Abstract
Purpose
The objective of this analysis is to examine the relationship between Fragile X Mental Retardation 1 gene (FMR1) cytosine-guanine-guanine (CGG) repeat number and ovarian reserve, with a particular focus exclusively on the range of CGG repeat number below the premutation (PM) range (<55 CGG repeats).
Methods
Our study included female patients who underwent assessment of FMR1 CGG repeat number and serum anti-Mullerian hormone (AMH) in 2009–2014. To examine the association between FMR1 repeat number and serum AMH, we created three summary measures of CGG repeat number for the two alleles—“Sum,” “Max,” and “Gap” (absolute difference). Using multivariable regression models, controlling for age, we then analyzed the impact of these summary measures on AMH.
Results
A total of 566 patients were included in our study. Using multivariable regression models, we found that the relationship between CGG repeat number and AMH differed depending on age. Specifically, in younger women, AMH increased by 7–8 % (Sum p < 0.01, Max p = 0.04) for every 1 unit increase in CGG repeat number. In contrast, starting at age 40, there was a 3 to 5 % decline in AMH for every 1 unit increase in CGG repeat number (Sum p < 0.01, Max p = 0.04).
Conclusions
This is the first study to report a statistically significant correlation of ovarian reserve and CGG repeat number in women with <55 CGG repeats. Although these women are generally considered to have a normal phenotype, our data suggest that increasing CGG repeat number within this normal range is associated with a more rapid decline in ovarian reserve.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-015-0577-0) contains supplementary material, which is available to authorized users.
Keywords: FMR1, CGG repeat, Ovarian reserve, AMH
Introduction
Although majority of causes of primary ovarian insufficiency (POI) remain unknown, it is well established that the most significant single gene associated with premature ovarian failure (POF) is the Fragile X Mental Retardation 1 gene (FMR1) at Xq27.3 [1]. Caused by expansion of cytosine-guanine-guanine (CGG) repeats at the 5′ untranslated region of the FMR1 gene, inheritance of greater than 200 repeats leads to Fragile X syndrome (FXS), the most common cause of inherited mental retardation. The number of cytosine-guanine-guanine (CGG) repeats in the Fragile X mental retardation (FMR1) gene has commonly been defined as normal (<45), intermediate (45–54), premutation (PM) (55–200), and full mutation (>200) [2]. Because of hypermethylation and subsequent gene silencing, full mutation carriers exhibit normal ovarian function. Premutation carriers, however, maintain gene transcription. In fact, because of the increased numbers of CGG repeats, these patients exhibit abnormally high levels of FMR1 gene transcripts, which have been suggested to have a “gain of toxicity” effect at the level of the ovaries, leading to an increased risk of POI [1, 3]. With a peak risk for Fragile X-associated POI (FXPOI), between 80 and 100 CGG repeats [4], 16–24 % of PM carriers will experience POI in some severity [5, 6].
Given the continuous nature of POI and the established correlation of FMR1 PM carriers and premature ovarian aging, a controversy, as to whether or not patients with high normal (35–44) or intermediate repeats (45–54) exhibit evidence of POI, has arisen. Emerging data suggest that not only the PM but also the intermediate repeat length are associated with both overt and occult POI [3, 7–9]. One group of investigators has suggested that low repeat number (<26) may also contribute to diminished ovarian reserve (DOR); however, these data have not been replicated [10]. Subsequently, Pastore et al. reported that 14.5 % of DOR patients (n = 62) exhibited a high normal number of CGG repeats (35–44) [9]. More recently, researchers have been unable to reconfirm the aforementioned findings nor establish a correlation of CGG repeat number (<55) with measures of ovarian reserve [11, 12].
The objective of this study was to examine the relationship between CGG repeat number and ovarian reserve as described by serum anti-Mullerian hormone (AMH). To do so, we developed novel regression models including predictor variables which describe the sum of the CGG number and the difference between CGG repeat number for the two FMR1 alleles for each woman, with a focus exclusively on the range of CGG repeat number below the PM range (<55 CGG repeats).
Materials and methods
Patient population
Patients initially screened for inclusion in the study were all who had serum AMH collected at a single fertility center from December 2009 to February 2014 and sent to a single reference laboratory for AMH measurement. From this population, we identified patients who had both AMH and FMR1 CGG repeat number available. Screening for FMR1 repeat number occurred for 1 of 2 purposes: standard evaluation of primary ovarian insufficiency or preconception genetic screening, which was universally offered to all patients. Only women with CGG repeat number below the premutation range (<55) were eligible for inclusion.
