Abstract
AIM
To compare the effects of intravitreal injection of bevacizumab (IVB) with intravitreal triamcinolone acetonide (IVTA) on the treatment of cystoid macular edema (CME) secondary to retinal vein occlusion (RVO).
METHODS
A literature search was conducted using PubMed, the Cochrane Central Register of Controlled Trials, Web of Science and the Chinese Biomedical Database. The comparison was divided into two groups, group 1 conducted comparison in branch RVO (BRVO) or central RVO (CRVO), group 2 conducted comparison in ischemic-RVO or nonischemic-RVO. Pooled mean differences (MDs) for changes in visual acuity (VA), central macular thickness (CMT) and intraocular pressure (IOP) were calculated in groups at 4, 12 and 24wk after treatment respectively.
RESULTS
Eight studies comparing the efficacy of IVB with IVTA were included in the Meta-analysis. In group 1, in BRVO, significant difference was shown on the comparison of CMT at 24wk (MD, -45.66; 95% CI, -76.03 to -15.28; P=0.003), IVB was effective on BRVO for at least 24wk; no significant differences were found in the comparison of VA at each time points (P>0.05 respectively). In CRVO, no significant differences were found in the comparison of VA or CMT between IVB and IVTA at each time points (P>0.05, respectively). In group 2, in ischemic-RVO, significant differences were shown in the comparison of VA (MD, -0.28; 95% CI, -0.42 to -0.14; P<0.0001) and CMT (MD, -86.50; 95% CI, -151.18 to -22.43; P=0.008) at 24wk; In nonischemic-RVO, no significant differences were demonstrated in the comparison of VA or CMT between IVB and IVTA at each time points (P>0.05, respectively). The occurrence of high IOP was much lower in IVB group.
CONCLUSION
This Meta-analysis suggested that IVB was effective in decreasing CMT in BRVO for at least 24wk, IVB is more effective on improving VA and reducing CMT in ischemic-RVO. IVB is more promising on RVO than IVTA.
Keywords: bevacizumab, retinal vein occlusion, Meta-analysis, triamcinolone acetonide
INTRODUCTION
Retinal vein occlusion (RVO) is the second most common retinal vascular disorder after diabetic retinopathy (DR) and is considered to be an important cause of visual loss[1],[2]. Depending on the location of the obstruction, RVO can be divided into central RVO (CRVO) and branch RVO (BRVO). They are different on symptoms, pathogenesis, risk factors and treatment. Usually, BRVO has better visual prognosis than CRVO. It is fundamental to study BRVO and CRVO separately.
RVO can also be divided into two types, ischemic and nonischemic, no matter what it is BRVO or CRVO. Ischemic RVO is associated with a significant loss of visual acuity (VA) at presentation and a poor prognosis, suggesting that the damage is substantial and most often irreversible[3]. However, 16% of RVOs with perfusion can progress to ischemia in 4mo[4].
Bevacizumab is a full-length humanized monoclonal antibody directed against all biologically active forms of vascular endothelial growth factor (VEGF)[5],[6]. Triamcinolone acetonide is a multiple potency drug that have anti-inflammatory, anti-angiogenic properties and may inhibit the expression of VEGF and other proinflammatory cytokines such as interleukin-6 (IL-6); intercelluar adhesion molecule-1 (ICAM-1) and monocyte chemoattractant protein-1 (MCP-1)[7]. Many studies have suggested that intravitreal injection of bevacizumab (IVB) or intravitreal triamcinolone acetonide (IVTA) are effective in improvement on VA and reducing central macular thickness (CMT) in BRVO[8]–[12]. Most of them demonstrated both therapies are effective on CRVO[13]–[15], however, there are no exact conclusions of which one of them is preferred in the treatment of RVO. Herein we performed a Meta-analysis to quantify the effect of IVB versus IVTA on RVO. The comparisons were conducted in BRVO or CRVO, and in ischemic-RVO or nonischemic-RVO, in order to evaluate the efficacy and safety of IVB versus IVTA in the treatment of cystoid macular edema (CME) secondary to RVO.
