Abstract
IMPORTANCE
Diabetic macular nonperfusion leads to decreased perifoveal capillary blood flow, which in turn causes chronic ischemia of the retinal tissue. Using point-to-point correlation between spectral-domain optical coherence tomography (SD-OCT) and nonperfusion on fluorescein angiography, we observed that retinal capillary nonperfusion is associated with photoreceptor compromise on OCT. This study highlights a new concept of a possible contribution of the retinal deep capillary plexus to photoreceptor compromise in diabetic retinopathy in the absence of diabetic macular edema.
OBJECTIVE
To report outer retinal structural changes associated with enlargement of the foveal avascular zone and/or capillary nonperfusion in the macular area of diabetic patients.
DESIGN, SETTING, AND PARTICIPANTS
Retrospective observational cross-sectional study in 9 patients who were diagnosed as having diabetic retinopathy without diabetic macular edema and underwent fluorescein angiography and SD-OCT for diabetic retinopathy from July 8, 2014, to December 1, 2014, at a tertiary academic referral center. This analysis was conducted between December 2, 2014, and January 31, 2015.
MAIN OUTCOMES AND MEASURES
Outer retinal changes on SD-OCT in areas of macular ischemia.
RESULTS
The study included 13 eyes of 9 diabetic patients (4 men and 5 women aged 34–58 years) with a mean duration of diabetes mellitus of 14.5 years. Nine eyes showed outer retinal disruption revealed by SD-OCT that colocalized to areas of enlargement of the foveal avascular zone and macular capillary nonperfusion. Four fellow eyes with normal foveal avascular zones did not show any retinal changes on SD-OCT.
CONCLUSIONS AND RELEVANCE
Macular ischemia in diabetic patients can be associated with photoreceptor compromise. The presence of disruption of the photoreceptors on OCT in diabetic patients can be a manifestation of underlying capillary nonperfusion in eyes without diabetic macular edema. Ischemia at the deep capillary plexus may play an important role in these outer retinal changes.
The human retina has a high oxygen demand that is supplied by 2 nonoverlapping vascular layers. While the inner retina is exclusively supplied by the retinal circulation, the outer retina, which is avascular, obtains its oxygen supply primarily via diffusion from the choroidal circulation and only minimally from the retinal circulation.1,2 Retinal vascular supply in the macula consists of 3 capillary plexuses. The superficial capillary plexus lies in the retinal nerve fiber layer or ganglion cell layer, while the middle capillary plexus and deep capillary plexus (DCP) are located on the inner and outer borders of the inner nuclear layer, respectively. It has been reported that the DCP contributes 10% to 15% of the oxygen supply to the photoreceptor cells, especially during dark adaptation.3
Because the foveola and perifoveal macula are completely avascular, their oxygen supply depends mainly on the choriocapillaris,4 although lateral diffusion of oxygen from the surrounding retinal capillaries may also contribute. Indeed, this capillary-free zone is termed the foveal avascular zone (FAZ) on fluorescein angiography (FA) and can vary in size significantly even in healthy individuals.5,6
Macular ischemia and enlargement of the FAZ can occur in a variety of retinal vascular diseases, including diabetic retinopathy (DR), hypertensive retinopathy, retinal vein occlusion, and sickle cell disease,5,7–12 leading to decreased perifoveal capillary blood flow and in turn causing chronic ischemia of the retinal tissue.5 Several studies in the 1970s used FA to define the functional importance of macular ischemia,5,9,10 which is now considered an important predictor of poor functional outcome in patients with diabetes mellitus (DM).13
Recently, spectral-domain optical coherence tomography (SD-OCT) has allowed more direct in vivo assessment of the retinal microstructure. Several OCT studies have shown thinning of the inner retinal layers, including the ganglion cell layer and retinal nerve fiber layer, in patients with DM.14–21
We hypothesized that macular ischemia and enlargement of the FAZ may contribute to outer retinal and photoreceptor disruption on SD-OCT in diabetic patients in the absence of diabetic macular edema (DME), as a possible consequence of ischemia at the level of the DCP. To investigate our hypothesis, we performed detailed analysis of 13 eyes of 9 diabetic patients, focusing on 9 eyes with various degrees of macular ischemia and macular capillary nonperfusion without DME.
Methods
We performed a retrospective analysis of the imaging database to identify all patients with diabetic macular nonperfusion who underwent FA and SD-OCT examinations in the Department of Ophthalmology, Northwestern University, Chicago, Illinois, from July 8, 2014, to December 1, 2014. This analysis was conducted between December 2, 2014, and January 31, 2015. The institutional review board at Northwestern University approved this research protocol. Informed consent was not required owing to the retrospective nature of this study.
We included patients with enlargement of the FAZ or capillary nonperfusion within the macula in the setting of either nonproliferative DR (NPDR) or proliferative DR (PDR). Fellow eyes with a normal FAZ were included as controls.
We excluded eyes with DME and eyes with other associated retinal diseases, such as age-related macular degeneration or vitreomacular traction. We also excluded eyes that had undergone surgical retinal repair or intravitreal anti–vascular endothelial growth factor or steroid injection and patients who received laser treatment in the preceding 5 years and/or any laser treatment in the nonperfused area in the macula as revealed on FA. Patients with significant cataracts graded higher than NO3 or NC3 were excluded to avoid an optical artifact that may compromise SD-OCT image quality.