Patients with factors known to affect ovarian reserve or with records that lacked information necessary to rule out confounding factors were excluded. These factors which are known to affect ovarian reserve included surgical removal of either one or both ovaries, advanced stage endometriosis, chemotherapy or radiation therapy, Turner Syndrome or other X chromosome abnormalities, and autoimmune factors. In addition, any patient with an FMR1 repeat number of >55 CGG repeats was excluded.
Additionally, 249 patients, referred from outside institutions to the reference laboratory, who underwent an assessment of both AMH and FMR1 repeat number (<55), were also included.
Permission to perform this study was granted by Stanford’s institutional review board (IRB 27794) and by New England Independent Review Board (IRB 10–094).
Laboratory analyses
Serum specimens were sent to a single reference laboratory (ReproSource, Woburn, MA) for analysis. Each woman was represented only once, and the first serum AMH measurement was used in women with more than one serum AMH measurement. The ReproSource laboratory-developed AMH assay was calibrated to oocytes retrieved [13] and is based on research-use-only materials and reagents from Beckman Coulter-DSL (Chaska, MN) initially with the “Gen I” ELISA assay and subsequently with the “Gen II” ELISA assays using uniform calibration across serum specimens in the study [14]. Intra- and interassay coefficients of variation with serum controls were approximately 3–8 % and 4–10 %, respectively [14, 15].
CGG repeat size within the FMR1 gene was determined by polymerase chain reaction (PCR) of isolated DNA with reported CGG repeat sizes accurate to plus or minus one for repeats less than 60 in length. The PCR products were generated using fluorescence labeled primers and sized by capillary electrophoresis. If indicated, Southern blot was performed by hybridizing the probe StB12.3 to EcoRI- and EagI digested DNA. The sensitivity of both the PCR and Southern blot analyses was 99 % [16–19].
Statistical analysis
To explore the relationship between FMR1 CGG repeat length of each allele and serum AMH, we speculated what summary measures could best describe a subject’s repeat length: the maximum (Max) repeat length, the sum of the two repeat lengths (Sum), and the absolute difference between the repeat lengths (Gap). As it is well established that premutation carriers (maximum repeat length ranging between 55 and 200 CGG repeats) are at increased risk of POI, Max was considered a predictor of primary interest. Similarly, Sum was also considered a primary predictor, as it is unclear how much biologic influence the smaller allele has on phenotype. Gap was considered a potentially useful variable, particularly in the event of a large difference between alleles that may need to be considered jointly with the other summary measures. These summary measures were chosen in an attempt to collapse biallelic CGG repeats into a single number in a way that is meaningful and interpretable while preserving as much information as possible.
For the 41 women whose AMH levels were recorded as <0.1 ng/mL, we set their AMH level to 0.1 ng/mL in our main analysis and assessed sensitivity of results using values of 0.01 and 0.05. Because the distribution of AMH was right-skewed, the natural logarithm of AMH was used in the regression models so that the models would more closely adhere to assumptions of normality. Linear regression techniques were employed to characterize the association between the primary summary measures and AMH. As it is established that AMH is known to vary according to age, all models were adjusted for age. The following multivariable models were fit:
To evaluate whether age modified the association between the summary measures (Sum and Max) and AMH, we additionally fit the following models:
In above model equations, beta coefficients represent how much ln(AMH) was expected to change when a summary measure increased by 1 when holding other variables constant, and epsilon represents random error. All predictors were centered at their respective means to aid interpretability.
We then used estimated associations between summary measures of CGG repeats and AMH levels to evaluate the AMH levels, in combination with CGG repeat number, at different ages.
Results
A total of 1666 unique patients underwent serum assessment of AMH from December 2009 to February 2014, ordered by a single fertility center and analyzed by a single reference laboratory. Of those 1666 patients, 341 patients also underwent PCR amplification of their FMR1 gene. Of the 341 patients, 24 were omitted from analysis secondary to meeting the criteria for exclusion or incomplete records. Of those meeting exclusion criteria, 3 patients had a CGG repeat number >55, 4 patients had an abnormal karyotype, 3 had advanced staged endometriosis, 1 with a prior oophorectomy, and 1 with a history of chemo/radiation. In addition to the 317 patients included in this analysis, 249 patients referred from outside institutions to the same reference laboratory, with both AMH and FMR1 CGG repeat number reported, were also included.
