Abstract
Purpose
The present study aimed to gather information on the impact of Alpha/European Society of Human Reproduction and Embryology (ESHRE) consensus regarding oocytes with aggregates of smooth endoplasmic reticulum (SERa) on in vitro fertilization outcome. In particular, we investigated if patients undergoing intracytoplasmic sperm injection (ICSI) and whose oocytes are discarded due to SERa have a higher chance of embryo transfer cancellation compared to patients without SERa oocytes.
Methods
This is a nested case–control study drawn from the cohort of women referring for in vitro fertilization with ICSI. Cases were patients showing at least one oocyte with SERa at the time of injection. Controls were subsequent patients showing no SERa oocytes and matched ratio 1:1 for age, clinical indication to in vitro fertilization (IVF), and body mass index. The main outcome was the rate of embryo transfer cancellation.
Results
The percentage of women experiencing a transfer cancellation (absence of suitable oocytes or viable embryos) in their ICSI cycle were significantly higher in cases (18 %) compared to controls (8 %) (p = 0.02); however, adjusted odds ratio for FSH and number of SERa oocytes, of follicles, of retrieved oocytes, and of inseminated oocytes were not statistically significant.
Conclusions
We have shown that the exclusion of SERa oocytes from ICSI cycles causes an increased frequency of transfer cancellation. This effect is mostly due to the reduced number of available oocytes after exclusion of SERa oocytes.
Keywords: Smooth endoplasmic reticulum aggregates, Oocyte dysmorphism, Embryology, ICSI, Clinical outcome
Introduction
It is well known that after ovarian stimulation, a certain proportion of oocytes present cytoplasmic or extra-cytoplasmic dysmorphisms [1], but the significance and the developmental consequences of most of them are unclear. In particular, it has been suggested that oocyte morphology can predict outcome of in vitro fertilization (IVF) cycles, but due to the variety of experimental models and conflicting results, available literature largely fails to attribute a significant impact on pregnancy outcomes to specific dysmorphisms [2, 3]. Smooth endoplasmic reticulum aggregates (SERa) represent a cytoplasmic dysmorphism that has been individually studied for its impact on embryological and obstetric outcomes. SERa are smooth vacuoles appearing as round flat disks in the cytoplasm and correspond to large clusters of tubular SER surrounded by a dense population of mitochondria and by small aggregates of dense granules containing tiny vesicles [4]. In some studies, it has been reported that SERa+ cycles have significantly lower fertilization, embryo cleavage, blastocyst, and pregnancy rates compared to controls [4, 5], and even more interestingly, several reports have associated the presence of SERa oocytes or their use in IVF cycles to malformations or genetic abnormalities in the newborns such as Beckwith–Wiedmann syndrome [6], diaphragmatic hernia [7], multiple malformations [8], and cardiovascular defect [4].
In 2011, based on these data, the Alpha Scientists and European Society of Human Reproduction and Embryology (ESHRE) issued guidelines that, as a precaution, advised against the use of SERa gametes in IVF [9]. However, subsequent studies have shown that SERa oocytes can give birth to healthy babies [5, 10]. As a consequence, there is a lack of true consensus and policy of IVF centers toward affected oocytes is not homogeneous. A recent multicenter survey study [11] revealed that after the publication of Alpha/ESHRE consensus [9], 56 out of 118 IVF centers changed their attitude toward the fate of SERa oocytes but only about 14 % of centers discard SERa oocytes prior to ICSI and 43 % of centers that do not discard affected oocytes, register, and follow up neonatal data.
Even though the impact of this dysmorphism has not been fully elucidated, our center scrupulously follows the recommendations of the Alpha/ESHRE consensus and SERa oocytes are systematically eliminated from ICSI procedures. The present study aimed to gather more information on the impact of this limitation determining if patients undergoing ICSI and whose oocytes are discarded due to SERa have a higher chance of embryo transfer cancellation compared to patients without SERa oocytes.
