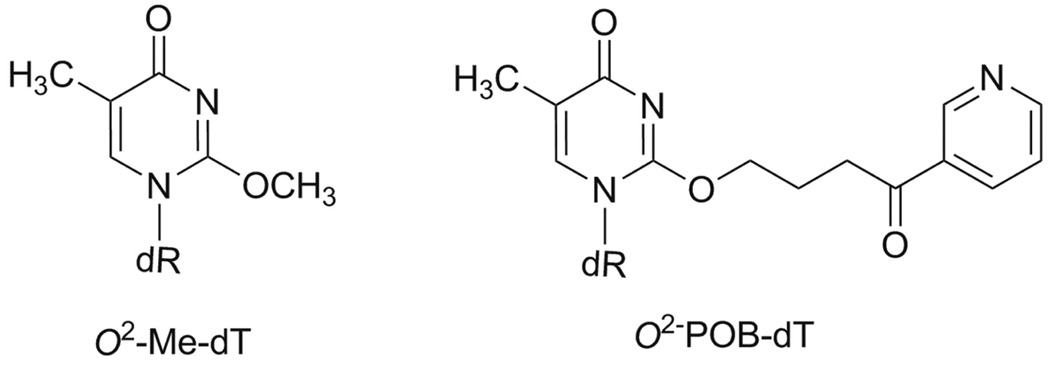
In vivo mutagenesis study of O2-Me-dT and O2-POB-dT (structures shown in Chart 1) showed that these DNA lesions are highly mutagenic in E. coli cells [22]. Both survival and the mutagenicity were increased with SOS induction, suggesting involvement of bacterial TLS pols for their bypass and mutagenesis. Replication of O2-alkylthymidine lesions with varying side chains in E. coli showed that bypass efficiency decreased as the chain length of the alkyl group increased [23] and pol V is indispensable for T→A mutations [22]. In vitro kinetics using exo-free Klenow fragment of E. coli DNA polymerase I (Kf−), Sulfolobus solfactaricus DNA polymerase IV (Dpo4), human polymerase κ (pol κ) and yeast polymerase eta (pol η) established that O2-Me-dT is a strong block to DNA synthesis and that the correct nucleotide dATP is preferentially incorporated opposite O2-Me-dT [24, 25]. Likewise, bypass of O2-POB-dT is inefficient by Kf− and Dpo4, but, when bypass occurs, the wrong nucleotide dTTP is preferentially incorporated opposite O2-POB-dT [25].
Chart 1.
Structures of O2-Me-dT and O2-POB-dT
To test the hypothesis that O2-POB-dT, the most abundant DNA adduct of the POB-pathway of NNK, is mutagenic, we have transfected a single-stranded (ss) pMS2 plasmid containing a single O2-Me-dT or O2-POB-dT in human embryonic kidney 293T cells (HEK293T). In addition, we investigated the involvement of TLS pols, including pol η, κ, ι, ζ and Rev1, for lesion bypass and mutagenesis by siRNA-induced knockdown of these TLS pols. We determined that both O2-Me-dT and O2-POB-dT are highly mutagenic in human cells, inducing predominantly T→A mutations. We also found that the lesion bypass is dependent on pol η, ζ, and Rev1.
2. Materials and methods
2.1. Materials
[γ-32P]ATP was purchased from Du Pont New England Nuclear (Boston, MA). All enzymes, including EcoRV restriction endonuclease, T4 DNA ligase, T4 polynucleotide kinase, uracil DNA glycosylase, and exonuclease III, were obtained from New England Bioloabs (Beverly, MA). HEK293T cells were purchased from the American Type Culture Collection (Manassas, VA). E. coli DH10B cells was purchased form Life Technologies, Inc. (Grand Island, NY)
siRNAs: Synthetic siRNA duplexes against POLH (SI02663619), POLK (SI04930884), POLI (SI03033310), REV1 (SI00115311), and All Stars negative control siRNA (1027280) were purchased from Qiagen (Valencia, CA). The siRNA for REV3 was purchased from Integrated DNA Technologies (Coralville, IA). Sequences of the siRNAs are listed in Table S1 of the Supporting Information.
2.2. Synthesis and characterization of Oligonucleotides
The modified oligonucleotides 5’-GTGCGT*GTTTGT-3’, (where T* represents O2-Me-dT or O2-POB-dT) containing the DNA sequence of p53 codons 272–275 in which the lesion was located in codon 273, were synthesized and characterized as reported [22]. Oligonucleotides were analyzed by MALDI-TOF MS analysis, which gave a molecular ion with a mass within 0.005% of theoretical. The M+1 for the O2-Me-dT oligodeoxynucleotide is 3713 and we found an m/z of 3712. The M+1 for the O2-POB-dT oligodeoxynucleotide is 3846 and we found an m/z of 3847. Unmodified oligonucleotides were analyzed by MALDI-TOF MS analysis, which gave a molecular ion with a mass within 0.005% of theoretical.
2.3. Construction and characterization of pMS2 vectors containing a single O2-Me-dT or O2-POB-dT
The single-stranded (ss) pMS2 shuttle vector, which contains its only EcoRV site in a hairpin region was prepared as described [26]. Synthesis and characterization of the lesion-containing and control constructs followed a protocol reported earlier [27]. The final constructs were dissolved in 1 mM Tris-HCl-0.1 mM EDTA, pH 8, and a portion was run on 1% agarose gel to assess the amount of circular DNA.
2.4. Replication and analysis in HEK 293T cells
The HEK293T cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 4 mM L-glutamine and adjusted to contain 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, and 10% fetal bovine serum. The cells were grown to ~90% confluency and 50 ng of each construct was transfected using 6 µL of Lipofectamine cationic lipid reagent (Invitrogen, Carlsbad, CA). After transfection with control or lesion containing pMS2, the cells were grown at 37 °C and 5% CO2 for 24 hours and the plasmid DNA was collected and purified by the method of Hirt [28]. Each progeny DNA was then transformed Escherichia coli DH10B, and the transformants were analyzed by oligonucleotide hybridization followed by DNA sequence analysis as described [27].
2.5. TLS assay in human cells
The lesion-containing or control pMS2 construct was mixed in a 2:1 ratio with a single-stranded pMS2 DNA containing the same DNA sequence as the construct, except in the 12mer insert it contained a C in place of G two nucleotides 5′ to the lesion site (i.e., 5′- GTCCGTGTTTGT- 3′). The mutant DNA was used as an internal control. The mixed DNA was used to transfect HEK293T cells and processed as described above. Oligonucleotide probes for the complementary sequences for both the lesion containing plasmid and the internal control plasmid were used to analyze the progeny. The internal control gave the same number of progeny as the control construct. Typically, three independent experiments were performed to determine the extent of TLS with each pol knockdown.