The FA images were evaluated by the agreement of 2 independent masked graders (L.M.J. and A.A.F.) to define enlargement of the FAZ and area of nonperfusion and to stage the DR using protocols and standard photographs from the Early Treatment Diabetic Retinopathy Study report number 11.22–25 Based on these criteria, diabetic macular ischemia was identified as absent or present. In situations of disagreement between graders, consensus was reached by an open discussion. Medical records were reviewed to gather information about visual acuity, data regarding slitlamp biomicroscopy, and history of retinal laser treatment.
Fluorescein angiography was performed according to the standard procedure (TRC-50DX; Topcon Corp). It was also used to identify angiographic macular edema defined as dye leakage within a 500-μm radius from the fovea or a 1500-μm radius in cases with hard exudates in this area. An early-phase FA (20–30 seconds) and a late-frame FA (3–5 minutes) were selected for this analysis.
Diabetic macular edema was diagnosed as either clinical or with SD-OCT or angiographic evidence of macular edema, and these eyes were excluded from this study.
The SD-OCT images were obtained using the Spectralis HRA+OCT imaging device (Heidelberg Engineering). The SD-OCT imaging protocol comprised 31 horizontal B-scans per volume scan of 20° × 20° in the macula, and each B-scan included an average of 30 frames. Retinal volume was measured using the incorporated software of the SD-OCT. The tracking feature of the Heidelberg Spectralis HRA+OCT was used. This feature allows every OCT B-scan to be registered to its exact location on the infrared image, which was superimposed onto the relevant FA frames, hence facilitating point-to-point correlations of the retinal imaging findings between the FA and SD-OCT.
Two observers (L.M.J. and A.A.F.), masked to any associated information and FA findings, analyzed the pathologic changes on the SD-OCT scans independently. For each patient, the scans were qualitatively evaluated as normal or with the presence of thinning of the inner retina and/or outer nuclear layer (ONL) or disruption of the external limiting membrane, inner segment–outer segment (IS/OS) junction, and OS–retinal pigment epithelium junction. In situations of disagreement between readers, consensus was reached by an open discussion.
Results
This study included a total of 13 eyes of 9 diabetic patients aged 34 to 58 years. The mean duration of DM was 14.5 years. Nine eyes showed either an enlargement of the FAZ and/or capillary nonperfusion in the macula, which tightly colocalized with outer retinal disruption on the SD-OCT. We did not see generalized outer retinal disruption in these patients, but rather a very close spatial correspondence between the outer retinal disruption and the region of FAZ enlargement and/or macular nonperfusion, suggesting a possible causal relationship. Four fellow eyes with a normal FAZ and macular perfusion served as controls and did not show any retinal abnormalities on SD-OCT. Five fellow eyes that did not meet the inclusion criteria were excluded. The demographic characteristics and ocular findings are summarized in the Table.
Table.
Demographic and Clinical Findings of Study Population
| Case No. | Sex/Age, y | DM Type | Duration of DM, y | Glycated Hb, % of Total Hb | HTN | HLD | KD | Study Eye | BCVA | DR Stage | Laser Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/early 50s | 2 | 12 | 12.3 | Yes | Yes | No | Right | 20/25 | PDR | Macular and PRP |
| 2 | F/mid-30s | 2 | 16 | 8.1 | Yes | No | No | Right (control) | 20/25 | Severe NPDR | None |
| Left | 20/20 | Severe NPDR | None | ||||||||
| 3 | M/late 50s | 2 | 6 | NA | Yes | No | No | Right | 20/30 | PDR | PRP |
| Left (control) | 20/30 | PDR | PRP | ||||||||
| 4 | F/late 50s | 2 | 20 | 9.2 | Yes | Yes | No | Left | 20/30 | PDR | Macular and PRP |
| 5 | M/early 40s | 2 | 15 | 5.9 | Yes | No | Yes | Right | 20/125 | PDR | PRP |
| 6 | M/early 50s | 2 | 12 | NA | Yes | No | No | Left | 20/50 | PDR | PRP |
| 7a | M/late 30s | 1 | 25 | 6.4 | Yes | No | No | Left | 20/30 | PDR | PRP |
| 8 | M/mid-50s | 2 | 10 | 8.4 | Yes | No | Yes | Right | 20/20 | PDR | PRP |
| Left (control) | 20/25 | PDR | PRP | ||||||||
| 9 | M/early 40s | 2 | 0.25 | NA | Yes | Yes | No | Right (control) | 20/20 | Moderate NPDR | None |
| Left | 20/30 | Moderate NPDR | None |
Abbreviations: BCVA, best-corrected visual acuity; DM, diabetes mellitus; DR, diabetic retinopathy; Hb, hemoglobin; HLD, hyperlipidemia; HTN, hypertension; KD, kidney disease; NA, not available; NPDR, nonproliferative DR; PDR, proliferative DR; PRP, panretinal photocoagulation.
SI conversion factor: To convert glycated Hb to proportion of total Hb, multiply by 0.01.
The patient had undergone pancreas and kidney transplantation 4 years prior to presentation.
Imaging Findings
The imaging findings in eyes with capillary nonperfusion are shown in Figure 1, Figure 2, and Figure 3. Figure 4 shows an example of a nonischemic control fellow eye (case 2).
