Abstract
Dark adaptation is a highly sensitive neural function and may be the first symptom of many status including the physiologic and pathologic entity, suggesting that it could be instrumental for diagnose. However, shortcomings such as the lack of standardized parameters, the long duration of examination, and subjective randomness would substantially impede the use of dark adaptation in clinical work. In this review we summarize the recent research about the dark adaptation, including two visual cycles-canonical and cone-specific visual cycle, affecting factors and the methods for measuring dark adaptation. In the opinions of authors, intensive investigations are needed to be done for the widely use of this significant visual function in clinic.
Keywords: dark adaptation, visual cycle, pigment regeneration, adaptometer
INTRODUCTION
Most vertebrates contain two types of photoreceptors, the rods and the cones, which have distinct functional properties and mutually contribute to our visual function including dark adaptation. Rods are several-hundred fold more sensitive than cones, but saturate at relatively low levels of light, while cones have an extremely high photon saturation threshold. Dark adaptation refers to how the eye recovers its sensitivity in the dark following exposure to bright lights. Most studies of dark adaptation concentrated on the two well-established pathways: the canonical cycle and the cone-specific cycle. The combined actions of two cycles are essential for the complete dark adaptation. Moreover, dark adaptation is a practical diagnostic aid because it is a highly sensitive neural function. However, dark adaptometry's utility has been impeded by the lack of standardized parameters, the long duration of examination and subjective randomness. Intensive investigations are searching for novel methods to this significant visual function.
In this review we summarized the recent research about the dark adaptation, including two visual cycles, affecting factors and the methods of measuring dark adaptation. The objective of this review is to strengthen the importance of dark adaptation, and attract more attention to investigate in order to monitor and protect this significant visual function.
VISUAL CYCLE
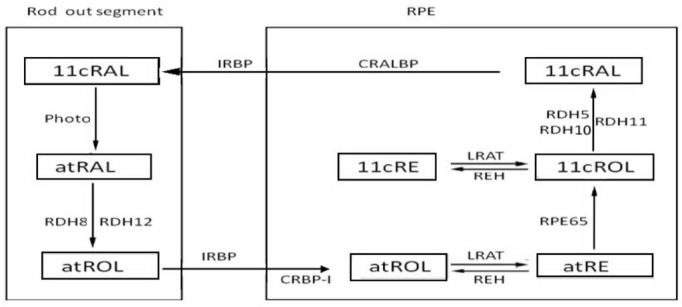
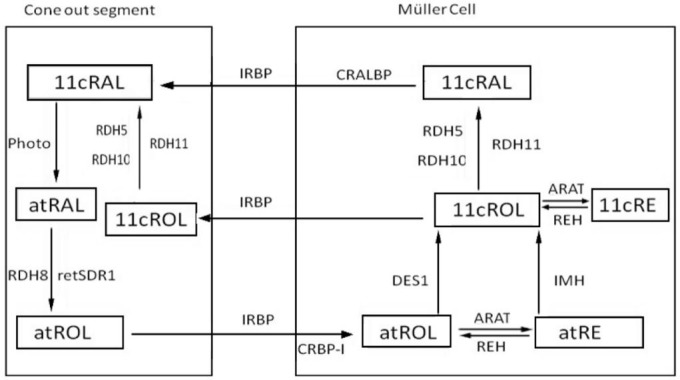
Dark adaptation is virtually a biochemical process of photopigments regeneration in the photoreceptors. Two visual cycles were well established for dark adaptation: the canonical visual cycle (Figure 1) and the cone-specific visual cycle (Figure 2). The former regenerates photopigments slowly through the retinal pigment epithelium (RPE) and provides for both rods and cones, while the latter regenerates photopigments rapidly through the Müller cells, other than the RPE, and just for the cones[1],[2].
Figure 1. The canonical visual cycle.
Figure 2. The cone-specific visual cycle.
Canonical Visual Cycle
The processes of photopigments regeneration in the canonical visual cycle occur in the photoreceptor cells, then transfer to the RPE, and finally back to photoreceptors. RPE plays a crucial role in this cycle which provides the cellular site and several key metabolic enzymes, including lecithin retinol acyltransferase (LRAT) and RPE-specific 65 kDa (RPE65).
Reaction in the photoreceptor
Photon absorption by the visual photopigments triggers off isomerization from 11-cis retinal (11cRAL) to all-trans retinal (atRAL), thereby activating the photo-transduction cascade. The atRAL is released into the bilayer of out segment disc membranes, and transported to the cytoplasm through diffusion by ATP-binding cassette transporter 4 (ABCA4)[3],[4]. Several evidences suggested that ABCA4 is expressed in both rods and cones and may be functional to transport atRAL across disk membranes [5],[6]. Then the released atRAL is reduced to all-trans retinol (atROL), which is catalyzed by NADPH dependent all-trans retinol dehydrogenase (RDH)[7],[8]. Both RDH8 (also known as photoreceptor RDH, prRDH) and RDH12 contribute to atRAL reduction[9], particularly the former is the main contributor to the total RDH activity[10],[11].
Transfer from photoreceptors to retinal pigment epithelium
The remaining steps of dark adaptation occur in RPE, thus it is required that the atROL should be transported into RPE. This process is promoted by the interphotoreceptor retinol binding protein (IRBP)[12],[13] that produced by the photoreceptors. Unlike other binding proteins which contain a single retinoid binding site, IRBP has at least three high affinity sites[14], which can bind several isomeric forms of retinol and retinal, but it has a favorite affinity for all-trans and 11-cis retinoid[15]. In addition to IRBP, there are two other ways for atROL transportation, the first is the endocytosis of holo-IRBP, and the other is the phagocytosis of RPE. Finally, RPE takes up atROL from photoreceptors.
Reaction within the retinal pigment epithelium
Within the RPE cells, atROL combined with another retinoid binding protein-the cellular retinol binding protein (CRBP), which has two isoforms: CRBP-I and CRBP-II, while only the CRBP-I was selectively expressed in the eye[16]. CRBP-I has a 100-fold higher affinity to binding with atROL than the IRBP, and this difference may facilitates atROL to dissociate from IRBP.
