Abstract
Rhabdomyosarcoma is a relatively common soft tissue sarcoma that frequently affects children and adolescents and may involve the head and neck. Rhabdomyosarcoma is defined by skeletal muscle differentiation which can be suggested by routine histology and confirmed by immunohistochemistry for the skeletal muscle-specific markers myogenin or myoD1. At the same time, it must be remembered that when it comes to head and neck malignancies, skeletal muscle differentiation is not limited to rhabdomyosarcoma. A lack of awareness of this phenomenon could lead to misdiagnosis and, subsequently, inappropriate therapeutic interventions. This review focuses on malignant neoplasms of the head and neck other than rhabdomyosarcoma that may exhibit rhabdomyoblastic differentiation, with an emphasis on strategies to resolve the diagnostic dilemmas these tumors may present. Axiomatically, no primary central nervous system tumors will be discussed.
Keywords: Rhabdomyosarcoma, Rhabdomyoblastic differentiation, Myogenin, MyoD1, Head and neck malignancies, Soft tissue sarcomas, Skeletal muscle differentiation
Introduction
Rhabdomyosarcoma (RMS) is a malignant mesenchymal neoplasm that exhibits skeletal muscle differentiation. RMS exists in two major forms: alveolar RMS which is a “small round cell tumor” that typically harbors t(2;13) or t(1;13) translocations resulting in PAX3-FOXO1A or PAX7- FOXO1A gene fusions; and embryonal RMS which is composed of round to spindle cells and harbors more complex genetic alterations (e.g., loss of the tumor suppressor CDKN2A, mutation/amplification of FGFR4, gain of GLI1, and mutations in the myogenic transcription factor MYOD1) [1–6] (Fig. 1). It is important to distinguish between the alveolar and embryonal types of RMS due to differing prognoses and treatment strategies [7, 8]. Other variants of RMS include sclerosing, spindled, and pleomorphic forms [5]. While RMS is typically encountered in children and young adults, it can also be seen in older adults, especially the alveolar subtype [5, 9, 10]. About 40 % of RMS affect the head and neck, in order of frequency: orbit, sinonasal tract, ear, and oral cavity, with other subsites rarely affected [5, 9, 11–14].
Fig. 1.
Rhabdomyosarcoma (RMS). Alveolar RMS grows between fibrous septa as nests of dyscohesive small round cells with high nuclear-cytoplasmic ratios (a). In the alveolar form of RMS, myogenin immunoexpression is diffuse (b). Embryonal RMS grows as round to spindle cells, often condensing beneath epithelial surfaces in a “cambium layer” (c). Myogenin is also positive in embryonal RMS, but the distribution is less diffuse than what is seen in the alveolar subtype (d)
All forms of RMS are defined, at least in part, by the presence of rhabdomyoblasts—densely eosinophilic polygonal or spindled cells with hyperchromatic nuclei and occasional cytoplasmic cross-striations. While skeletal muscle differentiation can be suggested by histology and desmin immunoreactivity, in the absence of clear-cut cross-striations it must be confirmed by nuclear immunohistochemical staining for myogenin and/or MyoD1, markers with high specificity for skeletal muscle differentiation [5, 15]. It must be remembered, however, that rhabdomyoblastic differentiation may be encountered in neoplasms other than RMS. This distinction is important because RMS is treated by specific chemotherapy protocols that may be different than those of other neoplasms in the differential diagnosis [8, 16]. This article reviews the malignant head and neck tumors other than RMS that may show rhabdomyoblastic differentiation, focusing on diagnostic strategies for distinguishing them from true RMS.
Malignant Triton Tumor
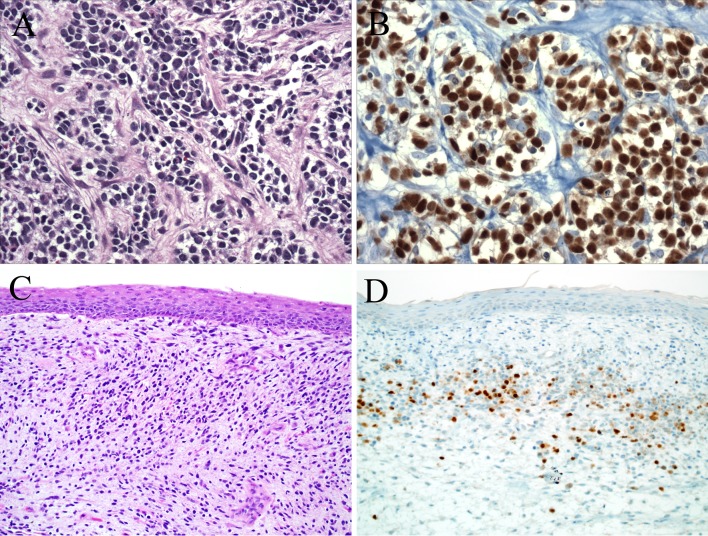
Malignant neoplasms arising in association with peripheral nerves or within pre-existing benign nerve sheath tumors (usually neurofibromas) are known as malignant peripheral nerve sheath tumors (MPNSTs) [17]. MPNSTs are uncommon, representing only 5-10 % of all sarcomas [17, 18]. MPNST typically arises in the deep soft tissue in adults, and may be sporadic or arise in the setting of neurofibromatosis [17]. The head and neck is one of the more common anatomic areas to be affected by MPNSTs [17]. MPNSTs typically grow in a herringbone-type fascicular pattern (“herringbone” refers to a repeating zigzag pattern where the fascicles are very regular and well defined). Classically, “dark” hypercellular areas alternate with “light,” less cellular ones, simulating fetal mesenchyme and leading to a so-called “marbleized” appearance [17, 19]. MPNSTs are highly cellular and exhibits nuclear hyperchromasia, pleomorphism, elevated mitotic rates, and necrosis (Fig. 2). By immunohistochemistry, the nerve sheath markers S100 protein and SOX10 are often positive, but classically are focal in distribution [17, 20]. CD34 is variably expressed, and pancytokeratin and BCL2 are negative or at most, focal [21].
Fig. 2.
Malignant Triton tumor. Malignant peripheral nerve sheath tumor often arises in the background of a benign nerve sheath tumor, usually neurofibroma (a). Malignant peripheral nerve sheath tumor often grows in a herringbone pattern of alternating fascicles. Often lighter staining areas alternate with darker areas creating a “marbleized” appearance (b). Eosinophilic rhabdomyoblasts stand out in the background of the pale staining malignant peripheral nerve sheath tumor (c). The rhabdomyoblasts are highlighted by a myogenin immunostain (d)
Up to 10–15 % of MPNSTs contain heterologous elements, the most frequent of which are rhabdomyoblastic foci [17, 22, 23]. Other reported heterologous elements include benign-appearing glands, islands of bone or cartilage, or angiosarcomatous foci [17, 22, 23]. MPNSTs with rhabdomyoblastic differentiation were first reported by Masson who considered the phenomenon as supporting the origin of these tumors from motor rather than sensory nerves [24]. They were dubbed malignant Triton tumors after early experiments in the Triton salamander in which dissection and ectopic re-implantation of the sciatic nerve was associated with formation of supernumerary ‘limbs’ containing skeletal muscle and bone [22–24]. The phenomenon is consistent with the capacity of neural-crest cells descendants to differentiate into both Schwann cells and various mesenchymal tissues [17, 25]. (As a brief aside, tumors referred to as “benign Triton tumors” are most likely hamartomas and are most likely unrelated.) [26] The majority of malignant Triton tumors occur in the setting of NF1, and as a result, the affected patient is typically young [22, 23]. About a third of malignant Triton tumors affect the head and neck where they can involve virtually any anatomic subsite [27–29]. The rhabdomyoblasts in malignant Triton tumors are typically focal, and they often stand out at low power as their abundant eosinophilic cytoplasm is distinctly different than the pale background Schwannian cells (Fig. 2). As expected, these cells (like the tumor cells of RMS) are positive for desmin and myogenin/MyoD1. Malignant Triton tumors are regarded to behave in an aggressive fashion, even more than usual MPNST, [22, 23, 30, 31] though head and neck cases described as “low-grade” have been reported [32, 33]. It has been suggested that tumors in the sinonasal tract have a more indolent course than those arising in other head and neck sites (however, at least some of those indolent “malignant Triton tumors” could in fact represent the newly-described “low-grade sinonasal sarcoma with neural and myogenic features,” a tumor that lacks myogenin/MyoD1 expression) [34, 35].