Materials and methods
This is a nested case–control study drawn from the cohort of women referring for IVF at the Fondazione Ca’ Granda, Ospedale Maggiore Policlinico. Data was obtained from both clinical and biological charts. Women older than 18 years and requiring ICSI were considered for study selection. Women failing to retrieve oocytes were excluded from both study groups. Cases (SERa+) were ICSI patients showing at least one oocyte with SERa at the time of insemination. Controls (SERa−) were subsequent patients showing no SERa oocytes and matched ratio 1:1 for age (±6 months), clinical indication for IVF, and body mass index (BMI) (±0.5 kg/m2). When several matched controls were found, the closest woman (in order of time) was selected. All patients performing an IVF cycle in the study period were considered for inclusion. Only one cycle per patient (the first performed in the study period) was included in the analysis.
A specific informed consent was not requested since this is a retrospective study. However, all women in our center were routinely requested to provide an informed consent for their data to be used for research purposes prior to initiate IVF, and those denying this consent were excluded. The study was accepted by the local ethical committee (Comitato Etico Milano Area B).
IVF-ICSI cycles were monitored and managed according to a standardized clinical protocol [12]. Briefly, the regimen used and the dose of gonadotropins were determined on an individual basis according to age, hormonal tests, antral follicle count, and data from previous IVF cycles. The patients underwent serial transvaginal ultrasounds, and when three or more leading follicles with a mean diameter >18 mm were visualized, human chorionic gonadotropin (hCG) was administered subcutaneously. Cycles were cancelled if follicular development was abnormal (premature ovulation or arrested follicular growth) or if it was deemed that the response could be significantly improved with the use of a different regimen or a different starting dose. This latter situation was mainly for a poor response (less than three follicles) since a “freeze-all” policy was preferred in case of ovarian hyperresponse. Oocyte retrieval was performed transvaginally 36 h after the hCG injection using a single lumen 17-gauge needle. Oocyte-cumulus complexes were immediately separated from follicular fluid washed and transferred to a 1-ml IVF medium (Sage In-Vitro Fertilization, Inc. Trumbull, CT, USA). Following a 3–4-h incubation at 37 °C in an atmosphere of 6 % CO2, oocytes were denuded using hyaluronidase 40 IU/ml (Sage In-Vitro Fertilization) and insemination were performed with ICSI: At that time, metaphase II oocytes (MII) were evaluated at 400× under a microscope (Eclipse TE200, Nikon Instruments) and those showing one or more SER aggregates (minimum detectable diameter ∼10 μm) were discarded according to Alpha/ESHRE consensus [9]. Oocytes were also discarded when immature (germinal vesicle or metaphase I stage), abnormal, or degenerated. Vitrification of MII oocytes was performed only for patients refusing embryo cryopreservation in case of ovarian hyperresponse or in case of supernumerary oocytes. Cycles performed with warmed oocytes or embryos were used to calculate cumulative implantation and delivery rates.
Inseminated oocytes were cultured at 37 °C in an atmosphere of 5 % O2 and 6 % CO2. A fertilization check for two pronuclei took place 16–18 h after ICSI. Embryo morphology was evaluated on days 2, 3, and/or 5, depending on the day of embryo transfer. Evaluation of day 2 embryos took place only for women undergoing embryo transfer on day 2. Embryos showing 4–6 cells on day 2 or 7–12 cells on day 3, no/low (<10 %) fragmentation and no multinucleation were classified as “good quality embryos.” Embryo transfer was generally performed 72 h after the oocyte collection (day 3). However, if the patient had only one to two fertilized oocytes, it could be done at day 2, and if she had at least four good quality embryos on day 3, day 5 transfer at the blastocyst stage could be considered. Non-transferred embryos were generally vitrified at blastocyst stage on day 5 or 6. Blastocysts were scored according to the Alpha/ESHRE consensus scoring system [9]. Blastocysts showing a degree of expansion classified as 3 or 4, an inner cell mass scored 1 or 2, and a trophectoderm type 1 to 2 were defined “good quality blastocysts.” Oocytes and embryos were vitrified with the Cryotop method [13].
Clinical pregnancy/implantation was defined as the sonographic documentation of at least one intrauterine fetus with heart beat 4–5 weeks after embryo transfer.
