Abstract
AIM
To investigate the effect of DSX, an active component extracted from Erigeron breviscapus, on the voltage-gated outward K+ channel currents in rat retinal ganglion cells (RGCs) by using electrophysiological method, and to explore the possible mechanisms of DSX on optic nerve protection.
METHODS
Outward K+ currents were recorded by using whole-cell patch-clamp techniques on acutely isolated rat RGCs. Outward K+ currents were induced by a series of depolarizing voltage pulses from a holding potential of -70 mV to +20 mV in an increment of 10 mV.
RESULTS
Extracellular application of DSX voltage-dependently suppressed both the steady-state and peak current amplitudes of outward K+ currents in rat RGCs. Furthermore, DSX reversibly and dose-dependently inhibited the amplitudes of outward K+ currents of the cells. At +20 mV membrane potential DSX at the concentrations of 0.02 g/L and 0.05 g/L showed no significant effects on the currents. In contrast, DSX at higher concentrations (0.1 g/L, 0.2 g/L and 0.5 g/L) significantly suppressed the current amplitudes.
CONCLUSION
These results suggest that DSX reversibly and dose-dependently suppress outward K+ channel currents in rat RGCs, which may be one of the possible mechanisms underlying Erigeron breviscapus prevents vision loss and RGC damage caused by glaucoma.
Keywords: Erigeron breviscapus, retinal ganglion cells, potassium channel
INTRODUCTION
Erigeron breviscapus (Vant.) Hand.-Mazz., belonging to genus erigeron and genera compositae, is an important herb in the traditional Chinese medicine (TCM). Erigeron breviscapus is mainly distributed in southwest of China, such as Yunnan, Guizhou, Guangxi, Sichuan and Tibet provinces, etc. As early as in 14th-15th centuries, Erigeron breviscapus had been collected in the book, namely “Southern Yunnan Materia Medica”, and has been widely used clinically in the TCM.
Erigeron breviscapus has distinct properties, such as tasting bitter, acrid and warm-natured, thus enduing its efficacy by promoting blood circulation, releasing pain, dispersing cold and chills, and removing body moisture. Erigeron breviscapus has been used successfully to treat stroke, hemiplegia, pain of obstruction, and reduce some symptoms such as headache and toothaches. Erigeron breviscapus is a mixture of flavonoid glycosides extracted from the Chinese herb Erigeron breviscapus (Vant.) Hand.-Mazz. Scutellarin is its primary active ingredient. Increased pharmacological evidence has demonstrated that Erigeron breviscapus could dilate blood vessels, reduce vascular resistance and blood viscosity, thus increasing blood flow and improving microcirculations. In addition, it could also inhibit the inflammatory factor release and protect vascular endothelial cells, etc[1],[2].
The preparations made by effective components of Erigeron breviscapus have been widely used clinically for treatment of diseases in the cardiovascular system, and diseases of liver, kidney and stroke. It is also used for treatment of ophthalmological diseases, such as glaucomatous optic nerve damage, diabetic retinopathy, retinal vein occlusion, etc[3],[4].
Retinal ganglion cells (RGCs) are output neurons of the retina. Apoptosis of RGCs in glaucoma is closely related to loss of visual field, optic nerve damage and thinned retinal nerve fiber layer[5],[6]. As the second leading cause of blindness, irreversibility of glaucoma has attracted more and more attention. Unfortunately, there are still no definite drugs that can reverse glaucomatous blindness. A survey, made by the Johns Hopkins University Hospital using an epidemiological model, claimed that the number of patients with glaucoma will rapidly increase, and most of these patients, with both acute close-angle glaucoma (ACG) and primary open angle glaucoma (OAG), will be in Asian[7].Glaucoma is a chronic and progressive optic neuropathy, which is characterized by loss of axons of RGCs that constitute the optic nerve[8]. Therefore, optic nerve protection, namely preventing RGC apoptosis, is particularly important to keep the vision of patients. For this purpose, many researchers have been made[9],[10].
Many animal experiments and clinical multi-center studies have demonstrated that Erigeron breviscapus and its extracts could promote the survival of RGCs, reduce the elevated intraocular pressure (IOP), thus protecting the optic nerve[11]–[14]. The present study was undertaken with an aim of exploring whether and how DSX, an Erigeron breviscapus extract, may modulate voltage-gated outward K+ channels in rat RGCs by using whole-cell patch-clamp techniques.