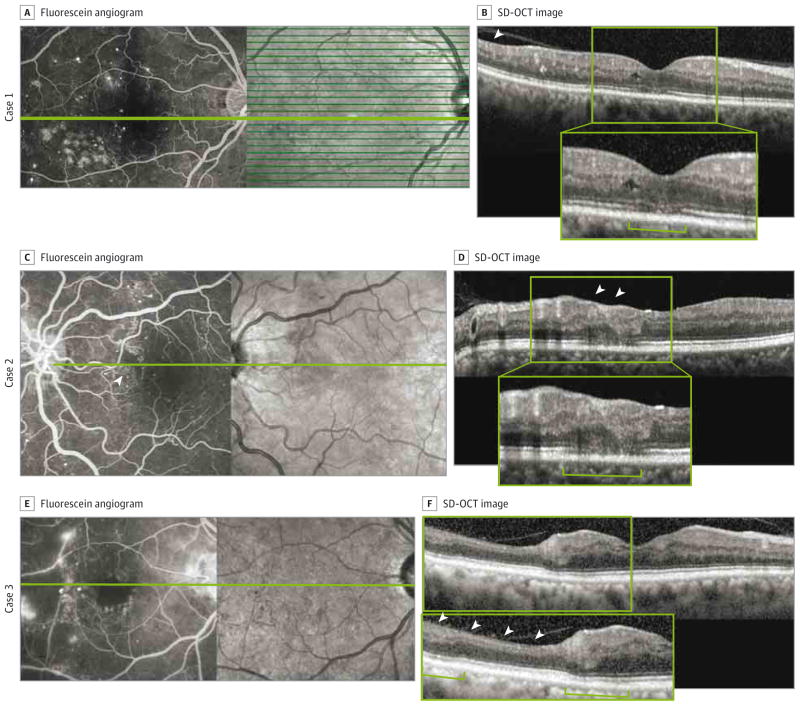
Figure 1. Macular Nonperfusion and Outer Retinal Changes in Cases 1, 2, and 3.
A-F, Macular nonperfusion and outer retinal changes are shown in cases 1 (A and B), 2 (C and D), and 3 (E and F). A, C, and E, Fluorescein angiography reveals enlargement of the foveal avascular zone and areas of capillary nonperfusion (arrowhead in C). B, D, and F, Spectral-domain optical coherence tomography (SD-OCT) shows thinning of the retinal nerve fiber layer and inner nuclear layer (arrowheads), thinning of the outer nuclear layer, disruption or decreased intensity of the inner segment–outer segment and outer segment–retinal pigment epithelium junctions, and shortening of the photoreceptor outer segments (brackets). Insets in B, D, and F show enlarged views of the SD-OCT images.
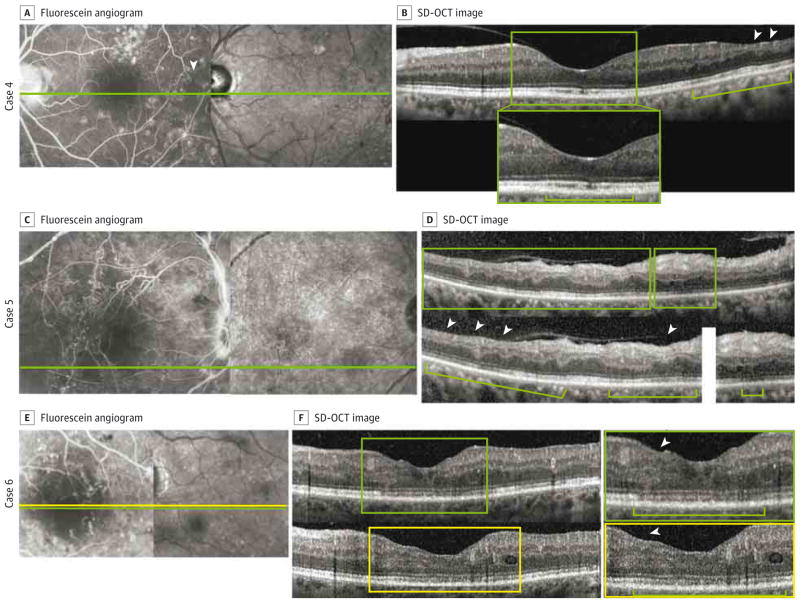
Figure 2. Macular Nonperfusion and Outer Retinal Changes in Cases 4, 5, and 6.
A–F, Macular nonperfusion and outer retinal changes are shown in cases 4 (A and B), 5 (C and D), and 6 (E and F). A, C, and E, Fluorescein angiography reveals enlargement of the foveal avascular zone and areas of capillary nonperfusion (arrowhead in A). B, D, and F, Spectral-domain optical coherence tomography (SD-OCT) shows thinning of the retinal nerve fiber layer and inner nuclear layer (arrowheads), shortening of the photoreceptor outer segments (brackets), and disruption of the inner segment–outer segment and outer segment–retinal pigment epithelium junctions (inset in B and top inset in F). Insets in B, D, and F show enlarged views of the SD-OCT images (top inset in F is from the scan at the green line in E, and bottom inset in F is from the scan at the yellow line in E).
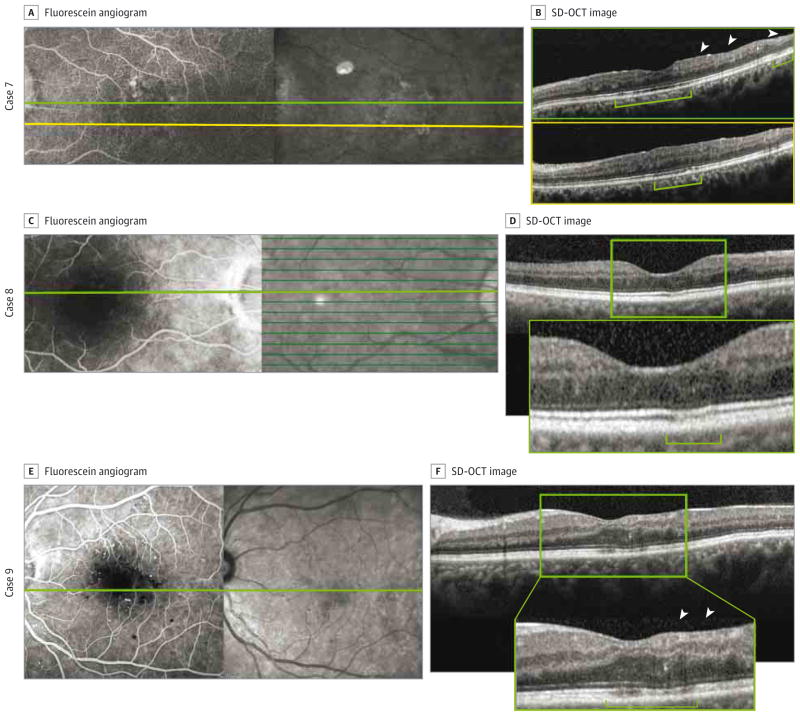
Figure 3. Macular Nonperfusion and Outer Retinal Changes in Cases 7, 8, and 9.