Differentiating malignant triton tumor with conspicuous rhabdomyoblasts from an embryonal or spindle cell RMS may be difficult on routinely stained histological sections. The challenge is compounded by the fact that NF1 patients are at increased risk for RMS, and some RMSs may express S100 protein [17, 36]. In contrast to a true RMS, the rhabdomyoblasts in malignant Triton tumors tend to be a relatively focal finding in a background of predominant Schwannian cells that are completely negative for desmin and myogenin and/or MyoD1. If present, a pre-existing benign nerve sheath tumor strongly supports the diagnosis of malignant Triton tumor. If on the other hand, the clinical, histologic, and immunophenotypic picture is most compatible with an embryonal RMS, the finding of some S100 protein positive cells should not dissuade a pathologist from that diagnosis [17].
Sarcomatoid Carcinoma
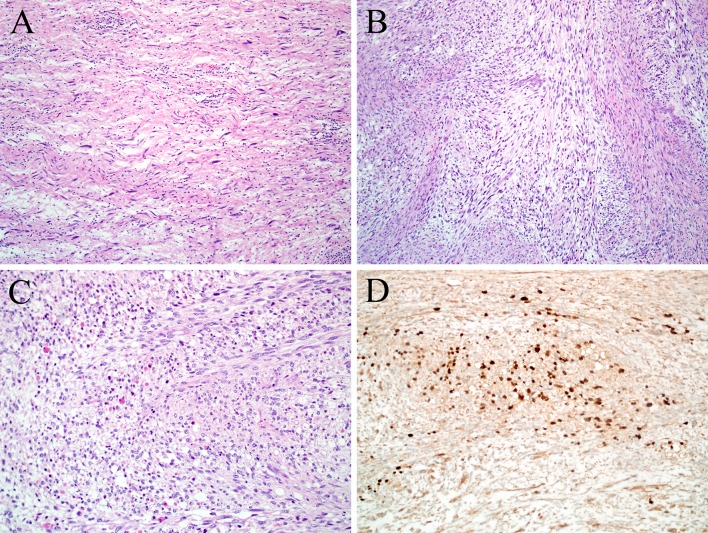
Sarcomatoid carcinoma (i.e., spindle cell variant of squamous cell carcinoma) is a variant of head and neck carcinoma characterized by a prominent or even exclusive population of malignant spindle or pleomorphic cells [37–41]. Once thought to represent a collision tumor between separately arising carcinoma and sarcoma, sarcomatoid carcinoma has since been shown to be a carcinoma derived from squamous epithelium that shows divergent differentiation into cells with mesenchymal features due to epithelial-mesenchymal transition [42–45]. While most sarcomatoid carcinomas demonstrate a haphazard, non-specific growth pattern in the sarcomatoid component of the tumor, 7–15 % of cases exhibit histologically definable heterologous mesenchymal elements like bone, cartilage, and rarely, skeletal muscle [40, 46, 47]. Sarcomatoid carcinomas in the oral cavity and oropharynx appear to be more aggressive, while those of the larynx, and particularly the true vocal cord, have a more favorable prognosis [40, 47–49]. Not surprisingly, the polypoid tumors, regardless of location, are more easily resected and tend to have a better prognosis [40, 50].
While rhabdomyoblastic differentiation is not uncommon in sarcomatoid carcinomas in other organs (especially malignant mixed Müllerian tumors of the uterus), it is quite rare in the mucosa of the head and neck, with only rare reported cases [51–55]. Interestingly, all the reported cases and those seen in our consultation practices arose in the larynx and all were biphasic, with a conventional squamous cell carcinoma component admixed with focal malignant spindle cells exhibiting rhabdomyoblastic features by routine histology and confirmed by immunohistochemistry (Fig. 3). In addition, one of these cases also showed neuroendocrine differentiation [54, 55].
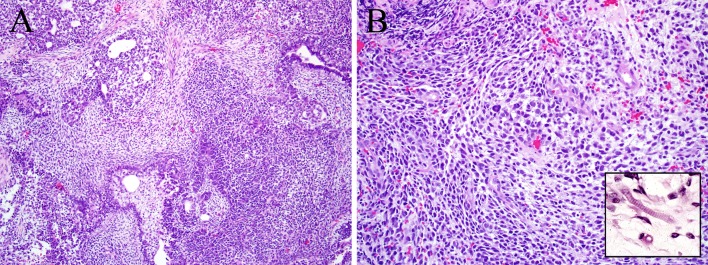
Fig. 3.
Sarcomatoid carcinoma. This laryngeal tumor demonstrates both epithelial differentiation in the form of invasive squamous cell carcinoma (center) as well as mesenchymal differentiation (a). Only the squamous nest is positive for cytokeratin immunohistochemistry (b), while the remaining spindle cell tumor component is positive for desmin (c) and myogenin (d), features diagnostic of rhabdomyoblastic differentiation
The diagnosis of sarcomatoid carcinoma relies on finding epithelial differentiation by routine morphology (i.e. squamous dysplasia or a component of squamous cell or other type of obvious carcinoma mixed with sarcomatoid tumor) or, if this is absent, by the demonstration of epithelial differentiation by immunohistochemistry for epithelial markers. However it should be emphasized that true RMS may express cytokeratins in up to 7 % of cases [21]. Even the newer squamous marker p63 can be positive in RMS, although all cases reported so far have been negative for p40, the more squamous-specific isoform of p63 [56, 57]. Further complicating the distinction from RMS or other sarcomas is that up to a third of sarcomatoid carcinomas are monophasic spindle cell neoplasms, and the sarcomatoid components of up to 74 % of sarcomatoid carcinomas are completely negative for epithelial markers [40, 42, 46, 47, 58]. Ultimately, a diagnosis of sarcomatoid carcinoma should be carefully considered before making the diagnosis of RMS in an older patient and in unusual mucosal locations like the larynx.
Undifferentiated (Anaplastic) Thyroid Carcinoma
Undifferentiated (anaplastic) thyroid carcinoma (UTC) is a highly aggressive malignant epithelial neoplasm of the thyroid. Morphologically the tumor grows in sheets of cells which are often spindled, pleomorphic/giant cell, or squamoid [59]. “Rhabdoid” cells have been identified in up to 10 % of UTC and one third of poorly differentiated thyroid carcinomas [60], however true skeletal muscle differentiation appears to be very rare in UTC with only 2 reported cases [61]. Distinguishing UTC from true sarcomas is aided immunohistochemically with cytokeratins or Pax-8, retained in about 75 % of UTC [62, 63]. Additionally, identification of an associated well-differentiated thyroid carcinoma, present in 30–70 % of UTC, supports dedifferentiation from a primary thyroid malignancy [59]. If skeletal muscle markers are present by immunohistochemical evaluation, focal or patchy distribution supports heterologous differentiation of UTC over a rare example of true RMS involving the thyroid gland [64, 65]. Moreover, the BRAF V600E mutation may also be identified in 20–30 % of UTC and can be utilized in select cases for supporting evidence toward thyroid [66, 67].