MATERIALS AND METHODS
Animals
Male Sprague Dawley rats, weighing 80-100 g, obtained from SLAC Laboratory Animal Co. Ltd. (Shanghai, China), were housed on a 12h light/dark schedule, no eye disease, with standard food and water provided ad libitum for at least 1wk before they were used for experiments. All experimental procedures dealing with the animals in the present work were in accordance with the National Institute of Health (NIH Publications No. 80-23) guidelines for the Care and Use of Laboratory Animals and the guidelines of Fudan University on the ethical use of animals. All efforts were made to minimize the number of animals used and their suffering.
Retrograde Labeling of Retinal Ganglion Cells
Retrograde labeling of RGCs was previously described in detail[15]–[17]. Briefly, after the rats were deeply anaesthetized with 40 mg/mL sodium pentobarbital (0.1 mL/100 g), 4% rhodamine-B-isothiocyanate (RITC) (List Biological Laboratories, Campbell, CA, USA) (2 µL) was injected into the superior colliculus bilaterally (6.0 mm posterior and 2.0 mm lateral to the bregma and 4-5 mm deep from the cortical surface). After a survival period of 5-7d, RGCs were clearly labeled for electrophysiological recording.
Preparation of Isolated Ganglion Cells for Electrophysiology
RGCs were acutely dissociated from retinas retrogradely labeled with RITC by enzymatic and mechanical methods as previously described[15]–[17]. Rats were anesthetized by 25% urethane (i.p., 4 mL/kg) and sacrificed by decapitation. The eyeballs were quickly removed and retinas. Isolated retinas were incubated in oxygenated Hank's solution containing the following (in mmol/L): NaCl 137, NaHCO3 0.5, NaH2PO4 1, KCl 3, CaCl2 2, MgSO4 1, HEPES 20, sodium pyruvate 1 and glucose 16 adjusted to pH 7.4 with NaOH, and then digested in 1.6 U/mL papain (Worthington Biochemical, Freehold, NJ, USA) containing Hank's solution, supplemented with 0.2 mg/mL L-cysteine and 0.2 mg/mL bovine serum albumin (BSA) for 35min at 35°C. The solution was bubbled continuously with 100% O2 and adjusted to pH 7.4 with NaOH. After several rinses in Hank's solution, the retinas were mechanically dissociated with fire-polished Pasteur pipettes and cell suspension was plated onto a culture dish mounted on an inverted microscope (IX 70; Olympus Optical, Tokyo, Japan). RITC-labeled RGCs, showing red fluorescence, were chosen for whole-cell patch-clamp recording within 2-3h after dissociation.
Whole-cell Recording
The dissociated cells were superfused continuously with the extracellular solution containing (mmol/L): NaCl 140, KCl 5, CaCl2 2, MgCl2 1, HEPES 10, and glucose 20, with 0.4 µmol/L tetrodotoxin (TTX) and 100 µmol/L CdCl2, pH adjusted to 7.4 with NaOH and to 290-300 mOsm/L. Patch pipettes were made by pulling borosilicate glass capillaries (BF150-86-10, Sutter Instrument Co., Novato, CA, USA) on a P-97 Flaming/Brown micropipette puller (Sutter Instrument Co.) and fire polished (Model MF-830; Narishige, Tokyo, Japan) for recording. The pipette resistance was typically 4-6 MΩ after filling with an internal solution consisting of (in mmol/L): KCl 140, NaCl 9, MgCl2 1, EGTA 0.2, HEPES 10, ethylene glycol-bis(β-aminoethyl ether) N, N, N', N'-tetraacetic acid (EGTA) 0.2, ATP-Mg 2, GTP-Na 0.25, 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) 10, and adjusted to pH 7.2 with KOH and to 290-300 mOsm/L. Whole-cell membrane currents of RGCs were recorded by voltage-clamp techniques[15],[17],[18], using a patch amplifier (Axonpatch 200B) with Digidata 1322A data acquisition board and pClamp 8.0 software (Molecular Devices, Foster City, CA, USA). Analog signals were sampled at 10 kHz, filtered at 1 kHz. Fast capacitance was fully cancelled and cell capacitance was partially cancelled as much as possible by the amplifier circuits. Seventy percent of the series resistance of the recording electrode was compensated. All recordings were made at room temperature (20°C-25°C).
