Abstract
Postpartum depression (PPD) is a common complication following childbirth experienced by one in every five new mothers. Although the neural basis of PPD remains unknown previous research in rats has shown that gestational stress, a risk factor for PPD, induces depressive-like behavior during the postpartum period. Moreover, the effect of gestational stress on postpartum mood is accompanied by structural modifications within the nucleus accumbens (NAc) and the medial prefrontal cortex (mPFC) – limbic regions that have been linked to PPD. Mothers diagnosed with PPD are often prescribed selective serotonin reuptake inhibitor (SSRI) antidepressant medications and yet little is known about their effects in models of PPD. Thus, here we investigated whether postpartum administration of Citalopram, an SSRI commonly used to treat PPD, would ameliorate the behavioral and morphological consequences of gestational stress. In addition, we examined the effects of gestational stress and postpartum administration of Citalopram on structural plasticity within the basolateral amygdala (BLA) which together with the mPFC and NAc forms a circuit that is sensitive to stress and is involved in mood regulation. Our results show that postpartum rats treated with Citalopram do not exhibit gestational stress-induced depressive-like behavior in the forced swim test. In addition, Citalopram was effective in reversing gestational stress-induced structural alterations in the postpartum NAc shell and mPFC. We also found that gestational stress increased spine density within the postpartum BLA, an effect which was not reversed by Citalopram treatment. Overall, these data highlight the usefulness of gestational stress as a valid and informative translational model for PPD. Furthermore, they suggest that structural alterations in the mPFC-NAc pathway may underlie stress-induced depressive-like behavior during the postpartum period and provide much needed information on how SSRIs may act in the maternal brain to treat PPD.
Keywords: amygdala, dendritic spines, maternal, mood, nucleus accumbens, prefrontal cortex
Introduction
Alterations in mood during the postpartum period are reported by approximately 40% of all new mothers with up to one in every five mothers developing the full phenotype of major depression known as postpartum depression (PPD; Gress-Smith et al., 2012; O’Hara, 2009; O’Hara and Wisner, 2014). Although numerous variables enhance vulnerability to PPD, epidemiological studies suggest that pregnancy stress is a major risk factor (Davey et al., 2011; Lancaster et al., 2010). Similar to humans, rats exposed to chronic stress during pregnancy exhibit depressive-like behaviors during the postpartum period (Haim et al., 2014; Leuner et al., 2014; O’Mahony et al., 2006; Smith et al., 2004). Recent studies have shown that gestational stress also induces structural modifications within brain areas that have been linked to PPD (Laurent and Ablow, 2012; McEwen et al., 2012; Moses-Kolko et al., 2010, 2011; Sacher et al., 2015) and which are implicated in the regulation of mood and stress including the NAc and mPFC (Price and Drevets, 2012; Russo and Nestler, 2013). Specifically, gestational stress reduces structural complexity and dendritic spine density on medium spiny neurons (MSNs) in the shell of the NAc (Haim et al., 2014), and in addition, diminishes spine density on pyramidal neurons within the mPFC (Leuner et al., 2014).
The negative consequences of PPD on the cognitive, emotional and social development of the offspring are well documented (Grace et al., 2003; Gress-Smith et al., 2012; Letourneau et al., 2012; Verbeek et al., 2012). As such, treatment of depressed mothers is critical and commonly achieved with administration of selective serotonin reuptake inhibitor (SSRI) antidepressant medications (Berle and Spigset, 2011; Logsdon et al., 2011). Other than one report showing increased neurogenesis in the hippocampus of gestationally stressed mothers following chronic postpartum fluoxetine administration (Pawluski et al., 2012), the ability of SSRI treatment to reverse stress-induced structural and behavioral changes in postpartum females hasn’t been assessed.
One of the most common SSRIs prescribed to patients diagnosed with mild to severe depression is Citalopram (Celexa©). Previous work has shown that Citalopram is safe for use in breastfeeding mothers (Rampono et al., 2006). Furthermore, Citalopram was shown to significantly improve mood in mothers diagnosed with PPD (Misri et al., 2012). Here we investigated whether chronic Citalopram administration during the postpartum period would reverse the adverse effects of gestational stress on postpartum mood and structural plasticity in the NAc shell and the mPFC. Given findings demonstrating amygdala dysregulation in PPD (Moses-Kolko et al., 2010; Silverman et al., 2011), we also investigated the effects of gestational stress as well as postpartum administration of Citalopram on structural complexity of neurons within the basolateral amygdala (BLA), another stress sensitive brain region that interconnects with both the NAc and mPFC to form a critical network involved in mood and emotion processing (Mitra et al., 2005; Stevenson and Gratton, 2003; Vialou et al., 2014).
Methods and Materials
Animals
Timed pregnant female Sprague-Dawley rats (Taconic, Albany, USA) arrived at our facility on gestation day 4 (GD4) and were individually housed in clear Plexiglas cages with unlimited access to food and water. Rats were kept in a temperature and humidity controlled environment maintained on 12h/12h light-dark cycle (lights on at 6AM). The day of pup delivery was designated as postpartum day 0 (PD0). On PD1, litters were culled to 8–10 pups (4–5 males, 4–5 females) in a randomized manner to minimize the possibility that the effects observed in the stressed mothers are driven by the characteristics of their pups. Pregnant rats were weighed daily from GD7-GD20 to verify the efficacy of our stress protocol since prior studies shave reported that gestational stress reduces gestational weight gain (Baker et al., 2008; Haim et al., 2014; Hillerer et al., 2011; Leuner et al., 2014). In addition, both mothers and litters were weighed daily throughout the postpartum period to assess any potential effect of antidepressant treatment or any possible lingering effect of stress as has been reported in some studies (Hillerer et al., 2014; Leuner et al., 2014). All experiments were performed in compliance with The Ohio State University Institutional Animal Care and Use Committee and the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Gestational stress protocol
Pregnant rats were randomly assigned to the stressed group or served as unstressed controls. Stressed rats were restrained in ventilated clear Plexiglas cylinders (21 cm long, 6 cm internal diameter) daily from GD7-GD20 in a variable randomized restraint protocol consisting of one of the following: 2 X 30 min, 3 X 45 min, 1 X 1 h and 1 X 2 h. All stress sessions took place between 10AM and 4PM. Multiple stress sessions within the same day were always separated by at least 1 h. Although multiple gestational stress paradigms have been used to induce postpartum-depressive like behavior (Haim et al., 2014; Leuner et al., 2104; O’Mahony et al., 2006; Smith et al., 2004), randomized variable restraint stress with different restraint durations is a component of chronic mild stress (CMS) paradigms (Isgor et al., 2004) and was used in this study to reduce stress predictability and potential habituation to the stress (Grissom and Bhatnagar, 2009). Unstressed controls were handled daily for 5 min.
Minipump implantation and antidepressant administration
Postpartum females were randomly assigned to receive Citalopram hydrobromide (H. Lundbeck, Copenhagen, DK) or saline vehicle resulting in a total of 4 groups: (1) No stress-Saline (n=8), (2) No stress-Citalopram (n=8), (3) Stress-Saline (n=7), (4) Stress-Citalopram (n=7). The dosage of Citalopram used here (10mg/kg/day) has been shown to ameliorate mood in women with postpartum depression (Misri et al., 2012) and in addition, has been used in chronic stress rat models to alleviate depressive-like behavior (Chen et al., 2012). Citalopram treatment was administered for 21 d via osmotic minipumps (2ML4, Alzet, Cupertino, CA) which were preloaded with 2 ml of saline or Citalopram and subcutaneously implanted between the scapulae on PD1 under Isoflurane anesthesia. During the surgery, litters remained in their home cages which were kept warm on a heating pad. Following implantation, mothers were placed back in their home cages and provided with ibuprofen (15mg/kg) via drinking water for 7 d. At the end the study, fluid from each minipump was aspirated to verify saline/drug delivery.
