Abstract
The Function Biomedical Informatics Research Network (FBIRN) developed methods and tools for conducting multi-scanner functional magnetic resonance imaging (fMRI) studies. Method and tool development were based on two major goals: 1) to assess the major sources of variation in fMRI studies conducted across scanners, including instrumentation, acquisition protocols, challenge tasks, and analysis methods, and 2) to provide a distributed network infrastructure and an associated federated database to host and query large, multi-site, fMRI and clinical datasets. In the process of achieving these goals the FBIRN test bed generated several multi-scanner brain imaging data sets to be shared with the wider scientific community via the BIRN Data Repository (BDR). The FBIRN Phase 1 dataset consists of a traveling subject study of 5 healthy subjects, each scanned on 10 different 1.5 to 4 Tesla scanners. The FBIRN Phase 2 and Phase 3 datasets consist of subjects with schizophrenia or schizoaffective disorder along with healthy comparison subjects scanned at multiple sites. In this paper, we provide concise descriptions of FBIRN’s multi-scanner brain imaging data sets and details about the BIRN Data Repository instance of the Human Imaging Database (HID) used to publicly share the data.
1. Introduction
The Function Biomedical Informatics Research Network (FBIRN) was a National Institutes of Health (NIH), National Center for Research Resources (NCRR) funded program designed to develop methods and tools to enable multi-center functional MRI studies. Multi-center studies can address several issues in medical research, including representative sampling and faster acquisition of large data sets of common2 or rare cases that are slow to acquire at individuals sites3. Representative sampling allows for broader generalization of findings, such as sampling based on census data1, an important problem given the typical demographic, ethnic, geographic, dietary, and co-morbidity variability found in many disorders. However, multi-scanner studies are only feasible if one can adequately control between-site variance.
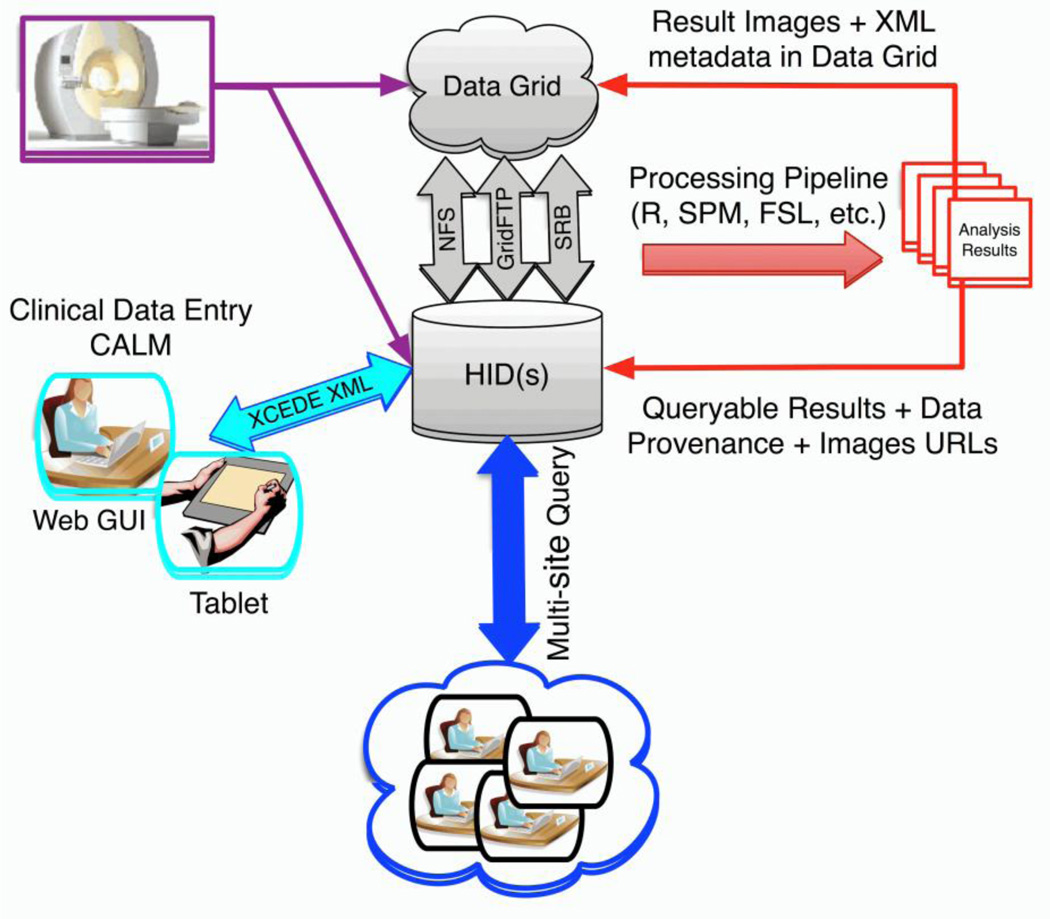
To enable multi-scanner fMRI studies, FBIRN developed the FBIRN agar phantom and associated software for scanner quality assurance4, 5, standardized fMRI scanner sequences and recommendations for multi-center functional imaging studies6, and the open-source Federated Informatics Research Environment (FIRE; Figure 1)7–9, which includes the BIRN Human Imaging Database (HID; www.nitrc.org/projects/hid) and web interface.