Salivary Carcinosarcoma (“True” Malignant Mixed Tumor)
Pleomorphic adenoma is the most common neoplasm (benign or malignant) of the salivary glands [68]. Occasionally, malignancies can arise within pleomorphic adenomas, and the most common form of this phenomenon is carcinoma ex-pleomorphic adenoma, where the malignant tumor that develops is a carcinoma [69]. Rare examples of the mesenchymal component also exhibiting malignant transformation are known as carcinosarcoma or so-called “true” malignant mixed tumor (the modifier “true” being applied in order to distinguish such tumors from carcinoma ex-pleomorphic adenoma and from benign metastasizing mixed tumor/pleomorphic adenoma) [70]. The most common type of mesenchymal malignancy in carcinosarcoma is chondrosarcoma, but other types include osteosarcoma, leiomyosarcoma, fibrosarcoma, and very rarely, RMS [71–75]. This finding is more of a curiosity than a true diagnostic challenge, because by definition, carcinosarcoma harbors a malignant epithelial component that will clue the observer into the correct diagnosis. The carcinomatous components in these cases of carcinosarcoma with rhabdomyoblastic differentiation have been adenocarcinoma not otherwise specified, salivary duct carcinoma, squamous cell carcinoma, large cell neuroendocrine carcinoma, and undifferentiated carcinoma [71–75]. Moreover, the focal rhabdomyosarcomatous components in all but one case were accompanied by other sarcomatous components which included sarcoma not otherwise specified, myxofibrosarcoma, chondrosarcoma, liposarcoma, and fibrosarcoma [71–75]. Four cases also featured a component of residual benign pleomorphic adenoma [72–75]. The prognosis of carcinosarcoma, regardless of the component malignancies, is poor [70].
Olfactory Neuroblastoma (Esthesioneuroblastoma)
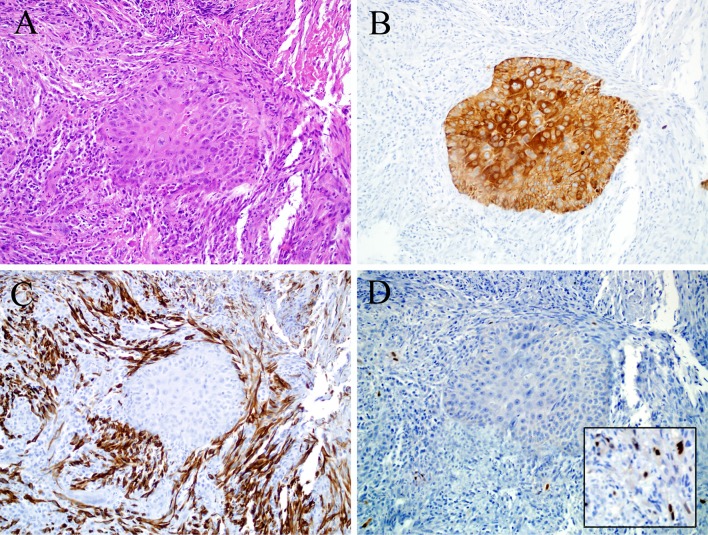
Olfactory neuroblastoma (ONB) is a malignant neoplasm that arises from the olfactory neuroepithelium of the superior nasal cavity and ethmoid sinus. The microscopic appearance of ONB depends on its grade. Low grade tumors have a lobular growth pattern, neurofibrillary matrix material, Homer Wright pseudorosettes, and a uniform population of round tumor cells with high nuclear-cytoplasmic ratios [76]. High grade ONBs show less of the lobular architecture and do not exhibit neurofibrillary matrix or Homer Wright pseudorosettes. High-grade ONBs instead have significant pleomorphism, high mitotic rates, and necrosis. They also may demonstrate true (Flexner-Wintersteiner) rosettes [76, 77]. By immunohistochemistry, ONB is positive for the neuroendocrine markers synaptophysin, chromogranin, and CD56 while S100 protein highlights the sustentacular supporting cells at the periphery of the tumor nests [76, 78]. Classically, other similar-appearing small round cell tumors such as lymphoma, melanoma, Ewing sarcoma/primitive neuroectodermal tumor, and RMS can be excluded because classic ONB shows a lobulated rather than diffuse growth pattern and is negative for CD45, CD99, desmin, myogenin, and HMB-45. Cytokeratins, while usually negative, may be focally positive in ONB, but EMA is consistently negative [78].
Occasionally, ONB may exhibit unusual forms of differentiation that may obscure the diagnosis. Most common is epithelial differentiation, where ONB may show cytokeratin and EMA immunoexpression. While this form of cyokeratin expression is typically focal, very rare examples of ONB may show foci of strong immunostaining and even glandular structures (though it is debatable whether these rare examples should be classified as another tumor type like neuroendocrine carcinoma or “olfactory carcinoma”) [79, 80]. Rarely, ONB may exhibit melanocytic or rhabdomyoblastic differentiation [81–83] (Fig. 4). Reports of this phenomenon are extremely limited, and as a result, the frequency and significance of this divergent differentiation cannot be determined (Table 1).
Fig. 4.
Olfactory neuroblastoma. This example of olfactory neuroblastoma grows in the typical fashion, as nests in the sinonasal submucosa (a). At high power, rhabdoid cells with abundant eccentric cytoplasm are evident (inset of a). As expected, this tumor was diffusely positive for synaptophysin (b) and had a peripheral (i.e., sustentacular) pattern of S100 immunostaining (c). The rhabdoid cells seen in areas of the tumor are confirmed to be rhabdomyoblasts by myogenin immunohistochemistry (d)
Table 1.
Head and neck malignancies that may exhibit rhabdomyoblastic differentiation
| Rhabdomyosarcoma |
| Malignant peripheral nerve sheath tumor (malignant Triton tumor) |
| Sarcomatoid carcinoma (spindle cell variant of squamous cell carcinoma) |
| Undifferentiated (anaplastic) thyroid carcinoma |
| Carcinosarcoma of the salivary glands |
| Olfactory neuroblastoma (esthesioneuroblastoma) |
| Teratocarcinosarcoma |
| Malignant teratoma |
| Melanoma |
| Liposarcoma |
It is important to distinguish ONB with rhabdomyoblastic differentiation from RMS, a problem compounded by both tumors being encountered in the sinonasal tract of young patients. Additionally, the alveolar subtype of RMS may express neuroendocrine markers, chromogranin and synaptophysin, in 20-30 % of tumors with nearly universal CD56 expression [21]. An important key to this dilemma is recognizing the nature of the myogenin expression. Alveolar RMS, a nested, small round cell tumor, generally demonstrates diffuse myogenin expression (Fig. 1), in contrast to the patchy distribution seen in ONB [15, 84] (Fig. 4). Moreover, even in the face of patchy rhabdomyoblastic differentiation, classic areas of ONB should be recognizable in the background. Finally, in a very difficult case, molecular studies for the t(2;13) or t(1;13) translocations of alveolar RMS may be useful. ONB are always translocation-negative and have complex cytogenetic alterations with deletions of 3p and overrepresentations of 17q in up to 100 % of cases [85].
