Abstract
AIM
To describe the clinical features, systemic associations, treatment and visual outcomes in Saudi patients with scleritis.
METHODS
A retrospective chart review was performed for patients with scleritis presenting to two tertiary care eye hospitals in Riyadh, Saudi Arabia, from 2001 to 2011. Data were collected on the clinical features of scleritis, subtypes of scleritis, associated systemic disease, history of previous ocular surgery and medical therapy, including the use of immunosuppressants. Treatment outcomes were evaluated based on best-corrected visual acuity (BCVA) and response to treatment.
RESULTS
Of the 52 patients included in the study, non-necrotizing anterior scleritis was the most common type of scleritis in 22 patients (42.3%), followed by posterior scleritis in 14 patients (26.9%). The majority of cases, 31 patients (59.6%), were idiopathic in nature. Systemic associations were present in 12 patients (23.1%). Infectious scleritis was confirmed in 6 patients (11.5%): 3 with bacterial scleritis after pterygium excision, 2 patients with scleritis related to tuberculosis and 1 patient with scleritis resulting from herpes simplex infection. For the various subtypes of scleritis, BCVA values after treatment and time to remission significantly differed (P<0.05, all cases). Systemic immunosuppressive therapies in addition to steroids were administered to 46.2% of all patients. The T-sign was present on B-scan ultrasonography in 9 (64.3%) of the 14 posterior scleritis patients.
CONCLUSION
Non-necrotizing anterior scleritis was the most common subtype of scleritis. Final visual outcome and time to remission differed among the various scleritis subtypes.
Keywords: autoimmune, scleritis, scleromalacia, episcleritis, necrotizing, immunosuppressive therapy
INTRODUCTION
Scleritis is a rare disease that is defined as inflammation of the white part of the eye (sclera), and it has a characteristic clinical picture. Based on the classification system developed by Watson and Hayreh[1] in the 1960s (and still in use today), scleritis can be classified as either anterior or posterior scleritis, depending on the anatomic site of the disease. Anterior scleritis can be divided into four subtypes: diffuse, nodular, necrotizing with inflammation and necrotizing without inflammation (scleromalacia perforans). Patients presenting with anterior scleritis typically complain of redness and globe tenderness. Patients with posterior scleritis may present with reduced vision, with or without pain. Patients older than 50y with posterior scleritis have an increased risk of associated systemic disease and associated visual loss; hence, these patients are more likely to require systemic immunosuppressive therapy to manage the disease[1]. The incidence of systemic disease is approximately 39% to 50% for patients with scleritis[2]–[4]. The prevalence of scleritis in the general population is about 6 cases per 100 000 people but increases for patients with rheumatoid arthritis (RA) (0.2%-6.3%) and for those with Wegener's granulomatosis (up to 7%) with no racial or geographic predilection[2],[3],[5]–[7]. The most common diseases associated with scleritis are RA, Wegener's disease, relapsing polychondritis, systemic lupus erythematosus, inflammatory bowel disease and polyarteritis nodosa[7],[8]. Trauma owing to surgery or other causes[9],[10] can lead to scleritis. Patients who have undergone pterygium surgery with adjunctive mitomycin C or beta irradiation have an increased risk for infectious scleritis[11]–[13]. Infections can cause scleritis in 4% to 18% of patients, with the most common causes being herpes zoster, tuberculosis, syphilis, leprosy and lyme borreliosis[7],[8],[14],[15].
Despite well-established treatments for scleritis, there are no published peer-reviewed studies on the types of scleritis and the response to treatment in the Saudi population. In this study, we examine the demographics, most common subtypes, clinical features, and response to treatment of patients with scleritis who presented to two major tertiary eye care hospitals in the Kingdom of Saudi Arabia from 2001 to 2011.
SUBJECTS AND METHODS
Patients, Inclusion and Exclusion Criteria, Data Collection
After obtaining approval from the Institutional Review Boards at King Khaled Eye Specialist Hospital (KKESH) and King Abdulaziz University Hospital (KAUH), we performed a retrospective chart review for patients who presented to KKESH and KAUH and were diagnosed with scleritis from January 2001 to January 2011. Inclusion criteria were patients aged 12y or older, a confirmed diagnosis of scleritis and a documented follow-up of 3mo or longer. Exclusion criteria were an unconfirmed diagnosis of scleritis (e.g. episcleritis, conjunctivitis, and uveitis), a follow-up of less than 3mo and patients with secondary scleritis (e.g. myositis, idiopathic orbital inflammatory disease). Cases with the diagnostic code for scleritis were selected from a preliminary chart review of patients who presented to KKESH within the selected time frame. A total of 250 patients were originally selected, of which 52 patients (65 eyes) met the criteria for inclusion.