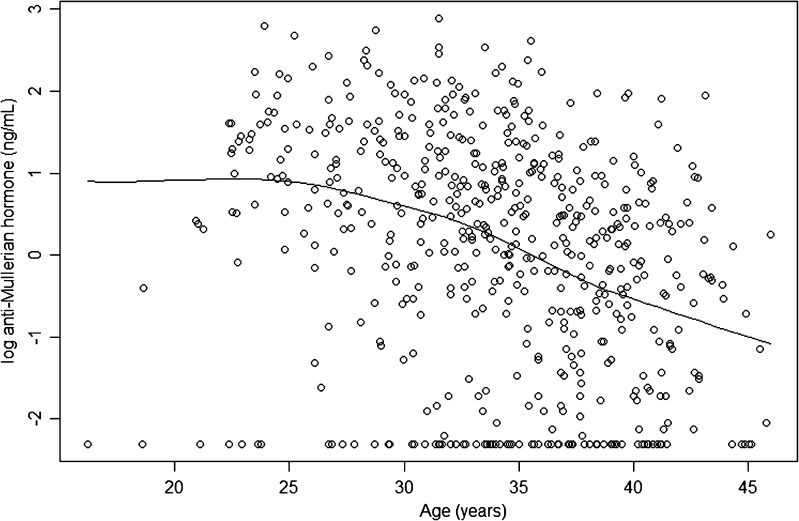
A total of 566 women were included in the analysis. Examining the baseline characteristics of these women, the mean age was 34.1 (median 34.5, range 16.2 to 45.9 years). The median AMH among all women was 1.47 ng/mL (range undetectable (<0.1 ng/mL) to 17.9 ng/mL). Consistent with past findings, we observed that ln(AMH) decreased with age in our sample (Fig. 1).
Fig. 1.
Correlation of log(serum anti-Mullerian hormone) with patient age. Upon plotting all log AMH values, a correlation of log AMH and age was established within our dataset
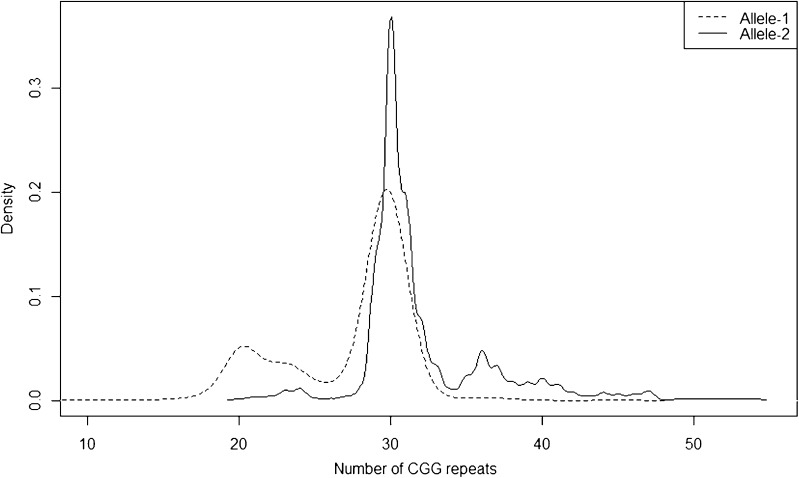
Next, we assessed the allelic distribution of FMR1 CGG repeat number among all of 566 patients (see Fig. 2). Because 46,XX females carry two copies of the FMR1 gene, we refer to allele 1 as the smaller of the 2 alleles in each patient and allele 2 as the larger of the pair. Ranging from 9 to 45, the median CGG repeat number on allele 1 was 29. Allele 2 exhibited a median CGG repeat number of 30, with a range of 20 to 54 repeats.
Fig. 2.