Forced swim test
The forced swim test (FST) was used to assess depressive-like behavior. Testing was performed during the light phase between 10AM and 12PM under 550 lux illumination. Briefly, Plexiglas cylinders (diameter: 30.5 cm, height: 49 cm) were filled to a depth of 30 cm with 25 ± 0.5°C water. On PD21, postpartum females were individually placed into the FST cylinders for 10 min, towel-dried and returned to their home cage. 24 h later (PD22), rats were returned to the same apparatus for 5 min and the session digitally recorded. The percentage of time spent immobile [(time spent floating in the water only making movements necessary to maintain the head above water/total test time) x 100] was later measured blind by a trained observer using BEST analysis software (Education Consulting Inc., Hobe Sound, FL).
Golgi staining
24 h following the FST, postpartum females (PD23) were deeply anesthetized, rapidly decapitated and brains removed for Golgi impregnation using the FD Rapid Golgi Stain kit (FD Neurotechnologies; Ellicot City, MD). Briefly, small blocks of tissue containing the NAc and mPFC and separate blocks containing the amygdala were placed in plastic scintillation vials filled with 10 ml of a potassium dichromate, mercuric chloride and potassium chromate solution (Solution A+B). Following two weeks of incubation in the dark at room temperature, brains were transferred to solution C and stored in the dark at 4°C for 2 d. Next, coronal sections (150 µm) were cut on a Vibratome, mounted onto gelatin-coated slides and dried at room temperature in the dark. Slides were then rinsed, developed in solutions D + E for 10 min, dehydrated, cleared with xylene and coverslipped with Permount.
Microscopic analyses
MSNs (1.7 mm and 1 mm anterior to Bregma; Paxinos and Watson, 1998) in the shell and core sub-regions of the NAc, pyramidal neurons in layer 2/3 of the prelimbic mPFC (3.7 mm and 2.2 mm anterior to Bregma; Paxinos and Watson, 1998) and principal neurons in the BLA (1.6 mm and 3.3 mm posterior to Bregma; Paxinos and Watson, 1998) were analyzed. The anterior commissure and ventricles were used as landmarks to identify and differentiate the shell and core subregions. Only neurons within these regions that were fully impregnated, not obscured by neighboring neurons and had no obviously truncated dendrites were chosen for analysis. For each animal, five randomly chosen, representative neurons in each region were completely traced. mPFC pyramidal neurons were traced at 10X whereas NAc shell and core MSNs and principal neurons in the BLA were traced at 20X using NIS elements software and a Nikon 90i microscope (Nikon Instruments, Melville, NY). From these traced neurons, total dendritic length and number of branch points (every point of bifurcation along dendritic branches) were measured. Further, on these neurons, dendritic spines were counted at 100X on five dendritic segments ~20 µm in length located at least 50 µm away from the cell body. For mPFC pyramidal neurons, morphological analyses were done separately for basal and apical branches. All analyses were done blind to experimental conditions.
Spine density was calculated by dividing the number of spines on a segment by the segment length and expressed as the numbers of dendritic spines per 10 µ m. The numbers of spines on five segments of a cell were averaged for a cell mean, and the five cells from each animal were then averaged for an animal mean. For dendritic length and branching, values for each of the five cells per animal were averaged to obtain an animal mean.
Statistical analyses
Group data are reported as the mean ± SEM. Student’s t-test was used to analyze the effect of gestational stress on percent weight gain during pregnancy. Postpartum body weight and litter weight parameters as well as percent immobility in the FST were analyzed using two-way ANOVA with drug (Saline vs. Citalopram) and stress condition (Stress vs. No stress) as independent variables. Neuronal morphology (i.e. total dendritic length, number of branch points and spine density) data was analyzed separately for the NAc shell, mPFC and BLA again using two-way ANOVA with drug and stress condition as independent variables. Bonferroni post-hoc tests were applied when necessary. All analyses were conducted using GraphPad Prism 5.0 software (La Jolla, CA) with significance set at p < 0.05.
Results
Effects of gestational stress on weight gain and litter characteristics
Percent body weight gain during pregnancy (No stress: 35.37 ± 1.59; Stress: 28.80 ± 2.40) was reduced by gestational stress [t(28) = 2.34, p < 0.05]. During the postpartum period, there were no main effects of gestational stress or drug and no stress x drug interaction on percent body weight gain (Table 1, p’s > 0.05).
Table 1.
Effects of gestational stress and chronic postpartum administration of Citalopram on percent weight gain during the postpartum period. Also shown are litter size, litter weight on PD1 and percent litter weight gain. Saline: no stress, n = 8; stress, n = 7. Citalopram; no stress, n = 8; stress, n = 7.
| Group | Postpartum weight gain (%) |
Litter size (pups) |
Litter weight on PD1 (g) |
Litter weight gain (%) |
|---|---|---|---|---|
| No stress Saline | 18.08 ± 1.75 | 10.25 ± 4.80 | 70.12 ± 10.89 | 507.08 ± 28.57 |
| No stress Citalopram | 21.41 ± 1.98 | 9.87 ± 3.13 | 72.62 ± 11.78 | 551.71 ± 30.78 |
| Stress Saline | 21.36 ± 1.15 | 12.00 ± 3.08 | 77.14 ± 9.68 | 532.63 ± 35.47 |
| Stress Citalopram | 19.60 ± 1.89 | 10.55 ± 5.15 | 68.57 ± 10.45 | 472.95 ± 32.49 |
With regard to the pups (Table 1), there was no main effect of stress or drug (p’s > 0.05) but a significant stress x drug interaction [F(1,30) = 4.24 p < 0.05] for percent litter weight gain. Post-hoc analysis, however, showed no significant differences between the groups. No significant main effects or interactions were found for litter size or litter weight on PD1 (p’s > 0.05).
Effects of chronic postpartum SSRI treatment on gestational stress-induced depressive-like behavior during the postpartum period
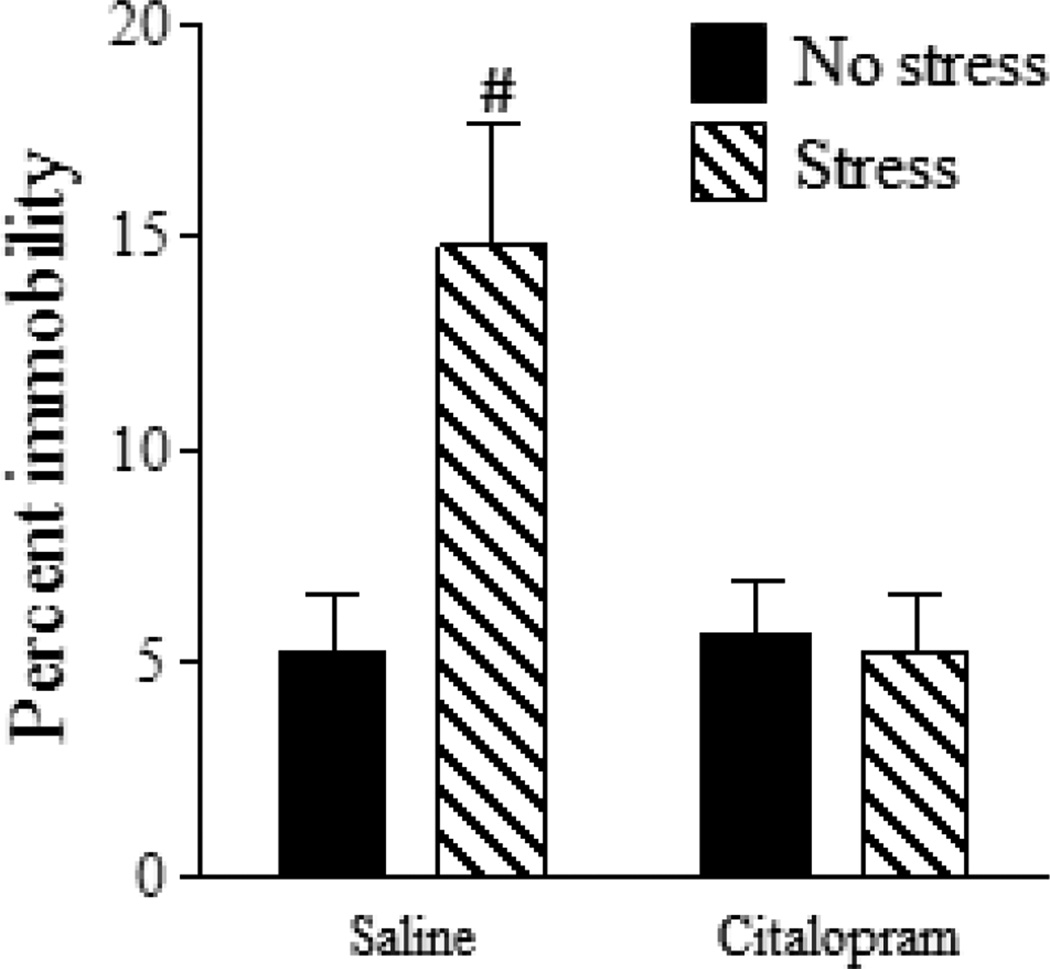
A significant main effect of both stress [F(1,26) = 6.61, p < 0.05] and drug [F(1,26) = 6.75, p < 0.05] as well as a significant stress x drug interaction [F(1,26) = 4.24, p < 0.05] was found for percent immobility in the FST (Fig. 1). Post-hoc analysis showed that the Stress-Saline group displayed more immobility in the FST as compared to all other groups (p < 0.5) which did not differ from one another (p’s > 0.05).