The atROL is esterified into all-trans retinyl ester (atRE) by the LRAT[17]. The LRAT rapidly and reversibly transfers an acyl group to atRE[18],[19], and then atRE is isomerized to 11-cis retinol (11cROL) in the isomerization cycle. The isomerization reaction is catalyzed by RPE65 [Isomerohydrolase (IMH), Isomerase I][20]–[22] and could be inhibited by its product[23]. Combined with another protein called cellular retinaldehyde binding protein (CRALBP), 11cROL is oxidized and transformed into retinal by the NADPH independent 11-cis RDH. During this process, RDH5 and RDH11 mutually contribute to the 11-cis RDH function in the RPE, and RDH5 plays the pivotal role while RDH11 is supplementary[9],[24]. It should be point out that RDH10 can substitute for RDH5 under experimental conditions[25].
In addition, the 11cROL can also be esterified into the 11-cis retinyl ester (11cRE) by LRAT. This is a reversible process, and the 11cRE can be reduced to 11cROL to replenish for the photopigments regeneration.
Transfer the 11-cis retinal back to the photoreceptor and subsequent reactions
IRBP is believed to be able to carry the 11cRAL and transfer it back to the outer segments for pigment regeneration[26]. The binding affinities of photopigments to IRBP followed this order: 11cRAL > atROL > atRAL > 11cROL[27]. Several studies showed that the retinal binding protein (RBP) might also participate in the transportation of 11cRAL[28].
After transported back to the photoreceptors, the 11cRAL combined with opsin to reconstitute functional pigments, and then light sensitivity regained (Figure 1).
Cone-specific Visual Cycle
The different function of photoreceptors required exact properties of their photopigments. After equal levels of bleaching, cones recover sensitivity approximately 10-fold faster than the rods. Several studies showed that cones might get access to a special source of photopigments which is exclusively independent of RPE[1],[29]. In addition, other investigations suggested that Müller cells could act as a cellular site for photopigments regeneration[1],[30]. These results strongly support the existence of a cone-specific visual cycle which involving the Müller cells other than the RPE.
Reaction in the photoreceptor and subsequent retinoid transportation
11cRAL is isomerized to atRAL by light absorption and then is transferred to the cytoplasm by ATP-binding cassette transporter(ABCR) The reaction in the cone-specific visual cycle is similar to the canonical visual cycle. The released atRAL is reduced to atROL by the NADPH dependent all-trans RDH. RDH8 and retina-derived short chain dehydrogenase/reductase-1 (retSDR1) mutually contribute to the reduction of atRAL[10].
Subsequently, the atROL is transported to Müller cells. IRBP should be responsible for this transportation of retinoid between cones and Müller cells[31],[32].
Reaction in the Müller cell
The atROL is bound to CRBP and transported to the Müller Cell. The following processes in the Müller cells are distinguishing from that in the RPE. The atROL reactions follow two ways: 1) atROL is directly isomerized to 11cROL by DES1 (dihydroceramide desaturase-1, Isomerase II)[33],[34]. Unlike RPE65 which uses atRE as substrate[35],[36], DES1 in retinas acts directly on the atROL[37]; 2) atROL is esterified to atRE, reversibly, atRE may also be hydrolyzed to atROL by retinyl ester hydrolases (REH). atRE could be isomerohydrolyzed to 11cROL directly by an isomerohydrolase (IMH)-based mechanism[38]. Then, the 11cROL has three paths to continue. One is the esterified path in which the 11cROL is transformed into 11cRE by acyl retinol acyl transferase (ARAT)[39]. The production of retinyl esters was highest in the presence of CRALBP, palmitoyl CoA, and 11cROL[30]. Interestingly, the rate of 11cRE synthesis is dependent on the concentration of atROL[37], suggesting that the two reactions might be correlated. Under dark adaptation, it can be reversed into 11cROL by 11-cis REH and be transferred to the photoreceptor for pigment regeneration[40]. The second path is that 11cROL is oxidized to 11cRAL by a retinol dehydrogenase in the Müller cell, the production will be bound to and protected by CRALBP. At last, 11cROL is released into cones directly through binding with CRALBP.
Transfer and reaction in the photoreceptors
IRBP is found to be able to carry 11cRAL and 11cROL and transfer them back to the outer segments for pigment regeneration[41],[42]. After transferred back to the photoreceptor, 11cRAL combines with opsin to reconstitute the cone pigment. Following the regeneration of pigment, 11cROL is oxidized to 11cRAL for cone pigment regeneration by a retinol dehydrogenase[43] (Figure 2).
AFFECTING FACTORS
Dark adaptation is susceptible to various factors in different ways.
Aging
Dark adaptation, especially the rod-mediated phase, is delayed in the older adults[44],[45]. An explanation is that the regeneration of photopigments could be disturbed with the incensement of age[46]. Several evidences found some changes in the RPE-Bruch's membrane complex of the older adults, including the accumulation of lipofuscin in the RPE, the altered structure of RPE, the accumulation of extracellular material between the RPE and Bruch's membrane[47], the thicken Bruch's membrane, and the reduced hydraulic conductivity of Bruch's membrane[48]. These changes may serve as barriers of the visual cycle. However, the degenerative changes of the photoreceptors should not be neglected, the rods are preferentially affected by aging and degenerate prior to the cones[49]. Taken together, the rod-mediated dark adaptation impairment was greater and faster than cone's.
Hypoxia
Hypoxia can occur in both physiological and pathological states. The former refers to the exposure to hypoxia at high altitude in mountaineers and pilots, while the latter refers to these diseases including cardiac diseases, or local occlusive vascular diseases. Oxygen supply of the retina derives from two independent vascular systems, the choroidal circulation which provides oxygen to the outer retina, while the retinal circulation which provides the oxygen to the inner retina.
The photoreceptor layer is completely free of blood vessels in all mammals. Oxygen supply of the photoreceptors is via the diffusion of oxygen from the adjacent vascular structures. But the contribution of oxygen to visual function depends on the conditions of light and dark. Under light conditions, all of the O2 come from the choroidal circulation. In contrast, under darkness condition, O2 diffuse from both the choroid and the retinal circulation[50]. Both the avascular nature of the outer retina and the high oxygen demands of the photoreceptors place this region at the risk of hypoxic insult. This risk exacerbate after dark adaptation due to the increasing oxygen consumption of photoreceptors[51].
Dark adaptation is highly sensitive to the hypoxia. Even in the normal people, dark adaptation sensitivity begins to drop when there is a slight reduction of inspired oxygen which is equivalent to ascending to 4000 feet (1219 m)[52]. Hypoxia might impair the regeneration of photopigment due to the lack of metabolic energy, which is dependent on the supply of oxygen[53]. However, one study on the systemic hypoxia with chronic respiratory insufficiency found that the dark adaptation was relatively normal. The differential vulnerability was partly caused by the effect of PaCO2 on the lumen of vessels. It has been shown that PaCO2 is a strong stimulator of hypercapnia and it can increase the blood flow in choroidal and retinal vessels to counteract the effect of hypoxia[54].