SUBJECTS AND METHODS
This study was conducted in accordance with the Declaration of Helsinki, and permission was granted by Shandong University. Using PubMed, the Cochrane Central Register of Controlled Trials, Web of Science and the Chinese Biomedical Database, we performed computerized literature searches with no language limitations, for relevant available articles published through December 2014. Searches comprised a combination of the following key words “RVO”, “bevacizumab” or “Avastin”, “triamcinolone acetonide”. Inclusion criteria comprised: 1) randomized control trials (RCTs), observational studies or case control studies; 2) interventional therapies for RVO consisting of IVB versus IVTA; 3) studies containing sufficient information on VA, CMT and intraocular pressure (IOP) outcomes. Exclusion criteria were: 1) studies with insufficient data analyses; 2) studies focused on combined therapy. All studies and analyses were in accordance with the Meta-analysis (PRISME) statement. Decisions regarding which trials to be included were made independently by reviewers. Disagreements were resolved by discussion.
Data abstraction was undertaken according to the predesigned data extraction form. Information regarding studies title, authors and journal; population characteristics (age, gender and number of patients and eyes); study designs; interventional groups and duration of follow-up were collected. We calculated pooled summary estimates for primary outcomes, and changes in VA (logMAR) and CMT (µm) measured at 4, 12 and 24wk post intervention. We also analyzed IOP (mm Hg) at 4, 12 and 24wk after injection.
The quality of RCTs was assessed using the Jadad scale. Cohort and case-control studies had to meet the criteria of the case, matched by the patient's characteristics. All studies were screened for quality and relevance.
The statistical analysis was performed by RevMan version 5.1.6 software (Review Manager, Copenhagen, Danmark). It was used in the present analysis to calculate relative risks, with 95% CIs of the primary outcomes of VA and CMT. Heterogeneity was assessed using the Chi-square test on Cochrane's Q statistic and by calculating I2. Weighted mean differences (MDs) were calculated based on either a fixed-effect model or random-effect model depending on the absence or presence of significant heterogeneity. P<0.05 and I2>50% were considered significant. All studies were pooled and overall efficacy of any duration was assessed. Subgroup analysis and asymmetry assessment of the funnel plot for publication biases were not conducted, as only a limited number of studies were involved in the final analysis.
RESULTS
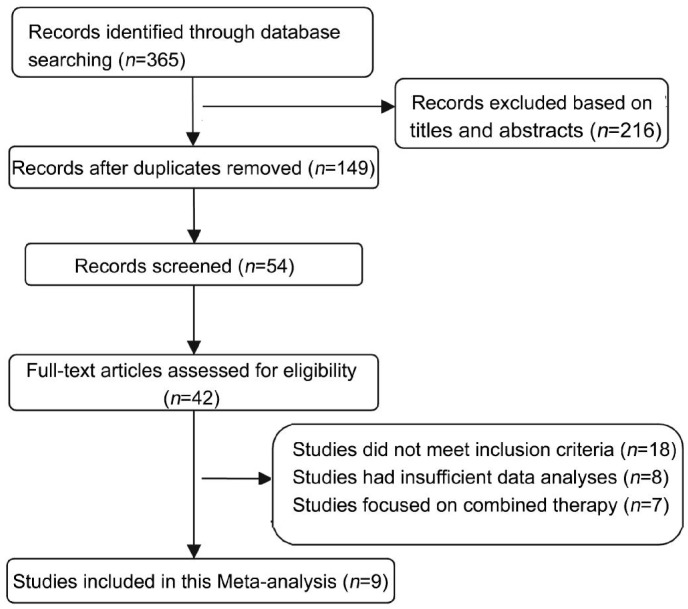
Eight studies included two RCTs and six case control studies, contained a total of 590 eyes, in which 271 eyes were treated with IVB and 319 eyes were treated with IVTA. Among these articles, six compared IVB (1.25 mg) with IVTA (4 mg) and two were IVB (1.25 mg) versus IVTA (2 mg) in the treatment (Figure 1).