Figure 1.
Chronic SSRI administration reverses gestational stress-induced depressive-like behavior during the postpartum period. Gestational stress significantly increased percent immobility in the FST indicative of depressive-like behavior. Postpartum females administered Citalopram did not exhibit increased immobility following gestational stress exposure. Saline: no stress, n = 8; stress, n = 7. Citalopram; no stress, n = 8; stress, n = 7. Bars represent mean ± SEM. # p < 0.05, Stress-Saline vs. all other groups.
Effects of gestational stress and chronic postpartum SSRI treatment on neuronal morphology within the postpartum NAc shell, NAc core, mPFC and BLA
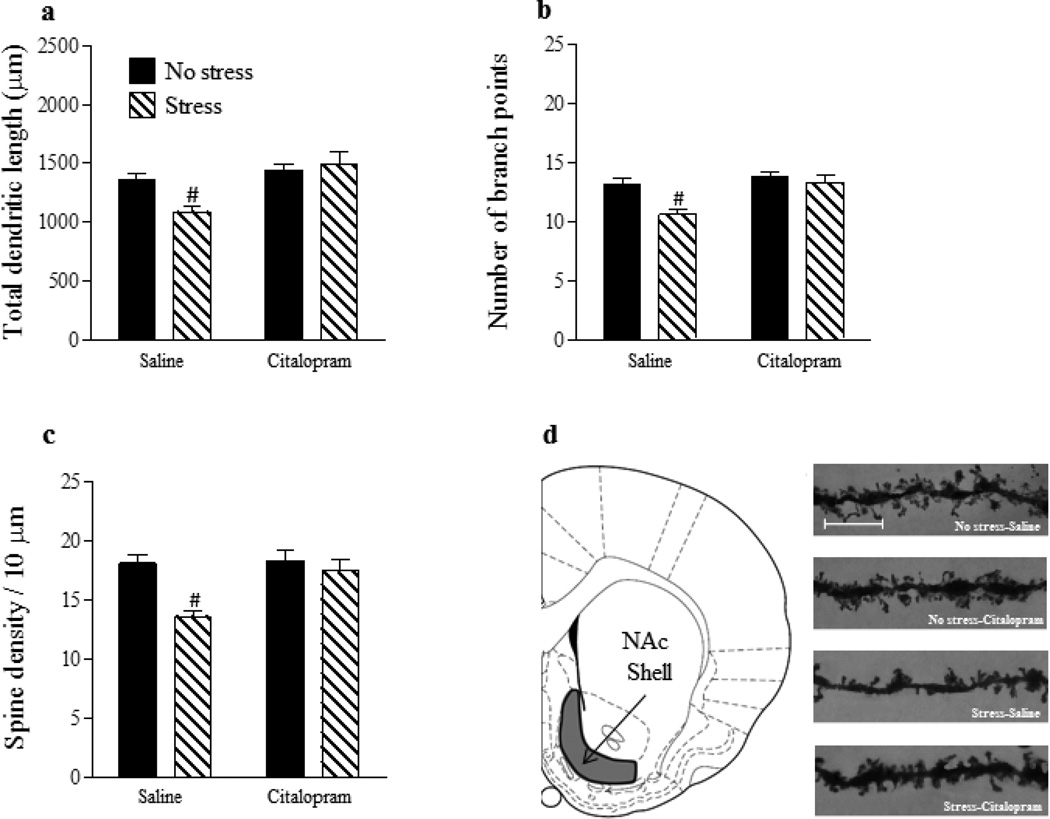
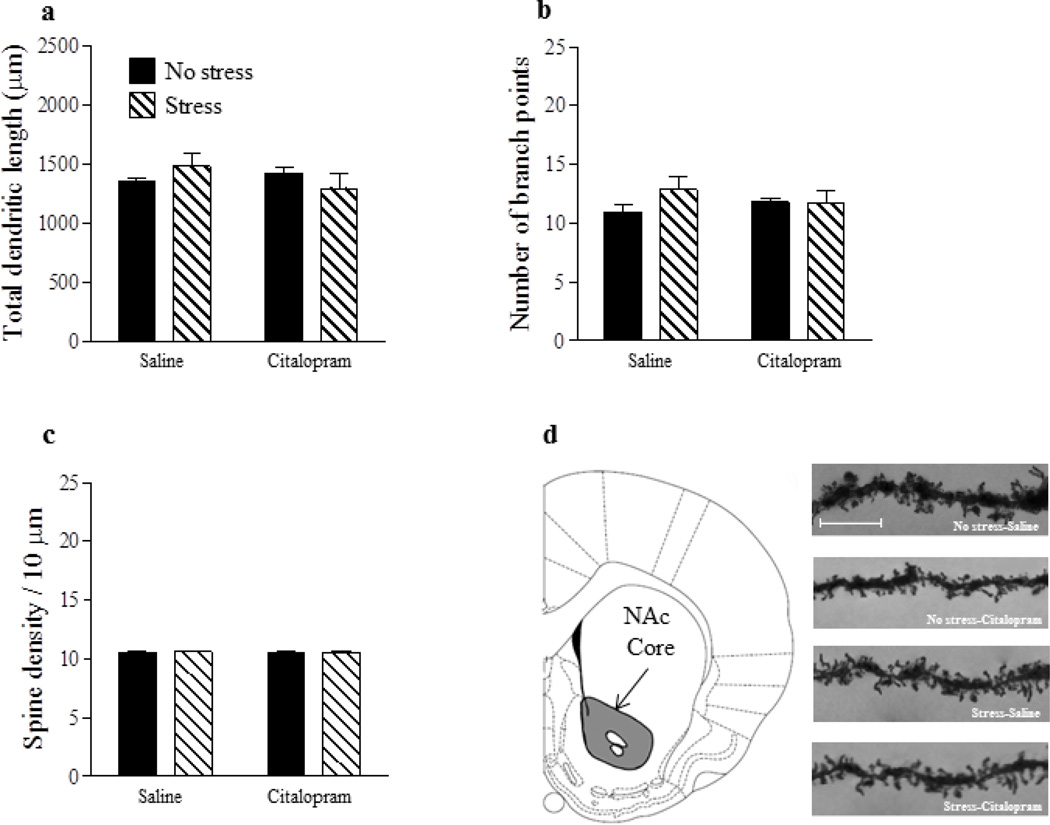
For total dendritic length of NAc shell MSNs (Fig. 2a), there was no main effect of stress (p > 0.05) but a significant main effect of drug [F(1,26) = 13.09, p < 0.01] and a significant stress x drug interaction [F(1,26) = 5.95, p < 0.05]. Post hoc analysis revealed that total dendritic length was significantly reduced in the Stress-Saline group in comparison with all other groups (p’s < 0.05) which did not differ (p’s > 0.05). For number of branch points on NAc shell MSNs (Fig. 2b), there were significant main effects of stress [F(1,26) = 10.04, p < 0.01] and drug [F(1,26) = 11.57, p < 0.01] as well as a significant interaction between the two factors [F(1,26) = 4.44, p < 0.05]. Post-hoc analysis revealed that dendritic branching was reduced in the Stress-Saline group in comparison with all other groups (p’s < 0.05) which did not differ from one another (p’s > 0.05). Lastly, for dendritic spine density on NAc shell MSNs (Fig. 2c), significant main effects of stress [F(1,26) = 12.35, p < 0.01] and drug [F(1,26) = 7.21, p < 0.05] as well as a significant stress x drug interaction [F(1,26) = 4.44, p < 0.05] were found. Once again, post-hoc analysis showed that spine density was reduced in the Stress-Saline group in comparison with all other groups (p’s < 0.05) which did not differ (p’s > 0.05). In contrast to the NAc shell, there were no significant main effects of stress or drug and no stress x drug interaction (p’s > 0.05) on total dendritic length, number of branch points, or spine density in the NAc core (Fig. 3a–c).