Figure 1.
FBIRN’s FIRE is an open-source and integrated set of tools for performing multi-site neuroimaging studies which includes: 1) centralized authentication and authorization services to support federated group management across resources, 2) support for both locally-mounted (e.g., NFS) and distributed file systems (i.e. Gridftp and the Storage Resource Broker), 3) a Clinical Assessment Layout Manager (CALM) for creating online data entry forms, 4) the Human Imaging Database (HID; www.nitrc.org/projects/hid) which provides all data management processes required for multi-center fMRI studies, 5) a standardized directory structure, and 6) the fBIRN image processing stream (http://www.nmr.mgh.harvard.edu/~greve/fbirn/fips/).
The HID provides the following capabilities: a) double data entry and validation (e.g., for clinical scale data), b) imaging and associated behavioral, and physiological (e.g., heart rate, respiration) data upload, and c) imaging, clinical, and derived data storage, query, and download7, 8. The HID was designed to manage federated data acquisition, hosting, and querying but can also be used as a centralized database for storing and sharing imaging and associated behavioral and clinical data with the broader research community.
This manuscript describes the FBIRN Data Repository (BDR), a simplified instance of the HID used for sharing FBIRN data, which were collected in the process of developing multi-center fMRI methods, with the wider research community. In the methods section we address several questions about the FBIRN BDR, including: 1) what was the BDR designed to do, 2), which data sets does it provide, 3) which data formats are available, 4) what quality control was performed on these data sets, 5) how do you access the BDR, 6) can new data be contributed to the BDR, and 7) what are the long term plans for the BDR? We conclude the manuscript referring to some of the published findings and works in progress based on data hosted on the BDR.
2 Methods
2.1 Purpose of the BIRN Data Repository
The BDR (fbirnbdr.nbirn.net:8080/BDR) was designed for the purpose of public sharing of FBIRN multi-modal (imaging, clinical, cognitive, and physiological) data with the research community. The BDR was built using a modified instance of the BIRN HID (version 1.6.2). To create the BDR, the full HID system was reduced. Query capabilities were limited to streamline use by those unfamiliar with the HID system and new data entry was disallowed. The data was collated by subject/project/site and linked on the HID home screen to enable easy downloads. Moreover, access control was modified to allow public access based on email addresses.
The BDR makes downloading large amounts of data simple. Users select and submit datasets for download using checkboxes. This process generates a request to the job management service, which handles transferring the bundle to the HID web server. The user can return later to check if the job has been completed. Once the bundle is available on the web server, the dataset can be downloaded to a local computer. The datasets will remain in the job management console until the user removes the entry or the BDR cache reaches capacity, at which time the oldest bundles will be deleted to free up space.
2.2 BIRN Data Repository Imaging Data Sets
This section describes the FBIRN Phase I, II, and III multi-center data sets. Currently, the BDR hosts FBIRN Phase I and Phase II data. The BDR FBIRN Phase III data will become publicly available in the fall of 2015. For information on sites involved in data collection for each project see S1, Table 7.
2.2.1 FBIRN Phase I Traveling Subjects Dataset
The FBIRN “Phase I” study objectives were to identify sources of inter-site differences in fMRI studies, to find ways to decrease variability by standardizing studies where possible, and to develop methods for correcting remaining inter-site differences4, 10, 11.
