Abstract
Purpose
The effect of age on telomere length heterogeneity in men has not been studied previously. Our aims were to determine the relationship between variation in sperm telomere length (STL), men’s age, and semen parameters in spermatozoa from men undergoing in vitro fertilization (IVF) treatment.
Methods
To perform this prospective cross-sectional pilot study, telomere length was estimated in 200 individual spermatozoa from men undergoing IVF treatment at the NYU Fertility Center. A novel single-cell telomere content assay (SCT-pqPCR) measured telomere length in individual spermatozoa.
Results
Telomere length among individual spermatozoa within an ejaculate varies markedly and increases with age. Older men not only have longer STL but also have more variable STL compared to younger men. STL from samples with normal semen parameters was significantly longer than that from samples with abnormal parameters, but STL did not differ between spermatozoa with normal versus abnormal morphology.
Conclusion
The marked increase in STL heterogeneity as men age is consistent with a role for ALT during spermatogenesis. No data have yet reported the effect of age on STL heterogeneity. Based on these results, future studies should expand this modest sample size to search for molecular evidence of ALT in human testes during spermatogenesis.
Keywords: Telomeres, Human spermatozoa, In vitro fertilization, Sperm morphology, Paternal age
Introduction
Telomeres are tandem DNA repeats (TTAGGG) which provide the substrate for specialized proteins to bind, cap, and protect eukaryotic chromosome ends. They prevent chromosome end joining, facilitate homologue pairing and chiasmata formation during early meiosis, and shorten with cell division and exposure to reactive oxygen [1]. Insufficient number of telomere repeats leads to chromosome uncapping, cell senescence, and death [1]. Telomere length (TL) is known to affect aging and longevity, cell division, meiosis, and fertility [2–6].
Spermatozoa are highly differentiated haploid cells which emerge from a multi-step process of spermatogenesis [7, 8]. Spermatogenesis takes place continuously throughout the life of the man, unlike women, who make oocytes only during a narrow window of fetal life [9]. In spermatozoa, telomeres are anchored to the nuclear membrane where they form dimers and tetramers and play a fundamental role in the organization of the sperm nucleus [8]. The importance of telomeres to reproduction has been demonstrated in mice, where telomerase knockout disrupts meiosis [2, 10, 11], but only after several generations in the telomerase null state, when telomeres have shortened to a critical length. Mice engineered to have short telomeres also display abnormal early embryo development [2]. Also, the importance of telomere homeostasis in fertility was recently demonstrated in mammalian germ cells [12, 13], where authors showed that telomere structure and homeostasis is compromised in spermatocytes from patients with idiopathic infertility [13].
Telomere length decreases with age in most cell types. Stem cells express telomerase to counter the effects of replicative senescence on telomere reserve. But unique among cells, telomeres actually elongate with age in the spermatozoa of men [9, 14–17]. Telomere elongation, especially of this magnitude, is unusual in nature.
Telomeres can elongate by two mechanisms—via the action of the reverse transcriptase telomerase and by a recombination-based alternative lengthening of telomeres (ALT) [18, 19]. It has generally been thought that telomere elongation in spermatozoa with age results from the continued effects of telomerase, a reverse transcriptase active in spermatogonia and spermatocytes [20]. However, even pluripotent stem cells, which are replete with telomerase, tend to maintain rather than elongate their telomeres.
Telomerase preferentially elongates the shortest telomeres, reducing telomere length heterogeneity [21]. ALT, which depends on recombination to elongate telomeres, robustly elongates telomeres, but produces marked telomere heterogeneity [19]. A previous study from Liu et al. reported that ALT underlies the marked telomere elongation that reprograms telomeres during early embryo development [22]. Some evidence suggests that telomeres in spermatozoa not only are longer than in somatic cells [14] and oocytes [unpublished data] but also are more heterogeneous [16]. Baird et al. [16] demonstrated that in human male germ-line telomeres are dynamic and highly variable in length. Using Universal STELA, they found extreme variation in TL in the male germ line. Severely truncated telomeres were more frequent than observed in somatic cells. The mechanisms underlying the telomeric instability reported by Baird et al. remain unclear. They invoked telomere-rapid deletion (TRD), which has been described in the meiotic yeast cells [23] and depends upon the MRX complex [24]. Other mechanisms that could produce extreme sperm telomere length variability include, telomeric sister chromatid exchange, a mechanism involved in ALT, previously described by us during early mammalian embryo development [22] and oxidative damage [25]. Oxidative DNA damage produces significant effects on spermatogenesis [26]. To our knowledge, the effect of paternal age on sperm telomere length heterogeneity has not been studied previously.