The main aim of the study was to compare the rate of embryo transfer cancellation in cases and controls. This outcome included women failing to obtain suitable oocytes or showing only non-viable embryos. According to Alpha/ESHRE consensus, a non-viable embryo is an embryo in which development has been arrested for at least 24 h or in which all the cells have degenerated or lysed due to complete fragmentation or cleavage arrest [9]. Our hypothesis was that the frequency of embryo transfer cancellation is doubled in cases compared to controls (30 vs 15 % according to our previous data). Therefore, 120 patients per group was the minimum sample needed, as revealed by the power study (α = 0.05, β = 0.2). An 18-month period (July 2012 to December 2013) was deemed sufficient to achieve this sample size.
Analysis of the data was carried out with the Statistics Package for Social Sciences (SPSS 18.0, Chicago, IL, USA). Data were compared using unpaired Student’s t test, chi-squared test, Fisher’s exact test, or Mann–Whitney test as appropriate. Exact Fisher’s confidence intervals were computed for rates and reported as 95 % confidence interval (95 % CI). A multivariate logistic regression model was used to assess the adjusted odds ratio (aOR) of embryo transfer cancellation. A p value ≤0.05 was considered statistically significant. Normally distributed variables are presented using means ± standard deviations, whereas skewed data are presented using medians and interquartile ranges (IQRs) between square brackets.
Results
The presence of SERa oocytes during ICSI was recorded in 130 out of 1092 ICSI cycles, corresponding to an incidence of 12 % (95 % CI 10–14 %). They were matched to 130 control cycles. Baseline characteristics of the enrolled patients did not significantly differ between cases and controls (Table 1). The median number of SERa discarded oocytes in cases was 1 [1, 2], range 1–12. Affected oocytes per women ranged between 4 and 100 % of the total number of retrieved oocytes with a median value of 18 % [11–33 %]. In four patients, the cycle was cancelled due to the exclusive presence of SERa oocytes: They recovered one oocyte each. Characteristics of the ICSI cycles in cases and in controls are reported in Table 2. Women with few oocytes retrieved were less common among cases. Overall, a similar number of suitable (unaffected) MII oocytes were observed in cases and controls: median 5 [3–8] and 5 [2–8], respectively (p = 0.820).
Table 1.
Baseline characteristics of cases and controls
| Characteristics | Cases (SERa+) | Controls (SERa−) | p |
|---|---|---|---|
| n = 130 | n = 130 | ||
| Age (years) | 36.5 ± 3.6 | 36.6 ± 3.7 | 0.81 |
| BMI (kg/m2) | 22.5 ± 3.9 | 22.3 ± 3.7 | 0.71 |
| Previous deliveries | 18 (14 %) | 25 (19 %) | 0.23 |
| Female indication | 1.00 | ||
| Unexplained | 81 (62 %) | 81 (62 %) | |
| Endometriosis | 29 (22 %) | 29 (22 %) | |
| Ovulatory | 5 (4 %) | 5 (4 %) | |
| Tubal | 15 (12 %) | 15 (12 %) | |
| Concomitant male factor of infertility | 38 (29 %) | 45 (35 %) | 0.28 |
| Duration of infertility (years) | 4.8 ± 2.8 | 4.1 ± 2.7 | 0.16 |
| Previous IVF cyles | 0.25 | ||
| None | 63 (48 %) | 73 (56 %) | |
| 1–2 | 57 (44 %) | 44 (34 %) | |
| ≥3 | 10 (8 %) | 13 (10 %) | |
| Day 3 serum FSH (IU/ml) | 7.7 ± 3.6 | 7.9 ± 3.4 | 0.51 |
| Serum AMH (ng/ml) | 2.6 ± 4.0 | 2.6 ± 4.9 | 0.94 |
| Total AFC | 10.1 ± 6.5 | 9.6 ± 7.0 | 0.56 |
| Previous ovarian surgery | 20 (15 %) | 16 (12 %) | 0.47 |
Data is reported as mean ± standard deviation or number (percentage)
BMI body mass index, FSH follicle-stimulating hormone, AMH anti-Müllerian hormone, AFC antral follicle count
Table 2.