Distribution of CGG repeat lengths by FMR1 allele in 566 patients. Plotted against CGG repeat length (x-axis) are two smoothed histograms representing the relative frequency (y-axis) of the occurrence of allele 1 (dashed line) and allele 2 (solid line), where allele 1 is the smaller of the two alleles
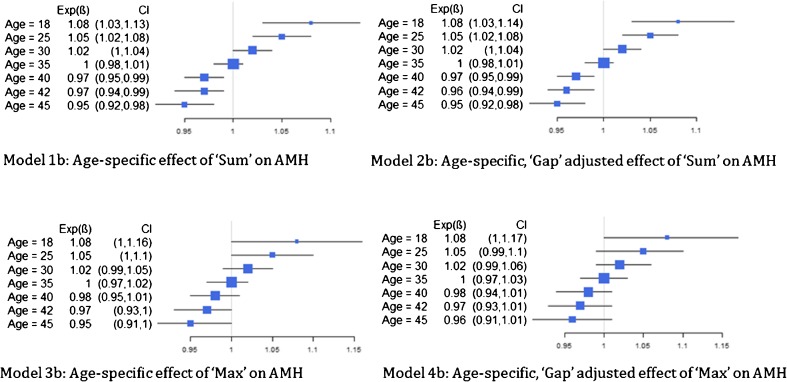
Models with and without interaction terms were evaluated. Because the interaction between age and our primary summary predictors were statistically significant, we based our findings on these models (model series b) (Fig. 1). More specifically, we found that the association between Sum and ln(AMH) (Model 1b) varied significantly by age (p < 0.01), where a 1 unit increase in the sum of the length of repeats across the two alleles was associated with an 8 % increase in AMH level for those aged 18 and a 5 % decrease in AMH level for those aged 45 (Fig. 3). A similar trend was observed for this association after adjusting for the gap between the alleles (Model 2b). Note that after adjusting for Sum (Models 1b and 2b), there is no association between the difference in length (Gap) of the two alleles and AMH level. The association between the maximum length of the alleles and AMH also varied significantly by age with and without adjustment for Gap (p = 0.041 for both Models 3b and 4b). For example, a 1-unit increase in the maximum length was associated with a 5 % increase in AMH level for those 25 years of age and a 2 % decrease in AMH level for those 40 years of age (Fig. 3). After adjusting for the maximum length, the trinucleotide Gap, itself, was not significantly associated with AMH.
Fig. 3.
Age-specific effect of summary measures of CGG repeat number on observed anti-Mullerian hormone. Each additional value of “Sum” and “Max” CGG repeat was associated with a positive difference in AMH for women under 35 and with a negative difference in AMH for women over 35. These age-specific associations were more pronounced for Sum than for Max, as indicated by confidence intervals that do not cross 1. Concurrent adjustment for “Gap” did not alter interpretation
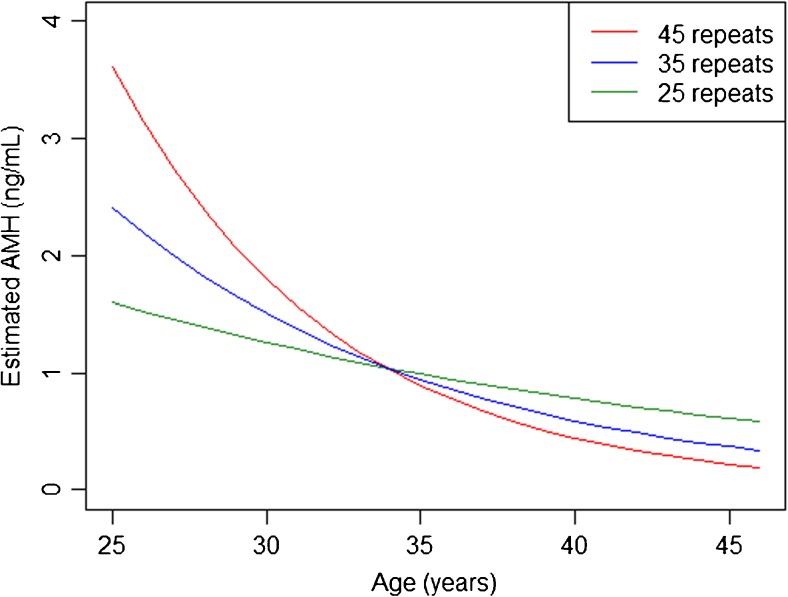
Given that our analysis was restricted to a single measurement of AMH, we are limited in our ability to determine the trajectory of AMH beyond that single measurement. However, to gain better visual insight into the variation in association between the max allelic CGG repeat number (allele 2) and AMH by age, we assessed fitted values from Model 4b for three chosen values of Max repeat CGG length: low (25 repeats), high (45 repeats), and average (35 repeats) (Fig. 4); patients with an elevated CGG repeat length modeled a trajectory of a more rapid decline in AMH as compared to women of the same age with low or average repeat numbers. It should be noted that our modeling of age and summary measures depicts each as continuous variables, and that all data obtained for this modeling is cross-sectional. Thus, within Fig. 4, the predicted AMH by age and Max exhibits the postulated affect of AMH based on a chosen maximum CGG length (i.e., 25 (low), 45 (high), or 35 (average)) repeats across various ages. Evaluation of the sum of allele 1 + allele 2 (Sum) yielded similar curves as well (data not shown). Based on current recommendations to cautiously interpret AMH in women below 25 years of age [20–22], Fig. 4 was restricted to women over 25. However, separate analysis using Model 4b, excluding women under 25 years of age, yielded similar results (data not shown).