The relationship between sperm telomere length (STL) and semen parameters remains controversial. Infertile men have shorter STL compared to fertile men [27] and oligozoospermic have shorter STL than normozoospermic men [28]. In this light, we hypothesized that in men undergoing in vitro fertilization (IVF), STL heterogeneity would increase with decreasing semen parameters. Our aims were to study the relationship between variation in STL, men’s age, and semen parameters. To determine the effect of age on sperm telomere length heterogeneity, we employed a robust, novel single-cell telomere length assay to measure TL in spermatozoa from men undergoing IVF.
Materials and methods
Ethical approval
This prospective cross-sectional pilot study was approved by the New York University Langone Medical Center Institutional Review Board (IRB). Each participant provided written informed consent.
Study population and sample preparation
To measure telomere length in individual spermatozoa, a micromanipulator was used to collect 200 spermatozoa drawn from semen samples—20 individual spermatozoa from ten men undergoing IVF treatment at the New York University Fertility Center (NYUFC). The demographic and clinical details of participants are presented in Table 1. Standard semen analyses (sperm concentration and total motility) were performed by the NYUFC Andrology Laboratory according to the World Health Organization (WHO) protocol [29] and participants’ information, as age and semen parameters was obtained from its records.
Table 1.
Demographic and clinical characteristics of patients
| Patients | 10 |
|---|---|
| Spermatozoa analyzed | 200 (20 per semen sample) |
| Age (years)a | 39.6 ± 6.0 (32–47) |
| Age (as categories) | |
| -Younger (≤35 years old) | 3 patients |
| -Older (>35 years old) | 7 patients |
| Semen parameters (count + motility) | |
| Normal | 120 spermatozoa (6 patients) |
| Abnormal | 80 spermatozoa (4 patients) |
| -Oligozoospermia | 2 patients |
| -Asthenozoospermia | 1 patient |
| -Oligoasthenozoospermia | 1 patient |
| Sperm concentration (×106/mL)a | 34.0 ± 32.2 (8–114) |
| Total motility (%)a | 76.2 ± 28.0 (30–100) |
| Sperm morphology | |
| Normal | 100 spermatozoa (10 patients) |
| Abnormal | 100 spermatozoa (10 patients) |
| Assisted reproduction technique | |
| ICSI (intracytoplasmic sperm injection) | 6 patients |
| IVF (in vitro fertilization) | 4 patients |
aMean ± SD (range).
After processing semen by swim-up technique, using an intracytoplasmic sperm injection (ICSI) plate, 10–20 μL (depending on the sperm count) of the processed sample were placed into a drop of 20 μL of phosphate buffered saline ((PBS) Invitrogen, Grand Island, NY, USA) solution containing 0.1 % of polyvinylpyrrolidone ((PVP) Sigma-Aldrich, St. Louis, MO, USA). Under the inverted microscope, the spermatozoon was observed with 40× objective and graded according to its morphology (normal or abnormal), following the WHO criteria [29]. The spermatozoon was captured using an ICSI micropipette (Origio, Trumbull, CT, USA), placed into a new drop of 1 μL 0.1 % PBS-PVP and finally, transferred into a sterile PCR microtube. We selected 10 normal and 10 abnormal spermatozoa from each subject. Sperm single-cell genomic DNA was obtained by adding 1 μL lysis buffer 2× (100 mM Tris · HCl pH 7.4, 300 mM NaCl, 0.8 mM EDTA, 2 % (vol/vol) Nonidet P-40, and 5 mM DTT) into the PCR microtube and heated at 75 °C for 5 min. Sperm single-cell genomic DNAs were stored at −20 °C until perform sperm telomere length assay.
