Abstract
AIM
To evaluate effects of Danhong Huayu Koufuye (DHK, a Chinese medicinal formulae) alone or combined with metformin on diabetic retinopathy (DR) in Zucker diabetic fatty (ZDF) rats, an animal model of obese type-2 diabetes, and then to investigate the mechanisms.
METHODS
ZDF (fa/fa) rats were administered with vehicle (distilled water), metformin, DHK, and DHK plus metformin. Electrophysiological and histological analysis were applied to evaluated effects of DHK alone or combined with metformin on DR. The levels of fasting blood glucose (FBG) and glycosylated hemoglobin (HbA1c) in blood were measured to evaluate the antihyperglycemic activity of DHK. Furthermore, levels of nitric oxide (NO), malondialdehyde (MDA) and activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) in serum were measured to study effects of DHK on oxidative stress in ZDF rats. In addition, body weight, lipidic indexes and insulin level were also assessed.
RESULTS
DHK combined with metformin significantly reversed the prolongation of latency times of flash electroretinogram (FERG) and oscillatory potentials (OPs) in diabetic rats. Furthermore, DHK alone or combined with metformin showed a remarkable suppression of retinal neovascularization and amelioration of retinal internal limiting membrane morphology. Moreover, DHK alone or plus metformin reduced FBG (P<0.05), HbA1c (P<0.01) and MDA (P<0.01) levels in diabetic rats. In addition, reductions in levels of triglycerides (TG) (P<0.01) and low density lipoprotein cholesterol (LDL-c) (P<0.01 and P<0.05, respectively) were also observed in diabetic rats treated with DHK alone or plus metformin.
CONCLUSION
DHK in combination with metformin had a preventive and therapeutic effect on DR in type-2 diabetic rats, and the possible mechanisms may be alleviating hyperglycemia, reducing oxidative stress and improving lipid metabolism.
Keywords: diabetic retinopathy, Danhong Huayu Koufuye, metformin, Zucker diabetic fatty rat, antihyperglycemic activity, oxidative stress
INTRODUCTION
Diabetic retinopathy (DR) is one of the most common and serious complications of diabetes. Its incidence reaches absolutely high level of 77.8% in individuals with type-2 diabetes (T2D) after 15 or more years' onset of diabetes[1]. DR is clinically characterized by retinal microvascular pathologic changes, such as microaneurisms, hemorrhages, capillary occlusion and neovascularization, finally leads to severe vision loss and irreversible blindness[2],[3]. DR is an important cause of blindness in working-age adults worldwide[4]. A number of therapeutics, such as anti-vascular endothelial growth factor (VEGF) therapy, long-acting steroids, and surgery with laser photocoagulation, has been applied clinically[5]. However, they were used for later stage of DR. Therefore, early treatments to delay DR progression are desperately needed and have great social and economic impacts.
Although the pathogenesis remains unclear, studies have indicated that many factors, including hyperglycemia, oxidative stress, and inflammatory cytokines, contribute to DR progression[6],[7]. Among these factors, hyperglycemia is accepted as the first initiating factor in the development of DR, which suggests that strict glycemic control is effective in delaying DR. In addition, oxidative stress, a result of hyperglycemia and a guide of inflammatory process, is also believed to play a critical role in the progression of DR.
Danhong Huayu Koufuye (DHK), a traditional Chinese prescription, contains Salvia miltiorrhiza radix, Angelicae Sinensis radix, Chuanxiong rhizoma, Persicae semen, Carthami flos, Bupleuri radix, as well as Aurantii fructus. Quantities of these ingredients are 29%, 11.5%, 15%, 11.5%, 11.5%, 11.5% and 10% of the total weight, respectively. DHK has capability to facilitate blood circulation to eliminate blood stasis and promote qi circulation to remove meridian obstruction. Therefore, DHK is applied for the treatment of blurred vision caused by stagnation of qi and blood stasis, and central retinal vein occlusion. In the view of traditional Chinese medicine, a vital energy or life force called qi circulates in the body through a system of pathways called meridians. Qi can propel the blood through the arteries and veins. The foundation of human health rests on the harmonious integration and balance that qi provides. Our previous research showed that DHK had antihyperglycemic activity, preventive and therapeutic effect on DR in streptozotocin-induced type-1 diabetic (T1D) rats[8].
To date, many rodent animal models of T2D have been set up. Among them, Zucker diabetic fatty (ZDF) rats are widely used as a genetic model for obese T2D. Male ZDF rats carry leptin receptor defect congenitally. They develop obesity and insulin resistance at young age, and progressively develop hyperglycemia with aging. Male diabetic ZDF rats are able to develop retinal abnormalities with increased expression of acellular capillaries, decreased number of pericytes, as well as increased thickness of basement membrane[9]–[11]. Thus, ZDF rats were applied in our study. The purpose of this study was to valuate effects of DHK alone or combined with metformin on DR in ZDF rats, and to investigate the underlying mechanisms.