Reagents and Drug Application
Dnase I, L-cysteine, BSA, TTX and cadmium chloride (CdCl2) were obtained from Sigma (Sigma-Aldrich, Inc., St. Louis, MO, USA). Erigeron breviscapus extract DSX lyophilized powder was provided by the National institute of medicine, Chengdu University of TCM. DSX was first dissolved in dimethyl sulfoxide (DMSO) and then added to the extracellular solution, with the final concentration of DMSO being less than 0.1% that had no effects on current of RGCs. The other chemicals were freshly dissolved in the extracellular solution. Drugs were delivered by a superfusion drug application system (DAD-8VCSP, ALA Scientific Instruments, Westbury, NY, USA), which has eight pressurized 5 mL reservoirs, each with its own control valve to feed fluid through an tubing manifold (500 µm inner diameter; ALA Scientific Instruments). The open/close switch of each valve was manually controlled. Once the valve was open, the solution in the corresponding reservoir was pressure ejected by nitrogen gas along the pipes. With the large flow pipes, 10%-90% whole-cell solution exchanges were achieved in less than 2.5ms.
Data Analysis
The data analysis was performed by Clampfit 8.0 (Molecular Devices, Foster City, CA, USA), Graphpad Prism 4.0 (Graphpad Software) and Igor 4.0 (WaveMetrics, Lake Oswego, OR, USA). The steady-state current amplitudes was determined by averaging the current amplitude between 300ms to 320ms in a 400ms depolarizing voltage pulse, and the peak K+ current amplitudes were measured maximum at 120-140ms of the voltage pulses. Data are all presented as mean ± S.E.M. Student's t-test was used for statistical analysis.
RESULTS
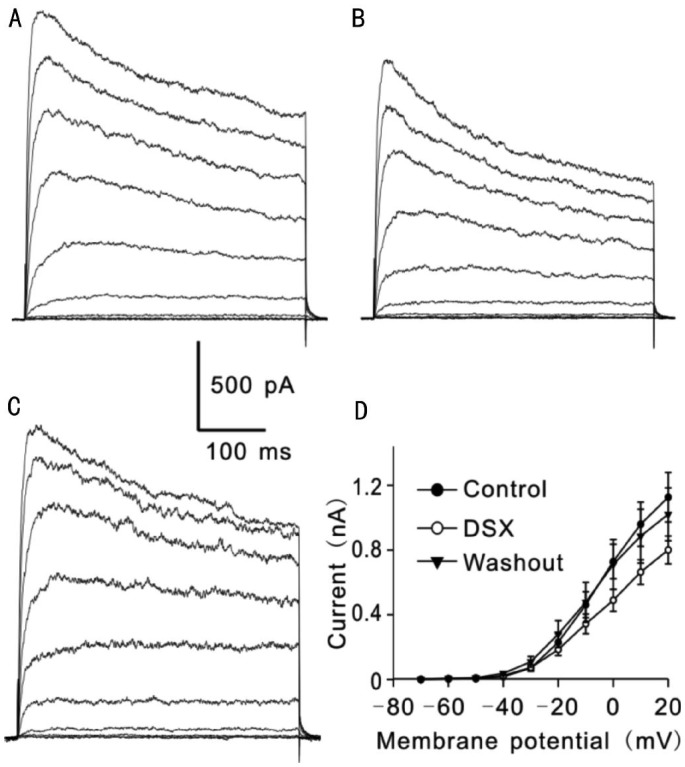
Effects of DSX on outward K+ currents of RGCs, induced by a series of 400ms depolarizing voltage pulses from a holding potential of -70 mV to +20 mV in increments of 10 mV, were first examined (Figure 1A). As shown in Figure 1B, 1C, extracellular application of DSX (0.1 g/L) significantly and reversibly suppressed the currents. Similar results were obtained in other six cells. Figure 1D shows current-voltage relationship curves of the steady-state current amplitudes under conditions of before (control), with DSX (0.1 g/L) and washout, revealing a voltage-dependent suppression of the currents (n=5).
Figure 1. DSX, an active component extracted from Erigeron breviscapus, suppressed outward potassium currents in rat retinal ganglion cells.
A: Outward K+ currents were induced by a series of depolarizing voltage pulses from a holding potential of -70 mV to +20 mV in an increment of 10 mV in a Ringer's bath solution containing TTX and CdCl2 (control); B: Extracellular application of DSX (0.1 g/L) significantly suppressed the currents; C: Washout with normal Ringer's solution brought the currents to control level; D: Current-voltage relationship (I-V) curves show voltage-dependent suppression of K+ current amplitudes by DSX (n=5).