Sperm telomere length assay in individual sperm cell
A single-cell telomere length assay (SCT-pqPCR), recently developed by Wang et al. [30], was used to measure telomere length in each spermatozoon. A key feature of this assay is a telomere pre-amplification step, performed before quantitative polymerase chain reaction (qPCR). Mean telomere length (T/R) was calculated by comparing the values of telomere DNA (T) and reference genes (Alu) (R) in individual spermatozoa. In brief, pre-PCR was performed using DNA Polymerase Hot Start Version (TAKARA). The reactions were set up by aliquoting 38 μL of master mix into the PCR microtubes containing 2 μL sperm single-cell genomic DNA. Each reaction was set up with 4 μL 10 × PCR buffer, 4 μL 2.5 mM dNTP, 0.25 μL DNA polymerase, 1 μL each of telomere forward and reverse primer (10 μM, TeloF: 5′ CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT 3′; TeloR: 5′ GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT 3′) and 1 μL each of reference genes forward and reverse primer (10 μm, AluF: 5′ GACCATCCCGGCTAAAACG 3′ e AluR: 5′ CGGGTTCACGCCATTCTC 3′), and water to a 40 μL final volume. Thermal cycler reaction conditions were the same described before [30]. PCR products were purified following the protocol of the purification kit (DNA clean and concentrator-5; Zymo Research, USA) and were eluted in 64 μL of double distilled water. Finally, the purified products from each individual spermatozoon were aliquoted into each well of a 96-well plate to perform the real-time PCR. Each sample was run in triplicate along with a target-specific no-template control (NTC). Positive controls were allocated to random wells on random plates to assure plate-to-plate concordance and DNA from HeLa cells (New England Biosciences, Ipswich, MA, USA) were serially diluted (20, 4, 0.8, 0.16, and 0.032 ng/μL) as a reference for standard curve calculation. After thermal cycling was completed, the CFX manager software was used to generate standard curves and Ct values for telomere signals and reference genes (Alu) signals. To ensure high reproducibility of samples, only assays with real-time PCR efficiencies between 95 and 105 % and intra-assay coefficient of variation (CoV) less than 1 % were included in analysis.
Statistical analysis
The STL was obtained from the mean value across the three replicates and represents the telomere to reference gene (T/R) ratio. STL was correlated to men’s age using the Spearman correlation test. Because data was not normally distributed, as ascertained by the Shapiro-Wilk test, only nonparametric tests were performed. The Mann-Whitney test was used to compare STL between semen parameters, sperm morphology, and men’s age (grouped into older men versus younger men). Levene’s test was used to test the equality of variances in the groups [31]. A p value less than 0.05 was considered significant. Statistics were performed on SPSS 20.0 software (IBM SPSS Software, Armonk, NY, USA), and graphs were plotted using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA).
Results
A total of 200 spermatozoa were analyzed from ten randomly selected subjects. The demographic and clinical characteristics of participants are presented in Table 1, and the results are shown as mean (SD).
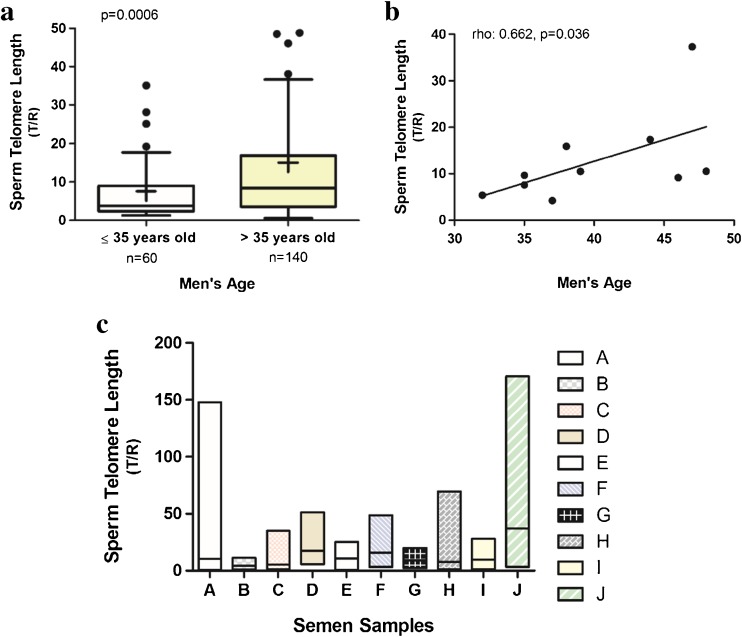
To analyze the effect of age on STL, we categorized men as older (age > 35 years old, n = 140 spermatozoa) and younger (age ≤ 35 years old, n = 60 spermatozoa). The 35-year-old cutoff was selected based on a previous study [32]. Consistent with prior studies, STL from older men was significantly longer (15.02 (24.07)) than that from younger men (age ≤ 35 years old, n = 60; (7.58 (10.62)), p = 0.0006, Mann-Whitney test, U = 5542.0 (Fig. 1a). The descriptive statistics for STL and age of each participant are presented in Table 2.