2.2.1.1 Study Design
The Phase I study included 5 right-handed, native English speaking, healthy male subjects between 20–29 years of age. Three subjects were Caucasian and two were African, each having at least 15 years of education. The subjects had no history of psychiatric or neurological illness, no MRI contraindications, and normal hearing. None of the subjects used nicotine, alcohol, caffeine, or regular medications. To measure inter-site, inter-subject, and intersession variance, the 5 traveling subjects were assessed with structural (sMRI) and functional (fMRI) scans on 2 separate days on ten different scanners across the country (see S1, Table 1,2). The scanner manufacturer, model, field strength (1.5-4 Tesla), head coil, and auditory, visual, and response devices varied across sites (see S1, Table 3). Since there is no obvious way to calibrate functional signals from cognitive tasks, FBIRN developed a sensorimotor (SM) task, to activate motor, visual, and auditory cortices, and a breath-hold (BH) task to measure vascular response.
2.2.1.2 Imaging Assessments
The imaging protocol at each site included: high-resolution T1-weighted, T2-weighted, and fMRI scans of Mismatch Negativity, Serial Item Recognition Paradigm (SIRP, 2 runs),12 SM10, resting state (REST), and BH13, 14 tasks. All tasks were performed in the same order at each site (see S1 Table 4). The tasks were programmed in E-Prime (www.pstnet.com), started by scanner trigger, and collected behavioral responses and response times. A basic in-scanner reaction time task was administered for comparison of response devices across sites.
2.2.1.3 Additional Assessments
All study participants were clinically assessed with the Structured Clinical Interview for the DSMN-on-Patient 15, the Beck Depression Inventory16, the Zung Self-Administered Anxiety Scale17, and the North American Adult Reading Test18. The Quick Mood Scale19 was also administered before and after each scan.
2.2.2 FBIRN Phase II Schizophrenia Dataset
The FBIRN Phase II study objective was to conduct a multi-center fMRI study in a clinical population to further develop multi-center methods to decrease inter-site variance in normal and clinical populations by standardization, building on the lessons learned during the FBIRN Phase I study.
2.2.2.1 Study Design
The Phase II study was able to share data from 87 individuals with DSM-IV schizophrenia or schizoaffective disorder diagnoses (SZ, 59 males) and 85 healthy comparison subjects (HC, 70 males) between the ages of 18–70. All subjects had normal hearing levels, sufficient eyesight to see visual displays, an IQ > 75, were fluent in English, were able to perform the study tasks, had no previous head injury or prolonged unconsciousness, substance and/or alcohol dependence, migraine treatments, or MRI contraindications. SZ subjects were excluded if they had a current or past history of a major medical illness, had significant extrapyramidal symptoms or tardive dyskinesia (measured by the Global section of the AIMS20), or were not clinically stable (had significant changes in their psychotropic medications in the previous two months). HC subjects were excluded if they had a current or past history of a major neurological or psychiatric medical illness or had a first degree relative with a psychotic illness diagnosis. Scanner field strengths ranged from 1.5 to 4.0 Tesla and included General Electric (GE), Siemens, and Marconi (Picker) imaging platforms. The scanner manufacturer, model, field strength, head coil, and auditory, visual, and response devices varied across sites (see S1 Table 5). Subjects had a normal night’s sleep, no more than one alcoholic drink the night before each scan, and abstained from drinking coffee 2 hours and smoking 40 minutes prior to scanning. Each scan session consisted of brief training to familiarize the subject with the paradigms and placement in the scanner for about 1.5 hours during which all images were collected. For each subject, the entire imaging session was repeated between 24 hours to 3 weeks later.
2.2.2.2 Imaging Assessments
The imaging protocol at each site included: T1-weighted and T2-weighted scans, B0 field mapping, the SM task (4 runs)10, a Serial Item Recognition Paradigm (SIRP; 3 runs)12, Auditory Oddball (AO) task (4 runs)21, 22, and a BH task23. All tasks were performed in the same order at each site. The tasks were programmed in E-Prime (www.pstnet.com) and recorded behavioral responses and response times. Stimulus responses were recorded using an SRBox response device. A basic in-scanner reaction time task was administered for comparison of response devices across sites. Visual stimuli were delivered using either rear-projection screens, front-projection screens with coil-mounted mirrors, or MRI-compatible goggles. Auditory stimuli were standardized as much as possible using an auditory setup program. The stimuli were delivered using pneumatic, sound-insulated headphones adjusted to a volume level that could be heard comfortably over the scanner noise. The relative volume was recorded, as it varied by scanner, subject, and headphones.
For every task, except AO, subjects completed one practice run prior to scanning. A behavioral analysis was run immediately to determine subjects were performing at acceptable levels (greater than 75% on the SIRP task). A reaction time task was also performed prior to scanning. In addition, the reaction time task was administered once subjects were inside the scanner, for comparison of response devices across sites. Responses were monitored using keypads, and reaction times were recorded for subsequent psychophysical analysis of timing and accuracy of task performance.
