Abstract
Purpose
The current research is aimed at finding potential non-invasive bio-markers that will help us learn more about the mechanisms at play in failed assisted reproduction treatment. This exploratory pilot study examined the relationship between cell-free DNA (CFD) in plasma and telomere length in lymphocytes among women undergoing in vitro fertilization (IVF) and compared telomere length and CFD levels to a healthy control group.
Methods
Blood of 20 women undergoing IVF was collected at three time points during the IVF cycle. We assessed the relationship between CFD and telomere length as well as controlling for morning cortisol levels. We also collected blood of 10 healthy controls at two time points (luteal and follicular phases of the menstrual cycle) and compared mean telomere length, CFD, and cortisol levels between the IVF patients and healthy controls.
Results
The results revealed an inverse relationship between CFD levels and telomere lengths at several time points that remained significant even after controlling for cortisol levels. Women undergoing IVF had statistically significant higher levels of CFD and shorter telomeres compared to healthy controls.
Conclusions
The relationship between telomere length and CFD should be further explored in larger studies in order to uncover potential mechanisms that cause both shortened telomere length and elevated CFD in women undergoing IVF.
Keywords: In vitro fertilization, Cell-free DNA, Telomere length, Cortisol
Introduction
Current trends in reproductive medicine research include a search for non-invasive bio-markers that will help improve in vitro fertilization (IVF) outcomes and expand our understanding of the mechanisms behind failed assisted reproduction treatment [1]. In previous research, a relationship between elevated cell-free DNA (CFD) and low pregnancy rates was found [2]. In addition, a recent study offered a telomere-based theory of reproductive aging connecting shortened telomere length to failed assisted reproduction treatment [3]. Thus, we conducted a pilot study to examine the relationship between these two biomarkers in women undergoing IVF treatment. Furthermore, we collected blood from a small sample of healthy women in order to examine if women undergoing IVF have CFD levels and telomere lengths that differ from a comparable set of women without fertility problems.
Cell-free DNA
CFDs are DNA fragments found outside the cell nucleus, evidence of apoptotic or necrotic processes. CFD has been found in the plasma of both healthy and diseased populations. Elevated levels have been associated with pathology in several medical conditions such as heart attack [4–7], sepsis [8], and preeclampsia [9–11]. Traver [12] concluded in a review that the concentration of CFD in serum from IVF patients or in embryo culture medium may potentially be used as a non-invasive biomarker of IVF outcome. We also found elevated plasma-CFD levels in women who did not conceive following IVF treatment [2]. Some research has connected apoptotic processes, plasma CFD, and inflammation [13]. Increased inflammation has also been associated with female infertility [14]; thus, reduction of inflammatory processes may be connected with increased conception rates.
The finding of embryo-toxic factors in the bloodstream of patients with repeated failure of IVF and repeated spontaneous abortions led researchers to examine CFD levels in women undergoing IVF treatment. However, no relationship was found between CFD levels in sera and conception rates in IVF patients in serum taken 1 week after embryo transfer [15].
In previous studies of our group, in addition to elevated CFD levels among non-conceiving women following IVF treatment [2], a CFD reduction following practicing stress reduction techniques was found [16]. In addition, women with high stress levels when starting IVF treatment were less than twice as unlikely to become pregnant following IVF treatment (author’s data).
Telomeres
Telomeres are TTAGGG nucleotide repeats located at the ends of the chromosome that function primarily to protect genomic DNA from damage during replication. In normal cell lines, telomere shortening can progress to a critical point which triggers cell death [17, 18]. Telomere length can be maintained by the enzyme telomerase, a ribonucleo-protein reverse transcriptase mainly expressed in stem cells, germ cells, and regenerating tissues. Somatic cells do not contain enough telomerase to indefinitely maintain telomere length. Therefore, telomeres shorten with age in most somatic tissues, with each cell division. Telomere length can be interpreted as a form of a biological clock. Telomere length is affected by recombination, epigenetic regulation, and genetic factors, as well as oxidative stress. Telomere length in peripheral leukocytes has been found to influence the risk of unexplained recurrent pregnancy loss [3] and cumulative inflammatory load [19]. There are no currently standardized telomere lengths that are considered norms for health; however, there are scientists currently working on developing a gold standard norm [20].