MATERIALS AND METHODS
Materials
Reagents
DHK and metformin [purity≥98.8%, detected by High Performance Liquid Chromatography (HPLC)] were kindly provided by Guangzhou Baiyunshan Hutchison Whampoa Chinese Medicine Co., Ltd. and Huainan Jiameng Pharmaceutical Co., Ltd., respectively. Hypromellose eye drops and dicaine hydrochloride eye drops were both from Zhongshan Ophthalmic Center of Sun Yet-Sen University. Compound tropicamide eye drops, pentobarbital sodium salt and xylazine hydrochloride injection were purchased from Shenyang Sinqi Pharmaceutical Co., Ltd., Merck Serono Co., Ltd. and Dunhua Shengda Pharmaceutical Co., Ltd., respectively.
Animals
Male ZDF (fa/fa) rats and congenic control rats (fa/+, lean) with 8wk of age were obtained from Charles River Laboratories (Beijing, China) and were kept until age of 31wk. All animals had free access to a diet of Purina 5008 rat chow (International Product Supplies Ltd., Shanghai, China) and water. They were maintained under a 12:12h cyclic lighting schedule with 21.0°C-23.0°C and 50%-60% humidity. All experiments were performed in accordance with the Animal Ethics Committee of Guangdong Pharmaceutical University and conformed to the ARVO Resolution for the use of animals in ophthalmic and vision research.
The lean rats were regarded as Lean+Vehicle group and treated with distilled water (p.o., 3.2 mL/kg). At 13wk of age, ZDF rats were with average fasting blood glucose (FBG) higher than 13.0 mmol/L, and were randomly divided into four groups (n=6): 1) ZDF+Vehicle group, ZDFs were administered with distilled water (p.o., 3.2 mL/kg); 2) ZDF+Met group, ZDFs were administered with metformin (p.o., 280 mg/kg); 3) ZDF+DHK group, ZDFs were administered with DHK (p.o., 3.2 mL/kg); 4) ZDF+DHK+Met group, ZDFs were administered with metformin and DHK (p.o., 280 mg/kg and 3.2 mL/kg, respectively). All rats were treated for 19wk. FBG were measured with FreeView Blood Glucose Monitoring Meter (Wondfo Biotech, Co., Ltd., Guangzhou, Guangdong Province, China).
Methods
Recording of flash electroretinogram and oscillatory potentials
At the end of the study, retinal function were detected by flash electroretinogram (FERG) and oscillatory potentials (OPs) as our described previously[12]. At age of 31wk, all rats were dark adapted overnight, and then anesthetized with pentobarbital sodium salt (3%, 0.6 mL/kg intraperitoneally) and xylazine hydrochloride injection (0.19 mL/kg intramuscularly). Drugs were given to rats each one and a half hour before recording. Pupils of all rats were dilated with compound tropicamide eye drops. Before recording, dicaine hydrochloride eye drops was given for surface anesthesia. Hypromellose eye drops was added to protect each eye and keep a good electrical connection.
FERG and OPs were recorded from both eyes simultaneously using two silver ring corneal electrodes, two forehead reference electrodes, and a ground electrode in the tail. The electroretinogram (ERG) machine (APS-2000AER) was purchased from Kanghua Rui Ming Technology Co., Ltd. FERG was recorded from 5 responses to flashes of unattenuated white light (20ms, 0.05 Hz) from a photic stimulator (light-emitting diodes) set at brightness of 6.325e−4 cd·s/m2 and filtered by a digital band-pass filter from 0.1-300 Hz. OPs was evaluated from 10 responses with an inter-stimulus interval of 20ms by filtering the original responses elicited by a stimulus luminance of 1.125e−3 cd·s/m2 by a frequency band-pass filter (50-200 Hz).
The amplitude of FERG a-wave was measured from the baseline to a-wave trough, and that of FERG b-wave was from a-wave trough to b-wave peak. The latency times of a-wave and b-wave were recorded the flash onset to the trough and peak of the wave. OPs 1 through 4 amplitudes and their latency times were derived from OPs wave forms. Amplitudes were measured from the trough immediately preceding the peak of each OP from OPs 1-4. Latency time for each OP was recorded from the flash onset to the peak of each OP.
Collection and preparation of blood samples
After recording FERG and OPs, individual blood sample was withdrawn from the carotid artery into vacutainer without anticoagulant for separating serum or with ethylene-diaminetetraacetic acid (EDTA)-2K for collecting hemolysate.
Histological studies of retina and pancreas
Eyes and pancreas were removed from rats and fixed with 4% paraformaldehyde in phosphate buffer saline. The paraffin sections (3 µm) were then stained with hematoxylin and eosin (HE). Pathological pictures of retinas and pancreas were taken at 400× and 200× under an optical microscope (OLYMPUS BX50), respectively. Retinal injury was evaluated by changes in retinal internal limiting membrane (ILM) of structure and neovascularization. Histological alterations of pancreas were analyzed by pathological changes in islets, such as hypertrophy, fibrosis and vacuolation.