2.6. Mutational analyses of TLS products from human cells with pol knockdowns
The procedure described in section 2.4 was adapted for the knockdown experiments as follows. Synthetic siRNA duplexes specific for TLS pols were transfected into HEK293T cells using Lipofectamine prior to transfection of the control and lesion containing vectors. HEK293T cells were plated in six-well plates at 50% confluence. After 24 h incubation, they were transfected with 100 pmol of the siRNA duplex mixed with Lipofectamine, diluted in Opti-MEM (Gibco), per well. One day before the transfection of the plasmid, cells were seeded in 24-well plates at 70% confluence. Cells were then co-transfected with another aliquot of siRNA and either control or lesion containing plasmid. After 24 h incubation period, progeny plasmids were isolated as described earlier.
2.7. Reverse transcription polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from the cells 72 h after the first transfection of siRNA duplexes, using the All Prep DNA/RNA/Protein Kit (Qiagen). One hundred nanograms of total RNA was used for RT-PCR analysis, performed with the One Step RT-PCR Kit (Qiagen) according to the manufacturer’s instructions. Primer sequences used for RT-PCR are listed in Table S2 of the Supporting Information. The conditions for RT-PCR and siRNA knockdown efficiency were determined as described elsewhere [29].
3. Results
3.1. Inhibition of replication by O2-Me-dT and O2-POB-dT adducts and contribution of pols η, ζ and Rev1 for lesion bypass
To investigate the mutagenic and replication blocking properties of the two O2-alkyl-dT adducts formed by the tobacco-specific nitrosamines, we have constructed ss pMS2 plasmids containing a single O2-Me-dT or O2-POB-dT, and the constructs were replicated in HEK293T cells. Replication of the lesion containing ss vectors allows for the study of the lesion in the absence of repair, as ss-plasmids are inefficiently repaired by DNA repair pathways. However, the mechanism of replication is believed to be similar to lagging strand synthesis [30].
To determine the roles of individual TLS pols in the bypass of these two lesions, we employed siRNA knockdown approach to suppress their expression [31]. In agreement with previous studies, the real time PCR and Western blot analysis showed that, for each pol, the knockdown was at least 70% efficient [29]. Prior to replication of the lesion containing vectors, HEK293T cell were treated with siRNA for a specific TLS pol and allowed 48 h to reduce their expression. A non-specific siRNA was used as the negative control. After 48 h, another aliquot of siRNA was added along with the lesion containing vectors, to ensure efficient knockdown of the desired pol during translesion replication.
The relative TLS efficiency of the lesion bypass was determined by a previously reported procedure using an internal control [29]. Briefly, the lesion-containing ss-pMS2 construct was mixed in a 2:1 ratio with another modified ss-pMS2 plasmid, in which the 12mer insert contained a C instead of G two nucleotides 5’ to the lesion site and the mixed DNA was transfected into siRNA treated cells. The unmodified vector was employed as an internal control to determine the TLS efficiency. After transfection, the plasmids were allowed to replicate for 24 h, the progeny plasmids were recovered, transformed to E. coli DH 10B cells, and the resulting colonies were analyzed by oligonucleotide hybridization [27, 29, 32]. TLS efficiency for lesion bypass was estimated as the percentage of the colonies originating from the lesion-containing plasmid relative to the same from the internal control.
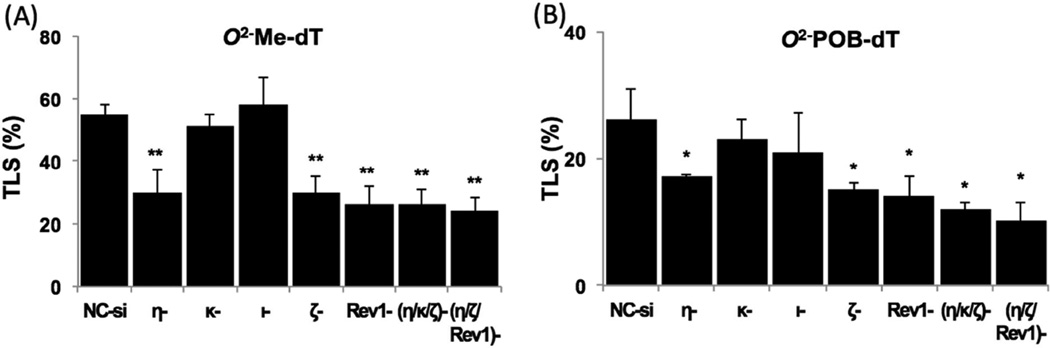
As shown in Figure 1 (and in Table S3 & S4 in the Supporting Information), the TLS efficiencies of O2-Me-dT and O2-POB-dT in HEK293T cells transfected with negative control (NC) siRNA were 55% and 26%, respectively, relative to 100% progeny generated from the control construct. This result is qualitatively similar to what we have observed in E. coli in that the bulkier O2-POB-dT is a stronger replication block [22]. In comparison to HEK293T cells that were treated with NC-siRNA, the TLS efficiency for O2-Me-dT decreased by 45–53% in pol η, ζ and Rev1 knockdown cells (Figure 1). Similarly, the relative %TLS of O2-POB-dT dropped by 35–46% in pol η, pol ζ and Rev1 knockdown cells (Figure 1). However, knockdown of pol κ or pol ι did not show substantial difference in TLS of either lesion (Figure 1). These results indicate that multiple TLS pols, notably pol η, pol ζ and Rev1, play important roles in bypassing O2-Me-dT and O2-POB-dT, albeit none of the pols are indispensable for lesion bypass, with the caveat that there was residual activity of the enzymes even after siRNA knockdowns. We also investigated the effects of knockdown of multiple pols simultaneously. Even when pol η, pol ζ and Rev1 were simultaneously knocked down, which individually had a major effect on the TLS, the additional lowering of TLS was modest. However, it is uncertain whether the remaining low concentrations of the enzymes in the knockdown cells were sufficient to carry out part of the TLS across the lesions.
Figure 1.