Figure 1. Flow diagram of the study selection for Meta-analysis.
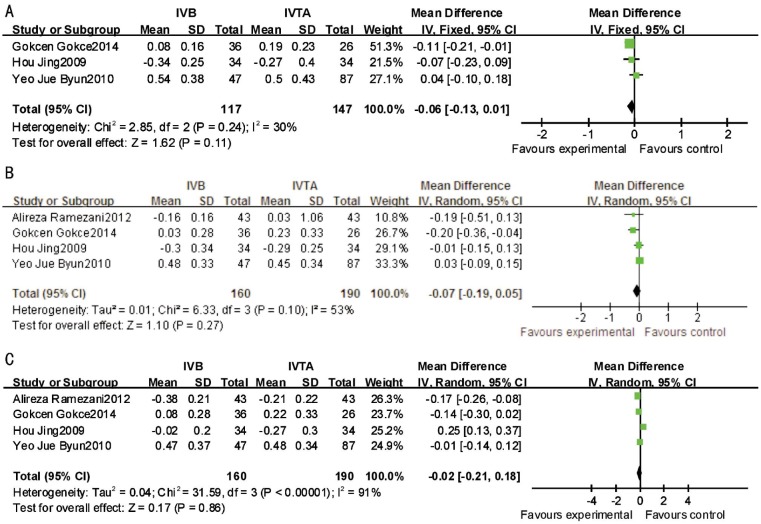
For BRVO, there were no significant differences between IVB and IVTA in VA at 4, 12, 24wk (P=0.11, I2=30%; P=0.27, I2=53%; P=0.86, I2=91%; respectively) after treatment (Figure 2). Significant difference was observed between IVB and IVTA in CMT at 24wk (MD, -45.66; 95% CI, -76.03 to -15.28; P=0.003). CMT was significantly reduced by IVB at that time point. No significant differences were found between the two therapeutic interventions at 4, 12wk (P=0.86, I2=70%; P=0.37, I2=94%; respectively) after treatment (Figure 3).
Figure 2. This forest plot from the Meta-analysis of VA (logMAR) for BRVO comparing IVB to IVTA at 4wk (A), 12wk (B) and 24wk (C) after treatment.
Figure 3. This forest plot from the Meta-analysis of CMT of BRVO comparing IVB to IVTA at 4wk (A), 12wk (B) and 24wk (C) after treatment.
For CRVO, there were no significant differences between IVB and IVTA in VA (P=0.92, I2=37%; P=0.33, I2=0; P=0.60, I2=86%; respectively) and CMT (P=0.59, I2=0; P=0.12, I2=37%; P=0.51, I2=82%; respectively) at 4, 12, 24wk after treatment.