Assays of lipidic profile
Serum total cholesterol (TC), triglycerides (TG), high density lipoprotein cholesterol (HDL-c) and low density lipoprotein cholesterol (LDL-c) were analyzed with an automatic biochemical analyzer (Hitachi 7180A, Japan). Glycosylated haemoglobin (HbA1c) level was analyzed with an automatic biochemical analyzer (Hitachi 7170A, Japan) with HPLC method. Serum insulin level was detected by using a rat insulin ELISA kit from Mercodia (Uppsala, Sweden). Insulin resistance, as an index of insulin sensitivity, was calculated as follows: homeostasis model assessment of insulin resistance (HOMA-IR)=fasting serum glucose (mmol/L)×fasting serum insulin (mIU/L)/22.5. β cell function in homeostasis model assessment (HOMA-β) was calculated as follows: HOMA-β (%)=100%×20×fasting serum insulin (mIU/L)/[fasting serum glucose (mmol/L)-3.5]. Fasting serum glucose in the formula was represented by FBG at week 31.
Assays of oxidative stress
Levels of nitric oxide (NO) and malondialdehyde (MDA), and activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) are effective indexes to assess the extent and condition of oxidative stress. Serum levels of NO and MDA were measured by commercial kits form R&D Systems (Minneapolis, USA) and Cayman Chemical (Michigan, USA), respectively. SOD and GSH-Px activities were both assayed by specific kits from Jiancheng Bioengineering Institute (Nanjing, Jiangsu Province, China).
Statistical Analysis
All data were expressed as mean±standard error of the mean (SEM) and analyzed by Statistical Package for the Social Sciences version 17.0 (SPSS 17.0). One-way analysis of variance (ANOVA) test was performed and post hoc multiple comparisons were conducted with LSD. P<0.05 was assumed to be significant.
RESULTS
Effects of Danhong Huayu Koufuye on Body Weight and Fasting Blood Glucose
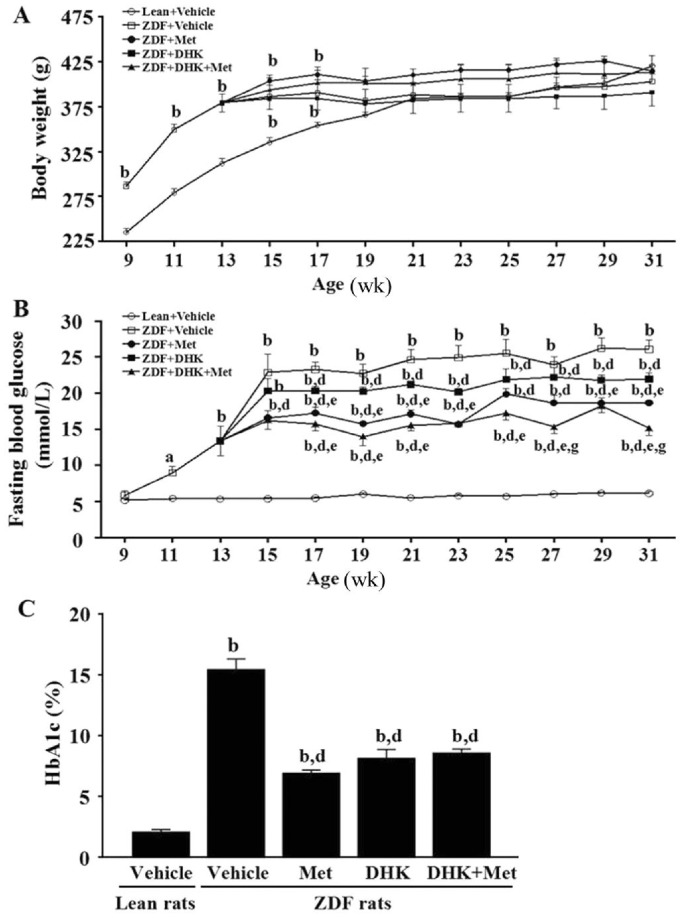
As shown in Figure 1A, body weights of ZDFs were greater than leans from week 9 to 17 (P<0.01). ZDFs and leans gained body weight quickly before the age of 15 and 21wk, respectively. At the end of study, there were no differences on body weight among these groups.
Figure 1. Effects of DHK on body weight (A), FBG (B) and HbA1c (C) of rats.
Data were expressed as mean±SEM, n=6. aP<0.05 and bP<0.01 vs Lean+Vehicle group; dP<0.01 vs ZDF+Vehicle group; eP<0.05 vs ZDF+DHK group; gP<0.05 vs ZDF+Met group.
All ZDFs were hyperglycemic in fasting state (13.4±2.0 mmol/L) at the age of 13wk (Figure 1B). FBG and HbA1c levels of vehicle-treated ZDFs were significantly higher than leans (P<0.01; Figure 1B and 1C), confirming the impaired glucose metabolism in diabetes. DHK or metformin alone markedly decreased FBG (P<0.01 vs ZDF+Vehicle group) from the age of 17wk or 15wk to 31wk, respectively. DHK in combination with metformin revealed more superior antihyperglycemic effect than DHK- or metformin-treated alone at the age of 27wk and 31wk. The HbA1c values of DHK, metformin and the combinated treatments revealed a significant decrease by 47.4%, 55.4% and 44.6% when compared with that of Vehicle-treated ZDFs, respectively.