Supplementary oxygen may increase the arterial oxygen tension, and then enhance the delivery of oxygen from the choroidal and retinal circulation to the photoreceptors. Some investigations showed that 100% oxygen could hasten rod-mediated adaptation[55]. From the practical perspective, it is necessary for the pilot or the mountaineers to breathe oxygen even on the ground, which will not only preserve the dark adaptation, but also hastens the process of dark adaptation.
Glare
When the eye is under dark adaptation, exposure to the glare can results in instantaneous bleaching of photopigments, while the regeneration of photopigments requires a few seconds to a few minutes. During this period, the eye cannot see certain objects or their details. Because vision impairment or vision loss is rapid and takes time to recover, glare can be hazardous. Aging, smoke and disease can increase the susceptibility to glare and prolong the recovery time from glare[54]. These phenomenon usually occur in the night flight and driving, which may lead to accidents and incidents[56],[57]. Accordingly, during night operations crew should be taught to avoid bright lights, such as look away from the bright lights, shield eyes if possible or keep one eye closed in order to protect the other, because the process of dark adaptation is independent of each eye.
Smoke
Many studies demonstrated that the dark adaptation could be impaired by smoking[58]. However, a few reports suggested there was no relationship existed between the dark vision and the smoking[59]. The discrepancy may be caused by the differences in the methodology and the duration of smoking. A study showed that recent smoking significantly affected the dark adaptation through reducing the blood flow of retinal circulation, increasing blood viscosity[60] and the vasoconstrictive action of nicotine[61]. Synchronously, the binding of CO to hemoglobin impaired the release of oxygen to the tissues which further compromised the micro-environmental retinal conditions, and all together created a state of hypoxia in retina. Another experiment in mice showed that the exposure to cigarette smoke caused a significant reduction in the function of both rods and cones, particularly under dark-adapted conditions[62]. It was seen that there were morphological changes in the RPE/Bruch's membrane complex, which further impaired the photoreceptor cell integrity. In addition, cigarette smoke exposure might reduce the amount of pigment and the rate of limiting enzyme (RPE65) necessary for the regeneration of photopigments.
DISEASES
Age-related Maculopathy
Age-related maculopathy (ARM) is a heterogeneous disorder affects the photoreceptors, RPE, Bruch's membrane and choriocapillaris, and causes irreversible blindness. It has been known that the dark adaptation was sensitive to these primary macular changes. Many studies found that the rod-mediated dark adaptation was impaired in early ARM[63],[64], including the delay of rod-cone break and the reduction of rod sensitivity. While the cone-mediated dark adaptation remained normal or near-normal[46],[65],[66]. Morphological changes of Bruch's membrane-RPE complex have been observed in AMD, such as the thickening of Bruch's membrane, the deposits in sub-RPE. These changes hampered the regeneration of photopigments and the nutrition of photoreceptors[67]. It was found that giving a high dose short-term course of retinol (preformed vitamin A) could improve the dark adaptation in persons with early ARM[68]. Not surprisingly, the dark adaptation delays do not occur in the cone system during early ARM, because cones have another cycle to regenerate the photopigments[2]. Recent studies reported that the cone dark adaptation was impaired in older adults with early AMD or in those at high-risk of early AMD[69]. It could be caused by the disturbances in the cone-specific retinoid cycle, and the competition with rods for retinoid in the canonical visual cycle[70].
Vitamin A Deficiency
Vitamin A is critical for photoreceptors function. It has been known that vitamin A deficiency led to a slower dark adaptation[71],[72]. In the initial stages of vitamin A deficiency (VAD), rod-mediated adaptation was impaired, while the final level of rhodopsin, the dark-adapted rod threshold, and the cone-mediated adaptation could be entirely normal. During moderate VAD, the rod-threshold would be elevated, and thereafter all rod function was lost, while the final cone-threshold still remained normal, except the rate of dark adaptation was slowed. With the precession of VAD, cone function showed abnormality which occurred in the rods[73],[74].
Effects of vitamin A treatment could be obvious and rapid. Many studies showed the effects could occur within a day after supplementation with vitamin A[75], as evidenced by the kinetics and final levels (both rod dark adaptation and rhodopsin) returned to normal after treatment. What is more interesting, the recovery of cone-mediated dark adaption was faster than that of rods after treatment[73]. Moreover, increased levels of vitamin A might accelerate the rate of transport between the RPE and the outer segments.
Retinitis pigmentosa
Retinitis pigmentosa (RP) is a group of heterogeneous disorders that characterized by the degeneration of the photoreceptors and RPE[76]. At the early stage, dark adaptation was slowed down and the final threshold was elevated with a biphasic dark adaptation curve[77],[78]. As the progress of disease, the rod-mediated dark adaption was totally lost and the curve showed monophasic[79]. It might be caused by the slow removal of bleaching byproducts that desensitize the rods and interfere with normal photopigments. Another possibility may be the slow photopigments regeneration kinetics and the degeneration of both the photoreceptors and the RPE. Theses together contribute to the abnormality of dark adaptation.
Sorsby Fundus Dystrophy
It is a progressive degeneration of the macula caused by the mutations in the tissue inhibitor metalloproteinases-3 (TIMP-3)[80]. Many experimental evidences demonstrated that both rod-mediated and cone-mediated dark adaptation was slow down, the rod-cone breaks were delayed, and finally the absolute threshold was raised in the sorsby fundus dystrophy's (SFD) retinas [81],[82]. The reversal of these effects by vitamin A supplementation suggested that the deficiency of vitamin A, which is caused by increased barriers between RPE and the choroidal circulation should be responsible for these features.
Fundus Albipunctatus
It is a type of congenital stationary night blindness (CSNB) caused by mutations in the 11-cis RDH5 gene[83]. Dark adaptation and rhodopsin regeneration rate are markedly delayed[84]. But after prolonged dark adaptation (>2h), rod sensitivity improves to the almost normal levels. In other words, the final rhodopsin levels and the final visual thresholds return to normal[85].