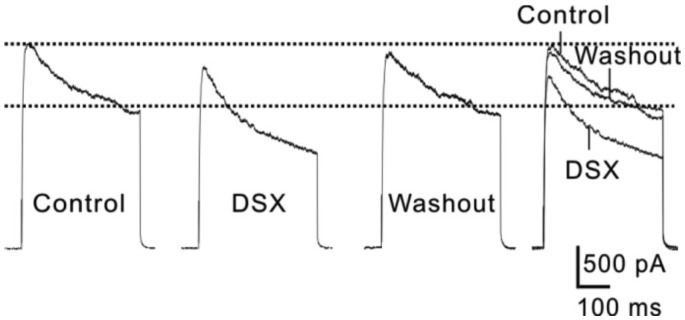
Since outward K+ currents recorded from rat RGCs may contain the contributions from different K+ channels[19]–[23], we analyzed changes of both the peak and steady-state current amplitudes before and after DSX application. Figure 2 shows representative current traces recorded from one RGC at +20 mV following a 2min perfusion of DSX (0.1 g/L). It was clear that DSX remarkably suppressed both the peak and steady-state current amplitudes, which was reversed by washing with normal extracellular solution (Figure 2).
Figure 2. DSX suppressed both peak and steady-state current amplitudes of outward K+ currents in rat retinal ganglion cells.
Representative current traces show that extracellular application of DSX (0.1 g/L) reversibly suppressed both the peak and steady-state current amplitudes at +20 mV.
Then, we further analyzed whether DSX at concentration of 0.1 g/L may affect the steady-state and peak amplitudes of the outward currents at different membrane potentials. As shown in Table 1, accompanying with depolarization of the cell membrane potentials, steady-state current amplitudes of outward K+ currents were progressively increased. When the membrane potentials were depolarized to values less than -20 mV (n=5, all P>0.05), extracellular application of DSX (0.1 g/L) did not show any effect on the steady-state current amplitudes. In contrast, at the membrane potentials ranging from -20 mV to +20 mV, DSX significantly and reversibly reduced the amplitudes of steady state currents (n=5, all P<0.05) (Table 1). Furthermore, DSX showed the similar effect on the peak current amplitudes of outward currents. That is, DSX had no significant influence on peak amplitudes of the outward currents at the membrane potentials between -70 mV to -10 mV (n=5, all P>0.05), and when the membrane potentials were more positive than -10 mV, a significant suppression of the peak current amplitudes by DSX was observed (n=5, P<0.05) in a reversible manner (Table 2). These results suggest that DSX suppressed the outward potassium currents in RGCs in a voltage-dependent manner.
Table 1. DSX (0.1 g/L) suppressed steady-state current amplitudes of outward K+ currents in rat RGCs.
| Voltage (mV) | Control | DSX (0.1 g/L) | Washout |
| -70 | 0.3±0.8 | -1.1±1.5 | 1.7±2.9 |
| -60 | 2.8±0.9 | 4.4±1.9 | 6.6±2.8 |
| -50 | 4.9±1.3 | 8.5±2.7 | 9.8±2.1 |
| -40 | 17.1±4.4 | 22.8±6.8 | 37.2±10.2 |
| -30 | 70.3±20.1 | 73.8±17.9 | 109.4±33.2 |
| -20 | 230.2±51.2 | 184.9±37.1a | 280.3±83.5 |
| -10 | 462.0±80.2 | 343.3±60.8a | 477.1±123.2 |
| 0 | 729.6±106.6 | 489.8±68.3a | 711.1±154.5 |
| 10 | 960.1±153.1 | 663.9±76.5a | 886.4±166.1 |
| 20 | 1027.2±153.1 | 801.0±85.7a | 1021.2±163.2 |
n = 5, aP <0.05 vs control.
x±s
Table 2. DSX (0.1 g/L) suppressed peak current amplitudes of outward K+ currents in rat RGCs.