Effects of Danhong Huayu Koufuye on Flash Electroretinogram and Oscillatory Potentials
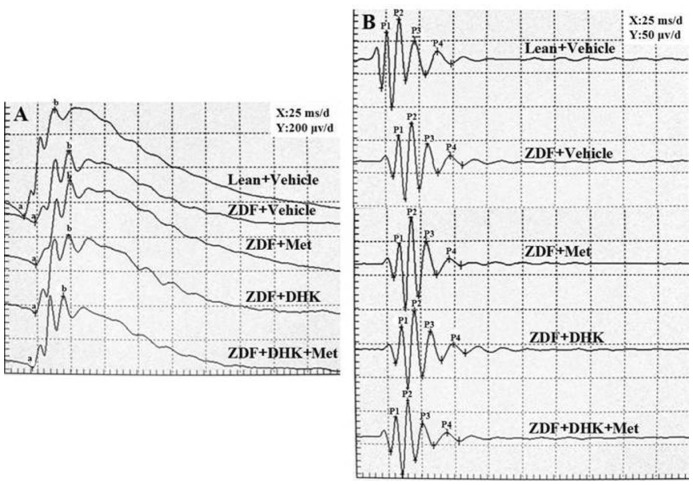
Decrease of FERG a-, b-wave (P<0.01) amplitudes and prolongation of a-, b-wave (P<0.01) latency times were observed in vehicle-treated ZDFs (Figure 2A, Table 1). Only DHK combined with metformin significantly reduced FERG a-, b-wave latency times by 14.7% and 6.1% as compared with vehicle-treated ZDFs. However, it had no effect on the decreased amplitude of FERG in diabetic rats. There were no differences in amplitudes of OPs among these groups (Figure 2B). But latency times of four OP waves were significantly increased in vehicle-treated ZDFs (P<0.01 vs Lean+Vehicle group; Figure 2B, Table 2). DHK combined with metformin remarkably reversed the prolongation of the four OP waves.
Figure 2. Representative FERG (A) and OPs (B) recording after administration of corresponding drugs in rats.
a: a-wave; b: b-wave; P1: OP1; P2: OP2; P3: OP3; P4: OP4.
Table 1. Effects of DHK on the amplitude and latency of FERG.
| Groups | Amplitude (µV) |
Latency (ms) |
||
| a-wave | b-wave | a-wave | b-wave | |
| Lean+Vehicle | 86.2±5.8 | 696.2±21.9 | 14.5±0.2 | 37.1±0.2 |
| ZDF+Vehicle | 54.3±5.6b | 445.1±24.6b | 23.5±0.5b | 49.1±0.6b |
| ZDF+Met | 48.1±3.7 | 452.4±39.3 | 23.7±0.5 | 49.8±1.4 |
| ZDF+DHK | 54.1±2.5 | 425.1±29.5 | 23.1±0.3 | 49.4±0.4 |
| ZDF+Met+DHK | 42.0±12.2 | 410.5±36.8 | 20.1±0.7d | 46.1±0.9c |
bP<0.01 vs Lean+Vehicle group; cP<0.05 and dP<0.01 vs ZDF+Vehicle group.
x±s, n=12
Table 2. Effects of DHK on the latency of OPs.
| Groups | Latency (ms) |
|||
| OP1 | OP2 | OP3 | OP4 | |
| Lean+Vehicle | 25.1±0.2 | 33.8±0.2 | 45.6±0.2 | 62.9±0.2 |
| ZDF+Vehicle | 34.9±0.5b | 44.5±0.5b | 55.9±0.6b | 72.9±0.5b |
| ZDF+Met | 35.7±1.0 | 43.5±0.1 | 55.2±0.3 | 71.9±0.4 |
| ZDF+DHK | 33.3±1.0 | 44.6±0.3 | 56.8±0.3 | 72.7±1.2 |
| ZDF+Met+DHK | 32.1±0.6c | 41.2±0.5d | 52.7±0.6d | 69.6±0.6c |
bP<0.01 vs Lean+Vehicle group; cP<0.05 and dP<0.01 vs ZDF+Vehicle group.
x±s, n=12
Effects of Danhong Huayu Koufuye on Retinal and Pancreatic Histology
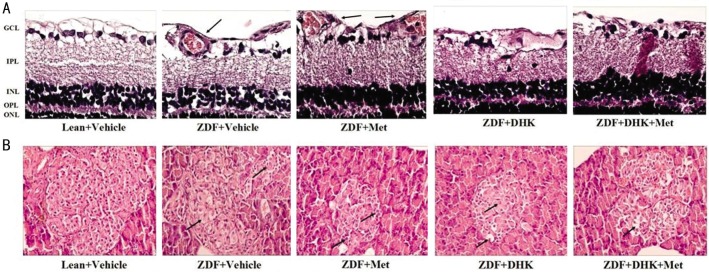
As shown in Figure 3A, the retinal layers of leans had clear structure, as well as tight and tidy cells. Moreover, retinal ILM remained smooth and integrity. However, as the arrows point out, swell ILM with protrudent capillary endothelial cells and new vessels were seen in vehicle-treated ZDFs. DHK treated alone or combined with metformin had an obvious suppression of retinal neovascularization and reduction in swelling of ILM. Figure 3B showed that, in the leans, Langerhans islets were normal with abundant cytoplasm and regular boundaries. Pathological changes of Langerhans islets were found in vehicle-treated ZDFs, including hypertrophy, atrophy, vacuolation and irregular boundaries as the arrows point out. There were no obvious amelioration in drug treatment groups.