We created a data collection form for the present study. Data were collected on patient demographics (e.g. age, gender, date of birth, and nationality), clinical features [visual acuity (VA) at presentation, symptoms at presentation, unilateral or bilateral disease, ultrasound features, previous ocular surgery, anatomic location of inflammation, ocular complications, and associated systemic disease], management (use of topical and/or systemic treatments and surgical interventions), and outcomes (VA at most recent visit and/or causes of poor outcome). We also retrospectively analyzed all the ocular findings and visual outcomes of patients with scleritis who presented to KKESH and KAUH over the past 10y.
Statistical Analysis
Data were entered into an Excel spreadsheet (Excel 2007®; Microsoft Corp., Redmond, WA, USA). Statistical analysis was performed with Statistical Product and Service Solutions (SPSS) version 19.0 (IBM Corp., Armonk, NY, USA) and MedCalc version 11.6 (MedCalc Software, Mariakerke, Belgium). Descriptive statistics, including mean, SD, and range values, were calculated to describe continuous variables, and categorical variables were expressed as frequencies [number (%)]. LogMAR visual acuity values at presentation and at most recent visit were compared using the Wilcoxon signed rank test. A P value less than 0.05 was considered statistically significant.
RESULTS
Patient demographics and other characteristics are summarized in Table 1. The mean age at onset of the disease was 50.8y (SD, 19.3y; range, 12-90y). There were 34 female patients (65.4%) and 18 male patients (34.6%) diagnosed with scleritis. Bilateral scleritis occurred in 15 patients (28.8%). There were 36 patients (69.2%) with anterior scleritis, 14 patients (26.9%) with posterior scleritis, and 2 patients (3.8%) with panscleritis (anterior and posterior scleritis).
Table 1. Patient characteristics according to scleritis subtype.
| Diagnosis/subtype | No. of patients | Mean age (a) | Duration of diagnosis (a) | Male patients n (%) | Female patients n (%) | Bilateral n (%) |
| Anterior scleritis | 36 | 55.3±18.1 (18-90) | 2.9±3.2 (1-14) | 15 (41.7) | 21 (58.3) | 7 (13.4) |
| Non-necrotizing scleritis | 22 | 53.0±17.7 (20-90) | 3.0±3.6 (1-14) | 10 (45.5) | 12 (54.5) | 5 (22.7) |
| Necrotizing scleritis | 12 | 59.3±18.6 (18-82) | 3.0±2.6 (1-9) | 4 (33.3) | 8 (66.7) | 0 (0.0) |
| Necrotizing without inflammation (scleromalacia perforans) | 2 | 60.6±13.7 (38-82) | 3.3±3.2 (1-9) | 1 (50.0) | 1 (50.0) | 2 (100.0) |
| Posterior scleritis | 14 | 40.6±18.4 (12-82) | 2.3±1.9 (1-7) | 2 (14.2) | 12 (85.7) | 7 (50.0) |
| Panscleritis (anterior+posterior scleritis) | 2 | 73.0±8.5 (18-90) | 5.0±4.2 (2-8) | 1 (50.0) | 1 (50.0) | 1 (50.0) |
| Total | 52 | 50.8±19.3 (12-90) | 2.6±2.8 (0-14) | 18 (34.6) | 34 (65.4) | 15 (28.8) |
x±s (range)
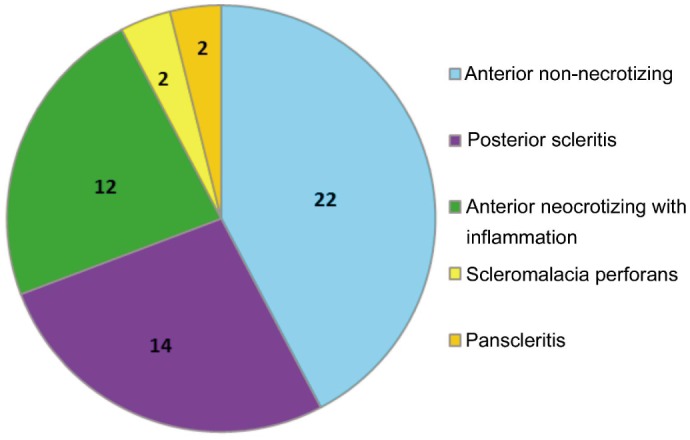
The most common subtype of scleritis was non-necrotizing anterior scleritis in 22 patients (42.3%) (18 patients with diffuse scleritis and 4 patients with nodular scleritis), followed by posterior scleritis in 14 patients (26.9%), necrotizing scleritis in 12 patients (23.1%), scleromalacia perforans in 2 patients (3.8%), and 2 patients had panscleritis (3.8%) (Figure 1).