| Voltage (mV) | Control | DSX (0.1 g/L) | Washout |
| -70 | 7.6±1.6 | 6.4±1.7 | 9.1±1.9 |
| -60 | 8.8±1.0 | 11.9±2.7 | 15.4±3.5 |
| -50 | 10.7±1.3 | 16.7±3.9 | 20.3±4.1 |
| -40 | 27.8±5.2 | 36.3±10.9 | 44.6±10.1 |
| -30 | 96.0±17.2 | 95.68±18.7 | 129.9±34.5 |
| -20 | 269.3±53.4 | 248.3±47.7 | 311.2±75.8 |
| -10 | 596.3±115.9 | 480.6±93.0 | 591.1±144.4 |
| 0 | 924.5±158.7 | 761.9±138.5a | 912.5±219.5 |
| 10 | 1228.7±185.5 | 1030.5±169.7a | 1184.1±274.6 |
| 20 | 1531.5±207.4 | 1265.0±199.1a | 1411.0±297.3 |
n = 5, aP<0.05 vs control.
x±s
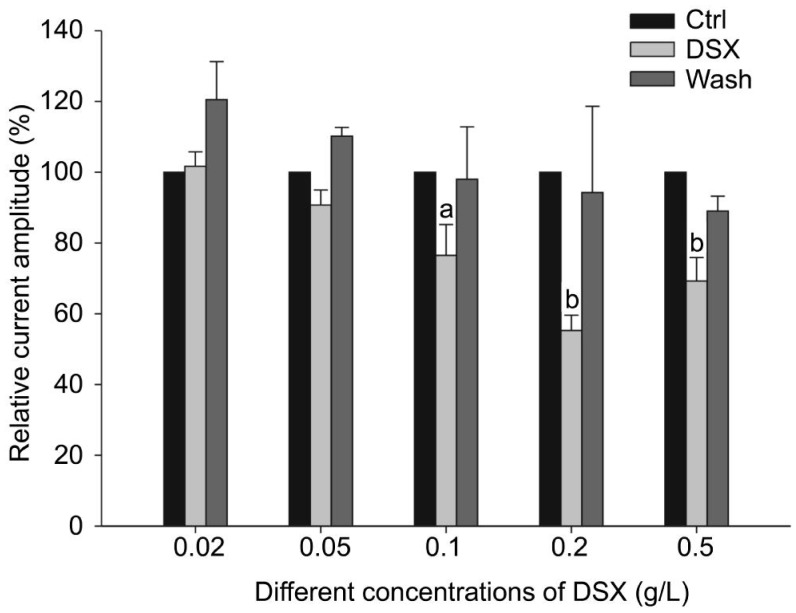
Finally, we examined whether the DSX effect on outward K+ currents in RGCs may be in a concentration-dependent manner. In these experiments, outward K+ currents were evoked by a single depolarizing voltage pulse from -70 mV to +20 mV. After stable control currents were obtained, DSX at different concentrations (0.02 g/L, 0.05 g/L, 0.1 g/L, 0.2 g/L and 0.5 g/L) was perfused to RGCs. As shown in Figure 3, the steady-state K+ current amplitudes were kept unchanged when DSX at concentrations of 0.02 g/L or 0.05 g/L was added, with the average amplitudes being 101.7%±4.1% of control (n=7, P>0.05) and 90.8%±4.2% of control (n=6, P>0.05) respectively. However, when the concentrations of DSX were increased to 0.1 g/L, 0.2 g/L and 0.5 g/L, a significant reduction of outward K+ current amplitudes was observed. The average steady-state current amplitudes at +20 mV were reduced to 76.8%±8.7% (n=5), 55.3%±4.3% (n=7) and 69.2%±6.7% of control (n=8), respectively (all P<0.05) (Figure 3). The effects of DSX were reversible since the current amplitudes recovered to control level (98.0%±14.8%, 94.3%±24.4% and 89.0%±4.2% of control, n=7, all P>0.05 vs control) following 5min washout (Figure 3). These results suggest that DSX dose-dependently suppresses outward K+ currents in rat RGCs.
Figure 3. DSX suppressed outward K+ currents of rat RGCs in a dose-dependent manner.
aP<0.05 and bP<0.01 vs control (Ctrl).
DISCUSSION
The present study demonstrates that DSX voltage-dependently suppresses outward K+ currents in rat RGCs. When the concentration of DSX increases to a higher level, the suppression extent does not further increase, suggesting that appropriate dose is important for clinical use of Erigeron breviscapus. Suppression of outward K+ channel currents in rat RGCs may be one of the possible mechanisms underlying Erigeron breviscapus prevents vision loss and RGC damage caused by glaucoma.