Figure 3. Morphologies of HE-stained retinas (A) and Langerhans islets (B).
GCL: Ganglion cell layer; IPL: Inner plexiform layer; INL: Inner nuclear layer; OPL: Outer plexiform layer; ONL: Outer nuclear layer. Pictures were at the magnification of ×400 and ×200, respectively.
Effects of Danhong Huayu Koufuye on Insulin Level, Homeostasis Model Assessment of Insulin Resistance and β Cell Function in Homeostasis Model Assessment
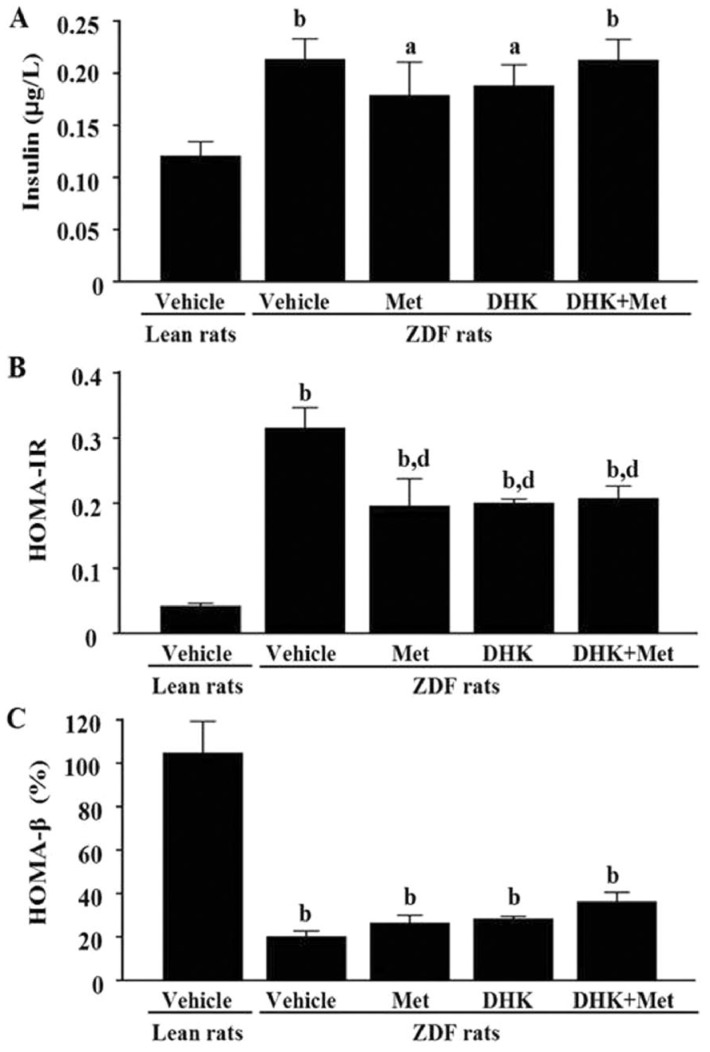
At the age of 31wk, the vehicle-treated ZDFs displayed an increase in insulin level (P<0.01 vs Lean+Vehicle group; Figure 4A), accompanied by obvious insulin resistance (Figure 4B). Although insulin levels had no significant differences among ZDFs, metformin alone (P<0.01), DHK alone (P<0.01), and the combined treatment (P<0.01) ameliorated insulin resistance as compared with vehicle-treated ZDFs. β-cell function was markedly reduced in ZDF+Vehicle group than Lean+Vehicle group (P<0.01; Figure 4C). There was no improvement in β-cell function in ZDF treated with drugs.
Figure 4. Effects of DHK on insulin level (A), HOMA-IR (B) and HOMA-β (C) of rats.
Data were expressed as mean±SEM, n=6. aP<0.05 and bP<0.01 vs Lean+Vehicle group; dP<0.01 vs ZDF+Vehicle group.
Effects of Danhong Huayu Koufuye on Lipidic Profile
Serum TC, TG, HDL-c and LDL-c levels of vehicle-treated ZDFs were significantly higher than those of leans (Table 3). DHK, and DHK plus metformin treatment significantly down-regulated serum TG and LDL-c levels as compared with vehicle-treated ZDFs.