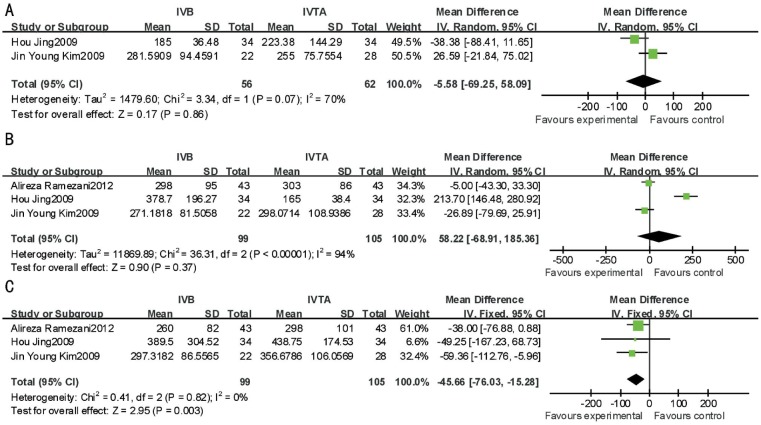
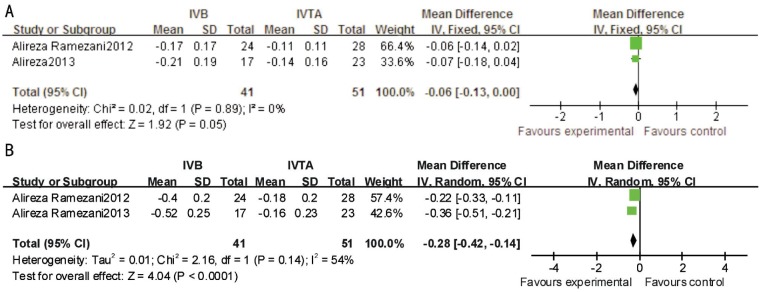
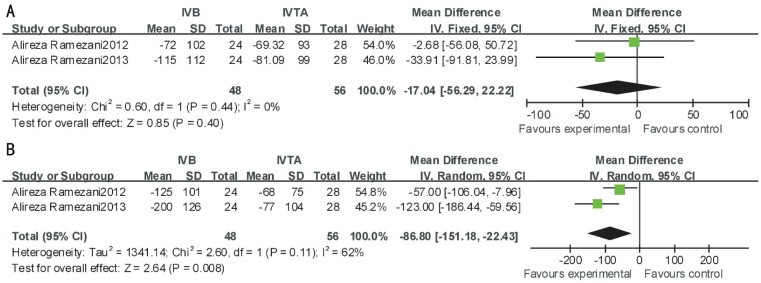
For ischemic-RVO, IVB was significantly more effective at 24wk between VA (MD, -0.28; 95% CI, -0.42 to -0.14; P<0.0001) and CMT (MD, -86.80; 95% CI,-151.18 to -22.43; P=0.008) after treatment. No significant difference was found between the two therapeutic interventions at 12wk between VA (P=0.05, I2=0) and CMT (P=0.40, I2=0) (Figures 4, 5).
Figure 4. This forest plot from the Meta-analysis of of VA (logMAR) for ischemic-RVO comparing IVB to IVTA at 12wk (A) and 24wk (B) after treatment.
Figure 5. This forest plot from the Meta-analysis of CMT for ischemic-RVO comparing IVB to IVTA at 12wk (A) and 24wk (B) after treatment.
For nonischemic-RVO, there were no significant difference between IVB and IVTA in VA at 12, 24wk (P=0.42, I2=0; P=0.13, I2=53%; respectively) after treatment. No significant difference between IVB and IVTA in CMT at 12, 24wk (P=0.17, I2=0; P=0.44, I2=39%; respectively) after treatment.
For IOP, four studies[8],[12],[14],[15] described the change of IOP in both IVB and IVTA groups. Compared with the IVB group, significant IOP increases were found in the IVTA group at 4wk (MD, -3.42; 95% CI, -5.28 to -1.55; P=0.0003), 12wk (MD, -2.29; 95% CI, -3.66 to -0.92; P=0.001) and 24wk (MD, -2.94; 95% CI, -4.33 to -1.55; P<0.0001) after treatment.
DISCUSSION
CME is the most common sight-threatening complication of RVO. It is the consequence of the inflammatory factors and increased VEGF, leading to vascular hyperpermeability[16]. That result in fluid and plasma constituents into retinal layers of the macular and thus edema[17],[18]. Antiangiogenic agents and corticosteroids have been beneficial for treating macular edema caused by RVO.
VA, a primary measure of treatment efficacy, is an exceedingly important outcome. On BRVO and CRVO, both bevacizumab and triamcinolone resulted in improvement in VA and reduction in CMT on optical coherence tomography (OCT). In the Meta-analysis, IVB and IVTA can improve VA of BRVO and CRVO at each time point, however, no significant differences were found between the two therapeutic regiments. These results were in agreement with other comparative studies between IVB and IVTA for the treatment of BRVO[9],[12],[19].