Effect of siRNA-induced knockdowns of TLS pol(s) on the replicative bypass of O2-Me-dT and O2-POB-dT in HEK293T cells. The %TLS in various pol knockdowns was estimated using an internal control of an unmodified plasmid containing a mutation two nucleotides 5’ to the lesion site. The data represent the mean and the standard deviation of results from at least 3 independent experiments (except for the triple knockdown experiments, which were performed twice). HEK293T cells were treated with negative control (NC-si) siRNA whereas the other single knockdowns are as indicated. The statistical significance between NC-siRNA treated HEK293T and TLS pols knockdowns were calculated using a two-tailed, unpaired Student’s t test (*p < 0.05: **p < 0.01).
3.2. Mutational specificity of O2-Me-dT and O2-POB-dT in HEK293T cells
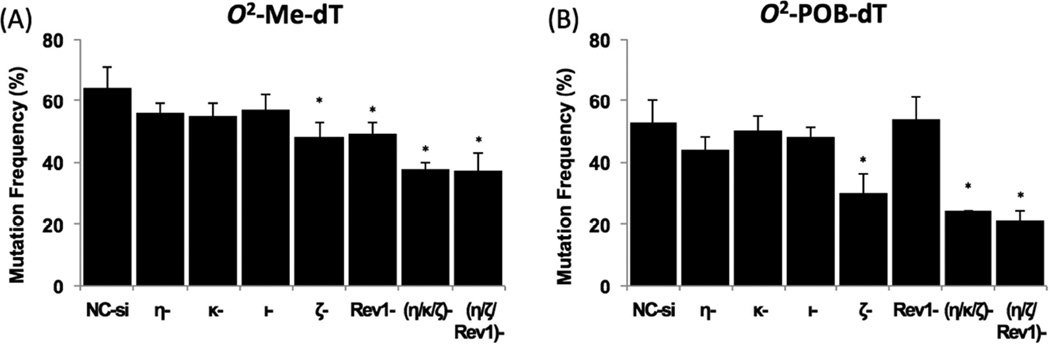
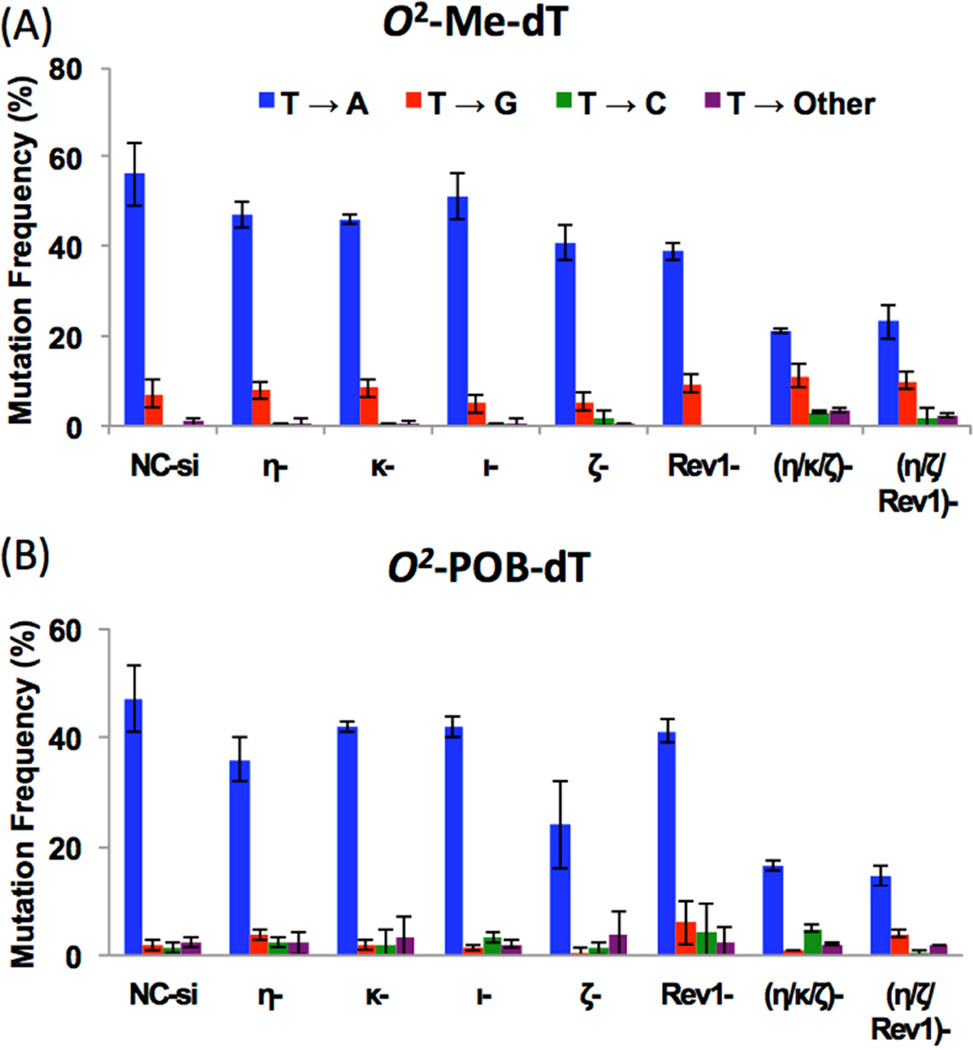
Figure 2 shows the DNA sequence analysis results of the TLS products of O2-Me-dT and O2-POB-dT plasmid constructs in the HEK293T cells, which showed that both O2-Me-dT and O2-POB-dT were highly mutagenic in human cells. For O2-Me-dT, 64% of the progeny analyzed were mutants and only ~36% progeny yielded accurate replication across the lesion in cells transfected with NC siRNA (Figure 2). Similarly, O2-POB-dT generated ~53% mutant progeny (Figure 2). Therefore, we conclude that, although O2-POB-dT is a strong block to DNA replication, its replication is 20% more accurate relative to O2-Me-dT in human cells. This result is different from the result in E. coli, in which O2-POB-dT was found more mutagenic than O2-Me-dT [22]. Most mutations in HEK293T cells were targeted one-base substitutions, while a low frequency of semi-targeted mutations (i.e., mutations near the lesion site) was also observed (Figure 3 and Tables S5–S8 in the Supporting Information). Unlike the most common T→G mutations observed in E. coli [22], the major type of mutations for both O2-Me-dT and O2-POB-dT in HEK293T cells were T→A mutations, detected in 56% and 47% frequency, respectively (Figure 3). dCMP incorporation opposite the O2-Me-dT lesion, which leads to T→G mutations at 7% frequency, was the second most prevalent mutagenic event; however, it occurred only at ~2% frequency for O2-POB-dT (Figure 3). A low level of T→C (1.5%) and semi-targeted (2.3%) mutations were observed for O2-POB-dT, but T→C events were not detected in the progeny from the O2-Me-dT construct (Figure 3). It is important to note that no mutations were detected within the 12mer sequence of the control construct in approximately 300 colonies that were analyzed (data not shown).