Characteristics of the ICSI cycles in cases and controls
| Characteristics | Cases (SERa+) | Controls (SERa−) | p |
|---|---|---|---|
| n = 130 | n = 130 | ||
| Protocol of ovarian stimulation | 0.30 | ||
| Protocol with GnRH agonists | 90 (69 %) | 81 (62 %) | |
| Protocol with GnRH antagonists | 40 (31 %) | 49 (38 %) | |
| Total amount of FSH used (IU) | 2908 ± 1316 | 2897 ± 1301 | 0.95 |
| Duration of stimulation (days) | 10.1 ± 2.2 | 9.7 ± 2.0 | 0.10 |
| Total number of follicles >10 mm | 10.6 ± 4.8 | 9.5 ± 5.8 | 0.12 |
| Number of follicles >15 mm | 7.3 ± 3.5 | 6.4 ± 3.7 | 0.05 |
| Estradiol at the end of stimulation (pg/ml) | 2404 ± 1296 | 1987 ± 1247 | 0.02 |
| Oocyte retrieved | 9.0 [5.0–11.0] | 6.5 [3.0–10.0] | 0.011 |
| 1–5 | 37 (29 %) | 53 (41 %) | 0.09 |
| 6–9 | 37 (29 %) | 35 (27 %) | |
| ≥10 | 56 (43 %) | 42 (32 %) | |
| Number of SERa metaphase II oocytes | 1 [1–2] | 0 [0–0] | 0.001 |
| Number of oocytes discarded | 447/1157 (39 %) | 268/999 (27 %) | 0.001 |
| SERa | 232/1157 (20 %) | 0/999 (0 %) | 0.001 |
| Number of oocytes cryopreserved/retrieved | 106/1157 (9 %) | 131/999 (13 %) | 0.003 |
| Number of inseminated oocytesa | 4.0 [2.0–7.0] | 4.0 [2.0–7.0] | 0.75 |
| Fertilization rate (%)a | 75 [50–89] | 83 [60–100] | 0.05 |
| Cancelled embryo transfera | 23 (18 %) | 10 (8 %) | 0.02 |
| Absence of suitable oocytes | 10 (8 %) | 1 (1 %) | 0.24 |
| Absence of fertilized oocytes | 6 (5 %) | 5 (4 %) | |
| Absence of viable embryos | 7 (5 %) | 4 (3 %) | |
| Delayed embryo transfer (freeze-all) | 14 (11 %) | 14 (11 %) | 1.00 |
| Rate of good quality embryosa | |||
| Day 2b | 32/76 (42 %) | 43/91 (47 %) | 0.55 |
| Day 3 | 132/268 (49 %) | 134/295 (45 %) | 0.40 |
| Day 5c | 42/133 (32 %) | 36/115 (31 %) | 1.00 |
| Day of embryo transfera | 0.29 | ||
| Day 2–3 | 78 (84 %) | 95 (90 %) | |
| Day 5 | 15 (16 %) | 11 (10 %) | |
| Number of embryos transferreda | 1.6 ± 0.6 | 1.7 ± 0.6 | 0.65 |
Data is reported as mean ± SD or number (%) or median [IQR] as appropriate
aIn the fresh cycle
bIncludes only patients undergoing day 2 embryo transfer
cIncludes only patients undergoing day 5 embryo transfer or day 5–6 freeze-all
The percentage of women experiencing embryo transfer cancellation in their ICSI cycle was significantly higher in cases (23/130, 18 %, 95 % CI 12–25 %) compared to controls (10/130, 8 %, 95 % CI 4–14 %) (p = 0.02) with an OR = 2.6 (95 % CI 1.2–5.7). This was mainly due to the absence of suitable oocytes after discarding SERa oocytes (Table 2). No absence of blastulation was observed among women scheduled for blastocyst transfer on day 5.
Selection of variables associated with embryo transfer cancellation showed a significant positive association for serum FSH levels (Exp(B) = 1.19, p = 0.001) and number of SERa oocytes (Exp(B) = 0.80, p = 0.03) and a negative association for total number of follicles (Exp(B) = 0.82, p = 0.001), number of retrieved oocytes (Exp(B) = 1.18, p = 0.001), and number of inseminated oocytes (Exp(B) = 0.74, p = 0.001). When adjusted for the abovementioned variables, aOR for embryo transfer cancellation in cases compared to controls was 2.4 (95 % CI 0.8–6.7).