A–F, Macular nonperfusion and outer retinal changes are shown in cases 7 (A and B), 8 (C and D), and 9 (E and F). A, C, and E, Fluorescein angiography reveals enlargement of the foveal avascular zone. B, D, and F, Spectral-domain optical coherence tomography (SD-OCT) shows thinning of the retinal nerve fiber layer and inner nuclear layer (arrowheads in B and F), disruption or decreased intensity of the inner segment–outer segment and outer segment–retinal pigment epithelium junctions (B and D), thinning of the outer nuclear layer (F), and increased visibility of the Henle fiber layer (brackets). B, Top image is from the scan at the green line in A, and bottom image is from the scan at the yellow line in A. Insets in D and F show enlarged views of the SD-OCT images.
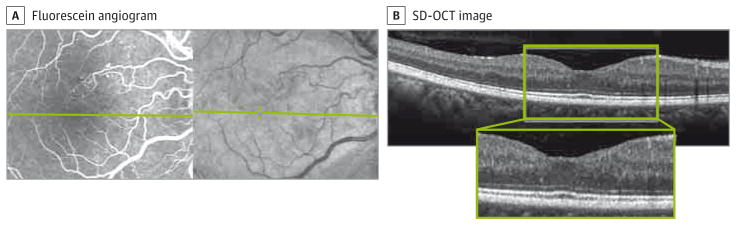
Figure 4. Normal Foveal Avascular Zone and Macular Perfusion With Normal Outer Retina on Optical Coherence Tomography.
Fellow eye of case 2, included in the study as a control. A, Fluorescein angiography shows a normal foveal avascular zone. B, Inner and outer retinal findings on spectral-domain optical coherence tomography (SD-OCT), with no foveal avascular zone abnormalities or capillary nonperfusion. Inset in B shows enlarged view of the SD-OCT image.
Case Summaries
Case 1
A man in his early 50s had a history of hypertension, hyperlipidemia, and type 2 DM treated with insulin and oral medications (pioglitazone hydrochloride and glipizide). The right eye, included in the study, had undergone macular laser treatment inferotemporal to the fovea 5 years prior. The macular laser treatment was outside the nonperfused area revealed by FA, and there was no evidence of DME either clinically or angiographically (Figure 1A and B). The left eye was excluded because of DME.
Case 2
A woman in her mid-30s had a history of hypertension and type 2 DM treated with insulin. Her DM was recently controlled with a rapid decrease in glycated hemoglobin from 11.3% to 8.1% of total hemoglobin (to convert to proportion of total hemoglobin, multiply by 0.01). Both eyes were included in the study. The left eye had severe NPDR and macular capillary nonperfusion (Figure 1C and D). The right eye served as a control because it showed normal macular perfusion (Figure 4).
Case 3
A man in his late 50s had a history of hypertension and type 2 DM treated with oral medications (nateglinide, sitagliptin phosphate, and glipizide). Both eyes were included in the study. In the right eye, FA showed an enlargement of the FAZ, a large area of nonperfusion, and the presence of fine retinal neovascularization temporal to the macula (Figure 1E and F). The left eye had a normal FAZ and no retinal abnormalities on SD-OCT scans and served as a control.
Case 4
A woman in her late 50s had a history of hypertension, hyperlipidemia, and type 2 DM treated with insulin. The right eye was excluded because of the presence of DME and previous anti–vascular endothelial growth factor injections. The left eye, included in the study, had undergone macular laser treatment superior to the fovea 5 years prior. The macular laser treatment was outside the area of macular nonperfusion on FA and the eye did not show any evidence of angiographic or clinical DME (Figure 2A and B).
Case 5
A man in his early 40s had a history of hypertension, chronic kidney disease, and type 2 DM treated with insulin. The right eye, included in the study, showed evidence of macular non-perfusion (Figure 2C and D). The left eye had undergone surgery for traction retinal detachment and was excluded.
Case 6
A man in his early 50s had a history of hypertension and type 2 DM treated with an oral medication (glipizide) and insulin. The right eye was excluded because of the presence of DME and previous anti–vascular endothelial growth factor injections. The left eye, included in the study, had macular ischemia in the setting of inactive PDR (Figure 2E and F).
Case 7
A man in his late 30s had a history of hypertension and type 1 DM and had undergone pancreas and kidney transplantation 4 years prior to presentation. The right eye was excluded because of the presence of a vitreous hemorrhage. The left eye, included in the study, had macular capillary nonperfusion in the setting of inactive PDR and a history of cataract extraction (Figure 3A and B).