Table 3. Effects of DHK on lipidic indexes.
| Groups | Lipidic index (mmol/L) |
|||
| TC | TG | HDL-c | LDL-c | |
| Lean+Vehicle | 2.6±0.1 | 0.7±0.1 | 0.8±0.0 | 0.2±0.0 |
| ZDF+Vehicle | 16.8±2.6b | 25.1±5.0b | 2.9±0.1b | 1.8±0.3b |
| ZDF+Met | 16.8±2.7 | 16.5±3.8 | 3.8±0.4d | 1.9±0.4 |
| ZDF+DHK | 10.0±1.0 | 10.5±1.5d | 2.6±0.1 | 0.8±0.2d |
| ZDF+Met+DHK | 12.9±2.1 | 8.7±0.9d | 3.1±0.3 | 1.3±0.3c |
bP<0.01 vs Lean+Vehicle group; cP<0.05 and dP<0.01 vs ZDF+Vehicle group.
x±s, n=6
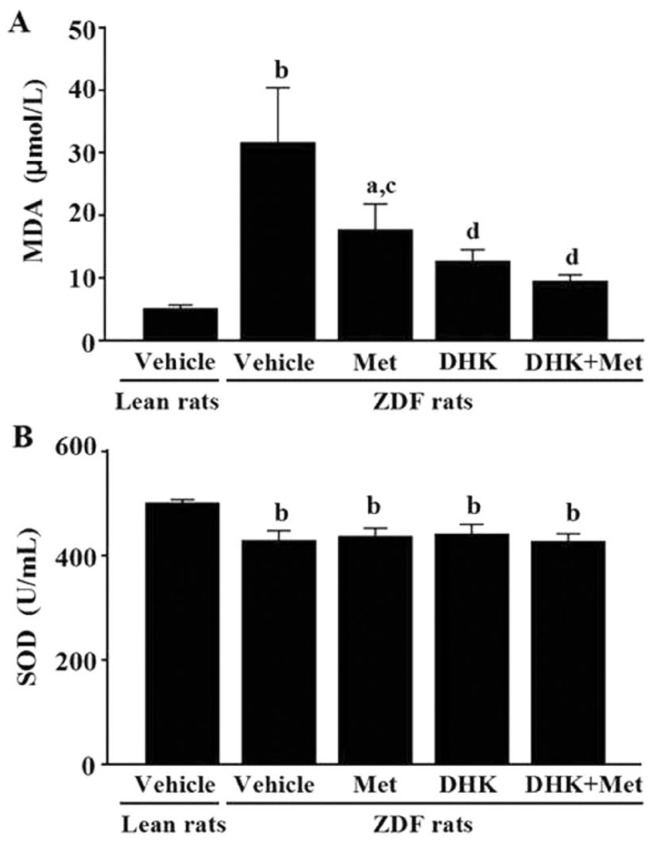
Effects of Danhong Huayu Koufuye on Oxidative Stress Profile
The vehicle-treated ZDFs exhibited greatly higher MDA level (P<0.01; Figure 5A) and lower SOD activity (P<0.01; Figure 5B) than those of leans in serum. Serum NO level and GSH-Px activity have no significant changes between the vehicle-treated ZDFs and leans (data not shown). After 19wk treatment, DHK significantly reduced MDA level in diabetic rats (P<0.01 vs ZDF+Vehicle group). Metformin alone, DHK alone and the combined treatments significantly reduced MDA levels (P<0.05, P<0.01, P<0.01 vs ZDF+Vehicle).
Figure 5. Effects of DHK on MDA level (A) and SOD activity (B) of rats.
Data were expressed as mean±SEM, n=6. aP<0.05 and bP<0.01 vs Lean+Vehicle group; cP<0.05 and dP<0.01 vs ZDF+Vehicle group.
DISCUSSION
Visual electrophysiological examination is an objective examination which reflected functional state of retina. FERG a-wave response reflects photoreceptor cell function, and b-wave reflects the bioelectrical activities of bipolar and Müller cells. The OP waves might originate from the inhibitory feedback circuits of the inner retinal layers and they are more sensitive to the changes of blood circulation in retina. In this study, decrease of FERG a-, b-wave amplitudes and prolongation of FERG a-, b-wave and OP waves latency times in ZDF+Vehicle group (Figure 2, Tables 1, 2), indicating the damage of retinal function and an animal model of T2D with DR had been successfully established. DHK in combination with metformin improved these parameters in ZDF rats, which indicates that DHK plus metformin had preventive and therapeutic effect on DR by reducing cellular damage and improving retinal blood circulation.
DHK alone had no effect on DR in ZDF rats (Figure 2, Tables 1, 2). However, our previous study showed that DHK alone had preventive effect on DR in T1D rats[8]. Differences in the diabetic type of research, choice of rodent strain, mode of diabetes induction and duration of diabetes may contribute to this different result.
Hyperglycemia, oxidative stress and inflammatory processes contribute to the progression of DR[6],[7]. Among these factors, hyperglycemia is accepted as the first initiating factor in the development of DR. Thus strict blood glucose control can effectively retard the development of DR. Our results showed that DHK or metformin alone had antihyperglycemic activity, and combined treatment displayed more significant effect on declining FBG (Figure 1A). However, only the combined treatment with DHK and metformin markedly improved retinal function (Tables 1, 2), which suggests that there are other underlying mechanisms other than antihyperglycemic activity.