Fig. 4.
Predicted pattern of decline in serum AMH with age using Model 4b and 3 different input examples of Max FMR1 repeat length. Predicted AMH values by age were calculated using Model 4b for three chosen CGG repeat lengths and are represented as follows: red line, 45 repeats; blue line, 35 repeats; and green line, 25 repeats. Because our models assume that both “Max” and age are continuous variables, each chosen value of Max depicts an age dependent, predicted effect on AMH
Sensitivity analyses that varied the value for undetectable AMH levels suggested no impact on results for different levels of AMH (data not shown).
Discussion
Our study provides evidence suggesting that increasing FMR1 CGG repeat lengths even within the currently considered normal range associates significantly with different patterns of declining ovarian reserve with age in a general infertile population. This adds evidence supporting theories which postulate FMR1 CGG repeat length below premutation ranges (i.e., <55 CGG repeats) may provide helpful information to improve prediction of serum AMH decline with age in women. Our study utilized age, FMR1 repeat length, and serum AMH measured from a single laboratory in a large cohort of women presenting for fertility evaluation and included only those with all FMR1 allele CGG repeat lengths below 55 repeats. We evaluated the strength of association between pattern of decline in serum AMH as women age and models which utilized summary measures of FMR1 CGG repeat length at each allele, such as the Max of both allele repeat lengths, the Sum of both allele repeat lengths, or the Gap between the allele repeat lengths. Compared to women with higher repeat lengths such as 45, our models indicate that women with a low number of repeats, such as 25, would be predicted to have serum AMH that is lower when aged between 20 and 30 years. As the age increases within this group, our cross-sectional model exhibits a slower rate of decline in AMH, such that the absolute serum AMH remains higher above 40 years of age (see Fig. 4). Conversely, women with higher repeat lengths such as 45 are predicted to have much higher serum AMH between 20 and 30 years of age relative to similarly aged women with fewer repeats but then, via rapid decline, are predicted to have lower AMH above age 40 years relative to women of the same age with fewer CGG repeats (see Fig. 4). Whereas Sum demonstrated the strongest association, namely that CGG repeat length associated with the pattern of serum AMH decline with age, both Max and Sum modeled similarly as summary measures. Thus, FMR1 repeat lengths under 55 appear important to understanding and possibly predicting the pattern of decline in ovarian reserve.
To date, an uncertainty in how to weigh and further interpret the potential association of each allele on ovarian reserve has been inconsistent. This variation in reporting creates an additional layer of complexity when comparing the existing literature. To reduce confusion in the literature through introduction of new terms such as Sum or Gap, until a new report suggest otherwise, our findings suggest that examining the role of the largest allele (Max), assuming methylation patterns and X inactivation are uniformly distributed, is reasonable for both clinical and research purposes.
We believe our research to be an important contribution; as to date, the literature regarding a correlation between FMR1 repeat number (or reference range below PM) and ovarian function is inconclusive. Karimov et al. analyzed 1056 patients (535 cases with low ovarian reserve and 521 infertile controls [unrelated to ovarian reserve] and oocyte donors) and reported that in addition to PM carriers, women who carried an intermediate ranged mutation (45–54 repeats) exhibited a higher prevalence of DOR [7]. Thereafter, four studies examined repeat lengths ranging from 35 to 54 and ovarian reserve or age at menopause [9, 23–25]. Both Pastore and Barosoain et al. reported that women with low (or diminished) ovarian reserve were more likely to have ≥35 CGG repeats as compared to their comparison groups (p = 0.0003 and p < 0.05) [9, 26]. Where the interpretation of the aforementioned [9, 24] is limited, secondary to small sample size, a recent study by De Geyter et al. reported no significant difference in carrier frequencies of either intermediate ranged mutations (defined as 35–44 and 45–54) or PM in a comparison of 372 cases of infertility (excluding male and tubal factors) vs. 199 fertile women in Switzerland [23]. Thereafter, analyzing more than 2000 women from the Breakthrough Generations Study with both FMR1 CGG repeat number and age of menopause, Murray et al. concluded that intermediate alleles were not significant risk factors for either early menopause or primary ovarian insufficiency [25].
