Abstract
AIM
To evaluate the relationship between intravitreal bevacizumab (IVB) treatment and the levels of vitreous vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and vitreous-retina surface fibrosis in patients with proliferative diabetic retinopathy (PDR).
METHODS
This study was a prospective, open-label, controlled, randomized clinical trial. Sixty-eight eyes of PDR patients (n=53) and macular hole patients (n=15) were enrolled in this study. Thirty-four eyes of the PDR patients received IVB before vitrectomy. Twenty-three of the 34 PDR patients received IVB treatment 5d before vitrectomy (subgroup a), and 11 of the 34 PDR patients received IVB treatment greater than 2wk prior to vitrectomy (subgroup b). Nineteen of the PDR patients did not receive IVB treatment at any time prior to vitrectomy. The levels of bFGF and VEGF in vitreous samples were measured using enzyme-linked immunosorbent assay (ELISA) and the degree of vitreoretinal fibrosis was characterized using clinical data and data obtained intra-operatively.
RESULTS
In PDR patients, VEGF and bFGF levels were significantly increased compared to non-PDR (control) subject's eyes (P<0.01). In PDR patients, vitreous VEGF levels were significantly decreased following IVB treatment compared to PDR patients that did not receive IVB treatment (P<0.01). The degree of vitreoretinal fibrosis was significantly increased in subgroup b compared to subgroup a(P<0.05) and to patients that did not receive IVB (P<0.05). Vitreous bFGF levels were significantly greater in subgroup b than subgroup a (P<0.01) or in patients who did not receive IVB treatment (P<0.05). A Spearman's rank correlation test indicated that higher levels of vitreous bFGF, but not VEGF, correlated with the degree of vitreoretinal fibrosis.
CONCLUSION
We found that bFGF levels increase in PDR patient's vitreous after IVB treatment longer than two weeks prior to vitrectomy and correlated with the degree of fibrosis after IVB treatment. These findings suggest vitreous fibrosis is increased in PDR patients after IVB treatment may be due to increased levels of bFGF.
Keywords: proliferative diabetic retinopathy, vascular endothelial growth factor, basic fibroblast growth factor, fibrosis, bevacizumab
INTRODUCTION
Diabetic retinopathy (DR) is the most common complication of diabetes and is one of the leading global causes of preventable blindness in adults[1]. The advanced stage of diabetic retinopathy known as proliferative diabetic retinopathy (PDR) is characterized by retinal ischemia and subsequent vitreoretinal fibrosis[2].
PDR itself is similar to a wound healing response in which fibrovascular membranes (FVMs) form on the surface of the retina. However, this can cause intravitreal hemorrhages and tractional retinal detachment (TRD) that may result in blindness[3].
The development of PDR is a complex process and involves several growth factors, including basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), hepatocyte growth factor (HGF), monocyte chemoattractant protein-1 (MCP-1), interleukin-8 (IL-8), and platelet-derived growth factor (PDGF)[4]–[6]. Wirostko et al[7] established VEGF as the primary mediator of retinal angiogenesis. bFGF is a 146-amino-acid protein that is widely expressed in tissues and stimulates cell proliferation, cell growth, tissue repair, fibroblasts and angiogenesis[8]. bFGF is also involved in epiretinal membrane formation in proliferative vitreoretinal disorders[9].
Bevacizumab (Avastin Genentech, San Francisco, California, USA) is a human-derived full-length monoclonal antibody with binding affinity to all isoforms of VEGF. Vitrectomy with preoperative intravitreal injection of bevacizumab (IVB) has been reported to decrease intraoperative hemorrhages, surgical time, and reduce postoperative vitreous hemorrhage (VH) rates[10].
However, some adverse consequences following IVB treatment have been reported. The progression or development of TRD has been observed following IVB treatment[11],[12]. Additionally, a profibrotic switch comprised of a significant reduction in the neovascular component and marked increase in the contractile elements of the proliferative membranes over time has been observed in diabetic fibrovascular proliferative membranes after IVB treatment[13].
Previous research has demonstrated that bFGF induces VEGF-A expression through ERK1/2 MPAK pathway, which is a central player in mediating both VEGF- and bFGF-induced angiogenesis[14]. A sudden decrease in VEGF due to treatment with bevacizumab may result in compensatory increases in bFGF[15].