Figure 2.
Chronic SSRI administration reverses the effects of gestational stress on structural plasticity within the postpartum NAc shell. Gestational stress significantly reduced total dendritic length (a), number of branch points (b) and dendritic spine density (c) on MSNs in the NAc shell. For all three morphological measurements, chronic postpartum administration of Citalopram reversed the effects of gestational stress. Coronal plate depicting the location of the NAc shell from which MSNs were sampled for morphological analysis (d). Representative images of dendritic segments from MSNs within the NAc shell, scale bar =10 µm (e). Saline: no stress, n = 8; stress, n = 7. Citalopram; no stress, n = 8; stress, n = 7. Bars represent mean ± SEM. # p < 0.05, Stress-Saline vs. all other groups.
Figure 3.
No effect of gestational stress or SSRI treatment on structural plasticity in the NAc core. Neither gestational stress nor Citalopram affected total dendritic length (a), number of branch points (b) or dendritic spine density (c) on MSNs in the NAc core. Coronal plate depicting the location of the NAc core from which MSNs were sampled for morphological analysis (d). Representative images of dendritic segments from MSNs within the NAc core, scale bar =10 µm (e). Saline: no stress, n = 8; stress, n = 7. Citalopram; no stress, n = 8; stress, n = 7. Bars represent mean ± SEM.
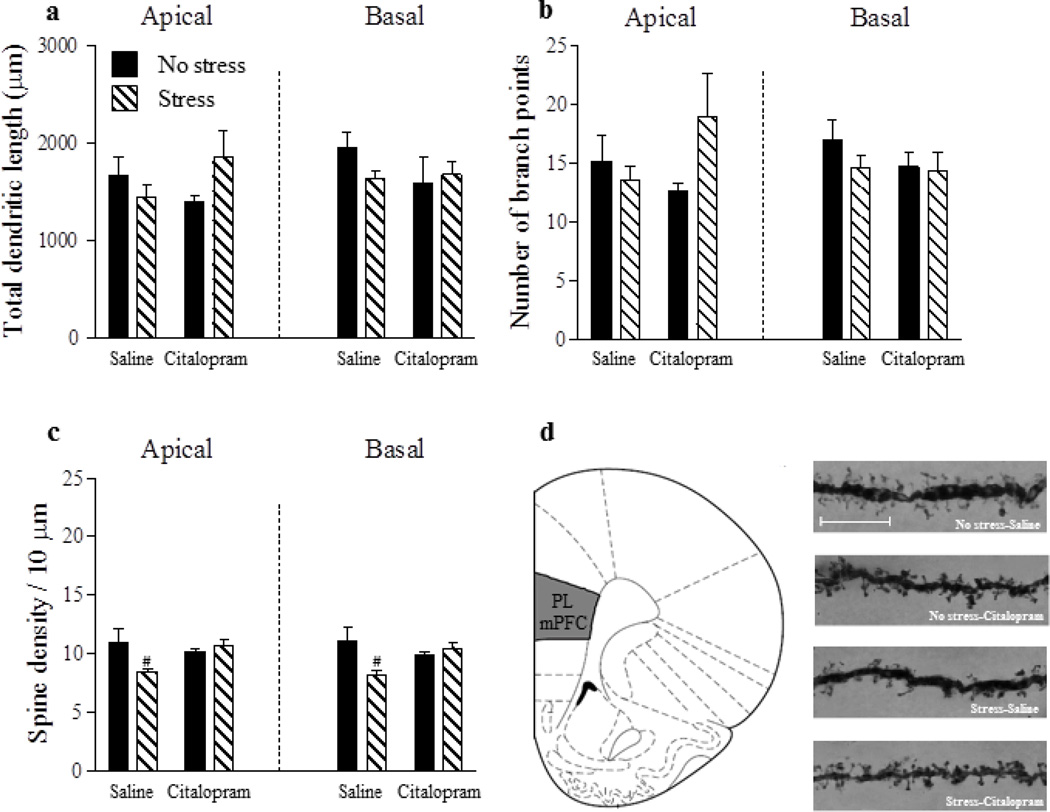
For apical and basal dendrites of mPFC pyramidal neurons (Fig. 4a, b), there were no significant main effects or interactions for either total dendritic length (p’s > 0.05) or branch points (p’s > 0.05). For apical and basal dendritic spine density (Fig. 4c), there were also no significant main effects for either stress or drug (p’s > 0.05). However, a significant stress x drug interaction for both apical [F(1,26) = 4.99, p < 0.05] and basal [F(26,1) = 5.63, p < 0.05] dendritic spine density was found. Post-hoc tests showed that spine density on apical and basal dendrites of mPFC pyramidal neurons was significantly reduced in the Stress-Saline group in comparison with all other groups (p’s < 0.05) which did not differ (p’s > 0.05).
Figure 4.
Reduced dendritic spine density in the mPFC of stressed mothers is reversed by chronic SSRI treatment during the postpartum period. Neither stress nor Citalopram altered total dendritic length or number of branch points (a,b). However, gestational stress significantly reduced spine density (c), an effect that was reversed by chronic postpartum administration of Citalopram. Coronal plate depicting the location of the prelimbic area of the mPFC from which pyramidal neurons were sampled for morphological analysis (d). Representative images of dendritic segments from pyramidal neurons within the prelimbic mPFC, scale bar = 10 µm (e). Saline: no stress, n = 8; stress, n = 7. Citalopram; no stress, n = 8; stress, n = 7. Bars represent mean ± SEM. #p < 0.05, Stress-Saline vs. all other groups.