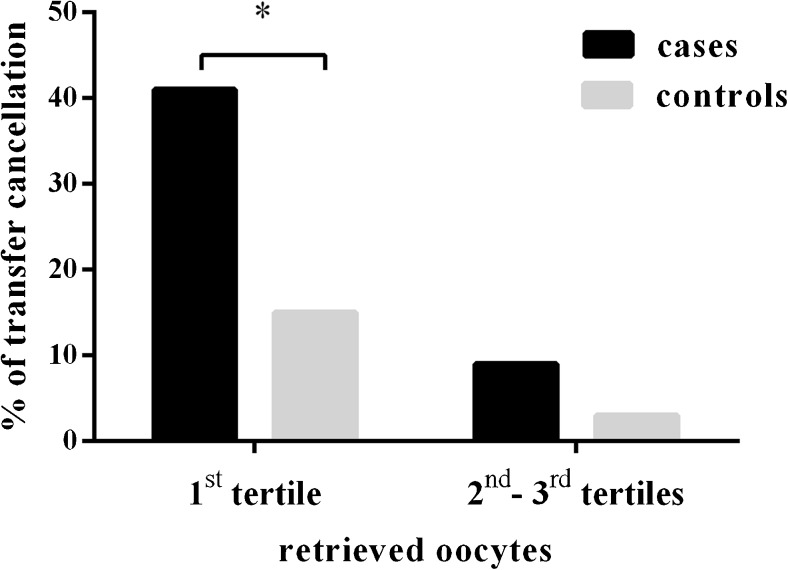
Tertiles of retrieved oocytes in cases were as follows: 1–5 (n = 37), 6–9 (n = 37), ≥10 (n = 56). Most of transfer cancellations in cases (15 out of 23) belonged to the first tertile with a statistically significant higher incidence compared to controls; on the contrary, the incidence of transfer cancellations in the second and third tertiles of retrieved oocytes was not statistically significant (Fig. 1).
Fig. 1.
Percentage of embryo transfer cancellation in cases (SERa+) and controls (SERa−) according to the number of retrieved oocytes (first tertile 1 ≤ n ≤ 5; second–third tertiles n ≥ 6). *p = 0.006
Clinical pregnancy, implantation, and delivery rates were not statistically different between the two groups, as reported in Table 3. One stillbirth was observed in the SERa+ group, and no major malformations were recorded among newborns.
Table 3.
Outcomes of the ICSI cycles in cases and controls
| Characteristics | Cases (SERa+) | Controls (SERa−) | p |
|---|---|---|---|
| n = 130 | n = 130 | ||
| Clinical pregnancy rate per patienta | 34/130 (26 %) | 38/130 (29 %) | 0.68 |
| Live births | 26 (77 %) | 30 (79 %) | 0.94 |
| Spontaneous abortions | 6 (18 %) | 7 (18 %) | |
| Stillbirths | 1 (3 %) | 0 (0 %) | |
| Therapeutic abortions (aneuploidy) | 1 (3 %) | 1 (3 %) | |
| Clinical implantation ratea | 41/151 (27 %) | 44/176 (25 %) | 0.71 |
| Cumulative clinical implantation rateb | 52/186 (28 %) | 55/215 (26 %) | 0.65 |
| Cumulative delivery rate with at least one live birthb | 32/130 (25 %) | 39/130 (30 %) | 0.33 |
| Weight at birth (g), singletonsb | 3002 ± 461 | 3176 ± 443 | 0.20 |
| Weight at birth (g), twinsb | 1863 ± 692 | 2182 ± 472 | 0.28 |
Data is reported as mean ± SD or number (%) or median [IQR] as appropriate
aIn the fresh cycle
bFresh + thawing cycles
At fertilization check (18 ± 1 h post-ICSI) in 22 cycles among cases, SERa were found also in a total of 53 out of 1157 (5 %, 95 % CI 3–6 %) previously unaffected oocytes. Only two of those oocytes showed normal signs of fertilization (4 %, 95 % CI 1–13 %). On the contrary, no SERa oocytes/zygotes were registered among controls at fertilization check.