Oxidative stress, as an unifying mechanism contributes to the development of DR, results in the activation of four accepted biochemical mechanisms, including polyol pathway, advanced glycation end products (AGEs) pathway, protein kinase C (PKC) pathway and hexosamine pathway[13]. Hyperglycemia causes overproduction of O2−, then may lead to increasing lipid peroxidation level and decreasing antioxidase activity, and thereby enhances oxidative stress in subjects with T2D. Oxidative stress thereafter results in an increase in thrombotic tendency and a reduction in prostacyclin stimulating factors in diabetics[14], which may contribute to DR. Our study showed an increased lipid peroxidation in terms of MDA and a decreased antioxidase activity in terms of SOD in diabetic rats (Figure 5). DHK significantly decreased MDA level, which might be one of the mechanisms of preventive effect on DR in ZDF rats.
Pathogenesis of obese T2D involves abnormalities in glucose and lipid metabolism. Duration of diabetes and level of LDL-c were risk factors for DR[15]. Most diabetic studies have found that HDL-c level was low in humans[16] and many rodent models of T2D, such as Wistar rats[17], SD rats[18] and db/db mice[19]. In our study, DHK decreased TG and LDL-c levels in diabetic rats, which might be another underlying way contributed to the preventive effect on DR. However, high HDL-c level was observed in diabetic ZDF rats in the present study. A special diet with Purina 5008 rat chow throughout the study might be the main reason[20].
The ingredients of DHK are Salvia miltiorrhiza radix, Angelicae Sinensis radix, Chuanxiong rhizoma, Persicae semen, Carthami flos, Bupleuri radix and Aurantii fructus. These ingredients were originally documented in the “Shennong's Herbal”, the oldest Chinese materia medica monographs. Tanshinone IIA is a major constituent of Salvia Miltiorrhiza Bunge, and have activities of anticoagulation[21], antioxidation[22], [23] and antiangiogenic[24], [25]. Angelicae Sinensis has been applied for disorders with blood deficiency for its capability to promote blood flow[26]. Tetramethylpyrazine, the predominant active component of Ligusticum Chuanxiong Hort, has been reported for its antioxidant proficiency on ischemic reperfusion[27] and atherosclerosis[28] in rats. Amygdalin and hydroxysafflor yellow A were believed to be the main components of Persicae semen and Carthamus in activating blood circulation to dissipate blood[29]. So many compounds in the prescription are the material basis and contributed to exerting anti-DR effect.
In summary, our study showed that DHK in combination with metformin improved retinal function in diabetic ZDF rats, an animal model of obese T2D. Alleviating hyperglycemia, reducing oxidative stress and improving lipid metabolism may be mechanisms contributed to preventing and delaying the procession of DR. This study suggests that DHK may be a valuable adjuvant therapy for DR.
Acknowledgments
Foundations: Supported by National Natural Science Fundation of China (No. 81303282); Central Finance of China in Support of the Development of Local Colleges and University [Educational Finance Grant No. 338 (2013/2014)]; Department of Education of Guangdong Province (No. Yq2013044); Science and Information Technology of Guangzhou (No. 2013J2200034).
Conflicts of Interest: Chen WP, None; Wang YD, None; Ma Y, None; Zhang ZY, None; Hu LY, None; Lin JL, None; Lin BQ, None.
REFERENCES
- 1.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102(4):520–526. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 2.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher EL, Phipps JA, Ward MM, Puthussery T, Wilkinson-Berka JL. Neuronal and glial cell abnormality as predictors of progression of diabetic retinopathy. Curr Pharm Des. 2007;13(26):2699–2712. doi: 10.2174/138161207781662920. [DOI] [PubMed] [Google Scholar]
- 4.Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14(4):179–183. doi: 10.1080/09286580701396720. [DOI] [PubMed] [Google Scholar]
- 5.Barot M, Gokulgandhi MR, Patel S, Mitra AK. Microvascular complications and diabetic retinopathy: recent advances and future implications. Future Med Chem. 2013;5(3):301–314. doi: 10.4155/fmc.12.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng L, Kern TS. Role of nitric oxide, superoxide, peroxynitrite and PARP in diabetic retinopathy. Front Biosci (Landmark ED) 2009;14:3974–3987. doi: 10.2741/3505. [DOI] [PubMed] [Google Scholar]
- 7.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30(5):343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin BQ, Zhou JY, Ma Y, Deng YJ, Zheng CJ, Lin JL. Preventive effect of danhong huayu koufuye on diabetic retinopathy in rats. Int J Ophthalmol. 2011;4(6):599–604. doi: 10.3980/j.issn.2222-3959.2011.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danis RP, Yang Y. Microvascular retinopathy in the Zucker diabetic fatty rat. Invest Ophthalmol Vis Sci. 1993;34(7):2367–2371. [PubMed] [Google Scholar]