Teratocarcinosarcoma
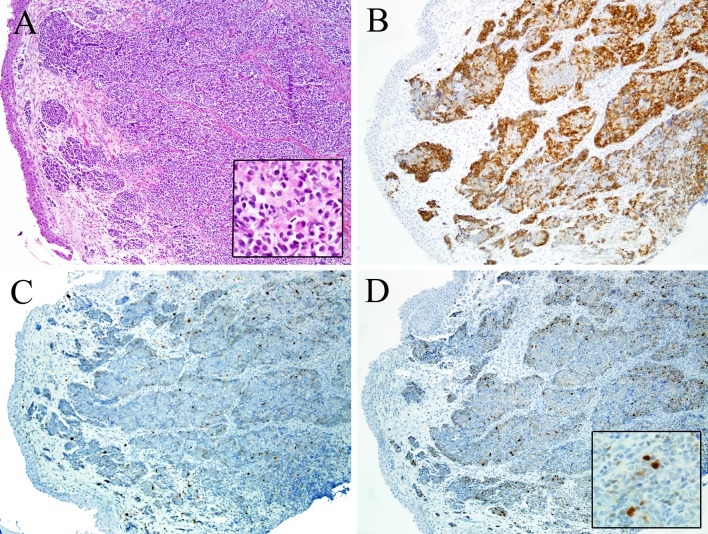
Teratocarcinosarcoma is a rare, peculiar sinonasal malignancy that features an admixture of epithelial, neuroectodermal/neuroendocrine, and mesenchymal elements showing varying degrees of maturation and cellular atypia (Fig. 5). The epithelial component may be either squamous or glandular, often with a cytologically bland, “fetal” appearance, reminiscent of what may be encountered in a teratoma [86–88]. The neuroectodermal/neuroendocrine tumor component is typically high-grade and primitive in its appearance, sometimes with rosette structures and/or neurofibrillary matrix. The tumor is set in a mesenchymal stroma that may be bland or overtly sarcomatous. This stroma can exhibit cartilaginous, smooth muscle, or skeletal muscle differentiation [86–88]. Immunostaining for skeletal muscle markers can facilitate the detection of small foci with crowding of rhabdomyoblasts in which cytoplasmic cross-striation may be easily missed in the initial evaluation with routine hematoxylin and eosin staining [89]. Components of seminoma, choriocarcinoma, and embryonal carcinoma are, by definition, not found. In fact, although teratocarcinosarcoma might suggest a germ cell tumor with teratomatous elements, it more likely arises from stem/progenitor cells of the neuroepithelium.
Fig. 5.
Teratocarcinosarcoma. Sinonasal teratocarcinosarcoma exhibits admixed zones of primitive small round cells, spindled cells, squamous epithelium with clear cytoplasm, and glands (a). Primitive and/or spindled components of teratocarcinosarcoma demonstrate rhabdomyoblastic differentiation (b) which is strongly suggested by cytoplasmic cross-striations (inset) and is confirmed by immunohistochemistry for myogenin or MyoD1
Because of its classically variable histologic appearance it is notoriously difficult to recognize teratocarcinosarcoma on biopsy [86–88]. Depending on which areas are sampled, teratocarcinosarcoma can be mistaken for ONB, chondrosarcoma, squamous cell carcinoma, adenocarcinoma, or even RMS. Distinguishing teratocarcinosarcoma from these other tumors relies primarily on adequate sampling to reveal the other tumor components and thus, the true tumor identity. Teratocarcinosarcoma is an aggressive neoplasm, though newer studies have shown that it is not as lethal as early reports had suggested. The mean survival is approximately 6 years [86–88, 90–92].
Malignant Teratoma
Teratoma is a neoplasm that consists of tumor differentiating into cells of all three embryonal germ cell layers (ectoderm, mesoderm, endoderm). Fewer than 5 % of teratomas arise in the head and neck, where the cervical soft tissue and nasopharynx are the most commonly affected sites [87]. Most teratomas occur in children, and many are detected in the perinatal period; they are rare in adults. The composition of teratomas is quite variable, and may include both mature and immature tissues of all three germ cell layers. It is important to remember that teratomas in children are uniformly benign, even when immature elements are present, although they may cause morbidity due to airway obstruction. On the other hand, when teratomas arise in the head and neck of an adult patient (typically in the cervical soft tissues and/or thyroid gland) their behavior does depend on the presence or absence of immature tissue elements. Unfortunately, most adult teratomas in the head and neck contain immature elements and they usually behave in an aggressive manner [93–96]. While focal, immature skeletal muscle may resemble RMS in isolation, it is unlikely to cause diagnostic difficulty when the other elements of a teratoma are present, which is almost invariably the case with malignant teratomas.
Melanoma
Rare examples of melanoma may show heterologous mesenchymal elements, including Schwannian, ganglionic, cartilaginous or osteoid differentiation [97]. In this context, it is not surprising that rare melanomas, including some arising in the head and neck region, have also exhibited focal rhabdomyoblastic differentiation [98–100]. The rhabdomyoblasts in these cases are positive for myogenin/MyoD1 but are negative for melanocytic markers [98–102]. These cases are very rare and it would be imprudent to attempt to draw any conclusions about the significance of the rhabdomyoblasts. Nevertheless, if abundant, they can be a diagnostic pitfall for the unwary. Clues to the diagnosis of melanoma include a cutaneous location (although melanomas can certainly arise from mucosal sites), older patient age, an overlying in situ melanoma within the epidermis/surface epithelium, and a more conventional melanoma component with prominent eosinophilic nucleoli, immunoreactivity for S100 and specific melanocytic markers like HMB45, Melan-A, and SOX-10, and in some cases, melanin pigment. It has been repeatedly emphasized that melanoma is incredibly variable in its appearance and as a result, diagnostic pathologists should have a very low threshold for keeping it in the differential diagnosis of any poorly differentiated head and neck tumor.
Liposarcoma
Liposarcoma is a malignant neoplasm of the soft tissues that demonstrates fatty differentiation. Liposarcomas are most common in the retroperitoneum and extremities, but may occasionally arise in the head and neck. Rare examples of liposarcoma have exhibited focal rhabdomyoblastic differentiation. Most of these were in the context of a liposarcoma undergoing de-differentiation/high-grade transformation, although rhabdomyoblasts have rarely been encountered in primary well-differentiated or myxoid liposarcomas, including two arising in the head and neck [103–108]. Again, the significance, if any, of this finding is unclear; and the rhabdomyoblasts have been merely a peculiar, focal finding in tumors that were otherwise straightforward liposarcomas, and did not pose a considerable diagnostic challenge. RMS lacks fatty differentiation.
Others
It should be noted that there is a handful of additional head and neck neoplasms such as de-differentiated chordoma [109], gnathic osteosarcoma [110], Merkel cell carcinoma [111], and small cell neuroendocrine carcinoma [112] where a single case with confirmed rhabdomyoblastic differentiation has been reported. It is difficult to draw any conclusions from these cases other than the idea that rhabdomyoblastic differentiation can be unexpectedly be encountered in a great variety of tumor types. In addition, there are a number of other head and neck neoplasms with rare reported examples of “rhabdomyoblastic” differentiation as defined hematoxylin and eosin and/or desmin immunohistochemistry, but not confirmed by myogenin and/or MyoD1 immunostaining. These include melanocytic neuroectodermal tumor of infancy [113] and the newly described low-grade sinonasal sarcoma with neural and myogenic features [35].
Conclusions
RMS frequently affects the head and neck but as we have demonstrated in this review, it is certainly not the only head and neck neoplasm that may exhibit rhabdomyoblastic differentiation. For malignant Triton tumor, An awareness of this phenomenon as well as attention to background tumor elements (i.e., away from the rhabdomyoblasts) and anatomic and demographic considerations (e.g., undifferentiated carcinoma is much more likely than RMS in the thyroid gland of an elderly patient) will help prevent misdiagnosis in most instances. Judicious use of molecular diagnostic tools can be helpful in select cases. When aberrant skeletal muscle differentiation is encountered, it is prudent to mention its presence in the diagnosis, along with a note clarifying that the neoplasm is not RMS.
Sample Diagnosis
NASAL CAVITY (BIOPSY): OLFACTORY NEUROBLASTOMA WITH FOCAL RHABDOMYOBLASTIC DIFFERENTIATION. SEE COMMENT.
COMMENT: The rhabdomyoblastic component is limited to a few scattered cells positive for desmin and myogenin, representing less than 5% of the tumor volume. This is not a rhabdomyosarcoma.