Figure 1. Distribution of various scleritis subtypes.
The majority of cases, 31 patients (59.6%), were idiopathic in nature. Systemic associations were present in 12 patients (23.1%); of these 12 patients, 6 patients had RA, 4 patients had Wegener's granulomatosis, and 2 patients had Vogt-Koyanagi-Harada (VKH) disease. Notably, 4 patients (7.6%) had a history of ocular surgery before scleritis diagnosis. Of these 4 patients, 3 patients had previously undergone pterygium excision, with all 3 of these patients being diagnosed with necrotizing scleritis caused by a bacterial infection. Infectious scleritis was documented in 6 patients (11.5%): 3 with bacterial scleritis, 2 patients with tuberculosis-related scleritis, and 1 patient with scleritis resulting from herpes simplex infection.
Ocular complications were noted during the follow-up period in 38 patients (73.1%), with some patients having more than one complication. According to the Standardization of Uveitis Nomenclature (SUN) grading scheme for cells[16], 21 patients (40.4%) had anterior chamber activity ranging between 1+ and 3+ cells. Cataract was the second most common complication, with 17 patients (32.7%) being diagnosed with it. Intraocular pressure (IOP) was elevated in 15 patients (28.8%). All these patients were managed successfully with topical antiglaucoma medications, and none required glaucoma surgery. Scleral thinning was noted in 10 patients. Cystoid macular edema was detected in 3 patients, with all 3 of these patients being diagnosed with posterior scleritis. The T-sign was present on B-scan ultrasonography in 9 (64.3%) of the 14 patients with posterior scleritis.
The initial VA and visual outcome for each eye with various subtypes of scleritis are summarized in Table 2. At the time of diagnosis, the BCVA was worse in patients with necrotizing scleritis and scleromalacia perforans than in those with diffuse scleritis and posterior scleritis. Patients with necrotizing scleritis had the worst BCVA at the most recent visit (1.5±1.8), followed by scleromalacia (1.2±1.6), posterior scleritis (0.6±1.2), diffuse scleritis (0.6±0.9), and pan scleritis (0.3±0.2). These differences in BCVA among the scleritis subtypes were statistically significant (P=0.05, all cases). The most common cause of poor visual outcome at the most recent follow-up visit was cataract, which was present in 17 patients (32.7%).
Table 2. Visual outcomes in different subtypes of scleritis.
| Characteristic | Anterior necrotizing | Anterior scleromalacia | Anterior non-necrotizing | Posterior scleritis | Pan scleritis | Total |
| Total (eyes) | 12 | 11 | 27 | 23 | 2 | 65 |
| Initial BCVA logMAR | 1.0±1.1 (0.2-4) | 0.7±0.8 (0.1-3) | 0.6±0.8 (0-3) | 0.9±1.2 (0-4) | 1.1±1.3 (0.2-2) | 0.8±1.0 (0-4) |
| Most recent visit BCVA logMAR | 1.5±1.8 (0-4) | 1.2±1.6 (0-4) | 0.6±0.9 (0-3) | 0.6±1.2 (0-4) | 0.3±0.2 (0.2-0.4) | 0.7±1.3 (0-4) |
BCVA: Best-corrected visual acuity.
x±s (range)
Oral steroids were administered to 34 patients (65.4%), and intravenous steroids were administered to 4 patients (7.7%). Treatment with non-steroidal anti-inflammatory drugs (NSAIDs) was provided to 21 patients (40.4%). A subconjunctival injection of triamcinolone acetonide was administered to 1 patient with posterior scleritis (1.9%). Treatment with immunosuppressive agents was provided to 24 patients (46.2%): 7 patients (13.5%) were treated with cyclosporine A (CsA), 6 patients (11.5%) with mycophenolate mofetil (CellCept), 6 patients (11.5%) with azathioprine, 3 patients (5.8%) with methotrexate, and 2 patients (3.8%) with infliximab.