Our previous study has shown that outward K+ currents of rat RGCs may contain multiple current components, such as TEA-sensitive K+ current, 4-AP sensitive K+ currents and glybenclamide sensitive K+ current, etc[17]. In the present work, DSX suppressed both the peak and steady-state current components, suggesting that DSX may modulate a variety of K+ channels in the cells. Indeed, it was reported that the active ingredient of Erigeron breviscapus inhibited Ito (transient outward K+ currents), but had no effect on Ik1 (inward rectifier K+ current) in cardiac cells[24]. Therefore, we will determine which one(s) of outward K+ currents in rat RGCs may mediate the DSX effect in our further study.
In the current stage, it is still difficult for early diagnosis of glaucoma. Most of patients, who are clinically diagnosed as glaucoma, always have irreversible visual impairment. Progressive visual damage is still occurred in some patients although the elevated IOP has been effectively reduced to normal level. On the other hand, in some glaucoma patients (primary open angle glaucoma or normal tension glaucoma), optic nervous degeneration and visual loss are gradually appeared even though their IOPs may be in normal level. Therefore, it is commonly believed that it is important to protect the surviving RGCs from further damage, thus protecting optic nerve and vision. For this ultimate aim, numerous efforts have been made to explore effective approaches in the nervous protection and study the underlying mechanisms by using a variety of innovative biological researches[25]–[30]. In regard to the cellular mechanisms of RGC damage, disorder of retinal energy metabolism and production of oxygen free radicals caused by intracellular Ca2+ overload, neurotrophic factor deprivation, cell messenger molecule NO-mediated glutamate release, glial cell reactivation[16], and activation of many apoptosis related genes may contribute to RGC apoptosis.
Based on these studies, many new drugs have been developed for treatment of glaucoma. However, these drugs need to be tested experimentally and clinically. In the present study, we found that DSX reversibly suppresses outward K+ currents in RGCs. We speculate that DSX may bind directly to the extracellular sites and allosterically change the gating properties of the channels[30],[31]. In addition, DSX is a liposoluble substance. It may enter to cytoplasm and directly inhibit the K+ channels, or non-specifically modulate intracellular signaling pathways, thus inhibiting the K+ channels[30],[32]. Moreover, DSX may also stimulate the release of endogenous potassium channel blockers, in turn inhibiting the K+ channels[30]. Nevertheless, these issues will be address in our further experiments.
Currently, preparations of Erigeron breviscapus have been widely used clinically, such as injection solution, tablets, capsules and granules, etc. It has been demonstrated that Erigeron breviscapus is effective for treatment glaucoma, although it may induce some moderate side effect, such as congestion. Nevertheless, many Chinese herbs in the TCM have their distinct advantages in neuroprotection through modulating immune responses[33]–[35]. However, the complexity and diversity of active components in the Chinese herbs make their effects via multi-targets and multi-approaches. Therefore, there are many unknown things remained to be explored in the optic nerve protection, concerning the Chinese herbs.
Acknowledgments
Foundations: Supported by National Major Science-Technology Project of Science and Technology Ministry-Major New Medicine Innovation (No. 2009ZX09103-369); Key Project of Chinese Ministry of Education; 2014 Sichuan Province Academic and Technology Leaders Training Funds.
Conflicts of Interest: Yin S, None; Wang ZF, None; Duan JG, None; Ji L, None; Lu XJ, None.