Fig. 1.
Sperm telomere length (STL) increases as men age and varies markedly within the same sample. a Older men’s STL was significantly longer than younger men’s. The box plot displays the first and third quartiles; the median is represented by the line that bisects the boxes and the mean is represented by +. The horizontal lines outside the box display minimum and maximum values. The outliers are represented by the black dots, but there were 5 outliers from the older men group (values: 170.5; 147.9; 115.2; 110.0; 51.2) and 2 outliers from the younger men group (values: 69.4; 63.4) not represented in the graph in order to highlight the behavior of the other values. b Correlation between STL and men’s age. The Spearman correlation coefficient (rho) and p value are shown. c STL of individual spermatozoa varied markedly within the same sample. The column bar’s borders display minimum and maximum values and the line that bisects the bars displays the mean
Table 2.
Descriptive statistics for STL and age of participants
| Patient | Age | Mean | Median | Min | Max | Variance (SD) |
|---|---|---|---|---|---|---|
| A | 39 | 10.48 | 1.90 | 0.60 | 147.97 | 32.51 |
| B | 37 | 4.21 | 3.27 | 1.13 | 11.31 | 2.83 |
| C | 32 | 5.39 | 3.44 | 1.47 | 35.13 | 7.28 |
| D | 44 | 17.37 | 13.71 | 5.80 | 51.26 | 13.32 |
| E | 48 | 10.50 | 6.95 | 0.71 | 25.21 | 7.79 |
| F | 38 | 15.92 | 15.52 | 3.22 | 48.75 | 9.81 |
| G | 46 | 9.15 | 8.98 | 2.20 | 19.94 | 5.45 |
| H | 35 | 7.59 | 3.31 | 1.31 | 69.43 | 14.97 |
| I | 35 | 9.66 | 9.41 | 1.48 | 28.12 | 8.11 |
| J | 47 | 37.3 | 23.06 | 3.19 | 170.59 | 45.32 |
Variances in STL were compared (homogeneity of variance) by Levene’s test. Significantly greater variance was noted in STL from older vs. younger men (p = 0.007). The Spearman correlation test showed a positive correlation between STL and age (rho = 0.662, p = 0.036 (Fig. 1b)). As shown in Fig. 1c, considerable variation in telomere length among sperm within individuals. STL of individual spermatozoa varied markedly within the same sample (rho = 0.660, p = 0.044).
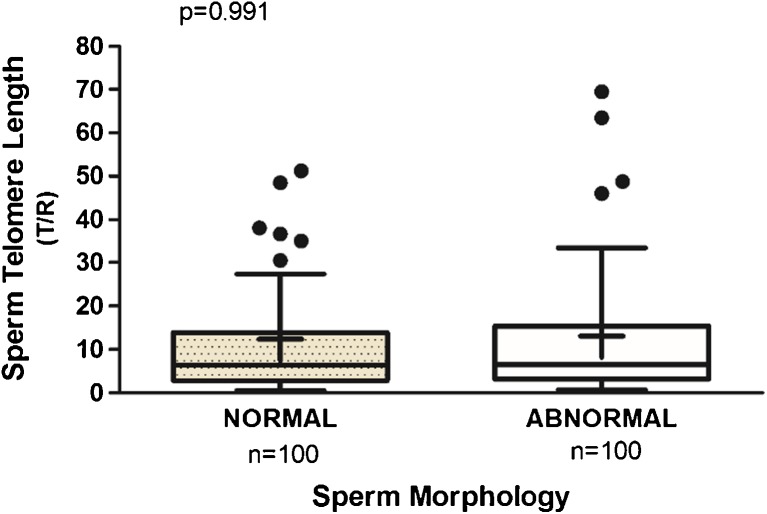
In order to test an association between STL and morphology of the spermatozoa, we compared 100 morphologically normal spermatozoa to 100 spermatozoa presenting abnormal morphology. STL from morphologically normal (12.40 (19.77)) did not differ significantly from abnormal spermatozoa (13.11 (22.56)), p = 0.991, Mann-Whitney test, U = 5005.0 (Fig. 2). The Levene’s test did not show differences in variance of STL between spermatozoa with normal and abnormal morphology in the sample size studied (p > 0.05).