METHODS OF MEASURING DARK ADAPTATION
Dark adaptation was mainly recorded by adaptometers in the living human eye, which can be divided into two groups: the canonical adaptometer and the rapid adaptometer. The former includes Goldmann-Weekers, Roland, Metro-vision, and YAK-II. After bleaching, the patient is given a stimulus which changes dynamically according to the response. The examination takes at least 30min. It is very exhausting and the patient may get sleepy. The standard for dark adaptation has long been the Goldmann-Weekers dark adaptometer, which can test different regions of the retina by using an 11° achromatic stimulus. While the methods was limited (5 ascending, 5 descending) and difficult to understand for some patients. The Roland adaptometer has a lot of possibilities how to do a measurement, such as threshold value (the lowest sensitivity without a bleaching) and full program (with a bleaching). The parameters including the fixation (20° by default), the bleaching (7000 cd/m2 is the default and optimal value), the stimulation phases and the intensity strategy can be changed/selected depending on needs, but the 7000 cd/m2 is too bright for many subjects in our experiment. One interesting thing is that the stimulus is red and green lights during the dark adaptation, which helps to identify the different sensitivity between the cone and rod. The Metro-vision has different illumination for each type instrument, during the dark phase, there are 10° white spot lights presented at the center of the screen which is larger than others. YAK-II adaptometer tests various functions of dark adapted eye, including the scotopic sensitivity, rapid dark adaptation and the absolute threshold, while the stimulus was limited.
Due to the long duration and high patient burden, the examination is impeded from the clinical use. There are some rapid adaptometers or methods accessible. Recently, a short-duration dark adaptation protocol was proposed. The AdaptDx adopts a short-high photoflash for bleach (0.8ms duration, 1.8×10[4] scotopic cd/m2 sec intensity), equivalent to 76% bleaching level for rods. Sensitivity measurements begin immediately after bleaching. The whole test only takes 6.5min, while the diagnostic sensitivity and specificity was similar to the longer duration protocols[86]. Others take the Purkinje shift as indicator for about 6min, which the sensitivity of eye changes from the red end of the visible spectrum toward the blue end when shifting from photopic to scotopic vision.
According to our literature, the dark adaptation of animal was detected mainly through optokinetic response (OKR), reflection densitometry, and electroretinogram (ERG).
OKR is a behavior that an animal vibrates its eyes to follow a rotating grating around it, which is known spatial frequency, contrast and velocity. It has been widely used to assess the visual functions in various animals[87]. In order to record a clean OKR without the interference of the vestibulo-ocular reflex (VOR), it is essential to prevent body movements during the optokinetic stimulation.
As reflection densitometry[88], a conventional way of monitoring regeneration of photopigments in the living eye, has been applied in a number of species, it has generally been found that rhodopsin regeneration in other mammalian species is slower than that in human, and sometimes more clearly rate-limited.
In addition to reflection densitometry, the dark adaptation of animal can also be monitored by the technique of ERG. The rod circulating current can be monitored in vivo by recording the scotopic a-wave of the ERG, while the cone visual function was assessed by delivering an initial flash that bleached virtually all rod and cone visual pigments and then following recovery of the cone-initiated b-wave under conditions in which rod signals were suppressed by a pretest flash. However, in laboratory animals such recordings require the use of general anaesthesia. Unfortunately, anaesthestics slow the time course of rhodopsin regeneration. Halothane completely blocks regeneration in mice, while the standard anaesthetics use for ERG experiments in rodents, a mixture of ketamine and xylazine, retard regeneration[89].
DISCUSSION
Dark adaptation mainly depends on the regeneration of the photopigments in both the canonical pathway and the cone-specific pathway. Two pathways are able to promote substantial cone pigment regeneration, the canonical pathway alone is sufficient for complete rod pigment regeneration, while the combined actions of rods and cones are essential for the complete dark adaptation. The elucidation of two visual cycles provides the basis for further investigations on the properties of dark adaptation. With manipulation of mouse genes became increasingly easy, many knockout (KO) strains lack proteins of retinoid cycle have been created, which have provided a powerful tool for investigation the nature and the roles of the proteins in the visual cycle, such as irbp KO mice[90], rpe65 KO mice, cralbp KO mice[91], abca4 KO mice[26] and lrat KO mice[92].
Dark adaptation has been known as a tool, particularly in the early diagnosis of diseases and screening for specific occupations, such as pilots and seamen, but has not received much attention in its clinic uses: 1) The long duration of the examination. As mention, the test usually takes at least 30min. It is very wearisome for the patient and can not apply to large-scale screening; 2) No standards parameters for dark adaptometers. The parameters are greatly different among all adaptometers, which resulted the outcomes cannot be comparable between different instruments and laboratories, such as the illumination and duration of light adaptation (Table 1) and the size of pupil (dilated or not), these all may lead to different levels of bleach and different rates of photopigments regeneration. During the dark phase, the color and size of stimulus also affect the process in dark phase; 3) It is a subjective examination. During the process, the patient should push the button when he/she can feel the light, which means the results depend on the patient's cooperation. These together would substantially impede the use of dark adaptation in clinical work. It will be of great interest to study how to monitor this function.
Table 1. The illumination and duration of light adaptation.
| Adaptometer | Illumination (cd/m2) | Duration |
| Goldmann-Weekers | 445-668 | 5min |
| Metro-vision | 780/250 | 5min |
| Roland | 7000 | 5min |
| YAK-II | 640 | 5min |
| AdaptDx | 1.8×104 | 0.8ms |
More and more studies investigate the properties of dark adaptation in animal diseases model through ERG. The CSNB rat lacked distinct b-wave of scotopic 3.0 ERG, and the animal models of glaucoma, diabetic retinopathy, and light induced retina damage showed significant reduced amplitude in scotopic 3.0 ERG. On the other hand, the parameters of adaptometers also attract more attention. We are trying to identify the effect of color and size of stimulus on the dark phase. In addition, we are trying to figure out some objective parameters to monitor this examination, such as pupil light reflex, which controls the diameter of the pupil, in response to the luminance of light that falls on the retina, thereby assisting in adaptation to various levels of lightness/darkness.
CONCLUSION
In numerous status, dark adaptation impairment, especially the rod-mediated is substantial and can be used as a practical diagnostic aid for the clinic use, with the advancement of the technology, more and more methods or instruments have been applied, but there are many shortcomings limited the clinic use of dark adaption. Future explorations are now trying to figure out some better way to understand and monitor this examination. Furthermore, the nature and the roles of proteins and kinetic properties of many visual enzymes in the visual cycle remain to be studied.