2.2.2.3 Additional Assessments
Prior to participating in scanning procedures, all subjects received extensive diagnostic evaluations by experienced raters. Subjects were diagnosed using the SCID15, 24. All participants received a demographics assessment, the Edinburgh Handedness Inventory25, Socio-economic Status Questionnaire (SES), Fagerstrom Test for Nicotine Dependence26 (FTND), Family Interview for Genetic Studies (FIGS), and the NAART18. In addition, all patients received the following symptom and side effect ratings: Scales for the Assessment of Positive (SAPS)27 and Negative Symptoms (SANS)28, a modified Positive and Negative Symptom Scale (PANSS)29, Calgary Depression Scale (CDS)30, Premorbid Adjustment Scale (PAS), Schedule for the Deficit Syndrome (SDS), the InterSePT Scale for Suicidal Thinking31, Abnormal Involuntary Movement Scale (AIMS)20, Barnes Akathisia Rating Scale (BARS)32, and Simpson-Angus Scale33 (SAS). All subjects completed an expanded version of the Quick Mood Scale immediately before/after each scanning session. The demographics assessment included age, gender, handedness, ethnicity, education (individual/family), occupation, living arrangements, number of children, and marital status.
The assessment data were collected electronically using forms designed in CALM and deployed on the HID. The forms were designed to mimic the physical layout of the corresponding paper assessments whenever possible. The data were double-entered into the HID and discrepancies resolved using a reconciliation interface. Each participating site entered the assessments into their local HID and data were made available to consortium members using the HID federated query interface.
2.2.3 FBIRN Phase III Schizophrenia Dataset
The FBIRN Phase III study objective was to conduct a large multi-center fMRI study in a clinical population, using the mature FBIRN multi-center methods6, 34 to decrease inter-site variance in both normal and clinical populations by standardization, building on the lessons learned and tools developed during the previous Phase I and II studies. The Phase III study was the second major multi-site FBIRN study of a clinical population, beginning in January of 2010.
2.2.3.1 Study Design
The dataset includes sMRI, fMRI, DTI, behavioral data, and demographic and clinical assessments, on 186 healthy controls and 176 individuals with schizophrenia from around the U.S. The goals of the Phase III study were similar to the Phase II study, to decrease inter-site variance by further standardization and by testing the methods for correction in normal and clinical populations developed in Phase l and ll. Study participants were between the ages of 18–62. The exclusion criteria were the same as those described for the Phase II study (see Section 2.2.2).
2.2.3.2 Imaging Assessments
The imaging protocol at each site included: localizer, T1-weighted, T2-weighted, B0 field mapping scans (4), arterial spin labeling, a diffusion tensor imaging scan (30 directions, 4 b=0), an Emotional Distracter Object Working Memory (7 runs), AO (2 runs), and two resting state (short+long) fMRI scans. All sites used 3.0 Tesla MRI scanners and included GE and Siemens imaging platforms. The order of the tasks was constant, unless technical difficulties precluded this and is provided with the data. All tasks started by scanner trigger. The stimuli and responses were presented and collected using Cigal (www.nitrc.org/projects/cigal)35, 36. The scanner manufacturer, model, head coil, and auditory, visual, and response devices varied across sites. The site IDs were kept constant relative to the Phase I and Phase II studies, enabling comparison of within site data across the data sets.
2.2.3.4 Additional Assessments
Subjects were diagnosed using the SCID15, 24 (modules A-E excluding anxiety disorders). All participants received a demographics assessment, the Edinburgh Handedness Inventory25, SES, FTND26, and the NAART18. In addition, all patients received the following symptom and side effects ratings, SAPS27, SANS28, PANSS29, SDS37, CDI30, Clinical Global Impression (CGI), AIMS20, BARS32, and SAS33. An expanded demographics assessment relative to the Phase II study was administered which included the following additional variables: drug history, diagnosis, body mass index, suicide attempts, and number of hospitalizations. An extensive neurocognitive tests battery was administered using the CMINDS™ platform38 (www.neurocomp.com/neurocomp/Solutions/Cminds; (see S1, Table 6).
The assessment data were collected electronically using forms deployed on a clinical assessment tablet system39. Once data was collected on the tablet, they were electronically imported into the local HID and made available to the entire consortium using the HID federated query interface.