A subgroup analysis was performed to compare, among cases, women who experienced embryo transfer cancellation and women who did not. Considering basal characteristics, the two groups differed for variables linked to ovarian reserve and responsiveness to controlled ovarian hyperstimulation: Basal FSH was 10.2 ± 4.9 vs 7.1 ± 3.0 IU/ml (p = 0.001), total amount of FSH used was 3585 ± 1661 vs 2763 ± 1189 IU (p = 0.03), total number of follicles at the end of stimulation was 7.3 ± 4.6 vs 11.3 ± 4.6, respectively (p = 0.001). While the median number of SERa oocytes were comparable between the two groups (median 1 [1, 2] in both groups), the median number of inseminated oocytes (1 [0–4] compared to 5 [3–8]) and the median fertilization rate (14 % [0–67 %] compared to 78 % [60–100 %]) were significantly lower in patients who had embryo transfer cancellation compared to women who had not, respectively (p = 0.001). The number of non-SERa metaphase II oocytes was an independent predictor of the chance of transfer cancellation with an Exp(B) = 0.65 (p = 0.001) implying that a one unit increase in the number of unaffected oocyte resulted in a 35 % reduction in the OR.
Discussion
In the present study, we have reported that IVF women with oocytes showing SER aggregates have a higher incidence of transfer cancellation when affected oocytes are excluded from insemination. This finding seems to be linked mainly to the reduced number of available oocytes after selection rather than to the quality of sibling oocytes. In fact, unaffected oocytes from cases give similar results compared to controls in terms of embryological variables and implantation rate with the exception of fertilization rate which was slightly reduced. Of note, most of the negative outcomes were registered among SERa+ women recovering five or less oocytes.
Our sample did not allow highlighting differences in terms of cumulative delivery rate per patient despite a trend favoring controls (30 vs 25 %).
The rate of SERa+ cycles in the literature is reported to be around 6–7 % [14]. However, the incidence in our series is nearly doubled. We believe that the explanation can be the inclusion of cycles with small or few SER aggregates together with our particular attention to this phenomenon.
Available evidence on the role of SERa oocytes in IVF was summarized in a recent review of the literature by Shaw-Jackson et al. [14]. The authors reported a 8.6 % perinatal complications in SERa+ cycles and suggested that transfers of affected embryos should be carried out with caution. The effect of SERa on fertilization, embryo quality, and implantation rates has been previously investigated and reviewed in the same paper. A trend toward lower performance of SERa oocytes compared to sibling unaffected ones is highlighted, but this decrease is statistically significant only in a few studies [4, 7]. Some authors reported that SERa+ cycles, when affected oocytes were generally discarded or preferentially not used for embryo transfer, have a lower fertilization rate [4] or pregnancy rate [6] compared to SERa− cycles. However and according to our data, most papers on these topics did not find differences between SERa+ and SERa− cycles [5, 7, 10]. Results are not conclusive because available studies are small and diverse, but, in general, it is concluded that SERa+ cycles do affect clinical and embryological outcomes to a limited extent [14].
SER aggregates have a negative impact on oocyte physiology, but the reason has not been fully elucidated.
Cortical and subcortical SER vesicles and associated mitochondria act as stores of calcium in normal oocytes: Since calcium has a key role during fertilization and early embryo development, it is likely that the disturbance of its release and oscillations due to the presence of abnormally large SER aggregates causes a developmental impairment in affected oocytes [4].
As recently shown by Van Beirs and colleagues [11], the decision concerning the fate of SERa oocytes or deriving embryos is affected by an important heterogeneity among IVF laboratories. Since the publication of Alpha/ESHRE Istanbul Consensus in 2011 on embryo evaluation [9], the attitude of embryologists toward affected oocytes has changed in about half of interviewed IVF centers but only a quarter of them systematically discard SERa oocytes as recommended by the workgroup. The main reason is probably that data regarding the association between SER aggregates and increased risk of an abnormal outcome [4, 6–8] are not fully convincing to most specialists and many centers, although aware of consensus, limit to record data regarding SERa. This position is supported by the publication of the retrospective analysis of 32 newborns from SERa+ only or SERa+/SERa− mixed cycles with the absence of malformations when only affected oocytes were used for embryo transfer [10]: Reported data do not support previous alarming reports. The same paper showed clinical and embryological data of ICSI cycles with at least one SERa oocyte and compared them with ICSI cycles without affected oocytes within the same period. Similarly to the present work, they found a higher number of recovered oocytes in cases compared to controls and no significant differences in clinical outcomes. However, despite a lower cycle efficiency in terms of viable embryos per fertilized oocyte in SERa+ cases, their rate of cancelled cycles was not influenced by the presence of affected oocytes; this may be due to the inclusion of SERa oocytes in the ICSI procedure and to the consequent higher number of treated oocytes per cycle.