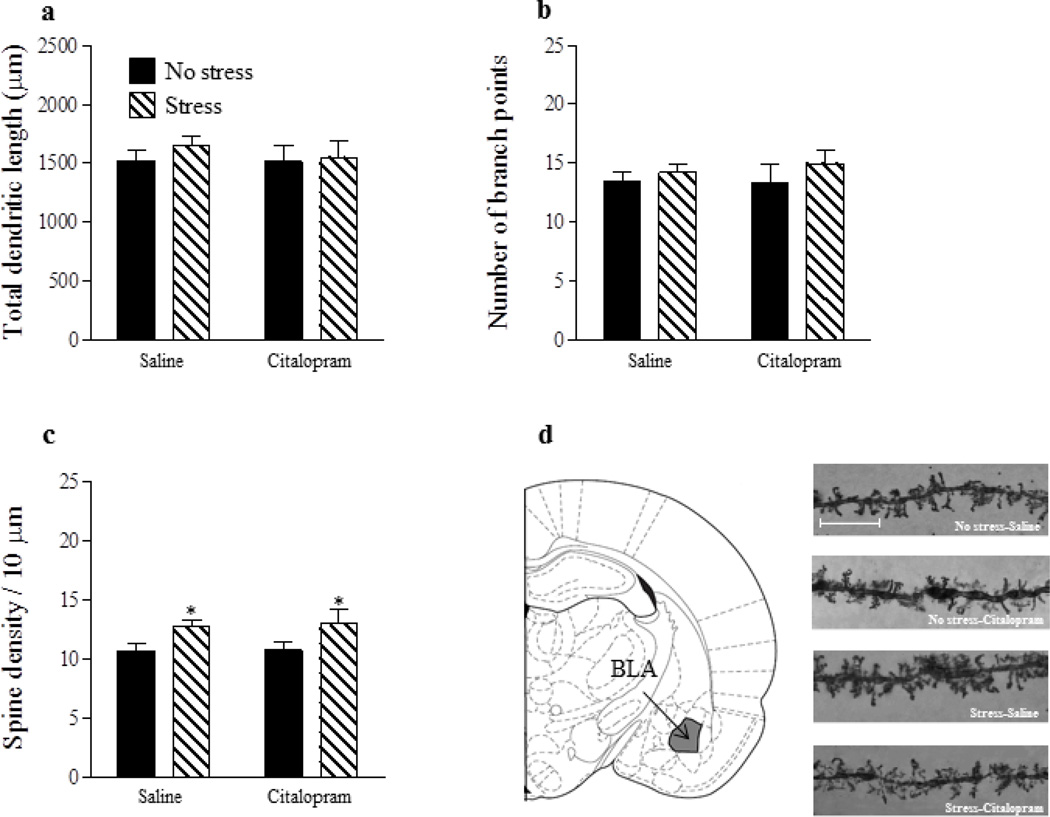
For neurons in the BLA (Fig. 5a, b), there were no significant main effects of stress or drug and no stress x drug interactions for either total dendritic length (p’s > 0.05) or number of branch points (p’s > 0.05). However, for spine density (Fig. 5c), there was a significant main effect of stress [F(1,26) = 8.17, p < 0.01] such that gestational stress significantly increased spine density in both the saline and Citalopram groups. There was no main effect of drug and no stress x drug interaction on BLA spine density (p’s > 0.05).
Figure 5.
Gestational stress increases spine density within the BLA. Neither stress nor Citalopram altered total dendritic length (a) or number of branch points (b) of principal neurons in the BLA. However, gestational stress significantly increased spine density regardless of postpartum Citalopram administration (c). Coronal plate depicting the location of the BLA from which neurons were sampled for morphological analysis (d). Representative images of dendritic segments from neurons within the BLA, scale bar = 10 µm (e). Saline: no stress, n = 8; stress, n = 7. Citalopram; no stress, n = 8; stress, n = 7. Bars represent mean ± SEM. *p < 0.05, main effect of stress.
Discussion
Here we show that postpartum administration of Citalopram, an SSRI commonly prescribed to mothers diagnosed with PPD, mitigated gestational stress-induced depressive-like behavior and reversed structural modifications within the NAc shell and mPFC during the late postpartum period of stressed mothers. We also show that in contrast to the NAc shell and mPFC, gestational stress increased spine density in the BLA, an effect which was not reversed by postpartum administration of Citalopram. Finally, no effects of stress or drug were identified in the NAc core. Although neuroimaging work has previously linked these brain regions to PPD (Laurent and Ablow, 2012; McEwen et al., 2012; Moses-Kolko et al., 2010, 2011; Sacher et al., 2015; Silverman et al., 2011), our data provide evidence that stress-induced neuroplastic changes in the postpartum mPFC and NAc shell may contribute to the pathophysiology of PPD and its pharmacologically induced recovery while structural changes in the BLA might underlie separate PPD symptomology beyond behavioral despair.
Gestational stress decreased percent pregnancy weight gain thus validating our stress paradigm. This result coincides with other studies which have demonstrated a decrease in pregnancy weight gain following gestational stress (Baker et al., 2008; Haim et al., 2014; Hillerer et al., 2011; Leuner et al., 2014) and supports research in humans showing a positive link between pregnancy stress and insufficient pregnancy weight gain (Brawarsky et al., 2005; Picone et al., 1982). In contrast with other studies (Hillerer et al., 2011; Leuner et al., 2014) however, we found no effect of gestational stress on litters’ or mothers’ weight gain during the postpartum period, a discrepancy likely attributable to different stress protocols or the randomized pup culling procedure used here which controls for possible prenatal stress exposure effects.
Although previous work has shown that chronic gestational stress increases depressive-like behavior during the postpartum period (Haim et al., 2014; Hillerer et al., 2011; Leuner et al., 2014; O’Mahony et al., 2006; Smith et al., 2004), our results demonstrate for the first time that chronic postpartum administration of the antidepressant Citalopram is effective in ameliorating postpartum depressive-like behavior in mothers exposed to gestational stress. Specifically, gestationally-stressed mothers treated with Citalopram during the postpartum period spent significantly less time immobile in the FST when compared to gestationally-stressed mothers that received saline and did not differ from mothers who were unstressed. Although, a similar reversal of stress-induced immobility following chronic Citalopram administration has been observed in male rats (Chen et al., 2012), the effects of chronic SSRI administration on depressive-like behavior in gestationally stressed mothers has only been examined in one other study which found no effect of gestational stress or antidepressant treatment on immobility in the FST (Pawluski et al., 2012). However, it is important to consider that a different SSRI (fluoxetine), stress protocol and stress duration were used and mothers were tested several days after weaning. Nonetheless, research in human mothers diagnosed with PPD indicates that SSRIs such as Citalopram and fluoxetine are generally effective in ameliorating mood (Berle and Spigset, 2011; Logsdon et al., 2011; Misri et al., 2012). Thus, Citalopram’s ability to reverse gestational stress-induced depressive-like behavior in postpartum rats increases the validity of gestational stress as a translational model for PPD.
In addition to ameliorating stress-induced depressive-like behavior, postpartum administration of Citalopram also reversed structural modifications in the NAc shell and mPFC of mothers exposed to chronic gestational stress thereby confirming and extending our prior work (Haim et al., 2014; Leuner et al., 2014). These included stress-induced reductions in total dendritic length, number of branch points and spine density on NAc shell MSNs as well as stress-induced reductions in spine density on mPFC pyramidal neurons. We also found that in addition to affecting structural plasticity in the mPFC and NAc shell, gestational stress had the opposite effect in the BLA and increased dendritic spine density. However, unlike the NAc shell and mPFC, chronic postpartum administration of Citalopram didn’t reverse increased spine density in the BLA of stressed mothers which raises at least three possible interpretations of the current results. One possibility is that enhanced depressive-like behavior in mothers exposed to gestational stress is mediated mainly by the mPFC-NAc pathway and is less affected by the BLA which instead may play a greater role in anxiety-like behavior (Belzung et al., 2014). Consistent with this are recent data showing that the cortico-NAc circuit is essential for depressive-like behavior while the cortico-BLA circuit is essential for anxiety-related behaviors (Vialou et al., 2014). Alternatively, our data may suggest that the postpartum BLA isn’t responsive to antidepressants as it is in male rats (Pillai et al., 2012) or that the postpartum BLA is more sensitive to stress. Future studies will be necessary to distinguish among these possibilities. Minimally, these data show that although the BLA is anatomically and functionally interconnected with the NAc and mPFC (Russo and Nestler, 2013; Stevenson and Gratton, 2003; Vialou et al., 2014), there are regionally specific effects of gestational stress and antidepressant treatment on the postpartum brain. Furthermore, our morphological analysis suggest that dendritic spines in the postpartum mPFC, NAc shell and BLA are particularly sensitive to stress since all three regions showed stress-induced changes in spine density. Determining the extent to which the morphological changes observed here function individually or together to affect behavior remains to be determined.