CMT is another strong prognositic measure of CME level. We illustrated decreasing CMTs in both IVB and IVTA groups at 4, 12 and 24wk post-treatment. Significant decreases in CMT were found in the IVB group compared with the IVTA group at 24wk of BRVO. While in BRVO and CRVO, both of bevacizumab and triamcinolone resulted in VA improvement and reduction in CMT on OCT at other follow-up points. While CMT at other follow-up points had no superiority in both BRVO and CRVO groups. IVB was able to decrease CMT in BRVO for at least 24wk.
In the Meta-analysis, we found a superior effect of IVB in VA and CMT of ischemic-RVO compared with the IVTA group at 24wk. VEGF expression increases under hypoxic condition and is upregulated in ischemic retinopathies such as DR[20],[21]. Abnormal high levels of VEGF have been found in intraocular fluid of patients with diabetic macular edema and macular edema secondary to RVO. While there were no significance difference between IVB and IVTA of nonischemic-RVO. The incidence of adverse events, however, was significantly greater in the IVT group than in the IVB group. IVB may be preferred over IVT for the treatment of macular edema in patients with non-ischemic CRVO[22].
VEGF is a core medication of intraocular neovascularization and macular edema, and animal experiments have shown that this molecule can promote ischemia caused by RVO. Furthermore, anti-VEGF seem to be a safe and effective treatment for RVO in terms of reducing ischemic progression and managing the complications, such as neovascular glaucoma[23]. However, IVTA has been less effective in the macular edema reduction for ischemic forms, not only in case report studies but also in prospective, comparative studies[24].
IVTA has several intravitreal injection-related complications, including increases in IOP (the most common), cataract progression, infection, retinal detachment, vitreous hemorrhage and endophthalmitis[25],[26]. In my Meta-analysis, comparing to IVB, the occurrence of IOP increase was higher when using IVTA. A quarter of eyes In IVTA group had IOP>21 mm Hg, in which required anti-glaucoma drugs and surgery[8],[12],[14],[15]. One sixth of eyes in IVTA group had cataract progression[10],[12],[14],[15]. Nevertheless, IVB has not only intravitreal-related ocular complications but also systemic adverse events, including mild blood pressure increases, transient ischemic attacks and venous thrombose[27]. However, the incidence of adverse effects were very low. So we used IVTA with caution because of its cumulative side-effects and potential toxicity of the drug itself.
There are several limitations in the present Meta-analysis that could affect the final outcome. First, a total of studies and the total number of subjects were relatively low. Second, There has been always a concern about worsening of retinal ischemia by using anti-VEGF, especially after repeated injections[28]. However, we observed the VA of ischemia-RVO by IVB is better than by IVTA. So we need further observation. Third, comparing to RCTs, the observational studies were prone to bias due to uncontrolled confounding. Four, most studies provided only crude-unadjusted data, which was probably the point of the high heterogeneity. Regression or stratification of study results could not be used to explore factors that could explain heterogeneities based on sample size or varying baseline levels.
In summary, we illustrated that IVB appears to be more effective on improving VA and reducing CMT in ischemic-RVO. IVB could keep longer efficiency on CMT than IVTA. So IVB is the promising therapy for RVO. Future multi-center controlled trials should be initiated to discover which patients benefit the most from IVB or IVTA.
Acknowledgments
The authors thank the researchers whose studies were involved in this Meta-analysis and provided useful data to us.
Conflicts of Interest: Sun Y, None; Qu Y, None.