However, it is unknown whether or not bFGF levels change in vitreous after IVB treatment or if changes in bFGF levels may be related to progression of TRD after IVB treatment. Therefore, we investigated vitreous VEGF and bFGF levels and the correlation between VEGF and bFGF levels with degree of fibrosis in a sample of PDR patients that had either undergone IVB treatment or were not treated with IVB prior to vitrectomy.
SUBJECTS AND METHODS
We investigated 68 vitreous samples from patients with PDR (n=53) and macular hole (n=15) that underwent 23G pars plana vitrectomy in Sir Run Run Shaw Hospital between June 2012 and April 2013. Thirty-four patients with PDR had received a single intravitreal injection of 1.25 mg/0.05 mL bevacizumab before vitrectomy (bevacizumab group). Twenty-three of the 34 patients were treated with bevacizumab and vitrectomies were performed 5d later (subgroup a). Eleven of them were treated with bevacizumab and were operated at least 14d later (subgroup b). The median time interval between bevacizumab injection and vitrectomy was 25d (range 14-44d). There were 19 patients with PDR that had not been treated with bevacizumab or any other anti-VEGF treatment (PDR group). Fifteen non-diabetic patients with macular hole were operated by 23G pars plana vitrectomy (control group). The control group was free of vitreous hemorrhage, proliferative viteroretinopathy, and retinal detachment.
Standardized forms were used to grade fibrosis from the pre-IVB and pre-operative ophthalmic examinations. Fibrosis was graded as 0 when there was no fibrosis, as limited when there was macular pucker, as 1 when there were a few pre-retinal membranes, as 2 when white preretinal fibrotic membranes with limited extension into the vitreous were present, and as 3 when abundant white membranes reaching into the vitreous body were observed[16].
Exclusion criteria included previous ocular surgery, a history of ocular inflammation, photocoagulation in the preceding 3mo, renal hematological diseases, uremia, prior chemotherapy, and chronic pathologies other than diabetes.
Informed consent was obtained from all patients, and the study was performed in accordance with the Helsinki Declaration with the approval of the Sir Run Run Shaw Hospital Ethics Committee.
Blood glucose of all patients was controlled prior to surgery. Target fasting blood glucose levels were <7.8 mmol/L, random blood glucose <10.0 mmol/L, and glycosylated hemoglobin <6.2%. At the time of the initial vitrectomy, samples of undiluted vitreous fluid (0.3-0.5 mL) were obtained from the mid vitreous transferred to sterile Eppendorf tubes and immediately frozen in liquid nitrogen. The samples were kept at -80°C until assayed. After thawing, vitreous samples were centrifuged at 20 000 g for 15min at 4°C, and supernatant was collected. Concentrations of VEGF and bFGF were determined by the Quantikine ELISA assay according to the manufacturer's protocol (R&D Systems, Minneapolis, Minnesota, USA). The ELISA plates were analyzed by measuring absorbance at 450 nm (reference at 570 nm) using a plate reader (Bio-Tek Instruments, Winooski, Vermont, USA).
Statistical Analysis
Statistical analysis was carried out using Predictive Analytics Software Statistics 19 software (SPSS 19.0, IBM Corporation, Armonk, NY, USA). The Pearson χ2 and Wilcoxon test were used to analyze categorical variables. For numerical variables in parametric distribution a one-way ANOVA and Student's t test was performed. The Spearman rank correlation test was used to test the correlation between treatment groups. Two-tailed P value less than 0.05 indicated statistical significance.
RESULTS
We evaluated the patient population using statistical methods and found that there was a significant difference in age between the patients in the control group compared to patients in the IVB treatment group (t=2.573, P<0.05). There was no significant difference in age between PDR patients that received IVB treatment (t=1.741, P=0.08) and PDR patients that did not receive treatment (t=1.447, P=0.17; Table 1). Statistical analysis demonstrated that there was no significant difference in the type of diabetes between PDR patients treated with IVB and the PDR group that was not treated with IVB (χ2=0.011, P=0.91; Table 1). There was also no significant difference in the history of diabetes between IVB group and PDR group (t=1.36, P=0.18; Table 1).