DISCUSSION
Scleritis may be associated with life-threatening systemic disease and accompanied by vision-threatening ocular complications. For these reasons, it is crucial to study the clinical features of the disease in different geographic areas and among multiple ethnicities so as to aid diagnosis.
In the Caucasian population, patients with scleritis are predominantly middle-aged, with a mean age at onset of 49y[17]. Female patients comprise 71% of all patients with scleritis[2] and 65% of patients with posterior scleritis[17]. In our study of a population of Saudi patients, there was a greater preponderance of female patients diagnosed with scleritis (65.4%), with a mean age of onset of 50y. Compared with other subtypes of scleritis in our series, posterior scleritis occurred earlier, with a mean age of onset of 40y. Our findings are in accordance with those of McCluskey et al[17] and Joysey et al[18], who previously reported a greater preponderance of female patients with scleritis. By contrast, only a slight predominance of women (55%) were diagnosed with scleritis in a study of a population of Japanese patients, with the mean age at presentation of 51y[19]. In addition, we found that anterior scleritis (69.2%) was much more common than posterior scleritis (26.9%) among the patients in the present study. Our research is in agreement with that of Watson and Hayreh[1], who reported a greater incidence of anterior scleritis compared with posterior scleritis in a population of British patients.
Systemic association was present in 23.0% of our scleritis patients. This result is much lower than the percentages of scleritis patients with systemic association (range, 40%-50%) reported in studies on populations in North America and Europe[1],[20],[21], but it is only slightly higher than the percentages of scleritis patients with systemic association (range, 15%-22%) reported in studies on a Japanese population[19],[22].
We found that visual outcome was dependent on the subtype of scleritis. Diffuse scleritis and posterior scleritis had better visual outcomes at presentation and at the most recent visit. Patients with necrotizing scleritis presented with poor VA and had poor vision after remission. Our findings are in agreement with those of Jabs et al[2], who found that visual outcomes were dependent on the subtype of scleritis. All our patients with scleritis who were diagnosed as having a bacterial infection had previously undergone pterygium excision. In a study of a Korean population, infectious scleritis was documented in 14.5% of patients with scleritis[23]; in our series, 11.5% of patients with scleritis were diagnosed with infectious scleritis. In their series, 5 patients were diagnosed with fungal scleritis, but none of our patients were diagnosed with a fungal infection. This difference in the percentage of patients with infectious scleritis could be explained by the difference in the climate where the two studies were conducted (Riyadh has a dry climate). The results of the current study suggest that a history of pterygium excision is a significant factor associated with poor prognosis in patients with scleritis owing to its strong association with necrotizing scleritis and infectious scleritis. Hence, patients with a history of pterygium surgery who present with necrotizing scleritis may require judicious use of immunosuppressants, provided that the results of a comprehensive microbiological laboratory investigation exclude the possibility of an infection.
In this study, 65.4% of patients with scleritis required treatment with systemic steroids, and 46.2% needed immunosuppressive medication to control scleral inflammation. The most commonly used medication was CsA, which was administered to 13.5% of patients, followed by mycophenolate mofetil (CellCept), which was administered to 11.5% of patients. By contrast, Keino et al[19] used methotrexate more frequently (19%) in their series of Japanese patients. In our series, methotrexate was used to treat 5.8% of all patients with scleritis. According to the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study, methotrexate and CsA were equally effective in controlling scleritis after 1y of treatment (58.3% and 52.8%, respectively); however, at 6mo, treatment with CsA produced better control in 52.8% of patients, whereas treatment with methotrexate produced only good control in 37.3% of patients[24],[25]. Because treatment with methotrexate produced fewer side effects, this type of treatment has a more reasonable safety record compared with treatment with CsA. The administration of mycophenolate mofetil may represent another treatment option. According to previous research, mycophenolate mofetil may be an effective corticosteroid-sparing agent in treating inflammatory eye disease[3]. Mycophenolate mofetil works by helping a corticosteroid to suppress the immune system, which allows the dose of the corticosteroid being administered to be lowered, thereby reducing the side effects associated with the corticosteroid. In our study, mycophenolate mofetil, a relatively new medication, was commonly administered to our newly diagnosed patients and seemed to have a good effect on scleritis control.