Acknowledgments
We wish to thank three anonymous referees for their critical review of the manuscript and many constructive suggestions.
Conflicts of Interest: Yang GQ, None; Chen T, None; Tao Y, None; Zhang ZM, None.
REFERENCES
- 1.Wang JS, Estevez ME, Cornwall MC, Kefalov VJ. Intra-retinal visual cycle required for rapid and complete cone dark adaptation. Nat Neurosci. 2009;12(3):295–302. doi: 10.1038/nn.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muniz A, Villazana-Espinoza ET, Hatch AL, Trevino SG, Allen DM, Tsin AT. A novel cone visual cycle in the cone-dominated retina. Exp Eye Res. 2007;85(2):175–184. doi: 10.1016/j.exer.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molday RS. ATP-binding cassette transporter ABCA4: molecular properties and role in vision and macular degeneration. J Bioenerg Biomembr. 2007;39(5–6):507–517. doi: 10.1007/s10863-007-9118-6. [DOI] [PubMed] [Google Scholar]
- 4.Quazi F, Lenevich S, Molday RS. ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer. Nat Commun. 2012;3:925. doi: 10.1038/ncomms1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molday LL, Rabin AR, Molday RS. ABCR expression in foveal cone photoreceptors and its role in Stargardt macular dystrophy. Nat Genet. 2000;25(3):257–258. doi: 10.1038/77004. [DOI] [PubMed] [Google Scholar]
- 6.Huang WC, Cideciyan AV, Roman AJ, Sumaroka A, Sheplock R, Schwartz SB, Stone EM, Jacobson SG. Inner and outer retinal changes in retinal degenerations associated with ABCA4 mutations. Invest Ophthalmol Vis Sci. 2014;55(3):1810–1822. doi: 10.1167/iovs.13-13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rattner A, Smallwood PM, Nathans J. Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol. J Biol Chem. 2000;275(15):11034–11043. doi: 10.1074/jbc.275.15.11034. [DOI] [PubMed] [Google Scholar]
- 8.Kolesnikov AV, Ala-Laurila P, Shukolyukov SA, Crouch RK, Wiggert B, Estevez ME, Govardovskii VI, Cornwall MC. Visual cycle and its metabolic support in gecko photoreceptors. Vision Res. 2007;47(3):363–374. doi: 10.1016/j.visres.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Haeseleer F, Jang GF, Imanishi Y, Driessen CA, Matsumura M, Nelson PS, Palczewski K. Dual-substrate specificity short chain retinol dehydrogenases from the vertebrate retina. J Biol Chem. 2002;277(47):45537–45546. doi: 10.1074/jbc.M208882200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker RO, Crouch RK. Retinol dehydrogenases (RDHs) in the visual cycle. Exp Eye Res. 2010;91(6):788–792. doi: 10.1016/j.exer.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C, Thompson DA, Koutalos Y. Reduction of all-trans-retinal in vertebrate rod photoreceptors requires the combined action of RDH8 and RDH12. J Biol Chem. 2012;287(29):24662–24670. doi: 10.1074/jbc.M112.354514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qtaishat NM, Wiggert B, Pepperberg DR. Interphotoreceptor retinoid-binding protein (IRBP) promotes the release of all-trans retinol from the isolated retina following rhodopsin bleaching illumination. Exp Eye Res. 2005;81(4):455–463. doi: 10.1016/j.exer.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Jin M, Li S, Nusinowitz S, Lloyd M, Hu J, Radu RA, Bok D, Travis GH. The role of interphotoreceptor retinoid-binding protein on the translocation of visual retinoids and function of cone photoreceptors. J Neurosci. 2009;29(5):1486–1495. doi: 10.1523/JNEUROSCI.3882-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw NS, Noy N. Interphotoreceptor retinoid-binding protein contains three retinoid binding sites. Exp Eye Res. 2001;72(2):183–190. doi: 10.1006/exer.2000.0945. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Fernandez F, Baer CA, Ghosh D. Module structure of interphotoreceptor retinoid-binding protein (IRBP) may provide bases for its complex role in the visual cycle - structure/function study of Xenopus IRBP. BMC Biochem. 2007;8:15. doi: 10.1186/1471-2091-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiser PD, Golczak M, Palczewski K. Chemistry of the retinoid (visual) cycle. Chem Rev. 2014;114(1):194–232. doi: 10.1021/cr400107q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu J, Bok D. Culture of highly differentiated human retinal pigment epithelium for analysis of the polarized uptake, processing, and secretion of retinoids. Methods Mol Biol. 2010;652:55–73. doi: 10.1007/978-1-60327-325-1_2. [DOI] [PubMed] [Google Scholar]
- 18.Canada FJ, Law WC, Rando RR, Yamamoto T, Derguini F, Nakanishi K. Substrate specificities and mechanism in the enzymatic processing of vitamin A into 11-cis-retinol. Biochemistry. 1990;29(41):9690–9697. doi: 10.1021/bi00493a026. [DOI] [PubMed] [Google Scholar]
- 19.Perusek L, Maeda T. Vitamin A derivatives as treatment options for retinal degenerative diseases. Nutrients. 2013;5(7):2646–2666. doi: 10.3390/nu5072646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moiseyev G, Takahashi Y, Chen Y, Gentleman S, Redmond TM, Crouch RK, Ma JX. RPE65 is an iron(II)-dependent isomerohydrolase in the retinoid visual cycle. J Biol Chem. 2006;281(5):2835–2840. doi: 10.1074/jbc.M508903200. [DOI] [PubMed] [Google Scholar]