- 10.Yang YS, Danis RP, Peterson RG, Dolan PL, Wu YQ. Acarbose partially inhibits microvascular retinopathy in the Zucker diabetic Fatty rat (ZDF/Gmi-fa) J Ocul Pharmacol Ther. 2000;16(5):471–479. doi: 10.1089/jop.2000.16.471. [DOI] [PubMed] [Google Scholar]
- 11.Wohlfart P, Lin J, Dietrich N, Kannt A, Elvert R, Herling AW, Hammes HP. Expression patterning reveals retinal inflammation as a minor factor in experimental retinopathy of ZDF rats. Acta Diabetol. 2014;51(4):553–558. doi: 10.1007/s00592-013-0550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin JL, Wang YD, Ma Y, Zhong CM, Zhu MR, Chen WP, Lin BQ. Protective effects of naringenin eye drops on N-methyl-N-nitrosourea-induced photoreceptor cell death in rats. Int J Ophthamol. 2014;7(3):391–396. doi: 10.3980/j.issn.2222-3959.2014.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 14.Jennings PE, McLaren M, Scott NA, Saniabadi AR, Belch JJ. The relationship of oxidative stress to thrombotic tendency in type 1 diabetic patients with retinopathy. Diabet Med. 1991;8(9):860–865. doi: 10.1111/j.1464-5491.1991.tb02125.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Cui X, Li F, Wang S, Liu X, Hui L, Song N, Li N. Association between diabetes mellitus with metabolic syndrome and diabetic microangiopathy. Exp Ther Med. 2014;8(6):1867–1873. doi: 10.3892/etm.2014.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siavash M, Amini M. Vitamin C may have similar beneficial effects to Gemfibrozil on serum high-density lipoprotein-cholesterol in type 2 diabetic patients. J Res Pharm Pract. 2014;3(3):77–82. doi: 10.4103/2279-042X.141075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar V, Bhandari U, Tripathi CD, Khanna G. Evaluation of antiobesity and cardioprotective effect of Gymnema sylvestre extract in murine model. Indian J Pharmacol. 2012;44(5):607–613. doi: 10.4103/0253-7613.100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierre W, Gildas AJ, Ulrich MC, Modeste WN, Benoit NT, Albert K. Hypoglycemic and hypolipidemic effects of Bersama engleriana leaves in nicotinamide/streptozotocin-induced type 2 diabetic rats. BMC Complement Altern Med. 2012;12:264. doi: 10.1186/1472-6882-12-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong WT, Gu L, Wang C, Sun HX, Liu X. Anti-hyperglycemic and hypolipidemic effects of Cistanche tubulosa in type 2 diabetic db/db mice. J Ethnopharmacol. 2013;150(3):935–945. doi: 10.1016/j.jep.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi T, Miyashita Y, Saiki A, Watanabe F, Watanabe H, Shirai, K Formula diet is effective for thereduction and differentiation of visceral adipose tissue in Zucker fatty rats. J Atheroscler Thromb. 2012;19(2):127–136. doi: 10.5551/jat.8466. [DOI] [PubMed] [Google Scholar]
- 21.Wu LC, Lin X, Sun H. Tanshinone IIA protects rabbits against LPS-induced disseminated intravascular coagulation (DIC) Acta Pharmacol Sin. 2012;33(10):1254–1259. doi: 10.1038/aps.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang HS, Wang SQ. Nrf2 is involved in the effect of tanshinone IIA on intracellular redox status in human aortic smooth muscle cells. Biochem Pharmacol. 2007;73(9):1358–1366. doi: 10.1016/j.bcp.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Zhang C, Guo Y, Su ZY, Yang Y, Shu L, Kong AN. Blocking of JB6 Cell Transformation by Tanshinone IIA: Epigenetic Reactivation of Nrf2 Antioxidative Stress Pathway. AAPS J. 2014;16(6):1214–1225. doi: 10.1208/s12248-014-9666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan XM, Ren GX, Liang QL, Wang YM, Luo GA. Study on inhibitory effects and mechanism of lipophilic components in Salvia miltiorrhiza on angiogenesis in vitro. Zhongguo Zhong Yao Za Zhi. 2014;39(4):744–747. [PubMed] [Google Scholar]
- 25.Xing Y, Tu J, Zheng L, Guo L, Xi T. Anti-angiogenic effect of tanshinone IIA involves inhibition of the VEGF/VEGFR2 pathway in vascular endothelial cells. Oncol Rep. 2015;33(1):163–170. doi: 10.3892/or.2014.3592. [DOI] [PubMed] [Google Scholar]
- 26.Wu YC, Hsieh CL. Pharmacological effects of Radix Angelica Sinensis (Danggui) on cerebral infarction. Chin Med. 2011;6:32. doi: 10.1186/1749-8546-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lv L, Jiang SS, Xu J, Gong JB, Cheng Y. Protective effect of ligustrazine against myocardial ischaemia reperfusion in rats: the role of endothelial nitric oxide synthase. Clin Exp Pharmacol Physiol. 2012;39(1):20–27. doi: 10.1111/j.1440-1681.2011.05628.x. [DOI] [PubMed] [Google Scholar]
- 28.Jiang F, Qian J, Chen S, Zhang W, Liu C. Ligustrazine improves atherosclerosis in rat via attenuation of oxidative stress. Pharm Biol. 2011;49(8):856–863. doi: 10.3109/13880209.2010.551776. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Duan JA, Tang Y, Guo J, Yang N, Ma H, Shi X. Taoren-Honghua herb pair and its main components promoting blood circulation through influencing on hemorheology, plasma coagulation and platelet aggregation. J Ethnopharmacol. 2012;139(2):381–387. doi: 10.1016/j.jep.2011.11.016. [DOI] [PubMed] [Google Scholar]