As previously reported (Haim et al., 2014), we found no effect of gestational stress on dendritic length, branching or spine density in the NAc core, a region which processes locomotor output associated with reward and motivation and which is involved to a lesser degree than the NAc shell in mood regulation (Malenka et al., 2009; Zahm, 1999). These data also coincide with other studies reporting a greater sensitivity of the shell to stress (Kalivas and Duffy, 1995; Wang et al., 2012). Since we also failed to find an effect of Citalopram on structural plasticity within the NAc core, it appears the effects of gestational stress and SSRI treatment are not widespread but instead are restricted to mood-relevant brain regions (Russo and Nestler, 2013).
The transition into motherhood is accompanied by dramatic behavioral and physiological changes, as the discoveries of Jay Rosenblatt and colleagues have shown (Rosenblatt, 1980; Rosenblatt et al., 1988). Indeed, Rosenblatt’s work contributed greatly to the identification of the multifaceted hormonal and neurochemical alterations that occur during the postpartum period (Numan and Insel, 2003; Rosenblatt, 1989). Thus, perhaps it should not be too surprising that the morphological findings reported here in postpartum females are complex and in some cases contrast stress effects, and the antidepressant response to those effects, that have been observed in virgin male and/or female rodents. For example, MSNs in the NAc of male rats or mice exposed to chronic stress display dendritic hypertrophy and increased spine density (Bessa et al., 2013; Christoffel et al., 2011), and these effects are reversed by SSRI antidepressant treatment (Bessa et al., 2013). Therefore, even though the fine structure of the NAc is differentially affected by stress in males versus postpartum females, both are responsive to antidepressants. With respect to the BLA, gestational stress increased spine density but had no effect on dendritic length or branching as it does in males (Padival et al., 2013; Vyas et al., 2004). Moreover, as noted above, unlike male rats (Pillai et al., 2012) increased spine density in the BLA of gestationally stressed mothers did not respond to antidepressant treatment. Finally, the postpartum mPFC also exhibited some unique responses to stress when compared to males, who in addition to showing reduced spine density also show dendritic atrophy (Shansky and Morrison, 2009). It should also be noted that unlike the NAc and BLA, sex differences in the effects of stress on mPFC structure have been investigated and these studies have shown that dendritic complexity of mPFC neurons is enhanced in female rats exposed to restraint stress (Garrett and Wellman, 2009; Shansky and Morrison, 2009). Thus, the impact of stress on neuronal morphology in the mPFC is not only sex dependent but is further modulated by reproductive state. At least in male rats, SSRIs (Bessa et al., 2013) and other classes of antidepressants (Licnzerski and Duman, 2013) are similarly able to reverse stress-induced structural modifications in the mPFC as we observed in postpartum females.
Chronic stress, which induces depressive-like behavior in rodents, has been shown to reduce central serotonin availability (Boyarskikh et al., 2013; Zhang et al., 2012). Moreover, changes in synaptic availability of serotonin are highly associated with neuronal remodeling (Sun and Schacher, 1998; Vetencourt et al., 2011). Thus, alterations in the serotonergic system following stress may be responsible, at least in part, for stress-induced morphological modifications in mood regulating brain regions. By reinstating synaptic availability of serotonin following chronic stress, Citalopram could thereby ameliorate stress-induced structural changes in the mPFC and NAc. Such restoration of impaired structural plasticity could in turn be a mechanism by which SSRIs mediate their therapeutic effects as has been previously suggested (Licznerski and Duman, 2013). The therapeutic effects of SSRIs in gestationally stressed mothers may also involve downstream effects on the oxytocin system (Emiliano et al., 2007), which has been linked to PPD (Zelkowitz et al., 2014). It should be noted that presynaptic serotonin transporters (5-HT1A, 5-HTB, and 5-HTD), the action sites of SSRIs, are expressed in the mPFC, NAc, as well as the amygdala (Celada et al., 2013; Selvaraj et al., 2014; Van Bockstaele and Pickel, 1993). Given that Citalopram was ineffective in reversing stress-induced structural changes in the postpartum BLA, it may suggest that gestational stress either alters the expression of presynaptic 5HT receptors in the postpartum BLA or interferes with the intracellular signaling of presynaptic 5HT receptors.
Another possible mechanism by which gestational stress and Citalopram could affect depressive-like behavior and structural plasticity is through the HPA axis (Nemeroff and Owens, 2004). Chronic restraint stress is known to elicit dysregulation in HPA axis activity (Bratt et al., 2001; Mizoguchi et al., 2008) which is implicated in depression (Pariante and Lightman, 2008; Swaab et al., 2005; Varghese and Brown, 2001) including PPD in both humans (Glynn et al., 2013; Holsen et al., 2013) and rodent models (Brummelte and Galea, 2010). High levels of stress hormones resulting from HPA hyperactivity are known to regulate structural plasticity throughout the brain including in the mPFC, NAc shell and BLA (Garrett and Wellman, 2009; Morales-Medina et al., 2009; Rodrigues et al., 2009) of virgin rats. Thus, reinstatement of normal HPA axis activity along with reductions in levels of stress hormones could underlie Citalopram’s positive effect on postpartum mood and structural plasticity noted in this study.
In conclusion, the present results demonstrate that gestational stress-induced depressive-like behavior during the postpartum period is accompanied by structural changes within mood and stress regulating areas, namely the NAc shell, mPFC and BLA. Importantly, postpartum administration of Citalopram was effective in reversing both depressive-like behavior and structural changes within the NAc shell and the mPFC, but not in the BLA. Together, these observations provide much needed insight into the effects of stress and antidepressant treatment on the postpartum brain and pave the way for future research using this translational model of PPD.
Highlights.