- 21.Kiser PD, Golczak M, Lodowski DT, Chance MR, Palczewski K. Crystal structure of native RPE65, the retinoid isomerase of the visual cycle. Proc Natl Acad Sci U S A. 2009;106(41):17325–17330. doi: 10.1073/pnas.0906600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi Y, Moiseyev G, Ma JX. Identification of key residues determining isomerohydrolase activity of human RPE65. J Biol Chem. 2014;289(39):26743–26751. doi: 10.1074/jbc.M114.558619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McBee JK, Van Hooser JP, Jang GF, Palczewski K. Isomerization of 11-cis-retinoids to all-trans-retinoids in vitro and in vivo. J Biol Chem. 2001;276(51):48483–48493. doi: 10.1074/jbc.M105840200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim TS, Maeda A, Maeda T, Heinlein C, Kedishvili N, Palczewski K, Nelson PS. Delayed dark adaptation in 11-cis-retinol dehydrogenase-deficient mice: a role of RDH11 in visual processes in vivo. J Biol Chem. 2005;280(10):8694–8704. doi: 10.1074/jbc.M413172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farjo KM, Moiseyev G, Takahashi Y, Crouch RK, Ma JX. The 11-cis-retinol dehydrogenase activity of RDH10 and its interaction with visual cycle proteins. Invest Ophthalmol Vis Sci. 2009;50(11):5089–5097. doi: 10.1167/iovs.09-3797. [DOI] [PubMed] [Google Scholar]
- 26.Quazi F, Molday RS. ATP-binding cassette transporter ABCA4 and chemical isomerization protect photoreceptor cells from the toxic accumulation of excess 11-cis-retinal. Proc Natl Acad Sci U S A. 2014;111(13):5024–5029. doi: 10.1073/pnas.1400780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Noy N. Retinoid specificity of interphotoreceptor retinoid-binding protein. Biochemistry. 1994;33(35):10658–10665. doi: 10.1021/bi00201a013. [DOI] [PubMed] [Google Scholar]
- 28.Adler AJ, Edwards RB. Human interphotoreceptor matrix contains serum albumin and retinol-binding protein. Exp Eye Res. 2000;70(2):227–234. doi: 10.1006/exer.1999.0780. [DOI] [PubMed] [Google Scholar]
- 29.Hood DC, Hock PA. Recovery of cone receptor activity in the frog's isolated retina. Vision Res. 1973;13(10):1943–1951. doi: 10.1016/0042-6989(73)90065-5. [DOI] [PubMed] [Google Scholar]
- 30.Muniz A, Villazana-Espinoza ET, Thackeray B, Tsin AT. 11-cis-Acyl-CoA:retinol O-acyltransferase activity in the primary culture of chicken Muller cells. Biochemistry. 2006;45(40):12265–12273. doi: 10.1021/bi060928p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garlipp MA, Nowak KR, Gonzalez-Fernandez F. Cone outer segment extracellular matrix as binding domain for interphotoreceptor retinoid-binding protein. J Comp Neurol. 2012;520(4):756–769. doi: 10.1002/cne.22773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Betts-Obregon BS, Gonzalez-Fernandez F, Tsin AT. Interphotoreceptor retinoid-binding protein (IRBP) promotes retinol uptake and release by rat Muller cells (rMC-1) in vitro: implications for the cone visual cycle. Invest Ophthalmol Vis Sci. 2014;55(10):6265–6271. doi: 10.1167/iovs.14-14721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaylor JJ, Yuan Q, Cook J, Sarfare S, Makshanoff J, Miu A, Kim A, Kim P, Habib S, Roybal CN, Xu T, Nusinowitz S, Travis GH. Identification of DES1 as a vitamin A isomerase in Muller glial cells of the retina. Nat Chem Biol. 2013;9(1):30–36. doi: 10.1038/nchembio.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaylor JJ, Cook JD, Makshanoff J, Bischoff N, Yong J, Travis GH. Identification of the 11-cis-specific retinyl-ester synthase in retinal Muller cells as multifunctional O-acyltransferase (MFAT) Proc Natl Acad Sci U S A. 2014;111(20):7302–7307. doi: 10.1073/pnas.1319142111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci U S A. 2005;102(38):13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci U S A. 2005;102(35):12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mata NL, Ruiz A, Radu RA, Bui TV, Travis GH. Chicken retinas contain a retinoid isomerase activity that catalyzes the direct conversion of all-trans-retinol to 11-cis-retinol. Biochemistry. 2005;44(35):11715–11721. doi: 10.1021/bi050942m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gollapalli DR, Rando RR. Molecular logic of 11-cis-retinoid biosynthesis in a cone-dominated species. Biochemistry. 2003;42(50):14921–14929. doi: 10.1021/bi0356505. [DOI] [PubMed] [Google Scholar]
- 39.Travis GH, Kaylor J, Yuan Q. Analysis of the retinoid isomerase activities in the retinal pigment epithelium and retina. Methods Mol Biol. 2010;652:329–339. doi: 10.1007/978-1-60327-325-1_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bustamante JJ, Ziari S, Ramirez RD, Tsin AT. Retinyl ester hydrolase and the visual cycle in the chicken eye. Am J Physiol. 1995;269(6 Pt 2):R1346–R1350. doi: 10.1152/ajpregu.1995.269.6.R1346. [DOI] [PubMed] [Google Scholar]
- 41.Parker R, Wang JS, Kefalov VJ, Crouch RK. Interphotoreceptor retinoid-binding protein as the physiologically relevant carrier of 11-cis-retinol in the cone visual cycle. J Neurosci. 2011;31(12):4714–4719. doi: 10.1523/JNEUROSCI.3722-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker RO, Crouch RK. The interphotoreceptor retinoid binding (IRBP) is essential for normal retinoid processing in cone photoreceptors. Adv Exp Med Biol. 2010;664:141–149. doi: 10.1007/978-1-4419-1399-9_17. [DOI] [PubMed] [Google Scholar]
- 43.Mata NL, Radu RA, Clemmons RC, Travis GH. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36(1):69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patryas L, Parry NR, Carden D, Baker DH, Kelly JM, Aslam T, Murray IJ. Assessment of age changes and repeatability for computer-based rod dark adaptation. Graefes Arch Clin Exp Ophthalmol. 2013;251(7):1821–1827. doi: 10.1007/s00417-013-2324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaffney AJ, Binns AM, Margrain TH. Aging and cone dark adaptation. Optom Vis Sci. 2012;89(8):1219–1224. doi: 10.1097/OPX.0b013e318263c6b1. [DOI] [PubMed] [Google Scholar]