Among the existing studies that specifically examine AMH levels in conjunction with FMR1 CGG repeat number, the collaborative findings are also uncertain. While some recent studies report no association of AMH with CGG repeat number [23, 27], one paper reported a positive association, controlling for age, between AMH level and FMR1 repeat number among 197 Korean women at risk for DOR with the largest CGG repeat number of 51 [28]. In contrast, Gleicher et al. reported that AMH was lower in women with 35–50 CGG (n = 35) than in women with <35 repeats (n = 122, p = 0.025) [29]. Additionally, Gleicher and Barad reported a similar trend in women with <26 repeats [10]. Although initially, we did not specifically look at the effect of <26 CGG repeats on AMH, when examining both the gap between alleles and the sum of alleles, we did not find any significant correlation with the gap between alleles (across all allele sizes), nor did we find a decline in AMH among women with a smaller numerical sum of alleles. What we did identify was that younger women with a low max CGG repeat number or sum of alleles modeled to maintain a low-normal AMH from a young age beyond that of their counterparts with a high Max or Sum (see Fig. 4), preserving their AMH over time. To explore this further, we modified models 3b and 4b to examine the relationship between the smaller allele “Min” and AMH (data not shown). Supporting our initial interpretation, we found that Min modeled similarly to that of Max and Sum, where younger women had a positive, age-related association with AMH with each numerical increase in CGG repeat number and older women exhibited a negative association (see Supplemental Figure 1). Although our findings differ from Gleicher et al. [10], our models behave similarly to those previously reported by Pastore et al., who reported a significantly steeper exponential decline of AMH with increasing age among women with DOR and ≥35 CGG repeats as compared to those with <35 CGG repeats (p = 0.035) [9].
The strength of this study is twofold. First, to our knowledge, this is the largest study to evaluate the relationship of ovarian reserve, specifically as measured by AMH at a single reference laboratory, with FMR1 repeat number, specifically among a large cohort of women with <55 CGG repeats. Second, supported by prior studies, our novel statistical models consistently suggest that FMR1 repeat number is associated with ovarian reserve, which requires further investigation. We are the first to explore biallelic contribution of the FMR1 gene by statistically evaluating the impact of the largest, smallest, gap, and sum of repeats. Our findings support prior reports that the larger allele appears to be the underlying driving force behind the associated patterns of ovarian reserve (AMH).
Despite a large sample size, our sample is a convenience sample that may be biased. Like many studies examining sub(in) fertility, our analysis is restricted to women who presented to an infertility clinic and therefore is not necessarily generalizable to the general population. Additionally, we currently have very little knowledge about the pattern in which AMH declines in individual women and can only conjecture about how averages in populations behave by age. Women may all decline in unison with the population average or may display various patterns such as steady then rapid fall off, starting high, and continuously falling at a rapid rate, etc. Given that our analysis was restricted to a single measurement of AMH, we are limited in our ability to determine the trajectory of AMH beyond that single measurement. Furthermore, our current model is unable to determine a specific CGG threshold, after which a patient would be at a higher likelihood for a predictable decline in AMH. Should a larger, more generalizable study confirm our findings, a threshold for “at risk” patients would be useful in recognizing, and ideally preventing, subfertility. Lastly, although AMH is a reliable and reproducible marker of ovarian reserve, response to ovarian stimulation and live birth rates would serve as a better correlate to ovarian reserve and fecundity.
Nonetheless, we believe this work to be an important contribution to what we currently know about the FMR1 gene and ovarian function. Although CGG repeats less than 55 are unlikely to expand to a full mutation in a single generation, a repeat size in this range appears to have clinical significance. Comparing our findings to the existing literature, we agree with the reports indicating a decline in ovarian reserve among women who have intermediate range CGG repeat numbers. We urge researchers to continue to examine this phenomenon, as testing for FMR1 CGG repeat number, in combination with traditional ovarian reserve parameters, may one day provide useful insight on a patient’s ovarian reserve, and perhaps reproductive potential, longitudinally.