Case 8
A man in his mid-50s had a history of hypertension and type 2 DM treated with an oral medication (glipizide) and insulin. Both eyes were included in the study. The right eye had no active PDR. Optical coherence tomography showed disruption of the outer retinal layers that colocalized with an enlargement of the FAZ (Figure 3C and D). The left eye had inactive PDR and served as a control because it showed normal macular perfusion.
Case 9
A man in his early 40s had a history of hypertension, hyperlipidemia, and recently diagnosed uncontrolled type 2 DM that was treated with oral medication (metformin hydrochloride). Both eyes were included in the study. The right eye had NPDR and served as a control because it showed a normal FAZ. The left eye had macular capillary nonperfusion in the setting of moderate NPDR (Figure 3E and F).
Discussion
The use of multimodal imaging in this series of 13 eyes of 9 diabetic patients highlights a possible contribution of macular non-perfusion to photoreceptor disruption in DR. In contrast with previous reports, our analysis illustrated that enlargement of the FAZ and capillary nonperfusion in the macula not only leads to thinning of the inner retina but can also be associated with outer retinal changes, including disruption of the external limiting membrane and the IS/OS junction as well as thinning of the ONL and photoreceptors, in diabetic patients. By confining our studies to eyes without DME, we excluded the potential confounding effects of chronic macular edema on the outer retina. In our series, we found no eyes in which the outer retina was completely normal on SD-OCT when there was an enlargement of the FAZ. The size of the FAZ is determined by the most central, deep capillaries; hence, as the FAZ expands, it is likely that the DCP is affected. The correlation we observed therefore suggests that deep capillary ischemia in the setting of DR might lead to disruption of the outer retina in diabetic patients with macular nonperfusion.
Wangsa-Wirawan and Linsenmeier26 reviewed the evidence that the photoreceptor ISs are the most important consumers of choroidal oxygen. The outer retinal oxygen gradient shows a steep decline with the nadir at the IS. Also, studies of cat retinas have highlighted that oxygen tension in the retina is highest at the choroid, rapidly decreases at the level of the ONL/ISs, and then increases again in the inner retinal layers.2,27,28 In the dark, the watershed retinal layers, where both the choroidal and retinal circulations might provide oxygen support, are where the oxygen tension decreases to its lowest level.3 The photoreceptor axon terminals reside in the outer plexiform layer (OPL) and are replete with mitochondria to supply their energy.29 The DCP is located at the inner aspect of the OPL and is unquestionably important for nutrition of photoreceptor synapses. However, the retinal circulation is also responsible for about 15% of the metabolic demand of the ISs, especially in darkness. Because of this, the photoreceptor and the OPL in this watershed zone may be particularly susceptible to ischemic insults affecting the choroid and/or DCP.
Currently, FA is the most widely used clinical tool to evaluate abnormalities of the smaller vessels and capillaries.30,31 Little is known about the influence of the size of the FAZ on photoreceptor layer integrity.
Using Adaptive Optics scanning laser ophthalmoscopy, Pinhas et al32 recently showed that healthy young individuals could have occult nonperfused retinal capillaries adjacent to the FAZ. They suggested these might represent normal variation and/or an early sign of pathology, more extensive in the vasculopathic eyes.32 It is important to note that FA (including Adaptive Optics scanning laser ophthalmoscopy–based FA) does not reveal features of the DCP,33 which has only recently been recognized as an important player in retinal pathogenesis.34 Thus, it is not surprising that previous studies could not reach definitive conclusions regarding the effect of angiographic capillary nonperfusion or macular ischemia on retinal sublayers in diabetic patients.35–38
Previous studies have revealed that macular ischemia in the setting of DME may be associated with photoreceptor OS shortening and disruption of the IS/OS junction.36,39 However, because visibility of the IS/OS junction is highly susceptible to OCT signal strength, the possibility that an indistinct IS/OS junction line could be related to an optical artifact of the overlying cystic changes could not be completely excluded in these studies. Furthermore, it is not clear in these studies whether these eyes had long-standing DME and hence secondary photoreceptor disruption39 or whether the thinning was potentially related to prior laser therapy. To this point, another more recent study did not find significant associations between FAZ size and the mean area of the disrupted IS/OS junction or the external limiting membrane in patients with treatment-naive DME.38
Several studies explored the relationship between the non-perfused areas on FA and thinning of the inner retinal layers on OCT in eyes with DR21,40 as well as in patients with retinal vein occlusion, but none of these studies reported the concurrent presence of outer retinal changes.41–43 In our series, all 9 eyes of 9 patients showed characteristics of photoreceptor disruption. Eight of these eyes showed thinning of the inner retinal layers as well. While the inner retinal findings are compatible with prior reports,32,37,38 we additionally reveal the association of diabetic capillary nonperfusion with outer retinal OCT changes in eyes without DME. Moreover, by overlaying the FA to the OCT, we confirm that these areas of outer retinal disruption on OCT correlated tightly to zones of capillary non-perfusion and showed no angiographic leakage in the late phase.
It is intriguing to speculate about the exact mechanism whereby capillary nonperfusion on FA can explain both the thinning of the inner retina and the photoreceptor changes. We can think of at least 3 additional mechanisms related to capillary nonperfusion in diabetic patients that could contribute to outer retinal changes in DR, including (1) concomitant vascular diseases, (2) acidosis in the outer retina following ischemia, and (3) “diabetic choroidopathy.” Furthermore, we know that FA is only able to show the distribution of the superficial capillary network, leading to a significant lack of information regarding the DCP network.32 The missing information about the DCP might be important in understanding the pathological changes in the outer retina on SD-OCT. We believe these outer retinal changes in the setting of capillary nonperfusion are likely specific to the metabolic derangements associated with DR. Indeed, cases of the most severe retinal capillary ischemia, namely central retinal artery occlusion, maintain a robust A-wave44 and an intact outer retinal structure on OCT. In cases of central retinal artery occlusion, the normal choroidal blood supply is sufficiently robust to meet the photoreceptor oxygen demand in these situations.