Ocular complications were detected in 73% of all our patients. Some of these patients also had more than one complication during the follow-up period. In a study by Keino et al[19], one or more ocular complications were observed in 78% of all patients, either at presentation or during the follow-up period. In their study, glaucoma was the most common complication, whereas in our series, anterior uveitis and cataract were more common than glaucoma.
One possible limitation of this study is that the data were collected retrospectively. Another possible limitation is that our study was conducted at two subspecialty tertiary eye care hospitals and, therefore, may not reflect the entire spectrum of scleritis observed and treated in the community. Moreover, files lacked information that may predict patients' response to treatment. Hence, it would be worthwhile to conduct a separate prospective study to analyze these factors. We believe that our results add new and important knowledge regarding the clinical features of patients with scleritis in a Saudi clinical setting.
In conclusion, our study of a population of Saudi patients showed that visual outcome depended on the subtype of scleritis. The most common type of scleritis in the patient population under investigation was anterior non-necrotizing scleritis. We also found that a greater preponderance of female patients presented with scleritis than male patients. Based on the association between infectious scleritis and previous history of ocular surgery found in the present study, we recommend that any postsurgical cases presenting with scleritis be required to undergo a thorough microbiologic workup so as to exclude an infectious etiology.
Acknowledgments
The authors thank Ms. Connie B. Unisa-Marfil for secretarial work.
Conflicts of Interest: Al Barqi M, None; Behrens A, None; Alfawaz AM, None.
REFERENCES
- 1.Watson PG, Hayreh SS. Scleritis and episcleritis. Br J Ophthalmol. 1976;60(3):163–191. doi: 10.1136/bjo.60.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jabs DA, Mudun A, Dunn JP, Marsh MJ. Episcleritis and scleritis: clinical features and treatment results. Am J Ophthalmol. 2000;130(4):469–476. doi: 10.1016/s0002-9394(00)00710-8. [DOI] [PubMed] [Google Scholar]
- 3.Daniel E, Thorne JE, Newcomb CW, Pujari SS, Kaçmaz RO, Levy-Clarke GA, Nussenblatt RB, Rosenbaum JT, Suhler EB, Foster CS, Jabs DA, Kempen JH. Mycophenolate mofetil for ocular inflammation. Am J Ophthalmol. 2010;149(3):423–432. doi: 10.1016/j.ajo.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCluskey PJ, Wakefield D. Ocular infection and immunity. In: Pepose JS, Holland GN, Wilhelmus KR, editors. Scleritis and episcleritis. St. Louis: Mosby; 1996. pp. 642–647. [Google Scholar]
- 5.Sainz de la Maza M, Foster CS, Jabbur NS. Scleritis associated with rheumatoid arthritis and with other systemic immune-mediated diseases. Ophthalmology. 1994;101(7):1281–1286; discussion 1287–1288. [PubMed] [Google Scholar]
- 6.Haynes BF, Fishman ML, Fauci AS, Wolff SM. The ocular manifestations of Wegener's granulomatosis. Fifteen years experience and review of the literature. Am J Med. 1977;63(1):131–141. doi: 10.1016/0002-9343(77)90125-5. [DOI] [PubMed] [Google Scholar]
- 7.Tarabishy AB, Schulte M, Papaliodis GN, Hoffman GS. Wegener's granulomatosis: clinical manifestations, differential diagnosis, and management of ocular and systemic disease. Surv Ophthalmol. 2010;55(5):429–444. doi: 10.1016/j.survophthal.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Smith JR, Mackensen F, Rosenbaum JT. Therapy insight: scleritis and its relationship to systemic autoimmune disease. Nat Clin Pract Rheumatol. 2007;3(4):219–226. doi: 10.1038/ncprheum0454. [DOI] [PubMed] [Google Scholar]