- 46.Owsley C, McGwin GJ, Jackson GR, Kallies K, Clark M. Cone- and rod-mediated dark adaptation impairment in age-related maculopathy. Ophthalmology. 2007;114(9):1728–1735. doi: 10.1016/j.ophtha.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 47.Curcio CA, Millican CL, Bailey T, Kruth HS. Accumulation of cholesterol with age in human Bruch's membrane. Invest Ophthalmol Vis Sci. 2001;42(1):265–274. [PubMed] [Google Scholar]
- 48.Starita C, Hussain AA, Pagliarini S, Marshall J. Hydrodynamics of ageing Bruch's membrane: implications for macular disease. Exp Eye Res. 1996;62(5):565–572. doi: 10.1006/exer.1996.0066. [DOI] [PubMed] [Google Scholar]
- 49.Jackson GR, Owsley C. Scotopic sensitivity during adulthood. Vision Res. 2000;40(18):2467–2473. doi: 10.1016/s0042-6989(00)00108-5. [DOI] [PubMed] [Google Scholar]
- 50.Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003;121(4):547–557. doi: 10.1001/archopht.121.4.547. [DOI] [PubMed] [Google Scholar]
- 51.Yu DY, Cringle SJ. Outer retinal anoxia during dark adaptation is not a general property of mammalian retinas. Comp Biochem Physiol A Mol Integr Physiol. 2002;132(1):47–52. doi: 10.1016/s1095-6433(01)00528-1. [DOI] [PubMed] [Google Scholar]
- 52.Petrassi FA, Hodkinson PD, Walters PL, Gaydos SJ. Hypoxic hypoxia at moderate altitudes: review of the state of the science. Aviat Space Environ Med. 2012;83(10):975–984. doi: 10.3357/asem.3315.2012. [DOI] [PubMed] [Google Scholar]
- 53.Connolly DM, Hosking SL. Aviation-related respiratory gas disturbances affect dark adaptation: a reappraisal. Vision Res. 2006;46(11):1784–1793. doi: 10.1016/j.visres.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 54.Thylefors J, Piitulainen E, Havelius U. Dark adaptation during systemic hypoxia induced by chronic respiratory insufficiency. Invest Ophthalmol Vis Sci. 2009;50(3):1307–1312. doi: 10.1167/iovs.08-2104. [DOI] [PubMed] [Google Scholar]
- 55.Mainster MA, Turner PL. Glare's causes, consequences, and clinical challenges after a century of ophthalmic study. Am J Ophthalmol. 2012;153(4):587–593. doi: 10.1016/j.ajo.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Nakagawara VB, Montgomery RW, Wood KJ. Aircraft accidents and incidents associated with visual effects from bright light exposures during low-light flight operations. Optometry. 2007;78(8):415–420. doi: 10.1016/j.optm.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 57.Nakagawara VB, Wood KJ, Montgomery RW. Laser exposure incidents: pilot ocular health and aviation safety issues. Optometry. 2008;79(9):518–524. doi: 10.1016/j.optm.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 58.Wiley RW. Dark adaptation and recovery from light adaptation: smokers versus nonsmokers. Mil Med. 1989;154(8):427–430. [PubMed] [Google Scholar]
- 59.Hammond BJ, Jr, Wenzel AJ, Luther MS, Rivera RO, King SJ, Choate ML. Scotopic sensitivity: relation to age, dietary patterns, and smoking status. Optom Vis Sci. 1998;75(12):867–872. doi: 10.1097/00006324-199812000-00008. [DOI] [PubMed] [Google Scholar]
- 60.Lowe GD, Drummond MM, Forbes CD, Barbenel JC. The effects of age and cigarette-smoking on blood and plasma viscosity in men. Scott Med J. 1980;25(1):13–17. doi: 10.1177/003693308002500103. [DOI] [PubMed] [Google Scholar]
- 61.Havelius U, Hansen F. Ocular vasodynamic changes in light and darkness in smokers. Invest Ophthalmol Vis Sci. 2005;46(5):1698–1705. doi: 10.1167/iovs.04-0756. [DOI] [PubMed] [Google Scholar]
- 62.Woodell A, Coughlin B, Kunchithapautham K, Casey S, Williamson T, Ferrell WD, Atkinson C, Jones BW, Rohrer B. Alternative complement pathway deficiency ameliorates chronic smoke-induced functional and morphological ocular injury. PLoS One. 2013;8(6):e67894. doi: 10.1371/journal.pone.0067894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iannaccone A. Measuring dark adaptation in the elderly: a predictor of who may develop macular degeneration? Invest Ophthalmol Vis Sci. 2014;55(8):4790. doi: 10.1167/iovs.14-15135. [DOI] [PubMed] [Google Scholar]
- 64.Owsley C, Huisingh C, Jackson GR, et al. Associations between abnormal rod-mediated dark adaptation and health and functioning in older adults with normal macular health. Invest Ophthalmol Vis Sci. 2014;55(8):4776–4789. doi: 10.1167/iovs.14-14502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steinmetz RL, Haimovici R, Jubb C, Fitzke FW, Bird AC. Symptomatic abnormalities of dark adaptation in patients with age-related Bruch's membrane change. Br J Ophthalmol. 1993;77(9):549–554. doi: 10.1136/bjo.77.9.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Owsley C, Jackson GR, White M, Feist R, Edwards D. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology. 2001;108(7):1196–1202. doi: 10.1016/s0161-6420(01)00580-2. [DOI] [PubMed] [Google Scholar]
- 67.Jackson GR, Owsley C, Curcio CA. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res Rev. 2002;1(3):381–396. doi: 10.1016/s1568-1637(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 68.Owsley C, McGwin G, Jackson GR, Heimburger DC, Piyathilake CJ, Klein R, White MF, Kallies K. Effect of short-term, high-dose retinol on dark adaptation in aging and early age-related maculopathy. Invest Ophthalmol Vis Sci. 2006;47(4):1310–1318. doi: 10.1167/iovs.05-1292. [DOI] [PubMed] [Google Scholar]
- 69.Dimitrov PN, Guymer RH, Zele AJ, Anderson AJ, Vingrys AJ. Measuring rod and cone dynamics in age-related maculopathy. Invest Ophthalmol Vis Sci. 2008;49(1):55–65. doi: 10.1167/iovs.06-1048. [DOI] [PubMed] [Google Scholar]
- 70.Gaffney AJ, Binns AM, Margrain TH. Topography of cone dark adaptation deficits in age-related maculopathy. Optom Vis Sci. 2011;88(9):1080–1087. doi: 10.1097/OPX.0b013e3182223697. [DOI] [PubMed] [Google Scholar]