REFERENCES
- 1.Ma L, Zhu BH, Zhou JG, et al. Effects of erigeron breviscapus on retinal blood flow changes in diabetic rats. Zhongshan Daxue Xuebao (medical sciences) 2004;25(6):554–556, 597. [Google Scholar]
- 2.Wu YB, Wu YH, Zhuang WD, et al. Influences of Erigeron Breviscapuse injection on vascular endothelial function in patients with acute cerebral infarction. Zhongguo Zhongxiyi Jiehe Jijiu Za zhi. 2006;13(1):6–8. [Google Scholar]
- 3.Xie DH. New progress of Erigeron Breviscapuse clinical application. Xin Yixue. 2007;38(6):408–410. [Google Scholar]
- 4.Guo WP. The clinical research and application of Erigeron breviscapus injection. Zhongxiyi Jiehe Xinnaoxueguanbing Zazhi. 2011;09(1):96–98. [Google Scholar]
- 5.Boland MV, Ervin AM, Friedman DS, Jampel HD, Hawkins BS, Vollenweider D, Chelladurai Y, Ward D, Suarez-Cuervo C, Robinson KA. Comparative effectiveness of treatments for open-angle glaucoma: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;158(4):271–279. doi: 10.7326/0003-4819-158-4-201302190-00008. [DOI] [PubMed] [Google Scholar]
- 6.Okisaka S, Murakami A, Mizukawa A, Ito J. Apoptosis in retinal ganglion cell decrease in human glaucomatous eyes. Jpn J Ophthalmol. 1997;41(2):84–88. doi: 10.1016/s0021-5155(97)00013-0. [DOI] [PubMed] [Google Scholar]
- 7.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Health Organ. 2004;82(11):887–888. [PMC free article] [PubMed] [Google Scholar]
- 9.Gong QH, Wu Q, Yang DL, Huang XN, Sun AS, Shi JS. Protective effects of Ginkgo biloba extract on aluminum-induced rat model of brain dysfunction. Sichuan Shengli Kexue Zazhi. 2004;26(4):176. [Google Scholar]
- 10.Wang H, Wang R, Thrimawithana T, Little PJ, Xu J, Feng ZP, Zheng W. The nerve growth factor signaling and its potential as therapeutic target for glaucoma. Biomed Res Int. 2014;2014:759473. doi: 10.1155/2014/759473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang ML, Fei ZG, Li H, He MN. Protective effect of Ginaton and fleabane on retinal ganglion cells. Guoji Yanke Zazhi( Int Eye Sci) 2010;10(5):850–852. [Google Scholar]
- 12.Wang NL, Sun XH, Li JZ, Wang JH, Chen XM, Lin D, Lü JH, Zhong YS, Zhang C, Guo WY. Neuroprotectiveeffects of erigeron breviscapus (vant) hand-mass on glaucoma-A multi-center clinical trial. Guoji Yanke Zazhi( Int Eye Sci) 2004;4(4):567–592. [Google Scholar]
- 13.Lu XJ, Zhang FW, Zhang Y, Duan JG, Liu AQ. Protective effect of total flavonoids of Erigeron breviscapus on optic nerve. Zhongyao Xinyao Yu Lingchuang Yaoli. 2011;22(5):510–514. [Google Scholar]
- 14.Lu XJ, Zhang FW, Cheng L, Liu AQ, Duan JG. Effect on multifocal electroretinogram in persistently elevated intraocular pressure by erigeron breviscapus extract. Int J Ophthalmol. 2011;4(4):349–352. doi: 10.3980/j.issn.2222-3959.2011.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao WJ, Zhang M, Miao Y, Yang XL, Wang Z. Melatonin potentiates glycine currents through a PLC/PKC signalling pathway in rat retinal ganglion cells. J Physiol(Lond) 2010;588(Pt 14):2605–2619. doi: 10.1113/jphysiol.2010.187641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji M, Miao Y, Dong LD, Chen J, Mo XF, Jiang SX, Sun XH, Yang XL, Wang Z. Group I mGluR-mediated inhibition of Kir channels contributes to retinal Muller cell gliosis in a rat chronic ocular hypertension model. J Neurosci. 2012;32(37):12744–12755. doi: 10.1523/JNEUROSCI.1291-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang CQ, Wu HJ, Wang SY, Yin S, Lu XJ, Miao Y, Wang XH, Yang XL, Wang Z. Suppression of outward K+ currents by WIN55212-2 in rat retinal ganglion cells is independent of CB1/CB2 receptors. Neuroscience. 2013;253:183–193. doi: 10.1016/j.neuroscience.2013.08.056. [DOI] [PubMed] [Google Scholar]