Electronic supplementary material
Comparing the association of summary measures Sum, Max, Gap, and Min with log(AMH). Fitting interaction terms in a linear model reveals a “trumpet” phenomenon for “Sum,” “Max,” and “Min”. The slope of each individual gray line varies based on age, such that the younger women have a positive association, and the older women have a negative association as the CGG repeat length increases. Gap does not exhibit this similar pattern, as it is not significantly associated with AMH at any CGG repeat length. The red line is the overall effect, without controlling for age. Gray lines darken with each increasing age category (<=30, 30–35, >35) (DOC 57 kb)
Footnotes
Capsule Using novel multivariate regression models, we observed a statistically significant correlation ofovarian reserve and FMR1 CGG repeat number in women with <55 CGG repeats. Our data suggests that increasing CGG repeat number within the normal rage is associated with a more rapid decline in ovarianreserve.
References
- 1.Sullivan SD, Welt C, Sherman S. FMR1 and the continuum of primary ovarian insufficiency. Semin Reprod Med. 2011;29(4):299–307. doi: 10.1055/s-0031-1280915. [DOI] [PubMed] [Google Scholar]
- 2.ACOG Committee opinion no. 469: carrier screening for fragile X syndrome. Obstet Gynecol. 2010;116(4):1008–10. doi: 10.1097/AOG.0b013e3181fae884. [DOI] [PubMed] [Google Scholar]
- 3.Bretherick KL, Fluker MR, Robinson WP. FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Hum Genet. 2005;117(4):376–82. doi: 10.1007/s00439-005-1326-8. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, et al. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20(2):402–12. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- 5.Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, Holden JJ, Yang KT, Lee C, et al. Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in Fragile X study—preliminary data. Am J Med Genet. 1999;83(4):322–5. doi: 10.1002/(SICI)1096-8628(19990402)83:4<322::AID-AJMG17>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherman SL. Premature ovarian failure in the fragile X syndrome. Am J Med Genet. 2000;97(3):189–94. doi: 10.1002/1096-8628(200023)97:3<189::AID-AJMG1036>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 7.Karimov CB, Moragianni VA, Cronister A, Srouji S, Petrozza J, Racowsky C, et al. Increased frequency of occult fragile X-associated primary ovarian insufficiency in infertile women with evidence of impaired ovarian function. Hum Reprod. 2011;26(8):2077–83. doi: 10.1093/humrep/der168. [DOI] [PubMed] [Google Scholar]
- 8.Bodega B, Bione S, Dalpra L, Toniolo D, Ornaghi F, Vegetti W, et al. Influence of intermediate and uninterrupted FMR1 CGG expansions in premature ovarian failure manifestation. Hum Reprod. 2006;21(4):952–7. doi: 10.1093/humrep/dei432. [DOI] [PubMed] [Google Scholar]
- 9.Pastore LM, Young SL, Baker VL, Karns LB, Williams CD, Silverman LM. Elevated prevalence of 35–44 FMR1 trinucleotide repeats in women with diminished ovarian reserve. Reprod Sci. 2012;19(11):1226–31. doi: 10.1177/1933719112446074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gleicher N, Barad DH. The FMR1 gene as regulator of ovarian recruitment and ovarian reserve. Obstet Gynecol Surv. 2010;65(8):523–30. doi: 10.1097/OGX.0b013e3181f8bdda. [DOI] [PubMed] [Google Scholar]
- 11.Kline JK, Kinney AM, Levin B, Brown SA, Hadd AG, Warburton D. Intermediate CGG repeat length at the FMR1 locus is not associated with hormonal indicators of ovarian age. Menopause. 2014;21(7):740–8. doi: 10.1097/GME.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voorhuis M, Onland-Moret NC, Janse F, Ploos van Amstel HK, Goverde AJ, Lambalk CB. The significance of fragile X mental retardation gene 1 CGG repeat sizes in the normal and intermediate range in women with primary ovarian insufficiency. Hum Reprod. 2014;29(7):1585–93. doi: 10.1093/humrep/deu095. [DOI] [PubMed] [Google Scholar]
- 13.Riggs RM, Duran EH, Baker MW, Kimble TD, Hobeika E, Yin L. Assessment of ovarian reserve with anti-Mullerian hormone: a comparison of the predictive value of anti-Mullerian hormone, follicle-stimulating hormone, inhibin B, and age. Am J Obstet Gynecol. 2008;199(2):202 e1–8. doi: 10.1016/j.ajog.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Seifer DB, Baker VL, Leader B. Age-specific serum anti-Mullerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril. 2011;95(2):747–50. doi: 10.1016/j.fertnstert.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Dolleman M, Faddy MJ, van Disseldorp J, van der Schouw YT, Messow CM, Leader B, et al. The relationship between anti-Mullerian hormone in women receiving fertility assessments and age at menopause in subfertile women: evidence from large population studies. J Clin Endocrinol Metabolism. 2013;98(5):1946–53. doi: 10.1210/jc.2012-4228. [DOI] [PubMed] [Google Scholar]