- 71.Abbott-Johnson WJ, Kerlin P, Abiad G, Clague AE, Cuneo RC. Dark adaptation in vitamin A-deficient adults awaiting liver transplantation: improvement with intramuscular vitamin A treatment. Br J Ophthalmol. 2011;95(4):544–548. doi: 10.1136/bjo.2009.179176. [DOI] [PubMed] [Google Scholar]
- 72.Abebe H, Abebe Y, Loha E, Stoecker BJ. Consumption of vitamin a rich foods and dark adaptation threshold of pregnant women at Damot Sore District, Wolayita, Southern Ethiopia. Ethiop J Health Sci. 2014;24(3):219–226. doi: 10.4314/ejhs.v24i3.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kemp CM, Jacobson SG, Faulkner DJ, Walt RW. Visual function and rhodopsin levels in humans with vitamin A deficiency. Exp Eye Res. 1988;46(2):185–197. doi: 10.1016/s0014-4835(88)80076-9. [DOI] [PubMed] [Google Scholar]
- 74.Collins CE, Koay P. Xerophthalmia because of dietary-induced vitamin a deficiency in a young Scottish man. Cornea. 2010;29(7):828–829. doi: 10.1097/ICO.0b013e3181bd9ed5. [DOI] [PubMed] [Google Scholar]
- 75.Congdon NG, Dreyfuss ML, Christian P, Navitsky RC, Sanchez AM, Wu LS, Khatry SK, Thapa MD, Humphrey J, Hazelwood D, West KJ. Responsiveness of dark-adaptation threshold to vitamin A and beta-carotene supplementation in pregnant and lactating women in Nepal. Am J Clin Nutr. 2000;72(4):1004–1009. doi: 10.1093/ajcn/72.4.1004. [DOI] [PubMed] [Google Scholar]
- 76.Haim M. Epidemiology of retinitis pigmentosa in Denmark. Acta Ophthalmol Scand Suppl. 2002;(233):1–34. doi: 10.1046/j.1395-3907.2002.00001.x. [DOI] [PubMed] [Google Scholar]
- 77.Herse P. Retinitis pigmentosa: visual function and multidisciplinary management. Clin Exp Optom. 2005;88(5):335–350. doi: 10.1111/j.1444-0938.2005.tb06717.x. [DOI] [PubMed] [Google Scholar]
- 78.Alexander KR, Fishman GA. Prolonged rod dark adaptation in retinitis pigmentosa. Br J Ophthalmol. 1984;68(8):561–569. doi: 10.1136/bjo.68.8.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Konieczka K, Flammer AJ, Todorova M, Meyer P, Flammer J. Retinitis pigmentosa and ocular blood flow. EPMA J. 2012;3(1):17. doi: 10.1186/1878-5085-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Langton KP, McKie N, Smith BM, Brown NJ, Barker MD. Sorsby's fundus dystrophy mutations impair turnover of TIMP-3 by retinal pigment epithelial cells. Hum Mol Genet. 2005;14(23):3579–3586. doi: 10.1093/hmg/ddi385. [DOI] [PubMed] [Google Scholar]
- 81.Steinmetz RL, Polkinghorne PC, Fitzke FW, Kemp CM, Bird AC. Abnormal dark adaptation and rhodopsin kinetics in Sorsby's fundus dystrophy. Invest Ophthalmol Vis Sci. 1992;33(5):1633–1636. [PubMed] [Google Scholar]
- 82.Cideciyan AV, Pugh EJ, Lamb TD, Huang Y, Jacobson SG. Rod plateaux during dark adaptation in Sorsby's fundus dystrophy and vitamin A deficiency. Invest Ophthalmol Vis Sci. 1997;38(9):1786–1794. [PubMed] [Google Scholar]
- 83.Gonzalez-Fernandez F, Kurz D, Bao Y, Newman S, Conway BP, Young JE, Han DP, Khani SC. 11-cis retinol dehydrogenase mutations as a major cause of the congenital night-blindness disorder known as fundus albipunctatus. Mol Vis. 1999;5:41. [PubMed] [Google Scholar]
- 84.Cideciyan AV, Haeseleer F, Fariss RN, Aleman TS, Jang GF, Verlinde CL, Marmor MF, Jacobson SG, Palczewski K. Rod and cone visual cycle consequences of a null mutation in the 11-cis-retinol dehydrogenase gene in man. Vis Neurosci. 2000;17(5):667–678. doi: 10.1017/s0952523800175029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Niwa Y, Kondo M, Ueno S, Nakamura M, Terasaki H, Miyake Y. Cone and rod dysfunction in fundus albipunctatus with RDH5 mutation: an electrophysiological study. Invest Ophthalmol Vis Sci. 2005;46(4):1480–1485. doi: 10.1167/iovs.04-0638. [DOI] [PubMed] [Google Scholar]
- 86.Jackson GR, Scott IU, Kim IK, Quillen DA, Iannaccone A, Edwards JG. Diagnostic sensitivity and specificity of dark adaptometry for detection of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55(3):1427–1431. doi: 10.1167/iovs.13-13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi Q, Stell WK. Die Fledermaus: regarding optokinetic contrast sensitivity and light-adaptation, chicks are mice with wings. PLoS One. 2013;8(9):e75375. doi: 10.1371/journal.pone.0075375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hagins WA, Rushton WA. The measurement of rhodopsin in the decerebrate albino rabbit. J Physiol. 1953;120(4):61P. [PubMed] [Google Scholar]
- 89.Keller C, Grimm C, Wenzel A, Hafezi F, Reme C. Protective effect of halothane anesthesia on retinal light damage: inhibition of metabolic rhodopsin regeneration. Invest Ophthalmol Vis Sci. 2001;42(2):476–480. [PubMed] [Google Scholar]
- 90.Sato K, Li S, Gordon WC, He J, Liou GI, Hill JM, Travis GH, Bazan NG, Jin M. Receptor interacting protein kinase-mediated necrosis contributes to cone and rod photoreceptor degeneration in the retina lacking interphotoreceptor retinoid-binding protein. J Neurosci. 2013;33(44):17458–17468. doi: 10.1523/JNEUROSCI.1380-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saari JC, Nawrot M, Kennedy BN, Garwin GG, Hurley JB, Huang J, Possin DE, Crabb JW. Visual cycle impairment in cellular retinaldehyde binding protein (CRALBP) knockout mice results in delayed dark adaptation. Neuron. 2001;29(3):739–748. doi: 10.1016/s0896-6273(01)00248-3. [DOI] [PubMed] [Google Scholar]
- 92.Fu Y, Zhang T. Pathophysilogical mechanism and treatment strategies for Leber congenital amaurosis. Adv Exp Med Biol. 2014;801:791–796. doi: 10.1007/978-1-4614-3209-8_99. [DOI] [PMC free article] [PubMed] [Google Scholar]