REFERENCES
- 1.Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–141; discussion 141–143. [PMC free article] [PubMed] [Google Scholar]
- 2.Rehak J, Rehak M. Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res. 2008;33(2):111–131. doi: 10.1080/02713680701851902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yau JW, Lee P, Wong TY, Best J, Jenkins A. Retinal vein occlusion: an approach to diagnosis, systemic risk factors and management. Intern Med J. 2008;38(12):904–910. doi: 10.1111/j.1445-5994.2008.01720.x. [DOI] [PubMed] [Google Scholar]
- 4.The Central Vein Occlusion Study Group Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol. 1997;115(4):486–491. doi: 10.1001/archopht.1997.01100150488006. [DOI] [PubMed] [Google Scholar]
- 5.Kreutzer TC, Alge CS, Wolf AH, Kook D, Burger J, Strauss R, Kunze C, Haritoglou C, Kampik A, Priglinger S. Intravitreal bevacizumab for the treatment of macular oedema secondary to branch retinal vein occlusion. Br J Ophthalmol. 2008;92(3):351–355. doi: 10.1136/bjo.2007.123513. [DOI] [PubMed] [Google Scholar]
- 6.Kriechbaum K, Michels S, Prager F, Georgopoulos M, Funk M, Geitzenauer W, Schmidt-Erfurth U. Intravitreal Avastin for macular oedema secondary to retinal vein occlusion: a prospective study. Br J Ophthalmol. 2008;92(4):518–522. doi: 10.1136/bjo.2007.127282. [DOI] [PubMed] [Google Scholar]
- 7.McAllister IL, Vijayasekaran S, Chen SD, Yu DY. Effect of triamcinolone acetonide on vascular endothelial growth factor and occludin levels in branch retinal vein occlusion. Am J Ophthalmol. 2009;147(5):838–846. doi: 10.1016/j.ajo.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Kim JY, Park SP. Comparison between intravitreal bevacizumab and triamcinolone for macular edema secondary to branch retinal vein occlusion. Korean J Ophthalmol. 2009;23(4):259–265. doi: 10.3341/kjo.2009.23.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou J, Tao Y, Jiang YR, Li XX, Gao L. Intravitreal bevacizumab versus triamcinolone acetonide for macular edema due to branch retinal vein occlusion: a matched study. Chin Med J (Engl) 2009;122(22):2695–2699. [PubMed] [Google Scholar]
- 10.Byun YJ, Roh ML, Lee SC, Koh HJ. Intravitreal triamcinolone acetonide versus bevacizumab therapy for macular edema associated with branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2010;248(7):963–971. doi: 10.1007/s00417-010-1320-2. [DOI] [PubMed] [Google Scholar]
- 11.Gokce G, Sobaci G, Durukan AH, Erdurman FC. The comparison of intravitreal triamcinolone and bevacizumab in patients with macular edema secondary to branch retinal vein occlusion. Clin Ophthalmol. 2014;8:355–362. doi: 10.2147/OPTH.S58468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramezani A, Esfandiari H, Entezari M, Moradian S, Soheilian M, Dehsarvi B, Yaseri M. Three intravitreal bevacizumab versus two intravitreal triamcinolone injections in recent-onset branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2012;250(8):1149–1160. doi: 10.1007/s00417-012-1941-8. [DOI] [PubMed] [Google Scholar]
- 13.Hu YJ. Intravitreal bevacizumab vs triamcinolone acetonide for macular oedema due to central retinal vein occlusion. Eye (Lond) 2010;24(8):1414–author reply 1414–1415. doi: 10.1038/eye.2010.35. [DOI] [PubMed] [Google Scholar]
- 14.Ding X, Li J, Hu X, Yu S, Pan J, Tang S. Prospective study of intravitreal triamcinolone acetonide versus bevacizumab for macular edema secondary to central retinal vein occlusion. Retina. 2011;31(5):838–845. doi: 10.1097/IAE.0b013e3181f4420d. [DOI] [PubMed] [Google Scholar]