Evaluating the SD-OCT of our patients, we find that the outer retinal findings on OCT appeared very similar to late findings in classic acute macular neuroretinopathy (Figure 1C and D, case 2). It has already been suggested34 that the initial OPL/ONL hyperreflectivity on SD-OCT in acute macular neuroretinopathy may reflect diffuse edema of photoreceptor cell bodies and their axons because of ischemia of DCP. Over time, these patients develop thinning of the ONL and disruption of the IS/OS and OS/retinal pigment epithelium junctions. We believe that in our series of diabetic patients, several systemic risk factors, such as hypertension, hyperlipidemia, kidney dysfunction, and hyperglycemia, contributed to severe ischemic insult of the retina as shown by the capillary nonperfusion on FA, resulting in ischemia involving all of the retinal capillary layers, particularly the DCP. However, because of the potential of OCT angiography to better visualize the DCP compared with FA, further studies are necessary to validate our findings in diabetic patients.45
Additionally, outer retinal acidosis can occur in the setting of ischemia and contribute to photoreceptor compromise. We cannot exclude its contribution to photoreceptor disruption on OCT in our series.46
Finally, while our study was not designed to examine the choroidal contribution, concurrent outer retinal ischemia related to ischemia of the choroid due to diabetic choroidopathy cannot be excluded.47–51
We acknowledge the inherent limitation of our retrospective series including relatively small numbers of eyes. This was mainly related to the strict exclusion criteria we applied to limit our study to eyes with macular nonperfusion in the absence of DME.
Conclusions
We believe that ischemic maculopathy with underlying ischemia of the DCP may play an important role in photoreceptor compromise in diabetic patients. Based on our findings on OCT and corresponding FA, we propose that clinicians carefully consider outer retinal structural changes on OCT in the setting of DR because these changes may be harbingers of overlying areas of retinal capillary nonperfusion. Future studies to investigate visual function, including microperimetry, are needed to delineate the functional consequences of photoreceptor compromise in this population.
At a Glance.
In 9 eyes diagnosed as having either nonproliferative or proliferative diabetic retinopathy, a possible association is shown between macular areas of capillary nonperfusion on fluorescein angiography and outer retinal disruption on spectral-domain optical coherence tomography.
In this setting, the retinal deep capillary plexus may play an important role in these photoreceptor abnormalities.
In diabetic retinopathy, areas of retinal nonperfusion can be associated with photoreceptor structural abnormalities.
The finding of outer retinal disruption in diabetic retinopathy on spectral-domain optical coherence tomography might predict overlying areas of capillary nonperfusion.
Acknowledgments
Funding/Support: This work was supported in part by grant R01EY019951 from the National Eye Institute (Dr Fawzi) and by a grant from Research to Prevent Blindness to the Department of Ophthalmology, Northwestern University.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Contributions: Drs Scarinci and Fawzi had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fawzi.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Scarinci.
Critical revision of the manuscript for important intellectual content: Jampol, Linsenmeier, Fawzi.
Obtained funding: Fawzi.
Administrative, technical, or material support: Scarinci, Fawzi.
Study supervision: Fawzi.
References
- 1.Haugh LM, Linsenmeier RA, Goldstick TK. Mathematical models of the spatial distribution of retinal oxygen tension and consumption, including changes upon illumination. Ann Biomed Eng. 1990;18(1):19–36. doi: 10.1007/BF02368415. [DOI] [PubMed] [Google Scholar]
- 2.Linsenmeier RA, Braun RD. Oxygen distribution and consumption in the cat retina during normoxia and hypoxemia. J Gen Physiol. 1992;99(2):177–197. doi: 10.1085/jgp.99.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birol G, Wang S, Budzynski E, Wangsa-Wirawan ND, Linsenmeier RA. Oxygen distribution and consumption in the macaque retina. Am J Physiol Heart Circ Physiol. 2007;293(3):H1696–H1704. doi: 10.1152/ajpheart.00221.2007. [DOI] [PubMed] [Google Scholar]
- 4.Hildebrand GD, Fielder AR. Anatomy and physiology of the retina. In: Reynolds J, Olitsky S, editors. Pediatric Retina. Berlin, Germany: Springer-Verlag Berlin Heidelberg; 2011. pp. 39–65. [Google Scholar]
- 5.Arend O, Wolf S, Jung F, et al. Retinal microcirculation in patients with diabetes mellitus: dynamic and morphological analysis of perifoveal capillary network. Br J Ophthalmol. 1991;75(9):514–518. doi: 10.1136/bjo.75.9.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu PK, Balaratnasingam C, Cringle SJ, McAllister IL, Provis J, Yu DY. Microstructure and network organization of the microvasculature in the human macula. Invest Ophthalmol Vis Sci. 2010;51(12):6735–6743. doi: 10.1167/iovs.10-5415. [DOI] [PubMed] [Google Scholar]