- 9.Doshi RR, Harocopos GJ, Schwab IR, Cunningham ET., Jr The spectrum of postoperative scleral necrosis. Surv Ophthalmol. 2013;58(6):620–633. doi: 10.1016/j.survophthal.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Johnson CC, Ohlstein DH. Peripheral ulcerative keratitis and necrotizing scleritis initiated by trauma in the setting of mixed cryoglobulinemia. Case Rep Ophthalmol. 2011;2(3):392–397. doi: 10.1159/000334496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cobo M. Inflammation of the sclera. Int Ophthalmol Clin. 1983;23(1):159–171. doi: 10.1097/00004397-198302310-00013. [DOI] [PubMed] [Google Scholar]
- 12.Moriarty AP, Crawford GJ, McAllister IL, Constable IJ. Fungal corneoscleritis complicating beta-irradiation-induced scleral necrosis following pterygium excision. Eye (Lond) 1993;7(Pt 4):525–528. doi: 10.1038/eye.1993.114. [DOI] [PubMed] [Google Scholar]
- 13.Moriarty AP, Crawford GJ, McAllister IL, Constable IJ. Bilateral streptococcal corneoscleritis complicating beta irradiation induced scleral necrosis. Br J Ophthalmol. 1993;77(4):251–252. doi: 10.1136/bjo.77.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okhravi N, Odufuwa B, McCluskey P, Lightman S. Scleritis. Surv Ophthalmol. 2005;50(4):351–363. doi: 10.1016/j.survophthal.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Tuft SJ, Watson PG. Progression of scleral disease. Ophthalmology. 1991;98(4):467–471. doi: 10.1016/s0161-6420(91)32269-3. [DOI] [PubMed] [Google Scholar]
- 16.Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCluskey PJ, Watson PG, Lightman S, Haybittle J, Restori M, Branley M. Posterior scleritis: clinical features, systemic associations, and outcome in a large series of patients. Ophthalmology. 1999;106(12):2380–2386. doi: 10.1016/S0161-6420(99)90543-2. [DOI] [PubMed] [Google Scholar]
- 18.Joysey VC, Roger JH, Ashworth F, Bullman W, Hazleman BL, Lachmann SM, Watson PG. Parallel studies of HLA antigens in patients with rheumatic heart disease and scleritis: comparisons with three control populations. J Rheumatol Suppl. 1977;3:84–88. [PubMed] [Google Scholar]
- 19.Keino H, Watanabe T, Taki W, Nakashima C, Okada AA. Clinical features and visual outcomes of Japanese patients with scleritis. Br J Ophthalmol. 2010;94(11):1459–1463. doi: 10.1136/bjo.2009.171744. [DOI] [PubMed] [Google Scholar]
- 20.Sainz de la Maza M, Jabbur NS, Foster CS. Severity of scleritis and episcleritis. Ophthalmology. 1994;101(2):389–396. doi: 10.1016/s0161-6420(94)31325-x. [DOI] [PubMed] [Google Scholar]
- 21.McGavin DD, Williamson J, Forrester JV, Foulds WS, Buchanan WW, Dick WC, Lee P, MacSween RN, Whaley K. Episcleritis and scleritis. A study of their clinical manifestations and association with rheumatoid arthritis. Br J Ophthalmol. 1976;60(3):192–226. doi: 10.1136/bjo.60.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakajima N, Hayasaka S. Clinical features of episcleritis and scleritis in some Japanese patients. Ophthalmologica. 1995;209(5):256–259. doi: 10.1159/000310626. [DOI] [PubMed] [Google Scholar]
- 23.Ahn SJ, Oh JY, Kim MK, Lee JH, Wee WR. Clinical features, predisposing factors, and treatment outcomes of scleritis in the Korean population. Korean J Ophthalmol. 2010;24(6):331–335. doi: 10.3341/kjo.2010.24.6.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gangaputra S, Newcomb CW, Liesegang TL, Kaçmaz RO, Jabs DA, Levy-Clarke GA, Nussenblatt RB, Rosenbaum JT, Suhler EB, Thorne JE, Foster CS, Kempen JH. S Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study. Methotrexate for ocular inflammatory diseases. Ophthalmology. 2009;116(11):2188–2198. doi: 10.1016/j.ophtha.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaçmaz RO, Kempen JH, Newcomb C, Daniel E, Gangaputra S, Nussenblatt RB, Rosenbaum JT, Suhler EB, Thorne JE, Jabs DA, Levy-Clarke GA, Foster CS. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 2010;117(3):576–584. doi: 10.1016/j.ophtha.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