Figure 2. Total mutational frequency (MF) of O2-Me-dT and O2-POB-dT in HEK293T cells treated with negative control siRNA (NC-si) or siRNA for TLS pol(s).
The data represent the mean and the standard deviation of results from at least 3 independent experiments (except for pol ι and the triple knockdown experiments, which were performed twice). The statistical significance between NC-siRNA treated HEK293T and TLS pols knockdowns were calculated using two-tailed, unpaired Student’s t test. (*p < 0.05).
Figure 3. Types of mutations observed in HEK293T cells treated with NC siRNA (NC-si) or siRNA for individual TLS pol(s).
The data represent the mean and the standard deviation of results from at least 3 independent experiments (except for pol ι and the triple knockdown experiments, which were performed twice).
3.3. Contribution of pols η, ζ and Rev1 in mutagenesis of O2-Me-dT and O2-POB-dT
To determine the possible roles of the TLS pols in O2-alkyl-dT mutagenesis, we analyzed the TLS products obtained from the TLS pol knockdown cells. MF for O2-Me-dT was reduced in either pol ζ or Rev1 knockdown cells by approximately 24–25% relative to HEK293T cells treated with NC siRNA (Figure 2). A substantial 44% reduction in MF was also observed in pol ζ knockdown cells for O2-POB-dT, but surprisingly no significant change was observed in Rev1 knockdown cells (Figure 2). For both O2-Me-dT and O2-POB-dT, the decrease in MF in different pol knockdown cells was mainly due to a reduction in T→A mutations (Figure 3). For both O2-POB-dT and O2-Me-dT, knockdown of pol η, pol κ, or pol ι resulted in little changes in MF (Figure 2). Since knockdown of pol ζ exhibited a substantial reduction in MF for both lesions, we have explored the effect of simultaneous knockdown of this pol with other bypass pols. For O2-Me-dT and O2-POB-dT, simultaneous knockdown of pol η, pol ζ and Rev1 resulted in 42% and 61% decrease, respectively, in MF (Figure 2). Simultaneous knockdown of pol η, pol ζ and pol κ, on the other hand, reduced MF of O2-Me-dT and O2-POB-dT, respectively, by 40% and 54% (Figure 2).
4. Discussion
4.1. Involvement of pol η, ζ and Rev1 for bypass of O2-Me-dT and O2-POB-dT
It is well established that NNK, a tobacco specific nitrosamine, is a potent carcinogen in experimental animals and humans [4, 33, 34]. During metabolic activation of NNK, multiple reactive intermediates are generated that react with DNA, forming adducts, which are presumed to play a role in chemical carcinogenesis [8]. In this study we focused on the replication properties of O2-Me-dT and O2-POB-dT. O2-POB-dT is the most abundant bulky adduct in human cells formed during metabolic activation of NNK. While O2-Me-dT has not been identified in NNK-treated animals, small amounts are undoubtedly formed, based upon the chemistry of the methylation pathway. In our study, O2-Me-dT serves as a model to dissect the relative importance of the altered Watson-Crick hydrogen bonding and bulkiness of O2-POB-dT. The TLS efficiency for O2-Me-dT and O2-POB-dT in NC-siRNA treated HEK293T cells were 55% and 26%, respectively (Figure 1). This is an order of magnitude higher than 4.5% and 2.7%, respectively, TLS of O2-Me-dT and O2-POB-dT in uninduced E. coli, which increase only 2–4-fold upon induction of SOS [22]. Comparable magnitude of increase in TLS efficiency in human cells compared to E. coli has been reported in TLS of the abasic site [35, 36]. It is conceivable that TLS efficiency for most replication-blocking DNA lesions is greater in human cells than in E. coli, presumably due to an abundance of bypass pols in the former. Furthermore, as in E. coli, the bulkier O2-POB-dT was more genotoxic and bypassed less efficiently in human cells than O2-Me-dT. In another study in E. coli, it was established that the TLS efficiency decreases with longer chain lengths of O2-alkyldT [23], which is consistent with the results of our current study.
The TLS efficiency for O2-Me-dT in pol η, pol ζ or Rev1 knockdown cells decreased by approximately 45–53%, whereas for O2-POB-dT it was decreased by 35–46% (Figure 1). This indicates involvement of multiple TLS pols for the lesion bypass, which supports the current hypothesis that more than one pol and multiple pol switches are involved during the TLS process [37–40]. The TLS efficiency dropped by 45% and 35%, respectively, for O2-Me-dT and O2-POB-dT in pol η knockdown cells (Figure 1). Pol η is considered one of the main pols that performs TLS of many lesions due to its capacious catalytic site [41–43]. Reduced TLS efficiency for both lesions in pol η knockdown cells indicates involvement of this pol in the lesion bypass. This is comparable to what we observed in E. coli, that is pol V, the ortholog of pol η, is essential for bypass of these lesions [22, 44].
We have established that pol ζ is critical for TLS of both O2-Me-dT and O2-POB-dT since the TLS efficiency was reduced in pol ζ knockdown cells by 45% and 42%, respectively (Figure 1). Pol ζ has been reported to perform extension after incorporation of a nucleotide opposite a lesion during TLS [45]. Therefore, it is conceivable that, it could function efficiently in the extension of the nucleotides that were preferentially incorporated by pol η and by pol κ or pol ι. Likewise, Rev1 is equally important for O2-Me-dT and O2-POB-dT bypass, as shown by 46–53% reduction in TLS in Rev1 knockdown cells. Based on experimental evidences it is accepted that Rev1 functionally interact with all other Y family pols during TLS process [46–48]. Therefore, it is likely that Rev1 acts as a structural element to form a bridge between the inserter, specifically pol η, and the extender, pol ζ, during TLS of O2-Me-dT and O2-POB-dT. However, when both pol η and pol ζ together with either pol κ or Rev1 were simultaneously knocked down, further reduction in TLS efficiency was modest. This suggests that, upon knockdown of these critical pols, part of TLS (~40%) may still be carried out by other TLS pols and the remaining fraction of these pols that was not knocked down.