Table 1. Patient characteristics.
| Patient groups | Age (a) | Sex (n, M/F) | Diabetes type (n, I/II) | Diabetes history (a) |
| IVB group | 48.9±11.2 | 19/15 | 5/29 | 7.24±3.51 |
| PDR group | 53.9±8.5 | 10/9 | 3/16 | 7.40±4.95 |
| Control group | 58.7±6.6 | 6/9 | - | - |
IVB: Intravitreal bevacizumab; PDR: Proliferative diabetic retinopathy; I: Type 1 diabetes; II: Type 2 diabetes.
x±s
We next examined the biological variations between the different groups and discovered that there was a statistically significant difference in intravitreal concentrations of VEGF between the IVB group compared to PDR group (t=14.145, P<0.01) and the control group compared to PDR group (t=8.518, P<0.01). There was no significant difference in intravitreal concentration of VEGF between the IVB treatment group and the control group (t=0.243, P=0.86). There was no significant difference in intravitreal concentrations of VEGF between subgroup a and subgroup b (t=1.346, P= 0.19) (Table 2).
Table 2. Comparisons of VEGF, bFGF levels in the vitreous and fibrosis in different group.
| Groups | VEGF (pg/mL) | bFGF (pg/mL) | Degree of fibrosis (n) |
|||
| 0 | 1 | 2 | 3 | |||
| IVB group (n=34) | 164.52±64.58 | 42.58±28.90 | 0 | 5 | 17 | 12 |
| Subgroup a (n=23) | 173.87±71.90 | 33.44±10.67 | 0 | 4 | 14 | 5 |
| Subgroup b (n=11) | 140.18±59.51 | 58.84±45.04 | 0 | 1 | 3 | 7 |
| PDR group (n=19) | 806.90±246.54 | 32.33±14.88 | 0 | 5 | 10 | 4 |
| Control (n=15) | 170.83±95.18 | 7.77±5.14 | - | |||
IVB: Intravitreal bevacizumab; PDR: Proliferative diabetic retinopathy.
x±s
Our analysis revealed a significant difference in intravitreal concentrations of bFGF between patients treated with IVB and PDR patients that received no treatment compared to the control group (t=4.12, P<0.01; t=5.49, P<0.01). There was no significant difference in intravitreal concentrations of bFGF between PDR patients treated with IVB with PDR patients that received no treatment (t=1.470, P=0.15). There was a significant difference in intravitreal concentrations of VEGF between subgroup b with subgroup a and with PDR patients that received no treatment (t=2.606, P<0.01; t=2.301, P<0.05). There was no significant difference in intravitreal concentrations of bFGF between subgroup a with PDR group (t=0.019, P=0.97). There was no significant difference between subgroup b with subgroup a in the diabetic time period (8.18±3.49y vs 6.74±3.43y, t=1.142, P=0.69), blood glucose control (6.19±0.54 mmol/L vs 6.04±0.74 mmol/L, t=0.586, P=0.21), HbA1c levels (5.15%±0.74% vs 5.20%±0.70%, t=0.157, P=0.66). There was no significant difference between subgroup b and the PDR control group in the diabetic time period (8.18±3.49y vs 7.47±5.07y, t=0.409, P=0.22), blood glucose control (6.19±0.54 mmol/L vs 5.83±0.88 mmol/L, t=1.224, P=0.21), and HbA1c levels (5.15%±0.74% vs 5.00%±0.77%, t=0.538, P=0.98).
There was no difference in degree of fibrosis between PDR patients treated with IVB or PDR patients that received no treatment (z=1.574, P=0.20). However, there was a significant difference in the degree of fibrosis between subgroup b and subgroup a (z=2.103, P< 0.05). There was also a significant difference in the degree of fibrosis between subgroup b and PDR patients that did not receive IVB treatment group (z=2.103, P< 0.05). There was no difference in degree of fibrosis between subgroup a with PDR group (z=1.13, P=0.44) (Table 2).
No patient in subgroup a exhibited fibrosis after IVB treatment. The degree of fibrosis increased in six patients in subgroup b (54.5%). Overall, the degree of fibrosis increased significantly after IVB in subgroup b (z=-2.449, P<0.05).
We used a Spearman rank correlation test to determine whether or not levels of bFGF or VEGF correlated with the degree of fibrosis. In all PDR patients, the levels of bFGF correlated with the degree of fibrosis (r=0.529, P<0.001; Figure 1). However, VEGF levels did not correlate with fibrosis (r=0.213, P=0.19) and the levels of VEGF and bFGF did not correlate across any group (r=0.131, P=0.30).