Footnotes
This paper was written by members of the International Head Neck Scientific Group (www.IHNSG.com).
References
- 1.Scrable HJ, Witte DP, Lampkin BC, et al. Chromosomal localization of the human rhabdomyosarcoma locus by mitotic recombination mapping. Nature. 1987;329:645–647. doi: 10.1038/329645a0. [DOI] [PubMed] [Google Scholar]
- 2.Bridge JA, Liu J, Qualman SJ, et al. Genomic gains and losses are similar in genetic and histologic subsets of rhabdomyosarcoma, whereas amplification predominates in embryonal with anaplasia and alveolar subtypes. Genes Chromosomes Cancer. 2002;33:310–321. doi: 10.1002/gcc.10026. [DOI] [PubMed] [Google Scholar]
- 3.Barr FG. Gene fusions involving PAX and FOX family members in alveolar rhabdomyosarcoma. Oncogene. 2001;20:5736–5746. doi: 10.1038/sj.onc.1204599. [DOI] [PubMed] [Google Scholar]
- 4.Barr FG, Galili N, Holick J, et al. Rearrangement of the PAX3 paired box gene in the paediatric solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;3:113–117. doi: 10.1038/ng0293-113. [DOI] [PubMed] [Google Scholar]
- 5.Miettinen M, Fetsch JF, Antonescu CR, et al. Tumors with skeletal muscle differentiation. AFIP atlas of tumor pathology: tumors of the soft tissues. Silver Spring: ARP Press; 2014. pp. 289–308. [Google Scholar]
- 6.Kohsaka S, Shukla N, Ameur N, et al. A recurrent neomorphic mutation in MYOD1 defines a clinically aggressive subset of embryonal rhabdomyosarcoma associated with PI3 K-AKT pathway mutations. Nat Genet. 2014;46:595–600. doi: 10.1038/ng.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parham DM, Barr FG. Classification of rhabdomyosarcoma and its molecular basis. Adv Anat Pathol. 2013;20:387–397. doi: 10.1097/PAP.0b013e3182a92d0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barr FG. New treatments for rhabdomyosarcoma: the importance of target practice. Clin Cancer Res. 2012;18:595–597. doi: 10.1158/1078-0432.CCR-11-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crist W, Gehan EA, Ragab AH, et al. The third intergroup rhabdomyosarcoma study. J Clin Oncol. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 10.Hicks J, Flaitz C. Rhabdomyosarcoma of the head and neck in children. Oral Oncol. 2002;38:450–459. doi: 10.1016/S1368-8375(01)00105-1. [DOI] [PubMed] [Google Scholar]
- 11.Pappo AS, Shapiro DN, Crist WM, et al. Biology and therapy of pediatric rhabdomyosarcoma. J Clin Oncol. 1995;13:2123–2139. doi: 10.1200/JCO.1995.13.8.2123. [DOI] [PubMed] [Google Scholar]
- 12.Nayar RC, Prudhomme F, Parise O, Jr, et al. Rhabdomyosarcoma of the head and neck in adults: a study of 26 patients. Laryngoscope. 1993;103:1362–1366. doi: 10.1288/00005537-199312000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Feldman BA. Rhabdomyosarcoma of the head and neck. Laryngoscope. 1982;92:424–440. doi: 10.1288/00005537-198204000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Wiener ES. Head and neck rhabdomyosarcoma. Semin Pediatr Surg. 1994;3:203–206. [PubMed] [Google Scholar]
- 15.Morotti RA, Nicol KK, Parham DM, et al. An immunohistochemical algorithm to facilitate diagnosis and subtyping of rhabdomyosarcoma: the Children’s Oncology Group experience. Am J Surg Pathol. 2006;30:962–968. doi: 10.1097/00000478-200608000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Walterhouse D, Watson A. Optimal management strategies for rhabdomyosarcoma in children. Paediatr Drugs. 2007;9:391–400. doi: 10.2165/00148581-200709060-00006. [DOI] [PubMed] [Google Scholar]
- 17.Antonescu CR, Scheithauer BW, Woodruff JM. Malignant tumors of peripheral nerves. AFIP atlas of tumor pathology: tumors of the peripheral nervous system. Silver Spring: ARP Press; 2013. pp. 381–474. [Google Scholar]
- 18.Lewis JJ, Brennan MF. Soft tissue sarcomas. Curr Probl Surg. 1996;33:817–872. doi: 10.1016/S0011-3840(96)80013-X. [DOI] [PubMed] [Google Scholar]
- 19.Reid RJ. The neural crest, its migrants, and cutaneous malignant neoplasms related to neurocristic derivatives. In: Lynch HT, Fusaro RM, editors. Cancer associated genodermatoses. New York: Nan Nostrand Reinhold; 1982. pp. 171–222. [Google Scholar]
- 20.Weiss SW, Langloss JM, Enzinger FM. Value of S-100 protein in the diagnosis of soft tissue tumors with particular reference to benign and malignant Schwann cell tumors. Lab Invest. 1983;49:299–308. [PubMed] [Google Scholar]
- 21.Bahrami A, Gown AM, Baird GS, et al. Aberrant expression of epithelial and neuroendocrine markers in alveolar rhabdomyosarcoma: a potentially serious diagnostic pitfall. Mod Pathol. 2008;21:795–806. doi: 10.1038/modpathol.2008.86. [DOI] [PubMed] [Google Scholar]
- 22.Woodruff JM, Perino G. Non-germ-cell or teratomatous malignant tumors showing additional rhabdomyoblastic differentiation, with emphasis on the malignant Triton tumor. Semin Diagn Pathol. 1994;11:69–81. [PubMed] [Google Scholar]
- 23.Woodruff JM, Chernik NL, Smith MC, et al. Peripheral nerve tumors with rhabdomyosarcomatous differentiation (malignant “Triton” tumors) Cancer. 1973;32:426–439. doi: 10.1002/1097-0142(197308)32:2<426::AID-CNCR2820320221>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 24.Masson P. Recklinghausen’s neurofibromatosis, sensory neuromas and motor neuromas. Contributions to the Medical Sciences in Honor of Dr Emanuel Libman by his Pupils, Friends and Colleagues. New York: International Press; 1932:793–802.