4.2. Mutagenicity of O2-Me-dT and O2-POB-dT and error-prone bypass by the TLS pols
Analysis of the progeny showed that both O2-Me-dT and O2-POB-dT are highly mutagenic lesions in HEK293T cells with MF of 65% and 53%, respectively (Figure 2). The relative MF of these two lesions was essentially reversed from what was reported in SOS-induced E. coli cells, where MF was found to be 21% and 56% for O2-Me-dT and O2-POB-dT, respectively [22]. In HEK293T cells, although bulkier O2-POB-dT was a stronger replication block, as reported in bacteria, it was bypassed slightly more accurately compared to O2-Me-dT. We suspect that, owing to the small size of the alkyl chain of the O2-Me-dT, it can be easily accommodated in the capacious catalytic site of the TLS pols, resulting in more efficient replication, albeit with low fidelity [49]. Even a relatively small methyl group at the 2 position of thymidine interferes with the base pairing modes with A and, based on thermal melting studies, G was found to be a better partner than A [50]. However, rather than T→C mutation, as suggested by the thermal melting experiments, the major type of mutations induced by both O2-Me-dT and O2-POB-dT was T→A transversions, at a frequency of 56% and 47%, respectively (Figure 3). The second most prevalent mutagenic event for O2-Me-dT was T→G (7%) followed by low levels of semi-targeted mutations (0.9%). O2-POB-dT generated approximately the same frequency of T→G, T→C and the semi-targeted mutations, which ranged from 1.5–2.3% (Figure 3). In NNK treated mice, AT→TA mutations occurred 2 to 3 times more frequently than AT→CG or AT→GC mutations [13, 51]. Furthermore, in vitro kinetic studies showed that dTTP incorporation opposite O2-POB-dT was more efficient than either dCTP or dGTP incorporation by both E. coli Kf− and Sulfolobus solfataricus DNA pol IV (Dpo4), two model DNA pols [25]. Our cellular results for O2-POB-dT are in agreement with the mutational spectra obtained from NNK-treated rodents as well as the in vitro kinetic data by purified model pols. In vitro replication study on a template containing O2-Me-dT, in a different sequence context, showed that it is a strong block of DNA replication by E. coli DNA polymerase I (Kf−), yeast pol η, and human pol κ [24]. However, Kf- and yeast pol η preferentially incorporated the correct nucleotide, dAMP, while human pol κ preferred dCMP opposite O2-Me-dT [24]. From our study, however, the greatest reduction in T→G mutations occurred when either pol ι or pol ζ was knocked down (Figure 3). Yet, when pol ζ as well as two other TLS pols were knocked down simultaneously, T→G mutations remain unaffected. This suggests that a part of T→G mutations stems from dCMP incorporation opposite O2-Me-dT by pol ι, but the mismatched pair can be extended either by pol ζ or another TLS pol.
The targeted T→A events changed in different pol knockdown cells for both O2-Me-dT and O2-POB-dT. Nevertheless, the pattern of the mutations remained comparable in most knockdown cells (Figure 3). Although the TLS efficiency was reduced in pol η knockdown cells, the MF was only slightly reduced for both lesions, suggesting that both error-free and erroneous TLS are catalyzed by pol η. Pol η was found to be involved in error-prone bypass of different DNA lesions [43], whereas it conducts accurate and efficient TLS of 7,8-dihydro-8-oxoguanine and cis-syn thymine-thymine dimer [52–54]. It is possible that it is involved in both correct nucleotide incorporation of dAMP and misincorporation of dTMP opposite both O2-alkyl-dT lesions. But pol ι and pol κ may also be involved in misincorporation opposite these lesions. As a result, it is challenging at this time to distinguish the roles of each TLS pol, as residual 10–30% enzyme may be able to carry out TLS across the lesions in the knockdown experiments. Future experiments preferably using a combination of in vitro assay and knockout cell lines will be needed to distinguish the roles of individual pol in the lesion bypass.
In pol ζ knockdown cells, the MF for O2-Me-dT and O2-POB-dT was decreased by 25% and 44%, respectively, in addition to a reduction in TLS efficiency (Figure 2). Pol ζ is relatively tolerant of abnormal structure at the primer terminus [55, 56]. Hence, we postulate that it takes a more active role in the extension of O2-alkyl-dT:dT mismatched base pair in comparison to the correct O2-alkyl-dT:dA pair, leading to error-prone replication of both lesions. The extension of O2-alkyl-dT:dT mismatch by pol ζ appeared to be more pronounced in the TLS of bulkier O2-POB-dT, presumably because the extension of the correct O2-POB-dT:A pair was even more inefficient (Figure 3). The MF in Rev1 knockdown cells was reduced to a similar extent as in pol ζ knockdown cells for O2-Me-dT, indicating that Rev1 plays an indispensable structural role in pol ζ dependent bypass of O2-Me-dT (Figure 2). It is unclear why it does not play a similar role in O2-POB-dT bypass, since MF of O2-POB-dT remained unaltered in Rev1 knockdown cells.
4.3. Mechanistic rationale for the error-prone TLS of O2-alkyl-dT
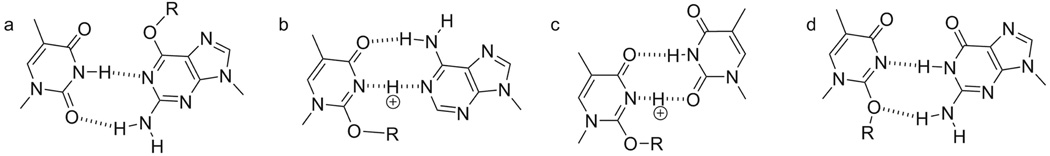
The mechanistic rationale for the preferential insertion of dT and dA opposite O2-alkyl-dT is not clear. The ability to form Watson-Crick-like structures has been proposed to explain the mutagenic potential of DNA adducts. This analysis worked well with O6-methylguanine, in which the Watson-Crick-like structure in Figure 4a, initially proposed by Loveless in 1969 [59], explains the mutagenic incorporation of dTTP [58–60]. However, the attempt to duplicate this analysis with O2-alkyl-dT is not as satisfying. Figure 4b shows a Watson-Crick-like structure between dA and O2-alkyl-dT. While the structure requires protonation, the alkyl group can also make a hydrophobic interaction with the 2-position of adenine stabilizing the structure [61]. Figure 4c displays a base-pair structure between dT and O2-alkyl-dT similar to the wobble structure that was observed in an oligodeoxynucleotide duplex containing a dT/dT mispair [62]. Evidently, this structure is not Watson-Crick-like. There are no noticeable features of Figure 4b and 4c that would explain why dA and dT are inserted opposite O2-alkyl-dT more often than dG in the absence of any structural studies. Figure 4d shows a potential Watson-Crick-like structure between dG and O2-alkyl-dT. This structure is very similar to Figure 4a, except that the alkyl group is in the minor groove. Perhaps this structure explains the mutagenic incorporation of dGTP opposite O2-alkyl-dT by pol V in E. coli [22, 63]. However, the preference for the incorporation of dT and dA opposite O2-alkyl-dG in humans cells is not evident. The different specificities in different kingdoms indicate that protein-DNA interactions play a large role in determining the identity of the nucleotide inserted opposite O2-alkyl-dTs.