- 16.Sherman S, Pletcher BA, Driscoll DA. Fragile X syndrome: diagnostic and carrier testing. Genet Med : Off J Am College Med Genet. 2005;7(8):584–7. doi: 10.109701.GIM.0000182468.22666.dd. [DOI] [PMC free article] [PubMed]
- 17.Garber KB, Visootsak J, Warren ST. Fragile X syndrome. Europ J Human Genet : EJHG. 2008;16(6):666–72. doi: 10.1038/ejhg.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wittenberger MD, Hagerman RJ, Sherman SL, McConkie-Rosell A, Welt CK, Rebar RW, et al. The FMR1 premutation and reproduction. Fertil Steril. 2007;87(3):456–65. doi: 10.1016/j.fertnstert.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Revenga L, Madrigal I, Pagonabarraga J, Xuncla M, Badenas C, Kulisevsky J, et al. Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Europ J Human Genet : EJHG. 2009;17(10):1359–62. doi: 10.1038/ejhg.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leader B, Baker VL. Maximizing the clinical utility of antimullerian hormone testing in women’s health. Curr Opin Obstetrics Gynecol. 2014;26(4):226–36. doi: 10.1097/GCO.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ, et al. Serum anti-mullerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metabolism. 2012;97(12):4650–5. doi: 10.1210/jc.2012-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. A validated model of serum anti-mullerian hormone from conception to menopause. PLoS One. 2011;6(7):e22024. doi: 10.1371/journal.pone.0022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Geyter C, M’Rabet N, De Geyter J, Zurcher S, Moffat R, Bosch N, et al. Similar prevalence of expanded CGG repeat lengths in the fragile X mental retardation I gene among infertile women and among women with proven fertility: a prospective study. Genet Med : Off J Am College Med Genet. 2014;16(5):374–8. doi: 10.1038/gim.2013.146. [DOI] [PubMed] [Google Scholar]
- 24.Barasoain M, Barrenetxea G, Huerta I, Telez M, Carrillo A, Perez C, et al. Study of FMR1 gene association with ovarian dysfunction in a sample from the Basque Country. Gene. 2013;521(1):145–9. doi: 10.1016/j.gene.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 25.Murray A, Schoemaker MJ, Bennett CE, Ennis S, Macpherson JN, Jones M, et al. Population-based estimates of the prevalence of FMR1 expansion mutations in women with early menopause and primary ovarian insufficiency. Genet Med : Off J Am College Med Genet. 2014;16(1):19–24. doi: 10.1038/gim.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pastore LM, Johnson J. The FMR1 gene, infertility, and reproductive decision-making: a review. Front Genet. 2014;5:195. doi: 10.3389/fgene.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kline JK, Kinney AM, Levin B, Brown SA, Hadd AG, Warburton D. Intermediate CGG repeat length at the FMR1 locus is not associated with hormonal indicators of ovarian age. Menopause. 2014 doi: 10.1097/GME.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choe SA, Kim KC, Lee JY, Kim CH, Hwang D, Jee BC. The relationship between the number of CGG repeats and serum level of anti-Mullerian hormone in women without FMR1 premutation. Eur J Obstet Gynecol Reprod Biol. 2013;169(2):275–8. doi: 10.1016/j.ejogrb.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Gleicher N, Weghofer A, Oktay K, Barad DH. Correlation of triple repeats on the FMR1 (fragile X) gene to ovarian reserve: a new infertility test? Acta Obstet Gynecol Scand. 2009;88(9):1024–30. doi: 10.1080/00016340903171058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparing the association of summary measures Sum, Max, Gap, and Min with log(AMH). Fitting interaction terms in a linear model reveals a “trumpet” phenomenon for “Sum,” “Max,” and “Min”. The slope of each individual gray line varies based on age, such that the younger women have a positive association, and the older women have a negative association as the CGG repeat length increases. Gap does not exhibit this similar pattern, as it is not significantly associated with AMH at any CGG repeat length. The red line is the overall effect, without controlling for age. Gray lines darken with each increasing age category (<=30, 30–35, >35) (DOC 57 kb)