Stress and telomeres
Shortened telomere length has been associated with hypo-cortisolemia [21]. In addition, greater cortisol responses to an acute laboratory stressor were associated with shorter telomeres, as were higher overnight urinary free cortisol levels and flatter daytime cortisol slopes [22]. Chronically stressed caregivers anticipate greater threat to a standardized stressor compared to controls, and this anticipatory threat is associated with shorter telomeres [23]. Shortened telomere length has been associated with depression and cumulative inflammatory load [19, 20, 24].
Exploring the relationship between CFD and telomere length may have implications for the field of reproductive medicine. We hypothesize a relationship between CFD and telomere lengths which have both been associated with apoptosis. An exploratory hypothesis is that the relationship between CFD and telomere length may be connected to plasma cortisol levels [25, 26].
Cortisol
Cortisol is the primary glucocorticoid secreted by the adrenal gland in response to ACTH stimulation. Cortisol has anti-inflammatory activity and is involved in gluconeogenesis, glycogen storage in the liver, immune regulation, mediation of physiologic stress responses, and nutrient metabolism. Under normal conditions, cortisol is secreted in a diurnal pattern in which levels peak after awakening and nadir during nighttime sleep [27–29]. Individuals experiencing chronic psychological stress have a blunted cortisol response in which morning levels are lower and continue to remain low with a decreased variability throughout the day. While the research results vary, it appears that individuals suffering from chronic stress show an initial hyperactivity of the HPA axis that over time turns into hypoactivity. This hypoactivity includes a blunted response to additional, acute stressors [27].
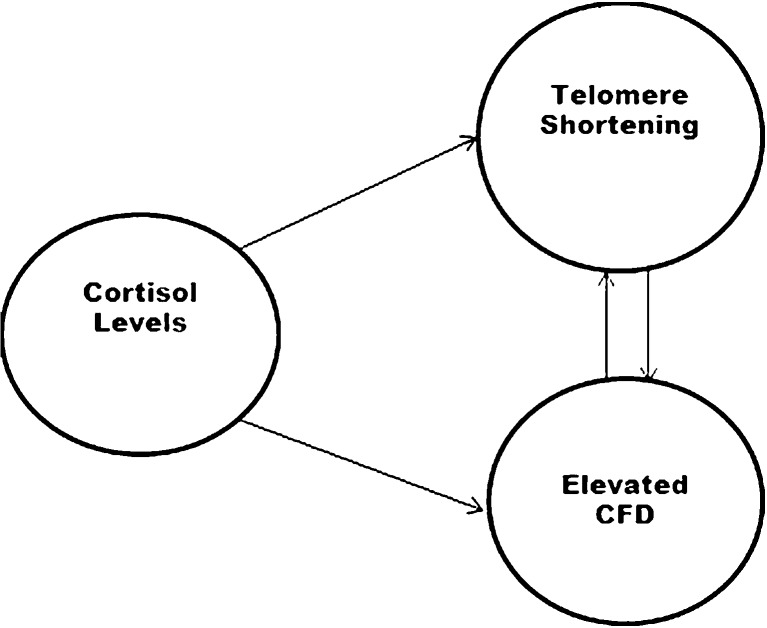
We propose a model in which the relationship between telomere length and CFD such that (1) psychological stress factors (both chronic from the infertility and acute from the invasive procedures of IVF; cortisol levels) result in elevated CFD, (2) the activation of the stress response via the HPA axis accelerates telomere shortening, and (3) enhanced apoptotic processes results in elevated CFD and telomere shortening (Fig. 1). The present study was designed to take a small step in evaluating this model. In addition, we hypothesized that there would be differences in CFD and telomere length between women undergoing IVF and women without fertility problems. We also believed that there may be differences in cortisol levels between women undergoing IVF and healthy women.
Fig. 1.
The theoretical model for the relationship between telemore length, CFD, and cortisol levels
Method
This is an exploratory pilot study that examined the relationship between CFD and telomere length and its relationship with plasma cortisol in a sample of 20 women undergoing IVF treatment. The study group comprised 20 women undergoing IVF–embryo transfer (ET) treatment at Soroka University Medical Center and 10 healthy women without fertility problems. The protocol was approved by the hospital’s ethics review board. The inclusion criteria were nulliparous women between the ages of 18–35 undergoing IVF–ET. We decided to limit the age group to 35 in order to avoid further complications related to an age related decline in fertility. In addition, this would add age as a confounding factor for telomere length, which declines with age naturally. The mean age of the IVF participants was 28 ± 4 years and the mean age of the healthy participants was 28 + 3 years. Further demographics in both groups are found in Table 1.