Figure 4.
Postulated base-pairing modes of (a) O6-alkyl-dG:dT by Loveless [57], (b) protonated O2-alkyl-dT:dA, (c) protonated O2-alkyl-dT:dT, and (d) O2-alkyl-dT:dG.
5. Conclusion
In conclusion, the O2-Me-dT and O2-POB-dT formed by the tobacco-specific nitrosamines were found to be strong blocks of DNA replication and highly mutagenic in HEK293T cells. This result is consistent with the POB-pathway playing an important role in NNK carcinogenesis. While the bulkier O2-POB-dT was a stronger replication block compared to O2-Me-dT, the latter was more mutagenic. The major type of mutations induced by these lesions was T→A, but low frequencies of T→G, T→C, and semi-targeted mutations also were detected. Though pols η, ζ and Rev1 play important roles in the error-prone replication of these lesions, siRNA knockdown experiments showed that pol ζ is a key enzyme for mutagenesis induced by O2-POB-dT.
Supplementary Material
Highlights.
O2-Me-dT and O2-POB-dT are replication blocking and highly mutagenic lesions in human cells.
Both O2-Me-dT and O2-POB-dT induce T→A as the major type of mutations in human cells.
Pols η, ζ and Rev1 play important roles in the TLS of O2-Me-dT and O2-POB-dT.
Pol ζ is a key enzyme for mutagenesis induced by O2-POB-dT.
Acknowledgements
This research was supported by the NIEHS grant ES021762.
Abbreviations
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- NNN
N'-nitrosonornicotine
- O2-Me-dT
O2-methylthymidine
- O2-POB-dT
O2-[4-(3-pyridyl-4-oxobut-1-yl]thymidine
- HEK293T
human embryonic kidney 293T
- TLS
translesion synthesis
- MF
mutation frequency
- pol
DNA polymerase
- Kf−
exo-free Klenow fragment of DNA polymerase I
- Dpo4
Sulfolobus solfactaricus DNA polymerase IV
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Hecht SS. Research opportunities related to establishing standards for tobacco products under the Family Smoking Prevention and Tobacco Control Act. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2012;14:18–28. doi: 10.1093/ntr/ntq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann D, Hoffmann I. The changing cigarette, 1950–1995. J Toxicol Environ Health. 1997;50:307–364. doi: 10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- 3.Hecht SS. Cigarette smoking: cancer risks, carcinogens, and mechanisms. Langenbeck's archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2006;391:603–613. doi: 10.1007/s00423-006-0111-z. [DOI] [PubMed] [Google Scholar]
- 4.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer, Nature reviews. Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Department of Health and Human Services. The health consequences of smoking-50 years of progress: A report of the surgeon general. Vol. 17. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 6.Xue J, Yang S, Seng S. Mechanisms of Cancer Induction by Tobacco-Specific NNK and NNN. Cancers. 2014;6:1138–1156. doi: 10.3390/cancers6021138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hecht SS. Progress and challenges in selected areas of tobacco carcinogenesis. Chem Res Toxicol. 2008;21:160–171. doi: 10.1021/tx7002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 9.Delaney JC, Essigmann JM. Biological properties of single chemical-DNA adducts: a twenty year perspective. Chem Res Toxicol. 2008;21:232–252. doi: 10.1021/tx700292a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morse MA, Amin SG, Hecht SS, Chung F-L. Effects of aromatic isothiocyanates on tumorigenicity, O6-methylguanine formation, and metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in A/J mouse lung. Cancer Res. 1989;49:2894–2897. [PubMed] [Google Scholar]
- 11.Peterson LA, Hecht SS. O6-methylguanine is a critical determinant of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone tumorigenesis in A/J mouse lung. Cancer Res. 1991;51:5557–5564. [PubMed] [Google Scholar]
- 12.Hecht SS, Jordan KG, Choi CI, Trushin N. Effects of deuterium substitution on the tumorigenicity of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in A/J mice. Carcinogenesis. 1990;11:1017–1020. doi: 10.1093/carcin/11.6.1017. [DOI] [PubMed] [Google Scholar]
- 13.Sandercock LE, Hahn JN, Li L, Luchman HA, Giesbrecht JL, Peterson LA, Jirik FR. Mgmt deficiency alters the in vivo mutational spectrum of tissues exposed to the tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) Carcinogenesis. 2008;29:866–874. doi: 10.1093/carcin/bgn030. [DOI] [PubMed] [Google Scholar]
- 14.Jalas JR, McIntee EJ, Kenney PM, Upadhyaya P, Peterson LA, Hecht SS. Stereospecific deuterium substitution attenuates the tumorigenicity and metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) Chem Res Toxicol. 2003;16:794–806. doi: 10.1021/tx034022l. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Spratt TE, Liu XK, Hecht SS, Pegg AE, Peterson LA. Pyridyloxobutyl adduct O6-[4-oxo-4-(3-pyridyl)butyl]guanine is present in 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone-treated DNA and is a substrate for O6-alkylguanine-DNA alkyltransferase. Chem Res Toxicol. 1997;10:562–567. doi: 10.1021/tx9602067. [DOI] [PubMed] [Google Scholar]
- 16.Wang M, Cheng G, Sturla SJ, Shi Y, McIntee EJ, Villalta PW, Upadhyaya P, Hecht SS. Identification of adducts formed by pyridyloxobutylation of deoxyguanosine and DNA by 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone, a chemically activated form of tobacco specific carcinogens. Chem Res Toxicol. 2003;16:616–626. doi: 10.1021/tx034003b. [DOI] [PubMed] [Google Scholar]
- 17.Hecht SS, Villalta PW, Sturla SJ, Cheng G, Yu N, Upadhyaya P, Wang M. Identification of O2-substituted pyrimidine adducts formed in reactions of 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone and 4-(acetoxymethylnitros-amino)-1-(3-pyridyl)-1-butanol with DNA. Chem Res Toxicol. 2004;17:588–597. doi: 10.1021/tx034263t. [DOI] [PubMed] [Google Scholar]