- 15.Ramezani A, Esfandiari H, Entezari M, Moradian S, Soheilian M, Dehsarvi B, Yaseri M. Three intravitreal bevacizumab versus two intravitreal triamcinolone injections in recent onset central retinal vein occlusion. Acta Ophthalmol. 2014;92(7):e530–539. doi: 10.1111/aos.12317. [DOI] [PubMed] [Google Scholar]
- 16.Glacet-Bernard A, Coscas G, Zourdani A, Soubrane G, Souied EH. Steroids and macular edema from retinal vein occlusion. Eur J Ophthalmol. 2011;21(Suppl. 6):S37–44. doi: 10.5301/EJO.2010.6053. [DOI] [PubMed] [Google Scholar]
- 17.Noma H, Funatsu H, Mimura T, Shimada K. Increase of aqueous inflammatory factors in macular edema with branch retinal vein occlusion: a case control study. J Inflamm (Lond) 2010;7:44. doi: 10.1186/1476-9255-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneda S, Miyazaki D, Sasaki S, Yakura K, Terasaka Y, Miyake K, Ikeda Y, Funakoshi T, Baba T, Yamasaki A, Inoue Y. Multivariate analyses of inflammatory cytokines in eyes with branch retinal vein occlusion: relationships to bevacizumab treatment. Invest Ophthalmol Vis Sci. 2011;52(6):2982–2988. doi: 10.1167/iovs.10-6299. [DOI] [PubMed] [Google Scholar]
- 19.Higashiyama T, Sawada O, Kakinoki M, Sawada T, Kawamura H, Ohji M. Prospective comparisons of intravitreal injections of triamcinolone acetonide and bevacizumab for macular oedema due to branch retinal vein occlusion. Acta Ophthalmol. 2013;91(4):318–324. doi: 10.1111/j.1755-3768.2011.02298.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaur C, Sivakumar V, Foulds WS. Early response of neurons and glial cells to hypoxia in the retina. Invest Ophthalmol Vis Sci. 2006;47(3):1126–1141. doi: 10.1167/iovs.05-0518. [DOI] [PubMed] [Google Scholar]
- 21.Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol. 1995;113(12):1538–1544. doi: 10.1001/archopht.1995.01100120068012. [DOI] [PubMed] [Google Scholar]
- 22.Demir M, Dirim B, Acar Z, Sendul Y, Oba E. Comparison of the effects of intravitreal bevacizuamab and triamcinolone acetonide in the treatment of macular edema secondary to central retinal vein occlusion. Indian J Ophthalmol. 2014;62(3):279–283. doi: 10.4103/0301-4738.105769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasanreisoglu M, Weinberger D, Mimouni K, Luski M, Bourla D, Kramer M, Robinson A, Axer-Siegel R. Intravitreal bevacizumab as an adjunct treatment for neovascular glaucoma. Eur J Ophthalmol. 2009;19(4):607–612. doi: 10.1177/112067210901900414. [DOI] [PubMed] [Google Scholar]
- 24.Jonas JB, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide as treatment of macular edema in central retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2002;240(9):782–783. doi: 10.1007/s00417-002-0529-0. Epub 2002 Aug 13. [DOI] [PubMed] [Google Scholar]
- 25.Fung AE, Bhisitkul RB. Safety monitoring with ocular anti-vascular endothelial growth factor therapies. Br J Ophthalmol. 2008;92(12):1573–1574. doi: 10.1136/bjo.2008.137604. [DOI] [PubMed] [Google Scholar]
- 26.Gillies MC, Simpson JM, Billson FA, Luo W, Penfold P, Chua W, Mithell P, Zhu M, Hunyor AB. Safety of an intravitreal injection of triamcinolone: results from a randomized clinical trial. Arch Ophthalmol. 2004;122(3):336–340. doi: 10.1001/archopht.122.3.336. [DOI] [PubMed] [Google Scholar]
- 27.Fung AE, Rosenfeld PJ, Reichel E. The international intravitreal bevacizumab safety survey: using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006;90(11):1344–1349. doi: 10.1136/bjo.2006.099598. Epub 2006 Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manousaridis K, Talks J. Macular ischaemia: a contraindication for anti-VEGF treatment in retinal vascular disease? Br J Ophthalmol. 2012;96(2):179–184. doi: 10.1136/bjophthalmol-2011-301087. [DOI] [PubMed] [Google Scholar]