2.3 Data Formats
All BDR imaging data are unprocessed and shared in NIfTI format. The fMRI task data are shared as E-Prime 1.0 Edat files (www.pstnet.com) or CIGAL text output (www.nitrc.org/projects/cigal) and the clinical data are shared in Microsoft Excel format. The BDR does not provide derived data, data processing pipelines, or citable DOIs or URIs.
2.4 Quality Control Performed on BDR Data Sets
Quality control (QC) of the FBIRN data was assessed during data collection. Our methods matured over the course of the projects, as we gained greater insight about previously unknown problems in collecting large imaging datasets in a distributed consortium. Here we summarize the primary QC components, see 4, 6 for additional information.
After acquisition the image data were uploaded to a locally installed HID data management system using the FBIRN data upload scripts (www.nitrc.org/projects/fbirn). The scripts include a graphical user interface to collect metadata about the scan (e.g. slice timing, project name, etc), which is saved in XCEDE (www.xcede.org) XML files. XCEDE is an extensive meta data hierarchy for storing, describing, and documenting data generated by scientific studies9. The XCEDE files were checked for consistency with respect to the consortium protocol using a Schematron validator (www.schematron.com). Image QC was then performed automatically after HID upload. The FBIRN developed a battery of QC metrics included with the XCEDE tools (www.nitrc.org/projects/bxh_xcede_tools)4. The results of the QC reports were reviewed by a data curator and posted on a Wiki. For Phase III, a study-tracking dashboard was created, tracking all steps of the data collection progress of each site, including entries for clinical assessments, imaging sessions, data availability, and QC reports. Data that did not pass QC was not made available through the BDR.
2.5 BIRN Data Repository Access and Download
The BDR is an open-access, public repository for the FBIRN data sets. It requires only an email address to login. A BDR user benefits from submitting a valid email address because notifications are sent to those email addresses when there is a change to the data archive or if notices about problem data sets needs to be communicated. Data collected as part of the FBIRN project are under the governance of the BIRN Data Usage Agreement (www.birncommunity.org/wp-content/uploads/2009/09/FBIRN_Data_Use_Agreement.pdf). The agreement specifies that publications using the FBIRN data sets cite the appropriate FBIRN grant. Redistribution of original Function BDR data is permitted so long as the data are redistributed under the same terms and conditions as described in the original document.
2.6 Contributing New Data to the BIRN Data Repository
The FBIRN Phase I, II, and III BDR data sets are static. While the BDR is actively maintained and additional data sets could theoretically be added to the BDR, there is no financial support for hosting other public data sets, but we are supportive of collaborations with research groups interested in hosting their public data in the BDR (e.g., BrainScape data hosted at fbirnbdr.nbirn.net:8080/BDR) or the HID (e.g., Autism Brain Imaging Data Exchange [ABIDE] hosted at computed2.bic.uci.edu:8080/abide).
2.7 Long Term Plans for the BIRN Data Repository
The BDR will continue to play a significant role in sharing both the FBIRN control-only and schizophrenia-control data sets with the research community into the foreseeable future, as long as there appears to be a demand for the data (data download rate is currently at about ~80 downloads/month). In addition, there is a plan to also make FBIRN’s schizophrenia-control data sets available via the SchizConnect resource (www.schizconnect.org)40. SchizConnect has been designed to federate schizophrenia data and provides an integrated domain model with other data resources. The FBIRN component of SchizConnect is managed through a HID database but the data are federated through the SchizConnect mediation service. The FBIRN Phase II schizophrenia data set is already available on SchizConnect and the Phase III schizophrenia data release is in progress.
3. Discussion and Conclusions
This paper describes the Function Biomedical Informatics Research Network Data Repository (BDR), which hosts the FBIRN Phase I and Phase II data and will host Phase III data for use by the wider scientific community. The Phase I and II imaging data sets have yielded numerous publications important to the fields of multi-center imaging and schizophrenia6, 10, 12, 21, 41–44.
The FBIRN consortium is actively working on publishing a first wave of scientific findings based on the multi-center Phase III clinical 45, cognitive, structural imaging46 and functional imaging47–49 data, which will be released to the public in the fall of 2015.
All the tools and methods developed by the Function Biomedical Informatics Research Network (FBIRN) are open source and open access or in the process of being made available in service of scientific teaching and discovery. The FBIRN data sets are freely available to the extent allowed by the member site’s IRBs. Many of the FBIRN tools continue to support multi-site research and some have formed the basis for state-of-the-art neuroimaging metadata tools such as the Neuroimaging Data Model (NIDM; nidm.nidash.org)50. The shared FBIRN data sets are a resource for the application of novel image analysis methods while also contributing to consortium meta-analyses51,52 and mega-analyses22, 53–55 efforts in identifying disease etiology.