- 25.Pytel P, Taxy JB, Krausz T. Divergent differentiation in malignant soft tissue neoplasms: the paradigm of liposarcoma and malignant peripheral nerve sheath tumor. Int J Surg Pathol. 2005;13:19–28. doi: 10.1177/106689690501300103. [DOI] [PubMed] [Google Scholar]
- 26.Tiffee JC, Barnes EL. Neuromuscular hamartomas of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124:212–216. doi: 10.1001/archotol.124.2.212. [DOI] [PubMed] [Google Scholar]
- 27.Sorensen KB, Godballe C, Krogdahl A. Malignant triton tumor (MTT) of the neck. Auris Nasus Larynx. 2006;33:89–91. doi: 10.1016/j.anl.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Bhatt S, Graeme-Cook F, Joseph MP, et al. Malignant triton tumor of the head and neck. Otolaryngol Head Neck Surg. 1991;105:738–742. doi: 10.1177/019459989110500518. [DOI] [PubMed] [Google Scholar]
- 29.Victoria L, McCulloch TM, Callaghan EJ, et al. Malignant triton tumor of the head and neck: a case report and review of the literature. Head Neck. 1999;21:663–670. doi: 10.1002/(SICI)1097-0347(199910)21:7<663::AID-HED12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Kamran SC, Howard SA, Shinagare AB, et al. Malignant peripheral nerve sheath tumors: prognostic impact of rhabdomyoblastic differentiation (malignant triton tumors), neurofibromatosis 1 status and location. Eur J Surg Oncol. 2013;39:46–52. doi: 10.1016/j.ejso.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 31.McConnell YJ, Giacomantonio CA. Malignant triton tumors-complete surgical resection and adjuvant radiotherapy associated with improved survival. J Surg Oncol. 2012;106:51–56. doi: 10.1002/jso.23042. [DOI] [PubMed] [Google Scholar]
- 32.James JA, Bali NS, Sloan P, et al. Low-grade malignant Triton tumor of the oral cavity: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:699–704. doi: 10.1067/moe.2003.140. [DOI] [PubMed] [Google Scholar]
- 33.Omar T, Raslan H, El Sheikh S, et al. Low-grade malignant triton tumor of the neck: a case report and review of the literature. Case Rep Pathol. 2014;2014:674094. doi: 10.1155/2014/674094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terzic A, Bode B, Gratz KW, et al. Prognostic factors for the malignant triton tumor of the head and neck. Head Neck. 2009;31:679–688. doi: 10.1002/hed.21051. [DOI] [PubMed] [Google Scholar]
- 35.Lewis JT, Oliveira AM, Nascimento AG, et al. Low-grade sinonasal sarcoma with neural and myogenic features: a clinicopathologic analysis of 28 cases. Am J Surg Pathol. 2012;36:517–525. doi: 10.1097/PAS.0b013e3182426886. [DOI] [PubMed] [Google Scholar]
- 36.Coindre JM, de Mascarel A, Trojani M, et al. Immunohistochemical study of rhabdomyosarcoma. Unexpected staining with S100 protein and cytokeratin. J Pathol. 1988;155:127–132. doi: 10.1002/path.1711550209. [DOI] [PubMed] [Google Scholar]
- 37.Nappi O, Wick MR. Sarcomatoid neoplasms of the respiratory tract. Semin Diagn Pathol. 1993;10:137–147. [PubMed] [Google Scholar]
- 38.Batsakis JG, Suarez P. Sarcomatoid carcinomas of the upper aerodigestive tracts. Adv Anat Pathol. 2000;7:282–293. doi: 10.1097/00125480-200007050-00002. [DOI] [PubMed] [Google Scholar]
- 39.Cardesa A, Zidar N, et al. Spindle cell carcinoma. In: Barnes L, Eveson JW, Reichart P, et al., editors. World Health Organization classification of tumours pathology and genetics of head and neck tumors. Lyon: IARC Press; 2005. pp. 127–128. [Google Scholar]
- 40.Thompson LD, Wieneke JA, Miettinen M, et al. Spindle cell (sarcomatoid) carcinomas of the larynx: a clinicopathologic study of 187 cases. Am J Surg Pathol. 2002;26:153–170. doi: 10.1097/00000478-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Lane N. Pseudosarcoma (polypoid sarcoma-like masses) associated with squamous-cell carcinoma of the mouth, fauces, and larynx. Cancer. 1957;10:19. doi: 10.1002/1097-0142(195701/02)10:1<19::AID-CNCR2820100104>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 42.Ansari-Lari MA, Hoque MO, Califano J, et al. Immunohistochemical p53 expression patterns in sarcomatoid carcinomas of the upper respiratory tract. Am J Surg Pathol. 2002;26:1024–1031. doi: 10.1097/00000478-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Kojc N, Zidar N, Gale N, et al. Transcription factors snail, slug, twist, and SIP1 in spindle cell carcinoma of the head and neck. Virchows Arch. 2009;454:549–555. doi: 10.1007/s00428-009-0771-5. [DOI] [PubMed] [Google Scholar]
- 44.Zidar N, Bostjancic E, Gale N, et al. Down-regulation of microRNAs of the miR-200 family and miR-205, and an altered expression of classic and desmosomal cadherins in spindle cell carcinoma of the head and neck-hallmark of epithelial-mesenchymal transition. Hum Pathol. 2011;42:482–488. doi: 10.1016/j.humpath.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 45.Zidar N, Gale N, Kojc N, et al. Cadherin–catenin complex and transcription factor Snail-1 in spindle cell carcinoma of the head and neck. Virchows Arch. 2008;453:267–274. doi: 10.1007/s00428-008-0649-y. [DOI] [PubMed] [Google Scholar]
- 46.Zarbo RJ, Crissman JD, Venkat H, et al. Spindle-cell carcinoma of the upper aerodigestive tract mucosa. An immunohistologic and ultrastructural study of 18 biphasic tumors and comparison with seven monophasic spindle-cell tumors. Am J Surg Pathol. 1986;10:741–753. doi: 10.1097/00000478-198611000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Lewis JE, Olsen KD, Sebo TJ. Spindle cell carcinoma of the larynx: review of 26 cases including DNA content and immunohistochemistry. Hum Pathol. 1997;28:664–673. doi: 10.1016/S0046-8177(97)90175-1. [DOI] [PubMed] [Google Scholar]
- 48.Batsakis JG, Rice DH, Howard DR. The pathology of head and neck tumors: spindle cell lesions (sarcomatoid carcinomas, nodular fasciitis, and fibrosarcoma) of the aerodigestive tracts, Part 14. Head Neck Surg. 1982;4:499–513. doi: 10.1002/hed.2890040609. [DOI] [PubMed] [Google Scholar]
- 49.Ellis GL, Corio RL. Spindle cell carcinoma of the oral cavity. A clinicopathologic assessment of fifty-nine cases. Oral Surg Oral Med Oral Pathol. 1980;50:523–533. doi: 10.1016/0030-4220(80)90436-3. [DOI] [PubMed] [Google Scholar]
- 50.Batsakis JG, Suarez P. Sarcomatoid carcinomas of the upper aerodigestive tracts. Adv Anatomic Pathol. 2000;7:282–293. doi: 10.1097/00125480-200007050-00002. [DOI] [PubMed] [Google Scholar]
- 51.Roy S, Purgina B, Seethala RR. Spindle cell carcinoma of the larynx with rhabdomyoblastic heterologous element: a rare form of divergent differentiation. Head Neck Pathol. 2013;7:263–267. doi: 10.1007/s12105-012-0402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldman RL, Weidner N. Pure squamous cell carcinoma of the larynx with cervical nodal metastasis showing rhabdomyosarcomatous differentiation. Clinical, pathologic, and immunohistochemical study of a unique example of divergent differentiation. Am J Surg Pathol. 1993;17:415–421. doi: 10.1097/00000478-199304000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Srinivasan U, Talvalkar GV. True carcinosarcoma of the larynx: a case report. J Laryngol Otol. 1979;93:1031–1035. doi: 10.1017/S002221510008806X. [DOI] [PubMed] [Google Scholar]