- 18.Urban AM, Upadhyaya P, Cao Q, Peterson LA. Formation and repair of pyridyloxobutyl DNA adducts and their relationship to tumor yield in A/J mice. Chem Res Toxicol. 2012;25:2167–2178. doi: 10.1021/tx300245w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balbo S, Upadhyaya P, Villalta PW, Qian X, Kassie F. DNA adducts in aldehyde dehydrogenase-positive lung stem cells of A/J mice treated with the tobacco specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) Chem Res Toxicol. 2013;26:511–513. doi: 10.1021/tx400054s. [DOI] [PubMed] [Google Scholar]
- 20.Lao Y, Yu N, Kassie F, Villalta PW, Hecht SS. Analysis of pyridyloxobutyl DNA adducts in F344 rats chronically treated with (R)- and (S)-N'-nitrosonornicotine. Chem Res Toxicol. 2007;20:246–256. doi: 10.1021/tx060208j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Perdigao J, Pegg AE, Lao Y, Hecht SS, Lindgren BR, Reardon JT, Sancar A, Wattenberg EV, Peterson LA. The influence of repair pathways on the cytotoxicity and mutagenicity induced by the pyridyloxobutylation pathway of tobacco-specific nitrosamines. Chem Res Toxicol. 2009;22:1464–1472. doi: 10.1021/tx9001572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jasti VP, Spratt TE, Basu AK. Tobacco-Specific Nitrosamine-Derived O2-Alkylthymidines Are Potent Mutagenic Lesions in SOS-Induced Escherichia coli. Chem Res Toxicol. 2011;24:1833–1835. doi: 10.1021/tx200435d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhai Q, Wang P, Cai Q, Wang Y. Syntheses and characterizations of the in vivo replicative bypass and mutagenic properties of the minor-groove O2-alkylthymidine lesions. Nucleic Acids Res. 2014;42:10529–10537. doi: 10.1093/nar/gku748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen N, Wang J, Wang P, Jiang Y, Wang Y. In-vitro replication studies on O2-methylthymidine and O4-methylthymidine. Chem Res Toxicol. 2012;25:2523–2531. doi: 10.1021/tx300325q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gowda AS, Krishnegowda G, Suo Z, Amin S, Spratt TE. Low fidelity bypass of O2-(3-pyridyl)-4-oxobutylthymine, the most persistent bulky adduct produced by the tobacco specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone by model DNA polymerases. Chem Res Toxicol. 2012;25:1195–1202. doi: 10.1021/tx200483g. [DOI] [PubMed] [Google Scholar]
- 26.Moriya M. Single-stranded shuttle phagemid for mutagenesis studies in mammalian cells: 8-oxoguanine in DNA induces targeted G.C-->T.A transversions in simian kidney cells. Proc Natl Acad Sci U S A. 1993;90:1122–1126. doi: 10.1073/pnas.90.3.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colis LC, Raychaudhury P, Basu AK. Mutational specificity of γ-radiation-induced guanine-thymine and thymine-guanine intrastrand cross-links in mammalian cells and translesion synthesis past the guanine-thymine lesion by human DNA polymerase η. Biochemistry. 2008;47:8070–8079. doi: 10.1021/bi800529f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 29.Pande P, Malik CK, Bose A, Jasti VP, Basu AK. Mutational analysis of the C8-guanine adduct of the environmental carcinogen 3-nitrobenzanthrone in human cells: critical roles of DNA polymerases η and κ and Rev1 in error-prone translesion synthesis. Biochemistry. 2014;53:5323–5331. doi: 10.1021/bi5007805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mechali M, Harland RM. DNA synthesis in a cell-free system from Xenopus eggs: priming and elongation on single-stranded DNA in vitro. Cell. 1982;30:93–101. doi: 10.1016/0092-8674(82)90015-0. [DOI] [PubMed] [Google Scholar]
- 31.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 32.Watt DL, Utzat CD, Hilario P, Basu AK. Mutagenicity of the 1-nitropyrene-DNA adduct N-(deoxyguanosin-8-yl)-1-aminopyrene in mammalian cells. Chem Res Toxicol. 2007;20:1658–1664. doi: 10.1021/tx700131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 34.Peterson LA. Formation, repair, and genotoxic properties of bulky DNA adducts formed from tobacco-specific nitrosamines. Journal of nucleic acids. 2010;2010 doi: 10.4061/2010/284935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avkin S, Adar S, Blander G, Livneh Z. Quantitative measurement of translesion replication in human cells: evidence for bypass of abasic sites by a replicative DNA polymerase. Proc Natl Acad Sci U S A. 2002;99:3764–3769. doi: 10.1073/pnas.062038699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weerasooriya S, Jasti VP, Basu AK. Replicative bypass of abasic site in Escherichia coli and human cells: similarities and differences. PloS one. 2014;9:e107915. doi: 10.1371/journal.pone.0107915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehmann AR, Niimi A, Ogi T, Brown S, Sabbioneda S, Wing JF, Kannouche PL, Green CM. Translesion synthesis: Y-family polymerases and the polymerase switch. DNA Repair. 2007;6:891–899. doi: 10.1016/j.dnarep.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Livneh Z, Ziv O, Shachar S. Multiple two-polymerase mechanisms in mammalian translesion DNA synthesis. Cell Cycle. 2010;9:729–735. doi: 10.4161/cc.9.4.10727. [DOI] [PubMed] [Google Scholar]
- 39.Friedberg EC, Lehmann AR, Fuchs RP. Trading places: how do DNA polymerases switch during translesion DNA synthesis? Mol Cell. 2005;18:499–505. doi: 10.1016/j.molcel.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 40.Boudsocq F, Kokoska RJ, Plosky BS, Vaisman A, Ling H, Kunkel TA, Yang W, Woodgate R. Investigating the role of the little finger domain of Y-family DNA polymerases in low fidelity synthesis and translesion replication. J Biol Chem. 2004;279:32932–32940. doi: 10.1074/jbc.M405249200. [DOI] [PubMed] [Google Scholar]