Supplementary Material
Highlights.
This manuscript presents Function Biomedical Informatics Research Network data
FBIRN data are shared via the BIRN Data Repository and SchizConnect
FBIRN shares data from individuals with schizophrenia and healthy controls
FBIRN shares structural and functional brain imaging, clinical, and cognitive data
Acknowledgements
We are thankful to Mrs. Liv McMillan for overall study coordination, to Ms. Shichun Ling for editorial assistance, and to the research subjects for their participation. This work was supported by the National Center for Research Resources at the National Institutes of Health (grant numbers: NIH 1 U24 RR021992 (Function Biomedical Informatics Research Network) and NIH 1 U24 RR025736-01 (Biomedical Informatics Research Network Coordinating Center; www.birncommunity.org). The funding sources had no role in the study design, data collection, or publication of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hudziak JJ, Albaugh MD, Ducharme S, et al. Cortical thickness maturation and duration of music training: health-promoting activities shape brain development. J Am Acad Child Adolesc Psychiatry. 2014 Nov;53(11):1153–1161. 1161, e1151–e1152. doi: 10.1016/j.jaac.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller SG, Weiner MW, Thal LJ, et al. Ways toward an early diagnosis in Alzheimer's disease: the Alzheimer's Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005 Jul;1(1):55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon TD, Chung Y, He G, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biological psychiatry. 2015 Jan 15;77(2):147–157. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman L, Glover GH. Report on a multicenter fMRI quality assurance protocol. J Magn Reson Imaging. 2006 Jun;23(6):827–839. doi: 10.1002/jmri.20583. [DOI] [PubMed] [Google Scholar]
- 5.Greve DN, Mueller BA, Liu T, et al. A novel method for quantifying scanner instability in fMRI. Magn Reson Med. 2011 Apr;65(4):1053–1061. doi: 10.1002/mrm.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glover GH, Mueller BA, Turner JA, et al. Function biomedical informatics research network recommendations for prospective multicenter functional MRI studies. J Magn Reson Imaging. 2012 Jul;36(1):39–54. doi: 10.1002/jmri.23572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozyurt IB, Keator DB, Wei D, et al. Federated web-accessible clinical data management within an extensible neuroimaging database. Neuroinformatics. 2010 Dec;8(4):231–249. doi: 10.1007/s12021-010-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keator DB, Wei D, Gadde S, et al. Derived Data Storage and Exchange Workflow for Large-Scale Neuroimaging Analyses on the BIRN Grid. Frontiers in neuroinformatics. 2009;3:30. doi: 10.3389/neuro.11.030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadde S, Aucoin N, Grethe JS, Keator DB, Marcus DS, Pieper S. XCEDE: an extensible schema for biomedical data. Neuroinformatics. 2011 Jan;10(1):19–32. doi: 10.1007/s12021-011-9119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman L, Glover GH. Reducing interscanner variability of activation in a multicenter fMRI study: controlling for signal-to-fluctuation-noise-ratio (SFNR) differences. NeuroImage. 2006 Nov 1;33(2):471–481. doi: 10.1016/j.neuroimage.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Friedman L, Glover GH, Krenz D, Magnotta V. Reducing inter-scanner variability of activation in a multicenter fMRI study: role of smoothness equalization. NeuroImage. 2006 Oct 1;32(4):1656–1668. doi: 10.1016/j.neuroimage.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 12.Potkin SG, Turner JA, Brown GG, et al. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophrenia bulletin. 2009 Jan;35(1):19–31. doi: 10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomason ME, Foland L, Glover GH. Paper presented at: ISMRM 12th Annual Meeting. Kyoto, Japan: 1999. Calibration of fMRI activation for the FIRST BIRN project. [Google Scholar]
- 14.Thomason ME, Glover GH. Controlled inspiration depth reduces variance in breath-holding-induced BOLD signal. NeuroImage. 2008 Jan 1;39(1):206–214. doi: 10.1016/j.neuroimage.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.First MB, Spitzer RL, Gibbon MG, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders - Non-Patient Edition (SCID-I/NP, 11/2002 revision) New York: 2002. [Google Scholar]