- 18.Yang XF, Miao Y, Ping Y, Wu HJ, Yang XL, Wang Z. Melatonin inhibits tetraethylammonium-sensitive potassium channels of rod ON type bipolar cells via MT2 receptors in rat retina. Neuroscience. 2011;173:19–29. doi: 10.1016/j.neuroscience.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 19.Lipton SA, Tauck DL. Voltage-dependent conductances of solitary ganglion cells dissociated from the rat retina. J Physiol(Lond) 1987;385:361–391. doi: 10.1113/jphysiol.1987.sp016497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ettaiche M, Heurteaux C, Blondeau N, Borsotto M, Tinel N, Lazdunski M. ATP-sensitive potassium channels (K(ATP)) in retina: a key role for delayed ischemic tolerance. Brain Res. 2001;890(1):118–129. doi: 10.1016/s0006-8993(00)03152-8. [DOI] [PubMed] [Google Scholar]
- 21.Clark BD, Kurth-Nelson ZL, Newman EA. Adenosine-evoked hyperpolarization of retinal ganglion cells is mediated by G-protein-coupled inwardly rectifying K+ and small conductance Ca2+-activated K+ channel activation. J Neurosci. 2009;29(36):11237–11245. doi: 10.1523/JNEUROSCI.2836-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fohlmeister JF, Cohen ED, Newman EA. Mechanisms and distribution of ion channels in retinal ganglion cells: using temperature as an independent variable. J Neurophysiol. 2010;103(3):1357–1374. doi: 10.1152/jn.00123.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koeberle PD, Wang Y, Schlichter LC. Kv1.1 and Kv1.3 channels contribute to the degeneration of retinal ganglion cells after optic nerve transection in vivo. Cell Death Differ. 2010;17(1):134–144. doi: 10.1038/cdd.2009.113. [DOI] [PubMed] [Google Scholar]
- 24.Deng CY, Tang CJ, Kuang SJ, et al. Effect of breviscapin on I_(to) and I_(K1) channel current in isolated Ventricular myocytes of rats. Chinese Journal of Pathophysiology. 2008;24(1):84–88. [Google Scholar]
- 25.Heiduschka P, Thanos S. Aurintricarboxylic acid promotes survival and regeneration of axotomised retinal ganglion cells in vivo. Neuropharmacology. 2000;39(5):889–902. doi: 10.1016/s0028-3908(99)00245-2. [DOI] [PubMed] [Google Scholar]
- 26.Wu MM, Zhu TT, Wang P, Kuang F, Hao DJ, You SW, Li YY. Dose-dependent protective effect of lithium chloride on retinal ganglion cells is interrelated with an upregulated intraretinal BDNF after optic nerve transection in adult rats. Int J Mol Sci. 2014;15(8):13550–13563. doi: 10.3390/ijms150813550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Wei A, Liu Y, He G, Zhou Z, Yu Z. IGF-1 protects retinal ganglion cells from hypoxia-induced apoptosis by activating the Erk-1/2 and Akt pathways. Mol Vis. 2013;19:1901–1912. [PMC free article] [PubMed] [Google Scholar]
- 28.Nakazawa T, Tamai M, Mori N. Brain-derived neurotrophic factor prevents axotomized retinal ganglion cell death through MAPK and PI3K signaling pathways. Invest Ophthalmol Vis Sci. 2002;43(10):3319–3326. [PubMed] [Google Scholar]
- 29.Shi J, Jiag Y, Liu X. Neuroprotective effect of erigeron breviscapus (vant) hand-mazz on NMDA-induced retinal neuron injury in the rats. Yan Ke Xue Bao. 2004;20(2):113–117. [PubMed] [Google Scholar]
- 30.Poling JS, Rogawski MA, Salem N, Vicini S. Anandamide, an endogenous cannabinoid, inhibits Shaker-related voltage-gated K+ channels. Neuropharmacology. 1996;35(7):983–991. doi: 10.1016/0028-3908(96)00130-x. [DOI] [PubMed] [Google Scholar]
- 31.Van den Bossche I, Vanheel B. Influence of cannabinoids on the delayed rectifier in freshly dissociated smooth muscle cells of the rat aorta. Br J Pharmacol. 2000;131(1):85–93. doi: 10.1038/sj.bjp.0703521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W, Cao X, Liu C, Liu L. Cannabinoid WIN 55,212-2 inhibits TRPV1 in trigeminal ganglion neurons via PKA and PKC pathways. Neurol Sci. 2012;33(1):79–85. doi: 10.1007/s10072-011-0620-6. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Wei YZ, Fan GZ. Research Status of herbal neuroprotective mechanism. Zhongguo Linchuang Kangfu. 2005;9(41):108–110. [Google Scholar]
- 34.Ren Q, Wang YM, Luo GA. Research rogress on Erigeron breviscapus. Jiangxi Zhongyi Xueyuan Xuebao. 2012;24(4):97–100. [Google Scholar]
- 35.Wang Y, Sun XH. Experimental study of neuroprotection in glaucoma. Yanke Xin Jinzhan. 2002;22(3):212–215. [Google Scholar]