Fig. 2.
STL in individual sperm did not differ significantly by sperm morphology. STL with normal morphology was 12.40 (19.77) vs abnormal 13.11 (22.56), p = 0.991. The box plot displays the first and third quartiles; the median is represented by the line that bisects the boxes, and the mean is represented by +. The horizontal lines outside the box display minimum and maximum values. The outliers are represented by the black dots, but there were 2 outliers from the normal group (147.9; 110.0) and 2 outliers from the abnormal group (170.5; 115.2) not represented in the graph in order to highlight the behavior of the other values
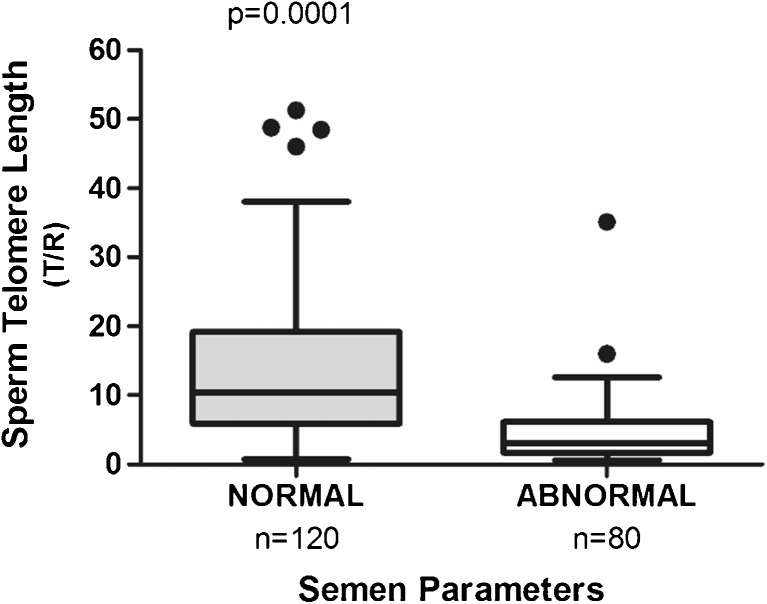
To understand the relationship between STL and semen parameters, we collected information regarding sperm concentration and total sperm motility. Sperm parameters were defined as “abnormal” according to the WHO criteria [29], when at least one category failed to fulfill the lower reference limit: 40 % for total motility and 15 × 106 spermatozoa per milliliter for sperm concentration. STL from samples with normal parameters was significantly longer (n = 120): (16.63 (22.29)) than that from samples with abnormal semen parameters (n = 80): (6.92 (18.13)), p = 0.0001, Mann-Whitney test, U = 1680.0 (Fig. 3). The nonparametric Levene’s test showed an equality of variance (p > 0.05).
Fig. 3.
STL is associated with semen parameters. Men with normal semen parameters presented significantly longer STL than men with abnormal semen parameters; p value is shown. The box plot displays the first and third quartiles, and the median is represented by the line that bisects the boxes. The horizontal lines outside the box display minimum and maximum values. The outliers are represented by the black dots, but there were 5 outliers from normal group (170.5; 147.9; 115.2; 110.0; 63.4) and 1 outlier from abnormal group (69.4) not represented in the graph in order to highlight the behavior of the other values
Discussion
Telomeres confer stability on chromosomes and preserve genomic stability. When telomeres reach a critical minimum length, cells no longer divide and the cell enters cell-cycle arrest or undergoes apoptosis [33]. Telomere attrition may contribute to segregation errors, apoptosis, reduced sperm count, and altered fertility [10].
Telomeres in spermatozoa are anchored to the nuclear membrane, where they play a fundamental role in meiosis [8]. Telomere length in sperm has been shown to increase with the age of the man [13–16], and our results add to the mounting evidence that this effect is robust and reproducible. The age-related increase in STL has been attributed to the continued effects of telomerase, but the magnitude of the increase in STL is unique even among pluripotent stem cells. This begs the question of whether alternative mechanism(s) might be at play in the age-related increase in STL.