- 16.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of personality assessment. 1996 Dec;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 17.Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971 Nov-Dec;12(6):371–379. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]
- 18.Uttl B. North American Adult Reading Test: age norms, reliability, and validity. Journal of clinical and experimental neuropsychology. 2002 Dec;24(8):1123–1137. doi: 10.1076/jcen.24.8.1123.8375. [DOI] [PubMed] [Google Scholar]
- 19.Woodruffe-Peacock C, Turnbull GM, Johnson MA, Elahi N, Preston GC. The Quick Mood Scale: development of a simple mood assessment scale for clinical pharmacology studies. Human Psychopharmacology: Clinical and Experimental. 1998;13(1):53–58. [Google Scholar]
- 20.Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: U.S. Department of Health, Education, and Education and Welfare, Public Health Service, Alcohol, Drug Abuse and Mental Health Administration; 1976. Abnormal Involuntary Movement Scale (AIMS) pp. 534–537. editor, ed. [Google Scholar]
- 21.Ford JM, Roach BJ, Jorgensen KW, et al. Tuning in to the voices: a multisite FMRI study of auditory hallucinations. Schizophrenia bulletin. 2009 Jan;35(1):58–66. doi: 10.1093/schbul/sbn140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DI, Mathalon DH, Ford JM, et al. Auditory oddball deficits in schizophrenia: an independent component analysis of the fMRI multisite function BIRN study. Schizophrenia bulletin. 2009 Jan;35(1):67–81. doi: 10.1093/schbul/sbn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kastrup A, Kruger G, Glover GH, Neumann-Haefelin T, Moseley ME. Regional variability of cerebral blood oxygenation response to hypercapnia. NeuroImage. 1999 Dec;10(6):675–681. doi: 10.1006/nimg.1999.0505. [DOI] [PubMed] [Google Scholar]
- 24.First MB, Spitzer RL, Gibbon MG, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders - Patient Edition (SCID-I/P, 11/2002 revision) New York: 2002. [Google Scholar]
- 25.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971 Mar;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 26.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991 Sep;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 27.Andreasen N. The scale for the assessment of positive symp-toms (SAPS) Iowa City: University of Iowa; 1984. [Google Scholar]
- 28.Andreasen N. The scale for the assessment of negative symptoms (SANS) Iowa City: University of Iowa; 1983. [Google Scholar]
- 29.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 30.Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophrenia research. 1990 Jul-Aug;3(4):247–251. doi: 10.1016/0920-9964(90)90005-r. [DOI] [PubMed] [Google Scholar]
- 31.Lindenmayer JP, Czobor P, Alphs L, et al. The InterSePT scale for suicidal thinking reliability and validity. Schizophrenia research. 2003 Sep 1;63(1–2):161–170. doi: 10.1016/s0920-9964(02)00335-3. [DOI] [PubMed] [Google Scholar]
- 32.Barnes TR. The Barnes Akathisia Rating Scale--revisited. J Psychopharmacol. 2003 Dec;17(4):365–370. doi: 10.1177/0269881103174013. [DOI] [PubMed] [Google Scholar]
- 33.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 34.Liu TT, Glover GH, Mueller BA, et al. Quality Assurance in Functional MRI. In: Ugurbil K, Berliner L, Uludag K, editors. fMRI: From Nuclear Spins to Brain Function. Springer; 2015. [Google Scholar]
- 35.Voyvodic JT. Real-time fMRI paradigm control, physiology, and behavior combined with near real-time statistical analysis. NeuroImage. 1999 Aug;10(2):91–106. doi: 10.1006/nimg.1999.0457. [DOI] [PubMed] [Google Scholar]
- 36.Voyvodic JT, Glover GH, Greve D, Gadde S. Automated real-time behavioral and physiological data acquisition and display integrated with stimulus presentation for FMRI. Frontiers in neuroinformatics. 2011;5:27. doi: 10.3389/fninf.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT., Jr The Schedule for the Deficit syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989 Nov;30(2):119–123. doi: 10.1016/0165-1781(89)90153-4. [DOI] [PubMed] [Google Scholar]