- 54.Ferlito A. Surgical pathology of laryngeal neoplasms. London: Chapman & Hall Medical; 1996. [Google Scholar]
- 55.Doglioni C, Ferlito A, Chiamenti C, et al. Laryngeal carcinoma showing multidirectional epithelial neuroendocrine and sarcomatous differentiation. ORL J Otorhinolaryngol Relat Spec. 1990;52:316–326. doi: 10.1159/000276158. [DOI] [PubMed] [Google Scholar]
- 56.Bishop JA, Montgomery EA, Westra WH. Use of p40 and p63 immunohistochemistry and human papillomavirus testing as ancillary tools for the recognition of head and neck sarcomatoid carcinoma and its distinction from benign and malignant mesenchymal processes. Am J Surg Pathol. 2014;38:257–264. doi: 10.1097/PAS.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jo VY, Fletcher CD. p63 immunohistochemical staining is limited in soft tissue tumors. Am J Clin Pathol. 2011;136:762–766. doi: 10.1309/AJCPXNUC7JZSKWEU. [DOI] [PubMed] [Google Scholar]
- 58.Lewis JS, Ritter JH, El-Mofty S. Alternative epithelial markers in sarcomatoid carcinomas of the head and neck, lung, and bladder-p63, MOC-31, and TTF-1. Mod Pathol. 2005;18:1471–1481. doi: 10.1038/modpathol.3800451. [DOI] [PubMed] [Google Scholar]
- 59.Rosai J, DeLellis RA, Carcangiu ML, et al. Undifferentiated (anaplastic) thyroid carcinoma. AFIP atlas of tumor pathology: tumors of the thyroid and parathyroid glands. Silver Spring: ARP Press; 2014. pp. 177–198. [Google Scholar]
- 60.Albores-Saavedra J, Hernandez M, Sanchez-Sosa S, et al. Histologic variants of papillary and follicular carcinomas associated with anaplastic spindle and giant cell carcinomas of the thyroid: an analysis of rhabdoid and thyroglobulin inclusions. Am J Surg Pathol. 2007;31:729–736. doi: 10.1097/01.pas.0000213417.00386.74. [DOI] [PubMed] [Google Scholar]
- 61.Carda C, Ferrer J, Vilanova M, et al. Anaplastic carcinoma of the thyroid with rhabdomyosarcomatous differentiation: a report of two cases. Virchows Arch. 2005;446:46–51. doi: 10.1007/s00428-004-1123-0. [DOI] [PubMed] [Google Scholar]
- 62.Bishop JA, Sharma R, Westra WH. PAX8 immunostaining of anaplastic thyroid carcinoma: a reliable means of discerning thyroid origin for undifferentiated tumors of the head and neck. Hum Pathol. 2011;42:1873–1877. doi: 10.1016/j.humpath.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Nonaka D, Tang Y, Chiriboga L, et al. Diagnostic utility of thyroid transcription factors Pax8 and TTF-2 (FoxE1) in thyroid epithelial neoplasms. Mod Pathol. 2008;21:192–200. doi: 10.1038/modpathol.3801002. [DOI] [PubMed] [Google Scholar]
- 64.Dutta M, Chatterjee I, Roy S, et al. Primary embryonal rhabdomyosarcoma of the anterior neck and thyroid: report of a new case with review of the literature. Laryngoscope. 2013;123:2072–2076. doi: 10.1002/lary.23794. [DOI] [PubMed] [Google Scholar]
- 65.Furze AD, Lehman DA, Roy S. Rhabdomyosarcoma presenting as an anterior neck mass and possible thyroid malignancy in a seven-month-old. Int J Pediatr Otorhinolaryngol. 2005;69:267–270. doi: 10.1016/j.ijporl.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 66.Begum S, Rosenbaum E, Henrique R, et al. BRAF mutations in anaplastic thyroid carcinoma: implications for tumor origin, diagnosis and treatment. Mod Pathol. 2004;17:1359–1363. doi: 10.1038/modpathol.3800198. [DOI] [PubMed] [Google Scholar]
- 67.Takano T, Ito Y, Hirokawa M, et al. BRAF V600E mutation in anaplastic thyroid carcinomas and their accompanying differentiated carcinomas. Br J Cancer. 2007;96:1549–1553. doi: 10.1038/sj.bjc.6603764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ellis GL, Auclair PL. Tumors of the salivary glands. Washington, D.C.: ARP Press; 2008. [Google Scholar]
- 69.Ellis GL, Auclair PL. Carcinoma ex pleomorphic adenoma. AFIP atlas of tumor pathology: tumors of the salivary glands. Washington, D.C.: ARP Press; 2008. [Google Scholar]
- 70.Ellis GL, Auclair PL. Carcinosarcoma. AFIP atlas of tumor pathology: tumors of the salivary glands. Washington, D.C.: ARP Press; 2008. pp. 363–368. [Google Scholar]
- 71.Ueo T, Kaku N, Kashima K, et al. Carcinosarcoma of the parotid gland: an unusual case with large-cell neuroendocrine carcinoma and rhabdomyosarcoma—Case report. Apmis. 2005;113:456–464. doi: 10.1111/j.1600-0463.2005.apm_231.x. [DOI] [PubMed] [Google Scholar]
- 72.Gandour-Edwards RF, Donald PJ, Vogt PJ, et al. Carcinosarcoma (malignant mixed tumor) of the parotid: report of a case with a pure rhabdomyosarcoma component. Head Neck. 1994;16:379–382. doi: 10.1002/hed.2880160414. [DOI] [PubMed] [Google Scholar]
- 73.Tanahashi J, Daa T, Kashima K, et al. Carcinosarcoma ex recurrent pleomorphic adenoma of the submandibular gland—Case report. Apmis. 2007;115:789–794. doi: 10.1111/j.1600-0463.2007.apm_647.x. [DOI] [PubMed] [Google Scholar]
- 74.Kwon MY, Gu M. True malignant mixed tumor (carcinosarcoma) of parotid gland with unusual mesenchymal component: a case report and review of the literature. Arch Pathol Lab Med. 2001;125:812–815. doi: 10.5858/2001-125-0812-TMMTCO. [DOI] [PubMed] [Google Scholar]
- 75.Yura S, Terahata S, Ohga N, et al. A case of carcinosarcoma arising in the submandibular gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2007;103:820–824. doi: 10.1016/j.tripleo.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 76.Wenig BM, Dulguerov P, Kapadia SB, et al. et al. Neuroectodermal tumours. In: Barnes L, Eveson JW, Reichart P, et al.et al., editors. World Health Organization classification of tumours pathology and genetics of head and neck tumors. Lyon: IARC Press; 2005. pp. 65–75. [Google Scholar]
- 77.Thompson LD. Olfactory neuroblastoma. Head Neck Pathol. 2009;3:252–259. doi: 10.1007/s12105-009-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frierson HF, Jr, Ross GW, Mills SE, et al. Olfactory neuroblastoma. Additional immunohistochemical characterization. Am J Clin Pathol. 1990;94:547–553. doi: 10.1093/ajcp/94.5.547. [DOI] [PubMed] [Google Scholar]
- 79.Miller DC, Goodman ML, Pilch BZ, et al. Mixed olfactory neuroblastoma and carcinoma. A report of two cases. Cancer. 1984;54:2019–2028. doi: 10.1002/1097-0142(19841101)54:9<2019::AID-CNCR2820540940>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 80.Sugita Y, Kusano K, Tokunaga O, et al. Olfactory neuroepithelioma: an immunohistochemical and ultrastructural study. Neuropathology. 2006;26:400–408. doi: 10.1111/j.1440-1789.2006.00703.x. [DOI] [PubMed] [Google Scholar]
- 81.Curtis JL, Rubinstein LJ. Pigmented olfactory neuroblastoma: a new example of melanotic neuroepithelial neoplasm. Cancer. 1982;49:2136–2143. doi: 10.1002/1097-0142(19820515)49:10<2136::AID-CNCR2820491025>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 82.Miyagami M, Katayama Y, Kinukawa N, et al. An ultrastructural and immunohistochemical study of olfactory neuroepithelioma with rhabdomyoblasts. Med Electron Microsc. 2002;35:160–166. doi: 10.1007/s007950200020. [DOI] [PubMed] [Google Scholar]