Given that SERa oocytes were discarded in our center, our data could not be used to compare embryological and clinical parameters between affected and unaffected oocytes. In particular, we could not investigate the relationship between major malformations and the use of SERa oocytes; however, our series suggests that sibling unaffected oocytes are not at increased risk of negative neonatal outcomes. In fact, no major malformations were recorded in cases and controls. Similarly, we found that, once the step of fertilization has been passed, cleavage and implantation rates are very similar between cases and controls.
The present work has focused on the impact of discarding SERa oocytes from an ICSI program in a case–control setting. Our study design allowed to compare the prognosis of SERa+ cycles to controls in order to evaluate the clinical effect of Alpha/ESHRE recommendations. The role of age and clinical characteristics of the patients on oocytes’ quality and fertility is crucial. For this reason, we designed a matched study including indication to infertility treatments, female age, and BMI. Of note, when adjusting for basal FSH, number of follicles, and available oocytes, we found that the risk for cycle cancellation in cases compared to unaffected matched ICSI cycles was not significantly increased. Moreover, the presence of SERa oocytes did not affect the chance of cumulative delivery per patient in the whole cohort of cases (25 % compared to 30 % of controls): however, it has to be recognized that our study was certainly underpowered to detect similar differences (power <15 %).
Our transfer cancellation rate due to absence of embryos was pretty high also in controls: This is probably due to the fact that the median number of inseminated oocytes was as low as 4 and 75 % of couples had less than eight oocytes to be inseminated.
In the subgroup analysis, we showed that, among cases, women with transfer cancellation had reduced ovarian reserve and poorer ovarian response to controlled ovarian hyperstimulation compared to women undergoing embryo transfer. In particular, SERa+ patients with a negative IVF prognosis, such as absence of viable embryos, had a median of only one inseminated oocyte. We believe that this information is of importance for counseling patients about their chance of success once the embryologist has evaluated the whole cohort of available oocytes. In view of the facts that recent data do suggest that healthy babies can originate from affected oocytes [5, 10] and that SERa+ cycles have shown to be recurrent in up to 40 % of cycles [4, 7], poor responders with a positive cycle might opt for the transfer of an embryo originating from a SERa oocyte in order to conceive with their own gametes.
Several studies have demonstrated that the length of ovarian stimulation, the estradiol levels, and the total amount of gonadotrophins administered were higher for cycles with affected oocytes [4–7]; our data confirm previous observations even though some variables, despite a clear trend, do not significantly differ between cases and controls. It has yet to be clarified whether these differences reflect differences in patient characteristics or, conversely, whether they are consequent to ovary hyperstimulation and to delay of oocyte retrieval [6, 14].
Some limitations and experimental choices of our study deserve to be commented. Firstly, this is a retrospective analysis, and although it was done in a matched case–control setting with comparable basal characteristics, we cannot exclude that untested variables could influence the results. A randomized prospective study would be more informative, but according to Alpha/ESHRE consensus, our center is recommended to discard affected oocytes. For the same reason, we cannot add valuable information to the current literature regarding developmental potential of SERa oocytes compared to sibling unaffected gametes. Moreover, the study was designed with the main outcome of transfer cancellation. This is a widely used parameter for cycle evaluation and counseling of couples, but it has to be mentioned that when studying the effect of oocyte morphology on clinical outcome, the preferable endpoint should at least be childbirth. Of note, this endpoint requires very large sample sizes when differences between cases and controls are small.
In conclusion, we have shown that the exclusion of SERa oocytes from ICSI cycles causes an increased frequency of embryo transfer cancellation: This effect is mostly seen in patients with a reduced number of available oocytes. We believe that in the absence of a clear association between SERa and fetal malformations, caution should be used when affected oocytes are recovered, and in the hypothesis of the most rigorous strategy, patients should be properly counseled about their altered chances of success. In particular, if Alpha/ESHRE consensus has to be followed, some couples with a limited number of available oocytes would have very reduced chances of conceiving with their own oocytes.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Capsule The exclusion of SERa oocytes from ICSI cycles causes an increased frequency of transfer cancellation.