- 7.Konno S, Feke GT, Yoshida A, Fujio N, Goger DG, Buzney SM. Retinal blood flow changes in type I diabetes: a long-term follow-up study. Invest Ophthalmol Vis Sci. 1996;37(6):1140–1148. [PubMed] [Google Scholar]
- 8.Nguyen TT, Wang JJ, Sharrett AR, et al. Relationship of retinal vascular caliber with diabetes and retinopathy: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2008;31(3):544–549. doi: 10.2337/dc07-1528. [DOI] [PubMed] [Google Scholar]
- 9.Sakata K, Funatsu H, Harino S, Noma H, Hori S. Relationship between macular microcirculation and progression of diabetic macular edema. Ophthalmology. 2006;113(8):1385–1391. doi: 10.1016/j.ophtha.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Kohner EM, Henkind P. Correlation of fluorescein angiogram and retinal digest in diabetic retinopathy. Am J Ophthalmol. 1970;69(3):403–414. doi: 10.1016/0002-9394(70)92273-7. [DOI] [PubMed] [Google Scholar]
- 11.Yeung L, Lima VC, Garcia P, Landa G, Rosen RB. Correlation between spectral domain optical coherence tomography findings and fluorescein angiography patterns in diabetic macular edema. Ophthalmology. 2009;116(6):1158–1167. doi: 10.1016/j.ophtha.2008.12.063. [DOI] [PubMed] [Google Scholar]
- 12.Bresnick GH, Engerman R, Davis MD, de Venecia G, Myers FL. Patterns of ischemia in diabetic retinopathy. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1976;81(4 pt 1):OP694–OP709. [PubMed] [Google Scholar]
- 13.Sim DA, Keane PA, Zarranz-Ventura J, et al. The effects of macular ischemia on visual acuity in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54(3):2353–2360. doi: 10.1167/iovs.12-11103. [DOI] [PubMed] [Google Scholar]
- 14.Bresnick GH, De Venecia G, Myers FL, Harris JA, Davis MD. Retinal ischemia in diabetic retinopathy. Arch Ophthalmol. 1975;93(12):1300–1310. doi: 10.1001/archopht.1975.01010020934002. [DOI] [PubMed] [Google Scholar]
- 15.Byeon SH, Chu YK, Lee H, Lee SY, Kwon OW. Foveal ganglion cell layer damage in ischemic diabetic maculopathy: correlation of optical coherence tomographic and anatomic changes. Ophthalmology. 2009;116(10):1949–1959. e8. doi: 10.1016/j.ophtha.2009.06.066. [DOI] [PubMed] [Google Scholar]
- 16.Reznicek L, Kernt M, Haritoglou C, Kampik A, Ulbig M, Neubauer AS. In vivo characterization of ischemic retina in diabetic retinopathy. Clin Ophthalmol. 2010;5:31–35. doi: 10.2147/OPTH.S13850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dijk HW, Verbraak FD, Stehouwer M, et al. Association of visual function and ganglion cell layer thickness in patients with diabetes mellitus type 1 and no or minimal diabetic retinopathy. Vision Res. 2011;51(2):224–228. doi: 10.1016/j.visres.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Dijk HW, Verbraak FD, Kok PH, et al. Early neurodegeneration in the retina of type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2012;53(6):2715–2719. doi: 10.1167/iovs.11-8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugimoto M, Sasoh M, Ido M, Narushima C, Uji Y. Retinal nerve fiber layer decrease during glycemic control in type 2 diabetes. J Ophthalmol. doi: 10.1155/2010/569215. published online August 8, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shahidi AM, Sampson GP, Pritchard N, et al. Retinal nerve fibre layer thinning associated with diabetic peripheral neuropathy. Diabet Med. 2012;29(7):e106–e111. doi: 10.1111/j.1464-5491.2012.03588.x. [DOI] [PubMed] [Google Scholar]
- 21.Sim DA, Keane PA, Fung S, et al. Quantitative analysis of diabetic macular ischemia using optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55(1):417–423. doi: 10.1167/iovs.13-12677. [DOI] [PubMed] [Google Scholar]
- 22.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103(12):1796–1806. [PubMed] [Google Scholar]
- 23.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy: ETDRS report number 12. Ophthalmology. 1991;98(5 suppl):823–833. [PubMed] [Google Scholar]
- 24.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs: an extension of the modified Airlie House classification: ETDRS report number 10. Ophthalmology. 1991;98(5 suppl):786–806. [PubMed] [Google Scholar]
- 25.Early Treatment Diabetic Retinopathy Study Research Group. Classification of diabetic retinopathy from fluorescein angiograms: ETDRS report number 11. Ophthalmology. 1991;98(5 suppl):807–822. [PubMed] [Google Scholar]
- 26.Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003;121(4):547–557. doi: 10.1001/archopht.121.4.547. [DOI] [PubMed] [Google Scholar]
- 27.Alder VA, Cringle SJ, Constable IJ. The retinal oxygen profile in cats. Invest Ophthalmol Vis Sci. 1983;24(1):30–36. [PubMed] [Google Scholar]
- 28.Alder VA, Cringle SJ. Vitreal and retinal oxygenation. Graefes Arch Clin Exp Ophthalmol. 1990;228(2):151–157. doi: 10.1007/BF00935725. [DOI] [PubMed] [Google Scholar]
- 29.Stone J, van Driel D, Valter K, Rees S, Provis J. The locations of mitochondria in mammalian photoreceptors: relation to retinal vasculature. Brain Res. 2008;1189:58–69. doi: 10.1016/j.brainres.2007.10.083. [DOI] [PubMed] [Google Scholar]