Figure 1. Boxplots showing the intravitreal concentrations of bFGF degree of fibrosis correlated significantly.
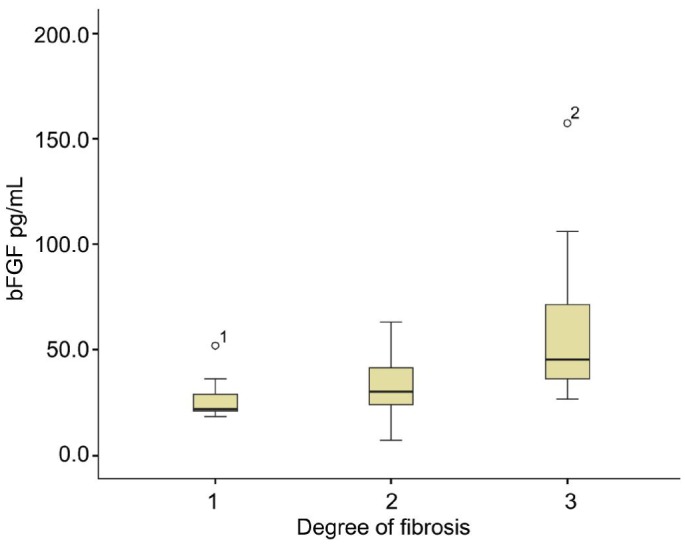
(r=0.529, P<0.001).
A separate analysis was performed to determine if there was any correlation between the levels of bFGF or VEGF and the degree of fibrosis in PDR patients in subgroup a or subgroup b. Our analysis revealed that there was no correlation between the degree of fibrosis and VEGF levels in PDR patients that received IVB treatment and the PDR patients that were not treated with IVB (r=0.221, P>0.05). However, the bFGF levels correlated with the degree of fibrosis in subgroup b (r=0.810, P<0.05). There was no correlation between bFGF levels and the degree of fibrosis in subgroup a or PDR group (r=0.368, P=0.08; r=0.339, P=0.16).
DISCUSSION
In our study, we found that the levels of two cytokines (bFGF and VEGF) were significantly raised in vitreous samples from eyes with PDR compared to eyes with macular hole. In agreement with previous studies we have shown that anti-VEGF treatment caused vitreous concentrations of VEGF to decrease significantly even 25d following treatment. We also found bFGF levels were increased in vitreous samples following IVB treatment longer than 14d prior to vitrectomy. However, bFGF levels were not increased when IVB treatment was done only 5d prior to vitrectomy. From these observations we postulate that IVB treatment longer than 2wk before vitrectomy may cause vitreous bFGF levels increase in PDR patients. Previous research by other laboratories have found that continued anti-angiogenic therapy targeting only the VEGF-VEGFR system might affect pro-angiogenic factors other than VEGF, such as bFGF[17]. Blockade of bFGF by anti-FGF antibodies may provide a greater benefit regarding antiangiogenesis when combined treatment with anti-VEGF[18].
Six of 11 patients who received IVB treatment and were operated at least 14d later (subgroup b) showed an increased degree of fibrosis. In those patients that received IVB treatment only 5d prior to surgery (subgroup a), no progression of fibrosis was observed. We believe that this may be due to the short time interval between bevacizumab injection and vitrectomy. This suggests that the chance that fibrosis develops or progresses increases with time and is may be related to increases in bFGF levels. bFGF has been shown to enhance the proliferation of Muller cells, retinal astrocytes, and retinal pigment epithelial cells in vivo[19]. bFGF is produced and stored in epiretinal membranes and bFGF may play a role in the auto- and paracrine control of the proliferative at the vitreoretinal interface[9]. This evidence and our observation that increased levels of bFGF, but not VEGF, correlated with the degree of fibrosis suggest that bFGF regulates the development of fibrosis in the eye after IVB treatment at least 14d before surgery.