- 38.O'Halloran JP, Kemp AS, Gooch KN, et al. Psychometric comparison of computerized and standard administration of the neurocognitive assessment instruments selected by the CATIE and MATRICS consortia among patients with schizophrenia. Schizophrenia research. 2008 Nov;106(1):33–41. doi: 10.1016/j.schres.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Turner JA, Lane SR, Bockholt HJ, Calhoun VD. The clinical assessment and remote administration tablet. Frontiers in neuroinformatics. 2011;5:31. doi: 10.3389/fninf.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Alpert K, Calhoun V, et al. SchizConnect: Mediating Schizophrenia Neuroimaging Databases for Large-Scale Integration. Neuroimage Special Issue on Brain Imaging Repositories. doi: 10.1016/j.neuroimage.2015.06.065. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Potkin SG, Turner JA, Guffanti G, et al. A genome-wide association study of schizophrenia using brain activation as a quantitative phenotype. Schizophrenia bulletin. 2009 Jan;35(1):96–108. doi: 10.1093/schbul/sbn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potkin SG, Turner JA, Fallon JA, et al. Gene discovery through imaging genetics: identification of two novel genes associated with schizophrenia. Molecular psychiatry. 2009 Apr;14(4):416–428. doi: 10.1038/mp.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potkin SG, Macciardi F, Guffanti G, et al. Identifying gene regulatory networks in schizophrenia. NeuroImage. 2010 Nov 15;53(3):839–847. doi: 10.1016/j.neuroimage.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Erp TG, Guella I, Vawter MP, et al. Schizophrenia miR-137 locus risk genotype is associated with dorsolateral prefrontal cortex hyperactivation. Biological psychiatry. 2014 Mar 1;75(5):398–405. doi: 10.1016/j.biopsych.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Erp TG, Preda A, Nguyen D, et al. Converting positive and negative symptom scores between PANSS and SAPS/SANS. Schizophrenia research. 2014 Jan;152(1):289–294. doi: 10.1016/j.schres.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Erp TG, Greve DN, Rasmussen J, et al. A multi-scanner study of subcortical brain volume abnormalities in schizophrenia. Psychiatry Res. 2014 Apr 30;222(1–2):10–16. doi: 10.1016/j.pscychresns.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner JA, Damaraju E, van Erp TG, et al. A multi-site resting state fMRI study on the amplitude of low frequency fluctuations in schizophrenia. Frontiers in neuroscience. 2013;7:137. doi: 10.3389/fnins.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ford JM, Palzes VA, Roach BJ, et al. Visual hallucinations are associated with hyperconnectivity between the amygdala and visual cortex in people with a diagnosis of schizophrenia. Schizophrenia bulletin. 2014 Jan;41(1):223–232. doi: 10.1093/schbul/sbu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Damaraju E, Allen EA, Belger A, et al. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clin. 2014;5:298–308. doi: 10.1016/j.nicl.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keator DB, Helmer K, Steffener J, et al. Towards structured sharing of raw and derived neuroimaging data across existing resources. NeuroImage. 2013 Nov 15;82:647–661. doi: 10.1016/j.neuroimage.2013.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stein JL, Medland SE, Vasquez AA, et al. Identification of common variants associated with human hippocampal and intracranial volumes. Nature genetics. 2012 May;44(5):552–561. doi: 10.1038/ng.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, Agartz I, Westlye LT, Haukvik UK, Dale AM, Melle I, Hartberg CB, Gruber O, Kraemer B, Zilles D, Donohoe G, Kelly S, McDonald C, Morris DW, Cannon DM, Corvin A, Machielsen MW, Koenders L, de Haan L, Veltman DJ, Satterthwaite TD, Wolf DH, Gur RC, Gur RE, Potkin SG, Mathalon DH, Mueller BA, Preda A, Macciardi F, Ehrlich S, Walton E, Hass J, Calhoun VD, Bockholt HJ, Sponheim SR, Shoemaker JM, van Haren NE, Pol HE, Ophoff RA, Kahn RS, Roiz-Santianez R, Crespo-Facorro B, Wang L, Alpert KI, Jonsson EG, Dimitrova R, Bois C, Whalley HC, McIntosh AM, Lawrie SM, Hashimoto R, Thompson PM, Turner JA. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2015 [Google Scholar]
- 53.Gupta CN, Calhoun VD, Rachakonda S, et al. Patterns of Gray Matter Abnormalities in Schizophrenia Based on an International Mega-analysis. Schizophrenia bulletin. 2014 Dec 28; doi: 10.1093/schbul/sbu177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segall JM, Turner JA, van Erp TG, et al. Voxel-based morphometric multisite collaborative study on schizophrenia. Schizophrenia bulletin. 2009 Jan;35(1):82–95. doi: 10.1093/schbul/sbn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turner JA, Calhoun VD, Michael A, et al. Heritability of multivariate gray matter measures in schizophrenia. Twin Res Hum Genet. 2012 Jun;15(3):324–335. doi: 10.1017/thg.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.