Gestational stress induced depressive-like behavior during the postpartum period
Gestational stress induced structural modifications in the NAc shell, mPFC and BLA
Postpartum SSRI administration ameliorated depressive-like behavior
In the NAc and mPFC, structural changes were reversed by postpartum SSRI treatment
Structural changes in the BLA were not affected by postpartum SSRI treatment
Acknowledgments
We thank H. Lundbeck for generously providing the Citalopram Hydrobromide and Orin Hemminger for technical assistance. This work was funded by a grant from the National Institute of Mental Health (R0084148) to B.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker S, Chebli M, Rees S, LeMarec N, Godbout R, Bielajew C. Effects of gestational stress: 1. evaluation of maternal and juvenile offspring behavior. Brain Res. 2008;12:98–110. doi: 10.1016/j.brainres.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Belzung C, Turiault M, Griebel G. Optogenetics to study the circuits of fear-and depression-like behaviors: a critical analysis. Pharmacol. Biochem. Behav. 2014;122:144–157. doi: 10.1016/j.pbb.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Berle JO, Spigset O. Antidepressant use during breastfeeding. Curr. Womens Health Rev. 2011;7:28–34. doi: 10.2174/157340411794474784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa JM, Morais M, Marques F, Pinto L, Palha JA, Almeida OF, Sousa N. Stress-induced anhedonia is associated with hypertrophy of medium spiny neurons of the nucleus accumbens. Transl. Psychiatry. 2013;3:266. doi: 10.1038/tp.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol. Psychiatry. 2009;14:764–773. doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- Boyarskikh UA, Bondar NP, Filipenko ML, Kudryavtseva NN. Dowregulation of serotonergic gene expression in the raphe nuclei of the midbrain under chronic social defeat stress in male mice. Mol. Neurobiol. 2013;48:13–21. doi: 10.1007/s12035-013-8413-y. [DOI] [PubMed] [Google Scholar]
- Bratt AM, Kelley SP, Knowles JP, Barrett J, Davis K, Davis M, Mittleman G. Long term modulation of the HPA axis by the hippocampus. Behavioral, biochemical and immunological endpoints in rats exposed to chronic mild stress. Psychoneuroendocrinology. 2001;26:141–145. doi: 10.1016/s0306-4530(00)00033-0. [DOI] [PubMed] [Google Scholar]
- Brawarsky P, Stotland NE, Jackson RA, Fuentes-Afflick E, Escobar GJ, Rubashkin N, Haas JS. Pre-pregnancy and pregnancy-related factors and the risk of excessive or inadequate gestational weight gain. Int. J. Gynaecol. Obstet. 2005;91:125–131. doi: 10.1016/j.ijgo.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LAM. Depression during pregnancy and postpartum: contribution of stress and ovarian hormones. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:766–776. doi: 10.1016/j.pnpbp.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Artigas F. Serotonin modulation of cortical neurons and networks. Front. Integr. Neurosci. 2013;7:25. doi: 10.3389/fnint.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Tan QR, Dang W, Wang HN, Zhang RB, Li ZY, Lin H, Liu R. The effects of Citalopram on chronic stress-induced depressive-like behavior in rats through GSK3β/β-catenin activation in the medial prefrontal cortex. Brain Res. Bull. 2012;88:338–344. doi: 10.1016/j.brainresbull.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robinson AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL, Eisch AJ, Dietz DM, Ferguson D, Neve RL, Greengard P, Kim Y, Morrison JH, Russo SJ. IkB kinase regulates social defeat-induced synaptic and behavioral plasticity. J. Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey HL, Tough SC, Adair CE, Benzies KM. Risk factors for sub-clinical and major postpartum depression among a community cohort of Canadian women. Mat. Child Health J. 2011;15:866–875. doi: 10.1007/s10995-008-0314-8. [DOI] [PubMed] [Google Scholar]
- Emiliano ABF, Cruz T, Pannoni V, Fudge JL. The interface of oxytocin-labeled cells and serotonin transporter containing fibers in the priamet hypothalamus: a substrate for SSRIs therapeutic effects? Neuropsychopharmacology. 2007;32:977–988. doi: 10.1038/sj.npp.1301206. [DOI] [PubMed] [Google Scholar]
- Garrett JE, Wellman CL. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 2009;162:195–207. doi: 10.1016/j.neuroscience.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, Davis E, Sandman CA. New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides. 2013;47:363–370. doi: 10.1016/j.npep.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Grace SL, Evindar A, Steward DE. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Arch. Womens Ment. Health. 2003;6:263–274. doi: 10.1007/s00737-003-0024-6. [DOI] [PubMed] [Google Scholar]
- Gress-Smith JL, Luecken LJ, Lemery-Chalfant K, Howe R. Postpartum depression prevalence and impact on infant health, weight, and sleep in low-income and ethnic minority women and infants. Mat. Child Health J. 2012;16:887–893. doi: 10.1007/s10995-011-0812-y. [DOI] [PubMed] [Google Scholar]
- Grissom N, Bhatnagar S. Habituation to stress: get used to it. Neurobiol. Learn. Mem. 2008;92:215–224. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haim A, Sherer MS, Leuner B. Gestational stress induces persistent depressive-like behavior and structural modifications within the postpartum nucleus accumbens. Eur. J. Neurosci. 2014;40:3766–3773. doi: 10.1111/ejn.12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillerer KM, Reber SO, Neumann ID, Slattery DA. Exposure to chronic pregnancy stress reverses peripartum-associated adaptations: Implications for postpartum anxiety and mood disorders. Endocrinology. 2011;152:3930–3940. doi: 10.1210/en.2011-1091. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Lancaster K, Klibanski K, Whitfield- Gabrieli S, Cherkerzian S, Buka S, Goldstein JM. HPA-axis hormone modulation of stress response circuitry activity in women with remitted major depression. Neuroscience. 2013;250:733–742. doi: 10.1016/j.neuroscience.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res. 1995;675:325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- Lancaster CA, Gold KJ, Flynn HA, Yoo H, Marcus SM, Davis MM. Risk factors for depressive symptoms during pregnancy: a systematic review. Am. J. Obstet. Gynecol. 2010;202:5–14. doi: 10.1016/j.ajog.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC. A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Soc. Cogn. Affect. Neurosci. 2012;7:125–134. doi: 10.1093/scan/nsq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau NL, Dennis CL, Benzies K, Duffett-Leger L, Stewart M, Tryphonopoulous PD, Este D, Watson W. Postpartum depression is a family affair: addressing the impact on mothers, fathers, and children. Issues Ment. Health Nurs. 2012;3:445–457. doi: 10.3109/01612840.2012.673054. [DOI] [PubMed] [Google Scholar]
- Leuner B, Fredericks PJ, Nealer C, Albin-Brooks C. Chronic gestational stress leads to depressive-like behavior and compromises medial prefrontal cortex structure and function during the postpartum period. Plos ONE. 2014;9:89912. doi: 10.1371/journal.pone.0089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licznerski P, Duman RS. Remodeling of axo-spinous synapses in the pathophysiology and treatment of depression. Neuroscience. 2013;251:33–50. doi: 10.1016/j.neuroscience.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon MC, Wisner K, Sit D, Luther JF, Wisniewski SR. Depression treatment and maternal functioning. Depress. Anxiety. 2011;28:1020–1026. doi: 10.1002/da.20892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Nestler EJ, Hyman SE. Molecular neuropharmacology: a foundation for clinical neuroscience. NY: McGraw-Hill Medical; 2009. [Google Scholar]