Another mechanism of telomere length expansion, ALT, can produce large increments in TL in relatively few cell cycles. An important distinction between telomerase activity and ALT is that telomerase preferentially adds telomere repeats to the shortest telomeres, thus minimizing telomere length heterogeneity. ALT, on the other hand, promotes extensive TL heterogeneity. To explore a possible role for ALT in the age-related increase in STL, we employed a novel, single-cell telomere length assay to allow measurement of STL variation as well as mean STL. Intriguingly, we confirmed the findings by Baird et al. [16] of extensive heterogeneity in STL and extended their work by demonstrating that STL heterogeneity increases as men age. This finding is consistent with ALT contributing to the age associated increase in telomere length in men. How age would enhance ALT in the male germ line is intriguing question, but accumulated effects of DNA damage with age would be expected to promote the recombination-based DNA damage response characteristic of ALT.
We also found that men with normal semen parameters present longer STL compared to those with abnormal semen parameters. We did not, however, find differences in mean STL between spermatozoa with normal versus abnormal morphology. This finding partly agrees with Turner and Hartshorne [32], who found mean STL was not associated with clinical or semen parameters in 45 samples. Only one study in the literature [28] found a significant, positive correlation between mean STL and sperm concentration, with significantly shorter STL in oligozoospermic men compared to normozoospermic specimens. This study stratified samples by age and used well-defined semen parameters. One recent study [27] suggested a possible contribution of shorter mean STL to unexplained male infertility. By analyzing mean STL in men with idiopathic infertility and controls, the authors found shorter STLs in infertile men, but their sample size was small (32 infertile men and 25 fertile controls). They also found that age and mean STL did not correlate, nor did any sperm parameters correlate with STL.
STL among individual spermatozoa within an ejaculate varies markedly, perhaps explaining the poor correlation between STL and sperm morphology. The modest sample size in this sperm single-cell study should be expanded to further study the effects of aging on mean and variation in STL.
In conclusion, we confirm the many prior studies showing increasing STL as men age. We also confirm the prior finding that STL is highly variable. We extend this work by demonstrating that STL heterogeneity increases with age. Moreover, mean STL is higher in normal semen parameters compared to abnormal semen parameters. STL heterogeneity, however, did not differ between normal and abnormal sperm morphology, in the sample size studied. Study of variation in STL may shed light on the mechanisms of spermatozoa elongation with age in men. Future studies should perform the comparison between STL pooled from raw sperm samples with STL pooled from processed samples in order to reflect the STL of the sperm present in the ejaculate, besides to access the human testes to develop the appropriate assays for ALT in this tissue, to explore the possibility that ALT or some other recombination-based mechanism contributes to the increased mean STL and increased STL heterogeneity observed with age.
Acknowledgements
We thank Dr. Cheongeun Oh (NYU) and Professor Licinio da Silva (UFF) for assistance during the statistical analysis.
Funding was provided by CAPES Foundation, Ministry of Education of Brazil; the NYU Department of Obstetrics and Gynecology; and the Clinical and Translational Sciences Institute at NYU [NIH: #1UL1RR029893].
Ethical approval
This prospective cross-sectional pilot study was approved by the New York University Langone Medical Center Institutional Review Board (IRB).
Informed consent
Each participant provided written informed consent.
Footnotes
Capsule The marked increase in STL heterogeneity as men age is consistent with a role for ALT during spermatogenesis.