- 30.Weinhaus RS, Burke JM, Delori FC, Snodderly DM. Comparison of fluorescein angiography with microvascular anatomy of macaque retinas. Exp Eye Res. 1995;61(1):1–16. doi: 10.1016/s0014-4835(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 31.Mendis KR, Balaratnasingam C, Yu P, et al. Correlation of histologic and clinical images to determine the diagnostic value of fluorescein angiography for studying retinal capillary detail. Invest Ophthalmol Vis Sci. 2010;51(11):5864–5869. doi: 10.1167/iovs.10-5333. [DOI] [PubMed] [Google Scholar]
- 32.Pinhas A, Razeen M, Dubow M, et al. Assessment of perfused foveal microvascular density and identification of nonperfused capillaries in healthy and vasculopathic eyes. Invest Ophthalmol Vis Sci. 2014;55(12):8056–8066. doi: 10.1167/iovs.14-15136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snodderly DM, Weinhaus RS, Choi JC. Neural-vascular relationships in central retina of macaque monkeys (Macaca fascicularis) J Neurosci. 1992;12(4):1169–1193. doi: 10.1523/JNEUROSCI.12-04-01169.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fawzi AA, Pappuru RR, Sarraf D, et al. Acute macular neuroretinopathy: long-term insights revealed by multimodal imaging. Retina. 2012;32(8):1500–1513. doi: 10.1097/IAE.0b013e318263d0c3. [DOI] [PubMed] [Google Scholar]
- 35.Sun JK, Lin MM, Lammer J, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132(11):1309–1316. doi: 10.1001/jamaophthalmol.2014.2350. [DOI] [PubMed] [Google Scholar]
- 36.Lee DH, Kim JT, Jung DW, Joe SG, Yoon YH. The relationship between foveal ischemia and spectral-domain optical coherence tomography findings in ischemic diabetic macular edema. Invest Ophthalmol Vis Sci. 2013;54(2):1080–1085. doi: 10.1167/iovs.12-10503. [DOI] [PubMed] [Google Scholar]
- 37.Dmuchowska DA, Krasnicki P, Mariak Z. Can optical coherence tomography replace fluorescein angiography in detection of ischemic diabetic maculopathy? Graefes Arch Clin Exp Ophthalmol. 2014;252(5):731–738. doi: 10.1007/s00417-013-2518-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benitez-Herreros J, Lopez-Guajardo L, Camara-Gonzalez C, Vazquez-Blanco M, Castro-Rebollo M. Association between macular perfusion and photoreceptor layer status in diabetic macular edema. Retina. 2015;35(2):288–293. doi: 10.1097/IAE.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 39.Murakami T, Nishijima K, Akagi T, et al. Optical coherence tomographic reflectivity of photoreceptors beneath cystoid spaces in diabetic macular edema. Invest Ophthalmol Vis Sci. 2012;53(3):1506–1511. doi: 10.1167/iovs.11-9231. [DOI] [PubMed] [Google Scholar]
- 40.Dodo Y, Murakami T, Uji A, Yoshitake S, Yoshimura N. Disorganized retinal lamellar structures in nonperfused areas of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015;56(3):2012–2020. doi: 10.1167/iovs.14-15924. [DOI] [PubMed] [Google Scholar]
- 41.Bowers DK, Finkelstein D, Wolff SM, Green WR. Branch retinal vein occlusion: a clinicopathologic case report. Retina. 1987;7(4):252–259. doi: 10.1097/00006982-198707040-00011. [DOI] [PubMed] [Google Scholar]
- 42.Frangieh GT, Green WR, Barraquer-Somers E, Finkelstein D. Histopathologic study of nine branch retinal vein occlusions. Arch Ophthalmol. 1982;100(7):1132–1140. doi: 10.1001/archopht.1982.01030040110020. [DOI] [PubMed] [Google Scholar]
- 43.Green WR, Chan CC, Hutchins GM, Terry JM. Central retinal vein occlusion: a prospective histopathologic study of 29 eyes in 28 cases. Retina. 1981;1(1):27–55. [PubMed] [Google Scholar]
- 44.Gouras P, Carr RE. Light-induced DC responses of monkey retina before and after central retinal artery interruption. Invest Ophthalmol. 1965;4:310–317. [PubMed] [Google Scholar]
- 45.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20(4):4710–4725. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Birol G, Budzynski E, Wangsa-Wirawan ND, Linsenmeier RA. Retinal arterial occlusion leads to acidosis in the cat. Exp Eye Res. 2005;80(4):527–533. doi: 10.1016/j.exer.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Hidayat AA, Fine BS. Diabetic choroidopathy: light and electron microscopic observations of seven cases. Ophthalmology. 1985;92(4):512–522. [PubMed] [Google Scholar]
- 48.Nagaoka T, Kitaya N, Sugawara R, et al. Alteration of choroidal circulation in the foveal region in patients with type 2 diabetes. Br J Ophthalmol. 2004;88(8):1060–1063. doi: 10.1136/bjo.2003.035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schocket LS, Brucker AJ, Niknam RM, Grunwald JE, DuPont J, Brucker AJ. Foveolar choroidal hemodynamics in proliferative diabetic retinopathy. Int Ophthalmol. 2004;25(2):89–94. doi: 10.1023/b:inte.0000031744.93778.60. [DOI] [PubMed] [Google Scholar]
- 50.Adhi M, Brewer E, Waheed NK, Duker JS. Analysis of morphological features and vascular layers of choroid in diabetic retinopathy using spectral-domain optical coherence tomography. JAMA Ophthalmol. 2013;131(10):1267–1274. doi: 10.1001/jamaophthalmol.2013.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hua R, Liu L, Wang X, Chen L. Imaging evidence of diabetic choroidopathy in vivo: angiographic pathoanatomy and choroidal-enhanced depth imaging. PLoS One. 2013;8(12):e83494. doi: 10.1371/journal.pone.0083494. [DOI] [PMC free article] [PubMed] [Google Scholar]