A significant advance in the management of PDR was the introduction of anti-VEGF therapy. However, vitreoretinal fibrosis can develop as a consequence of anti-VEGF treatment[20]. The fibrotic phenomenon has been observed previously in age-related macular degeneration after IVB[21]. In a prospective study, eleven of 213 PDR patients displayed progression of TRD after IVB[12], TRD was observed after a mean of 13d after bevacizumab when researchers found collagen and smooth muscle actin significantly and progressively more expressed in preretinal fibrovascular proliferative membranes[13]. These observations could explain why the degree of fibrosis increased in subgroup b in the current study. Based on our present findings, we argue that decreased VEGF levels combined with the elevated bFGF levels that we observed in subgroup b may have accelerated the fibrotic response in these patients.
Evidence to support our argument that decreased VEGF levels increase bFGF and fibrosis comes from our observations that bFGF levels are strongly correlated with the degree of vitreoretinal fibrosis in PDR patients. However, our statistical analysis showed that only subgroup b bFGF levels are strongly correlated with the degree of vitreoretinal fibrosis. In subgroup a or PDR group, there were no statistically significant correlations with IVB treatment and fibrosis. We speculate that when bFGF levels increase after IVB treatment more than 14d prior to surgery, preretinal fibrovascular proliferative membranes was active too and caused increased fibrosis in a short time.
In conclusion, the results of our study suggest the time of anti-VEGF therapy is an essential factor in the development of fibrosis. Longer periods following IVB treatment cause bFGF levels to increase that may have correlated to harmful acceleration of fibrosis in PDR.
Although, anti-VEGF therapy has made a significant advance in the management of PDR, vitreoretinal fibrosis can increase or progress when IVB is used as an adjuvant to vitrectomy. This may be associated with poorly controlled diabetes mellitus associated with elevated HbA1c, insulin administration, and a longer time interval between intravitreal bevacizumab and vitrectomy[12]. Previous research has found that the vitreous levels of bFGF were significantly greater in not receiving insulin treatment patients[22]. Our results suggest that a longer time interval between IVB treatment and vitrectomy increases bFGF levels in vitreous and is correlated with the degree of fibrosis. This could explain the number of patients reported by Arevalo et al[12] that developed or had progression of TRD. Therefore, we advocate that adjuvant IVB may have more beneficial effects if it is done within 5d before surgery.
Acknowledgments
Foundation: Supported by Public Interest Research on Social Development Projects in Zhejiang Province (No. 2011c23019).
Conflicts of Interest: Li JK, None; Wei F, None; Jin XH, None; Dai YM, None; Cui HS, None; Li YM, None.
REFERENCES
- 1.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 2.Fong DS, Aiello LP, Ferris FL, 3rd, Klein R. Diabetic retinopathy. Diabetes Care. 2004;27(10):2540–2553. doi: 10.2337/diacare.27.10.2540. [DOI] [PubMed] [Google Scholar]
- 3.Hiscott P, Wong D, Grierson I. Challenges in ophthalmic pathology: the vitreoretinal membrane biopsy. Eye(Lond) 2000;14(4):549–559. doi: 10.1038/eye.2000.142. [DOI] [PubMed] [Google Scholar]
- 4.Mohan N, Monickaraj F, Balasubramanyam M, Rema M, Mohan V. Imbalanced levels of angiogenic and angiostatic factors in vitreous, plasma and postmortem retinal tissue of patients with proliferative diabetic retinopathy. J Diabetes Complications. 2012;26(5):435–441. doi: 10.1016/j.jdiacomp.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J, Wang S, Xia X. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr Eye Res. 2012;37(5):416–420. doi: 10.3109/02713683.2012.661114. [DOI] [PubMed] [Google Scholar]
- 6.Cui JZ, Chiu A, Maberley D, Ma P, Samad A, Matsubara JA. Stage specificity of novel growth factor expression during development of proliferative vitreoretinopathy. Eye(Lond) 2007;21(2):200–208. doi: 10.1038/sj.eye.6702169. [DOI] [PubMed] [Google Scholar]