References
- 1.Vanblerkom J. Occurrence and developmental consequences of aberrant cellular-organization in meiotically mature human oocytes after exogenous ovarian hyperstimulation. J Electron Microsc Tech. 1990;16(4):324–46. doi: 10.1002/jemt.1060160405. [DOI] [PubMed] [Google Scholar]
- 2.Serhal PF, Ranieri DM, Kinis A, Marchant S, Davies M, Khadum IM. Oocyte morphology predicts outcome of intracytoplasmic sperm injection. Hum Reprod. 1997;12(6):1267–70. doi: 10.1093/humrep/12.6.1267. [DOI] [PubMed] [Google Scholar]
- 3.Rienzi L, Vajta G, Ubaldi F. Predictive value of oocyte morphology in human IVF: a systematic review of the literature. Hum Reprod Update. 2011;17(1):34–45. doi: 10.1093/humupd/dmq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sa R, Cunha M, Silva J, Luis A, Oliveira C, da Silva JT, et al. Ultrastructure of tubular smooth endoplasmic reticulum aggregates in human metaphase II oocytes and clinical implications. Fertil Steril. 2011;96(1):143–9. doi: 10.1016/j.fertnstert.2011.04.088. [DOI] [PubMed] [Google Scholar]
- 5.Hattori H, Nakamura Y, Nakajo Y, Araki Y, Kyono K. Deliveries of babies with normal health derived from oocytes with smooth endoplasmic reticulum clusters. J Assist Reprod Genet. 2014;31(11):1461–7. doi: 10.1007/s10815-014-0323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otsuki J, Okada A, Morimoto K, Nagai Y, Kubo H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod. 2004;19(7):1591–7. doi: 10.1093/humrep/deh258. [DOI] [PubMed] [Google Scholar]
- 7.Ebner T, Moser M, Shebl O, Sommerguber M, Tews G. Prognosis of oocytes showing aggregation of smooth endoplasmic reticulum. Reprod Biomed Online. 2008;16(1):113–8. doi: 10.1016/S1472-6483(10)60563-9. [DOI] [PubMed] [Google Scholar]
- 8.Akarsu C, Caglar G, Vicdan K, Sozen E, Biberoglu K. Smooth endoplasmic reticulum aggregations in all retrieved oocytes causing recurrent multiple anomalies: case report. Fertil Steril. 2009;92(4):1496e1–3. doi: 10.1016/j.fertnstert.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 9.Alpha Scientists in Reproductive Medicine and ESI The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270–83. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 10.Mateizel I, Van Landuyt L, Tournaye H, Verheyen G. Deliveries of normal healthy babies from embryos originating from oocytes showing the presence of smooth endoplasmic reticulum aggregates. Hum Reprod. 2013;28(8):2111–7. doi: 10.1093/humrep/det241. [DOI] [PubMed] [Google Scholar]
- 11.Van Beirs N, Shaw-Jackson C, Rozenberg S, Autin C. Policy of IVF centres towards oocytes affected by Smooth Endoplasmic Reticulum aggregates: a multicentre survey study. J Assist Reprod Genet. 2015;32(6):945–50. doi: 10.1007/s10815-015-0473-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benaglia L, Bermejo A, Somigliana E, Faulisi S, Ragni G, Fedele L, et al. In vitro fertilization outcome in women with unoperated bilateral endometriomas. Fertil Steril. 2013;99(6):1714–9. doi: 10.1016/j.fertnstert.2013.01.110. [DOI] [PubMed] [Google Scholar]
- 13.Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67(1):73–80. doi: 10.1016/j.theriogenology.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Shaw-Jackson C, Van Beirs N, Thomas A-L, Rozenberg S, Autin C. Can healthy babies originate from oocytes with smooth endoplasmic reticulum aggregates? A systematic mini-review. Hum Reprod. 2014;29(7):1380–6. doi: 10.1093/humrep/deu101. [DOI] [PubMed] [Google Scholar]