- McEwen AM, Burgess DT, Hanstcok CC, Seres P, Khalili P, Newman SC, Baker GB, Mitchell ND, Khudabux-Der J, Allen PS, LeMelledo JM. Increased glutamate levels in the medial prefrontal cortex in patients with postpartum depression. Neuropsychopharmacology. 2012;37:2428–2435. doi: 10.1038/npp.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misri S, Abizadeh J, Albert G, Carter D, Ryan D. Restoration of functionality in postpartum depressed mothers: an open label study with escitalopram. J. Clin. Psychopharmacol. 2012;32:729–732. doi: 10.1097/JCP.0b013e31826867c9. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas S, Chattarji A. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl. Acad. Sci. USA. 2005;26:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi K, Shoji H, Ikeda R, Tanaka Y, Tabira T. Persistent depressive state after chronic stress in rats is accompanied by HPA axis dysregulation and reduced prefrontal dopaminergic neurotransmission. Pharmacol. Biochem. Behav. 2008;91:170–175. doi: 10.1016/j.pbb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Morales-Medina JC, Sanchez F, Flores G, Dumont Y, Quirion R. Morphological reorganization after repeated corticosterone administration in the hippocampus, nucleus accumbens and amygdala in the rat. J. Chem. Neuroanat. 2009;38:266–272. doi: 10.1016/j.jchemneu.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, Phillips ML. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am. J. Psychiatry. 2010;167:1373–1380. doi: 10.1176/appi.ajp.2010.09081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Fraser D, Wisner KL, James JA, Saul AT, Fiez JA, Phillips ML. Rapid habituation of ventral striatal response to reward receipt in postpartum depression. Biol. Psychiatry. 2011;70:395–399. doi: 10.1016/j.biopsych.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Owens MJ. Pharmacologic differences among the SSRIs: focus on monoamine transporters and the HPA axis. CNS Spectr. 2004;9:23–31. doi: 10.1017/s1092852900025475. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel T. The Neurobiology of Parental behavior. New York: Springer; 2003. [Google Scholar]
- O’Hara MW. Postpartum depression: what we know. J. Clin. Psychol. 2009;65:1258–1269. doi: 10.1002/jclp.20644. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, Wisner KL. Perinatal mental illness: definition, description and aetiology. Best. Pract. Res. Clin. Obstet. Gynaecol. 2014;28:3–12. doi: 10.1016/j.bpobgyn.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony SM, Myint AM, van den Hove D, Desbonnet L, Steinbusch H, Leonard BE. Gestational stress leads to depressive-like behavioural and immunological changes in the rat. Neuroimmunomodulation. 2006;13:82–88. doi: 10.1159/000096090. [DOI] [PubMed] [Google Scholar]
- Padival MA, Blume SR, Rosenkranz JA. Repeated restraint stress exerts different impact on structure of neurons in the lateral and basal nuclei of the amygdala. Neuroscience. 2013;246:230–242. doi: 10.1016/j.neuroscience.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends. Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Charlier TD, Fillet M, Houbart V, Crispin HT, Steinbusch HW, van den Hove DL. Chronic fluoxetine treatment and maternal adversity differentially alter neurobehavioral outcomes in the rat dam. Behav. Brain Res. 2012;228:159–168. doi: 10.1016/j.bbr.2011.11.043. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Picone TA, Allen JH, Schramm MM, Olsen PN. Pregnancy outcome in North American women. I. Effects of diet, cigarette smoking, and psychological stress on maternal weight gain. Am. J. Clin. Nutr. 1982;36:1214–1224. doi: 10.1093/ajcn/36.6.1205. [DOI] [PubMed] [Google Scholar]
- Pillai AG, Anilkumar S, Chattarji S. The same antidepressant elicits contrasting patterns of synaptic changes in the amygdala vs hippocampus. Neuropsychopharmacology. 2012;37:2702–2711. doi: 10.1038/npp.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends. Cog. Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Rampono J, Hackett LP, Kristensen JH, Kohan R, Page-Sharp M, Ilett KF. Transfer of escitalopram and its metabolite demethylescitalopram into breast milk. Br. J. Clin. Pharmacol. 2006;62:316–322. doi: 10.1111/j.1365-2125.2006.02659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Ann. Rev. Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS. Hormonal and nonhormonal regulation of maternal behavior: a theoretical survey. Reprod. Nutr. Dev. 1980;20:791–800. doi: 10.1051/rnd:19800505. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, Mayer AD, Giordano AL. Hormonal basis during pregnancy for the onset of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13:29–46. doi: 10.1016/0306-4530(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS. The physiological and evolutionary background of maternal responsiveness. New Dir. Child Dev. 1989;43:15–30. doi: 10.1002/cd.23219894304. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J, Rekkas PV, Wilson AA, Houle S, Romano L, Hamidi J, Rusjan P, Stewart DE, Meyer JH. Relationship of monoamine oxidase-a distribution volume to postpartum depression and postpartum crying. Neuropsychopharmacology. 2015;40:429–435. doi: 10.1038/npp.2014.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Mouchlianitis E, Faulkner P, Turkheimer F, Cowen PJ, Roiser JP, Howes O. Biol. Psychiatry. 2014. Presynaptic serotonergic regulation of emotional processing: a mutlimodal brain imaging study. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Morrison JH. Stress-induced dendritic remodeling in the medial prefrontal cortex: effects if circuit, hormones and rest. Brain Res. 2009;1293:108–113. doi: 10.1016/j.brainres.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman ME, Loudon H, Liu X, Mauro C, Leiter G, Goldstein MA. The neural processing of negative emotion postpartum: a preliminary study of amygdala function in postpartum depression. Arch. Womens. Men. Health. 2011;14:355–359. doi: 10.1007/s00737-011-0226-2. [DOI] [PubMed] [Google Scholar]
- Smith JW, Seckl JR, Evans AT, Costall B, Smythe JW. Gestational stress induces post-partum depression-like behavior and alters maternal care in rats. Psychoneuroendocrinology. 2004;29:227–244. doi: 10.1016/s0306-4530(03)00025-8. [DOI] [PubMed] [Google Scholar]
- Stevenson CW, Gratton A. Basolateral amygdala modulation of the nucleus accumbens dopamine response to stress: role of the medial prefrontal cortex. Eur. J. Neurosci. 2003;17:1287–1295. doi: 10.1046/j.1460-9568.2003.02560.x. [DOI] [PubMed] [Google Scholar]
- Sun ZY, Schacher S. Binding of serotonin to receptors at multiple sites is required for structural plasticity accompanying long-term facilitation of Aplysia sensorimotor synapses. J. Neurosci. 1998;18:3991–4000. doi: 10.1523/JNEUROSCI.18-11-03991.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res. Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pickel VM. Ultrastructure of serotonin-immunoreactive terminals in the core and shell of the rat nucleus accumbens: cellular substrates for interactions with catecholamine afferents. J. Comp. Neurol. 1993;334:603–617. doi: 10.1002/cne.903340408. [DOI] [PubMed] [Google Scholar]
- Varghese FP, Brown ES. The hypothalamic-pituitary-adrenal axis in major depressive disorder: a brief primer for primary care physicians. Prim. Care Companion J. Clin. Psychiatry. 2001;3:151–155. doi: 10.4088/pcc.v03n0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek T, Bockting CL, van Pampus MG, Ormel J, Meijer JL, Hartman CA, Burger H. Postpartum depression predicts offspring mental health problems in adolescence independently of parental lifetime psychopathology. J. Affect. Disord. 2012;136:948–954. doi: 10.1016/j.jad.2011.08.035. [DOI] [PubMed] [Google Scholar]
- Vetencourt JFM, Tiraboschi E, Spolidoro M, Castren E, Maffei L. Serotonin triggers a transient epigenetic mechanism that reinstates adult visual cortex plasticity in rats. Eur. J. Neurosci. 2011;33:49–57. doi: 10.1111/j.1460-9568.2010.07488.x. [DOI] [PubMed] [Google Scholar]
- Vialou V, Bagot RC, Cahill ME, Ferguson D, Robinson AJ, Dietz DM, Fallon B, Mazei-Robinson M, Ku SM, Harrigan E, Winstanley CA, Joshi T, Feng J, Berton O, Nestler EJ. Prefrontal cortical circuit for depression and anxiety related behaviors mediated by cholecystokinin: role of deltaFosB. J. Neurosci. 2014;34:3878–3887. doi: 10.1523/JNEUROSCI.1787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Wang YC, Ho UC, Ko MC, Liao CC, Lee LJ. Differential neuronal changes in medial prefrontal cortex, basolateral amygdala and nucleus accumbens after postweaning social isolation. Brain Struct. Funct. 2012;217:337–351. doi: 10.1007/s00429-011-0355-4. [DOI] [PubMed] [Google Scholar]
- Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann. N. Y. Acad. Sci. 1999;29:113–128. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]
- Zelkowitz P, Gold I, Feeley N, Hayton B, Carter CS, Tulandi T, Abenhaim HA, Levin P. Psychosocial stress moderates the relationships between oxytocin, perintal depression, and maternal behavior. Horm. Behav. 2014;66:351–360. doi: 10.1016/j.yhbeh.2014.06.014. [DOI] [PubMed] [Google Scholar]
- Zhang J, Fan Y, Li Y, Zhu H, Wang L, Zhu MY. Chronic social defeat up-regulates expression of the serotonin transporter in rat dorsal raphe nucleus and projection regions in a glucocorticoid-dependent manner. J. Neurochem. 2012;123:1054–1068. doi: 10.1111/jnc.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]