- 7.Wirostko B, Wong TY, Simó R. Vascular endothelial growth factor and diabetic complications. Prog Retin Eye Res. 2008;27(6):608–621. doi: 10.1016/j.preteyeres.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Beranek M, Kolar P, Tschoplova S, Kankova K, Vasku A. Genetic variation and plasma level of the basic fibroblast growth factor in proliferative diabetic retinopathy. Diabetes Res Clin Pract. 2008;79(2):362–367. doi: 10.1016/j.diabres.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Hueber A, Wiedemann P, Esser P, Heimann K. Basic fibroblast growth factor mRNA, bFGF peptide and FGF receptor in epiretinal membranes of intraocular proliferative disorders (PVR and PDR) Int Ophthalmol 1996- 1997;20(6):345–350. doi: 10.1007/BF00176889. [DOI] [PubMed] [Google Scholar]
- 10.Zhao LQ, Zhu H, Zhao PQ, Hu YQ. A systematic review and meta-analysis of clinical outcomes of vitrectomy with or without intravitreal bevacizumab pretreatment for severe diabetic retinopathy. Br J Ophthalmol. 2011;95(9):1216–1222. doi: 10.1136/bjo.2010.189514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.di Lauro R, De Ruggiero P, di Lauro R, di Lauro MT, Romano MR. Intravitreal bevacizumab for surgical treatment of severe proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248(6):785–791. doi: 10.1007/s00417-010-1303-3. [DOI] [PubMed] [Google Scholar]
- 12.Arevalo JF, Maia M, Flynn HW, Jr, Saravia M, Avery RL, Wu L, Eid Farah M, Pieramici DJ, Berrocal MH, Sanchez JG. Tractional retinal detachment followingintravitreal bevacizumab (Avastin) in patients with severe proliferative diabeticretinopathy. Br J Ophthalmol. 2008;92(2):213–216. doi: 10.1136/bjo.2007.127142. [DOI] [PubMed] [Google Scholar]
- 13.El-Sabagh HA, Abdelghaffar W, Labib AM, Mateo C, Hashem TM, Al-Tamimi DM, Selim AA. Preoperative intravitreal bevacizumab use as an adjuvant to diabetic vitrectomy: histopathologic findings and clinical implications. Ophthalmology. 2011;118(4):636–641. doi: 10.1016/j.ophtha.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 14.Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22(4):201–207. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- 15.Dowlati A, Gray R, Sandler AB, Schiller JH, Johnson DH. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab--an Eastern Cooperative Oncology Group Study. Clin Cancer Res. 2008;14(5):1407–1412. doi: 10.1158/1078-0432.CCR-07-1154. [DOI] [PubMed] [Google Scholar]
- 16.Kuiper EJ, Van Nieuwenhoven FA, de Smet MD, van Meurs JC, Tanck MW, Oliver N, Klaassen I, Van Noorden CJ, Goldschmeding R, Schlingemann RO. The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS One. 2008;3(7):e2675. doi: 10.1371/journal.pone.0002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arbab AS. Activation of alternative pathways of angiogenesis and involvement of stem cells following anti-angiogenesis treatment in glioma. Histol Histopathol. 2012;27(5):549–557. doi: 10.14670/hh-27.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Oliveira Dias JR, Rodrigues EB, Maia M, Magalhães O, Jr, Penha FM, Farah ME. Cytokines in neovascular age-related macular degeneration: fundamentals of targeted combination therapy. Br J Ophthalmol. 2011;95(12):1631–1637. doi: 10.1136/bjo.2010.186361. [DOI] [PubMed] [Google Scholar]
- 19.Okada-Ban M, Thiery JP, Jouanneau J. Fibroblast growth factor-2. Int J Biochem Cell Biol. 2000;32(3):263–267. doi: 10.1016/s1357-2725(99)00133-8. [DOI] [PubMed] [Google Scholar]
- 20.Sohn EH, He S, Kim LA, Salehi-Had H, Javaheri M, Spee C, Dustin L, Hinton DR, Eliott D. Angiofibrotic response to vascular endothelial growth factor inhibition in diabetic retinal detachment: report no.1. Arch Ophthalmol. 2012;130(9):1127–1134. doi: 10.1001/archophthalmol.2012.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang JC, Del Priore LV, Freund KB, Chang S, Iranmanesh R. Development of subretinal fibrosis after anti-VEGF treatment in neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2011;42(1):6–11. doi: 10.3928/15428877-20100924-01. [DOI] [PubMed] [Google Scholar]
- 22.Boulton M, Gregor Z, McLeod D, Charteris D, Jarvis-Evans J, Moriarty P, Khaliq A, Foreman D, Allamby D, Bardsley B. Intravitreal growth factors in proliferative diabetic retinopathy: correlation with neovascular activity and glycaemic management. Br J Ophthalmol. 1997;81(3):228–233. doi: 10.1136/bjo.81.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]