References
- 1.Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–6. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, et al. An essential role for functional telomeres in mouse germ cells during fertilization and early development. Dev Biol. 2002;249:74–84. doi: 10.1006/dbio.2002.0735. [DOI] [PubMed] [Google Scholar]
- 3.Keefe DL, et al. Telomere length predicts embryo fragmentation after in vitro fertilization in women—toward a telomere theory of reproductive aging in women. Am J Obst Gynecol. 2005;192:1256–60. doi: 10.1016/j.ajog.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 4.Sahin E, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–65. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaetzlein S, et al. Telomere length is reset during early mammalian embryogenesis. Proc Natl Acad Sci U S A. 2004;101:8034–8. doi: 10.1073/pnas.0402400101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalmbach KH, et al. Telomeres and human reproduction. Fertil Steril. 2013;99:23–9. doi: 10.1016/j.fertnstert.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krawetz SA. Paternal contribution: new insights and future challenges. Nat Rev Genet. 2005;6:633–42. doi: 10.1038/nrg1654. [DOI] [PubMed] [Google Scholar]
- 8.Moskovtsev SI, et al. Disruption of telomere-telomere interactions associated with DNA damage in human spermatozoa. Syst Biol Reprod Med. 2010;56:407–12. doi: 10.3109/19396368.2010.502587. [DOI] [PubMed] [Google Scholar]
- 9.Kimura M, et al. Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ioannou D, Griffin DK. Male fertility, chromosome abnormalities, and nuclear organization. Cytogenet Genome Res. 2011;133:269–79. doi: 10.1159/000322060. [DOI] [PubMed] [Google Scholar]
- 11.Lee HW, et al. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–74. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 12.Reig-Viader R, et al. Telomeric repeat-containing RNA (TERRA) and telomerase are components of telomeres during mammalian gametogenesis. Biol Reprod. 2014;90(5):103. doi: 10.1095/biolreprod.113.116954. [DOI] [PubMed] [Google Scholar]
- 13.Reig-Viader R, et al. Telomere homeostasis is compromised in spermatocytes from patients with idiopathic infertility. Fertil Steril. 2014;102(3):728–38. doi: 10.1016/j.fertnstert.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Allsopp RC, et al. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992;89:10114–8. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aston KI, et al. Divergence of sperm and leukocyte age-dependent telomere dynamics: implications for male-driven evolution of telomere length in humans. Mol Hum Reprod. 2012;18:517–22. doi: 10.1093/molehr/gas028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baird DM, et al. Telomere instability in the male germline. Hum Mol Genet. 2006;15:45–51. doi: 10.1093/hmg/ddi424. [DOI] [PubMed] [Google Scholar]
- 17.Sartorius GA, Nieschlag E. Paternal age and reproduction. Hum Reprod Update. 2010;16:65–79. doi: 10.1093/humupd/dmp027. [DOI] [PubMed] [Google Scholar]
- 18.Bryan TM, et al. The telomere lengthening mechanism in telomerase-negative immortal human cells does not involve the telomerase RNA subunit. Hum Mol Genet. 1997;6:921–6. doi: 10.1093/hmg/6.6.921. [DOI] [PubMed] [Google Scholar]
- 19.Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet. 2010;11:319–30. doi: 10.1038/nrg2763. [DOI] [PubMed] [Google Scholar]
- 20.Dolcetti R, De Rossi A. Telomere/telomerase interplay in virus-driven and virus-independent lymphomagenesis: pathogenic and clinical implications. Med Res Rev. 2012;32:233–53. doi: 10.1002/med.20211. [DOI] [PubMed] [Google Scholar]
- 21.Bryan TM, et al. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 1995;14:4240–8. doi: 10.1002/j.1460-2075.1995.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, et al. Telomere lengthening early in development. Nat Cell Biol. 2007;9:1436–41. doi: 10.1038/ncb1664. [DOI] [PubMed] [Google Scholar]
- 23.Li B, Lustig AJ. A novel mechanism for telomere size control in Saccharomyces cerevisiae. Genes Dev. 1996;10:1310–26. doi: 10.1101/gad.10.11.1310. [DOI] [PubMed] [Google Scholar]
- 24.Bucholc M, Park Y, Lustig AJ. Intrachromatid excision of telomeric DNA as a mechanism for telomere size control in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:6559–73. doi: 10.1128/MCB.21.19.6559-6573.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Zglinicki T, Saretzki G, Docke W, Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res. 1995;220:186–93. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- 26.Aitken RJ, et al. Oxidative stress and male reproductive health. Asian J Androl. 2014;16:31–8. doi: 10.4103/1008-682X.122203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thilagavathi J, et al. Analysis of sperm telomere length in men with idiopathic infertility. Arch Gynecol Obstet. 2013;287:803–7. doi: 10.1007/s00404-012-2632-8. [DOI] [PubMed] [Google Scholar]
- 28.Ferlin A, et al. In young men sperm telomere length is related to sperm number and parental age. Hum Reprod. 2013;28:3370–6. doi: 10.1093/humrep/det392. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 2010; 5th edition
- 30.Wang F, et al. Robust measurement of telomere length in single cells. Proc Natl Acad Sci U S A. 2013;110:E1906–12. doi: 10.1073/pnas.1306639110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nordstokke DW, Zumbo BD. A new nonparametric Levene test for equal variances. Psicológica. 2010;31:401–30. [Google Scholar]
- 32.Turner S, Hartshorne GM. Telomere lengths in human pronuclei, oocytes and spermatozoa. Mol Hum Reprod. 2013;19:510–8. doi: 10.1093/molehr/gat021. [DOI] [PubMed] [Google Scholar]
- 33.Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12:1133–8. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]