- 41.Waters LS, Minesinger BK, Wiltrout ME, D'Souza S, Woodruff RV, Walker GC. Eukaryotic Translesion Polymerases and Their Roles and Regulation in DNA Damage Tolerance. Microbiology and Molecular Biology Reviews. 2009;73:134–154. doi: 10.1128/MMBR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuda T, Bebenek K, Masutani C, Hanaoka F, Kunkel TA. Low fidelity DNA synthesis by human DNA polymerase η. Nature. 2000;404:1011–1013. doi: 10.1038/35010014. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Yuan F, Wu X, Rechkoblit O, Taylor JS, Geacintov NE, Wang Z. Error-prone lesion bypass by human DNA polymerase η. Nucleic Acids Res. 2000;28:4717–4724. doi: 10.1093/nar/28.23.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee CH, Chandani S, Loechler EL. Homology modeling of four Y-family, lesion-bypass DNA polymerases: the case that E coli Pol IV and human Pol κ are orthologs, and E coli Pol V and human Pol η are orthologs. Journal of molecular graphics & modelling. 2006;25:87–102. doi: 10.1016/j.jmgm.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Shachar S, Ziv O, Avkin S, Adar S, Wittschieben J, Reißner T, Chaney S, Friedberg EC, Wang Z, Carell T, Geacintov N, Livneh Z. Two-polymerase mechanisms dictate error-free and error-prone translesion DNA synthesis in mammals. The EMBO Journal. 2009;28:383–393. doi: 10.1038/emboj.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pustovalova Y, Bezsonova I, Korzhnev DM. The C-terminal domain of human Rev1 contains independent binding sites for DNA polymerase η and Rev7 subunit of polymerase ζ. FEBS Lett. 2012;586:3051–3056. doi: 10.1016/j.febslet.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo C, Fischhaber PL, Luk MJ, ÄêPaszyc, Masuda Y, Zhou J, Kamiya K, Kisker C, Friedberg EC. Mouse Rev1 protein interacts with multiple DNA polymerases involved in translesion DNA synthesis. The EMBO Journal. 2003;22:6621–6630. doi: 10.1093/emboj/cdg626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohashi E, Murakumo Y, Kanjo N, Akagi J, Masutani C, Hanaoka F, Ohmori H. Interaction of hREV1 with three human Y-family DNA polymerases. Genes Cells. 2004;9:523–531. doi: 10.1111/j.1356-9597.2004.00747.x. [DOI] [PubMed] [Google Scholar]
- 49.Sale JE, Lehmann AR, Woodgate R. Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nat Rev Mol Cell Bio. 2012;13:141–152. doi: 10.1038/nrm3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Y-Z, Swann PF. Oligodeoxynucleotides containing O2-alkylthymine: Synthesis and characterization. Tetrahedron Lett. 1994;35:303–306. [Google Scholar]
- 51.Hashimoto K, Ohsawa K-i, Kimura M. Mutations induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in the lacZ and cII genes of Muta™Mouse. Mutation Res/Genetic Toxicol and Environ Mutagenesis. 2004;560:119–131. doi: 10.1016/j.mrgentox.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 52.Haracska L, Yu SL, Johnson RE, Prakash L, Prakash S. Efficient and accurate replication in the presence of 7,8-dihydro-8-oxoguanine by DNA polymerase η. Nature Genetics. 2000;25:458–461. doi: 10.1038/78169. [DOI] [PubMed] [Google Scholar]
- 53.Prakash S, Johnson RE, Washington MT, Haracska L, Kondratick CM, Prakash L. Role of yeast and human DNA polymerase η in error-free replication of damaged DNA. Cold Spring Harb Sym. 2000;65:51–59. doi: 10.1101/sqb.2000.65.51. [DOI] [PubMed] [Google Scholar]
- 54.Washington MT, Johnson RE, Prakash S, Prakash L. Accuracy of thymine-thymine dimer bypass by Saccharomyces cerevisiae DNA polymerase η. P Natl Acad Sci USA. 2000;97:3094–3099. doi: 10.1073/pnas.050491997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kikuchi S, Hara K, Shimizu T, Sato M, Hashimoto H. Structural basis of recruitment of DNA polymerase ζ by interaction between REV1 and REV7 proteins. J Biol Chem. 2012;287:33847–33852. doi: 10.1074/jbc.M112.396838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hara K, Hashimoto H, Murakumo Y, Kobayashi S, Kogame T, Unzai S, Akashi S, Takeda S, Shimizu T, Sato M. Crystal structure of human REV7 in complex with a human REV3 fragment and structural implication of the interaction between DNA polymerase ζ and REV1. J Biol Chem. 2010;285:12299–12307. doi: 10.1074/jbc.M109.092403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969;223:206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- 58.Warren JJ, Forsberg LJ, Beese LS. The structural basis for the mutagenicity of O6 - methyl-guanine lesions. Proc.Natl.Acad.Sci.U.S.A. 2006;103:19701–19706. doi: 10.1073/pnas.0609580103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eoff RL, Angel KC, Egli M, Guengerich FP. Molecular Basis of Selectivity of Nucleoside Triphosphate Incorporation Opposite O6-Benzylguanine by Sulfolobus solfataricus DNA Polymerase Dpo4: STEADY-STATE AND PRE-STEADY-STATE KINETICS AND XRAY CRYSTALLOGRAPHY OF CORRECT AND INCORRECT PAIRING. J. Biol. Chem. 2007;282:13573–13584. doi: 10.1074/jbc.M700656200. [DOI] [PubMed] [Google Scholar]
- 60.Spratt TE, Levy DE. Structure of the hydrogen bonding complex of O6 -methylguanine with cytosine and thymine during DNA replication. Nucleic Acids Res. 1997;25:3354–3361. doi: 10.1093/nar/25.16.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loechler EL. Molecular modeling studies of O2-alkylthymines and O4-alkylthymines in DNA: Structures that may be pertinent to the incorporation of the corresponding dAlkTTP into DNA by DNA polymerases in vitro. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 1990;233:39–44. doi: 10.1016/0027-5107(90)90149-x. [DOI] [PubMed] [Google Scholar]
- 62.Gervais V, Cognet JA, Le Bret M, Sowers LC, Fazakerley GV. Solution structure of two mismatches A.A and T.T in the K-ras gene context by nuclear magnetic resonance and molecular dynamics. Eur.J.Biochem. 1995;228:279–290. [PubMed] [Google Scholar]
- 63.Zhai Q, Wang P, Cai Q, Wang Y. Syntheses and characterizations of the in vivo replicative bypass and mutagenic properties of the minor-groove O2-alkylthymidine lesions. Nucleic Acids Res. 2014;42:10529–10537. doi: 10.1093/nar/gku748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.