- 83.Slootweg PJ, Lubsen H. Rhabdomyoblasts in olfactory neuroblastoma. Histopathology. 1991;19:182–184. doi: 10.1111/j.1365-2559.1991.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 84.Dias P, Chen B, Dilday B, et al. Strong immunostaining for myogenin in rhabdomyosarcoma is significantly associated with tumors of the alveolar subclass. Am J Pathol. 2000;156:399–408. doi: 10.1016/S0002-9440(10)64743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bockmuhl U, You X, Pacyna-Gengelbach M, et al. CGH pattern of esthesioneuroblastoma and their metastases. Brain Pathol. 2004;14:158–163. doi: 10.1111/j.1750-3639.2004.tb00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heffner DK, Hyams VJ. Teratocarcinosarcoma (malignant teratoma?) of the nasal cavity and paranasal sinuses A clinicopathologic study of 20 cases. Cancer. 1984;53:2140–2154. doi: 10.1002/1097-0142(19840515)53:10<2140::AID-CNCR2820531025>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 87.Cardesa A, Luna MA, et al. Germ cell tumors. In: Barnes L, Eveson JW, Reichart P, et al., editors. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 76–79. [Google Scholar]
- 88.Smith SL, Hessel AC, Luna MA, et al. Sinonasal teratocarcinosarcoma of the head and neck: a report of 10 patients treated at a single institution and comparison with reported series. Arch Otolaryngol Head Neck Surg. 2008;134:592–595. doi: 10.1001/archotol.134.6.592. [DOI] [PubMed] [Google Scholar]
- 89.Fernandez PL, Cardesa A, Alos L, et al. Sinonasal teratocarcinosarcoma: an unusual neoplasm. Pathol Res Pract. 1995;191:166–171. doi: 10.1016/S0344-0338(11)80567-4. [DOI] [PubMed] [Google Scholar]
- 90.Misra P, Husain Q, Svider PF, et al. Management of sinonasal teratocarcinosarcoma: a systematic review. Am J Otolaryngol. 2014;35:5–11. doi: 10.1016/j.amjoto.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 91.Yang S, Sun R, Liang J, et al. Sinonasal teratocarcinosarcoma: a clinical and pathological analysis. Int J Surg Pathol. 2013;21:37–43. doi: 10.1177/1066896912457202. [DOI] [PubMed] [Google Scholar]
- 92.Budrukkar A, Agarwal JP, Kane S, et al. Management and clinical outcome of sinonasal teratocarcinosarcoma: single institution experience. J Laryngol Otol. 2010;124:739–743. doi: 10.1017/S0022215109992866. [DOI] [PubMed] [Google Scholar]
- 93.Batsakis JG, el-Naggar AK, Luna MA. Teratomas of the head and neck with emphasis on malignancy. Ann Otol Rhinol Laryngol. 1995;104:496–500. doi: 10.1177/000348949510400616. [DOI] [PubMed] [Google Scholar]
- 94.Colton JJ, Batsakis JG, Work WP. Teratomas of the neck in adults. Arch Otolaryngol. 1978;104:271–272. doi: 10.1001/archotol.1978.00790050037008. [DOI] [PubMed] [Google Scholar]
- 95.Thompson LD, Rosai J, Heffess CS. Primary thyroid teratomas: a clinicopathologic study of 30 cases. Cancer. 2000;88:1149–1158. doi: 10.1002/(SICI)1097-0142(20000301)88:5<1149::AID-CNCR27>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 96.Vujanic GM, Harach HR, Minic P, et al. Thyroid/cervical teratomas in children: immunohistochemical studies for specific thyroid epithelial cell markers. Pediatr Pathol. 1994;14:369–375. doi: 10.3109/15513819409024265. [DOI] [PubMed] [Google Scholar]
- 97.Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119–129. doi: 10.1111/j.1365-2559.2007.02823.x. [DOI] [PubMed] [Google Scholar]
- 98.Shenjere P, Fisher C, Rajab R, et al. Melanoma with rhabdomyosarcomatous differentiation: two further cases of a rare pathologic pitfall. Int J Surg Pathol. 2014;22:512–519. doi: 10.1177/1066896914531817. [DOI] [PubMed] [Google Scholar]
- 99.Pasz-Walczak G, Jesionek-Kupnicka D, Kubiak R, et al. Rhabdomyosarcomatous (myoblastic?) phenotype of metastatic malignant melanoma. A case report. Pol J Pathol. 2002;53:97–100. [PubMed] [Google Scholar]
- 100.Gharpuray-Pandit D, Coyne J, Eyden B, et al. Rhabdomyoblastic differentiation in malignant melanoma in adults: report of 2 cases. Int J Surg Pathol. 2007;15:20–25. doi: 10.1177/1066896906295775. [DOI] [PubMed] [Google Scholar]
- 101.Gattenlohner S, Brocker EB, Muller-Hermelink HK. Malignant melanoma with metastatic rhabdomyosarcomatoid transdifferentiation. N Engl J Med. 2008;358:649–650. doi: 10.1056/NEJMc0707079. [DOI] [PubMed] [Google Scholar]
- 102.Reilly DJ, Volchek M, Ting JW, et al. Rhabdomyoblastic differentiation in metastatic melanoma: making sense of a rare but complex form of mimicry. Int J Surg Pathol. 2014;22:520–524. doi: 10.1177/1066896913510031. [DOI] [PubMed] [Google Scholar]
- 103.Govender D, Pillay P. Primary myxoid liposarcoma with rhabdomyoblastic differentiation. Arch Pathol Lab Med. 1998;122:740–742. [PubMed] [Google Scholar]
- 104.Shanks JH, Banerjee SS, Eyden BP. Focal rhabdomyosarcomatous differentiation in primary liposarcoma. J Clin Pathol. 1996;49:770–772. doi: 10.1136/jcp.49.9.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McCormick D, Mentzel T, Beham A, et al. Dedifferentiated liposarcoma. Clinicopathologic analysis of 32 cases suggesting a better prognostic subgroup among pleomorphic sarcomas. Am J Surg Pathol. 1994;18:1213–1223. doi: 10.1097/00000478-199412000-00004. [DOI] [PubMed] [Google Scholar]
- 106.Longano A, DuGuesclin A, Mitchell C. Primary dedifferentiated liposarcoma of the lung with rhabdomyoblastic and chrondroblastic differentiation. Histopathology. 2014 doi: 10.1111/his.12410. [DOI] [PubMed] [Google Scholar]
- 107.Okamoto S, Machinami R, Tanizawa T, et al. Dedifferentiated liposarcoma with rhabdomyoblastic differentiation in an 8-year-old girl. Pathol Res Pract. 2010;206:191–196. doi: 10.1016/j.prp.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 108.Shimada S, Ishizawa T, Ishizawa K, et al. Dedifferentiated liposarcoma with rhabdomyoblastic differentiation. Virchows Arch. 2005;447:835–841. doi: 10.1007/s00428-005-0025-0. [DOI] [PubMed] [Google Scholar]
- 109.Chan AC, Tsang WY, Chan GP, et al. Dedifferentiated chordoma with rhabdomyoblastic differentiation. Pathology. 2007;39:277–280. doi: 10.1080/00313020701230716. [DOI] [PubMed] [Google Scholar]
- 110.Seethala RR, Sturgis EM, Raymond AK, et al. Postirradiation osteosarcoma of the mandible with heterologous differentiation. Arch Pathol Lab Med. 2006;130:385–388. doi: 10.5858/2006-130-385-POOTMW. [DOI] [PubMed] [Google Scholar]
- 111.Adhikari LA, McCalmont TH, Folpe AL. Merkel cell carcinoma with heterologous rhabdomyoblastic differentiation: the role of immunohistochemistry for Merkel cell polyomavirus large T-antigen in confirmation. J Cutan Pathol. 2012;39:47–51. doi: 10.1111/j.1600-0560.2011.01794.x. [DOI] [PubMed] [Google Scholar]
- 112.Eusebi V, Damiani S, Pasquinelli G, et al. Small cell neuroendocrine carcinoma with skeletal muscle differentiation: report of three cases. Am J Surg Pathol. 2000;24:223–230. doi: 10.1097/00000478-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 113.Pettinato G, Manivel JC, d’Amore ES, et al. Melanotic neuroectodermal tumor of infancy. A reexamination of a histogenetic problem based on immunohistochemical, flow cytometric, and ultrastructural study of 10 cases. Am J Surg Pathol. 1991;15:233–245. doi: 10.1097/00000478-199103000-00004. [DOI] [PubMed] [Google Scholar]