Table 1.
Demographics and health behaviors IVF vs. healthy controls
| Characteristics | IVF | Healthy control | |
|---|---|---|---|
| Age, years; mean (SD) | 29.3 (4.3) | 28.6 (3.4) | |
| Education**, years; mean (SD) | 13 (2.5) | 18 (1.6) | |
| Marital status** | Married (%) | 98 | 20 |
| Religiosity* | Religious (%) | 20 | 10 |
| Traditional (%) | 46 | 10 | |
| Secular (%) | 68 | 80 | |
| Economical difficulty | Very difficult (%) | 2 | 0 |
| Somewhat difficult (%) | 38 | 20 | |
| Not difficult at all (%) | 70 | 80 | |
| Study/work outside the home | Yes (%) | 88 | 80 |
| No (%) | 12 | 20 | |
| Born in Israel | Yes (%) | 90 | 80 |
| No (%) | 10 | 20 | |
| Type of living | City (%) | 76 | 100 |
| Village (%) | 8 | 0 | |
| Moshav (rural Jewish settlement) (%) | 4 | 0 | |
| Development town (%) | 10 | 0 | |
| Other (%) | 5 | 0 | |
| Smoking past or present (% yes) | 34 % | 30 % | |
| Currently smoke | 12 % | 0 | |
| Intensive exercise > times a week; mean (SD)* | 3.2 (0.91) | 2.12 (1.33) | |
*p < 0.05; **p < 0.001
IVF treatment
IVF patients were treated using a standard ovarian stimulation protocols including downregulation of the pituitary gland with a gonadotrophin-releasing hormone (GnRH) agonist (Decapeptyl 0.1; Ferring, Germany), followed by ovarian stimulation with exogenous FSH (Gonal-F; Merck-Serono, Switzerland; or Puregon; Schering-Plough, USA; or Menogon; Ferring). Oocyte retrieval was performed 36–38 h after the administration of 250 mg recombinant human chorionic gonadotrophin (HCG; Ovitrelle; Merck-Serono) when at least two or three follicles of 17–20 mm diameter were observed by ultrasound examination, and blood 17b-oestradiol concentrations reached at least 150–200 pg/ml per follicle over 17 mm diameter. Embryos were transferred using an abdominal ultrasound-guided technique on the second or third day after oocyte retrieval, using a Soft-Trans Embryo Transfer Catheter (K-Soft 5000; Cook, Ireland). Patients were instructed to start with luteal support using 4 mg/day Estrofem (Novo Company, Copenhagen, Denmark) and 600 mg/day Endometrin (Ferring Pharmaceuticals, Parsippany, NJ, USA) from the second day after oocyte retrieval until clinical pregnancy was determined. βhCG was determined 14 days after embryo transfer.
Research procedures
Blood was taken during routine blood draws from the patients including an additional 10 ml at three consecutive time points during the IVF cycle. The first time point (T1) was 2 weeks after GnRH agonist administration to ensure downregulation of the pituitary gland by the expected low estradiol concentrations. The second time point (T2) was during oocyte retrieval via the intravenous port placed for anesthesia, prior to its administration. The third time point (T3) was during the routine blood test for βhCG 2 weeks after embryo transfer. These time points were chosen in order to avoid additional pricks during the IVF cycle and represent baseline, middle, and endpoint of the IVF treatment cycle. Blood was also taken at two time points (luteal and follicular phases of the menstrual cycle) from 10 women with no infertility problems who reported good general health and were comparable in terms of demographics and age to our IVF patients.
CFD
The method for CFD analysis was a rapid direct fluorescent assay for quantification in biological fluids and a description can be found in Czamanski-Cohen [2]. After thawing, plasma was directly assayed for CFD without DNA extraction or amplification. Ten microliters of plasma was placed in each well of a 96-well plate along with 40 μl of SYBR® Gold Nucleic Acid Gel Stain (Invitrogen, Paisley, UK). SYBR® Gold Nucleic Acid Gel Stain is a proprietary unsymmetrical cyanine dye that detects single-or double-stranded DNA or RNA by exhibiting fluorescent enhancement upon binding to nucleic acids and has a high quantum yield (∼0.6) upon binding to double- or single-stranded DNA or to RNA. Each sample with one duplicate was read by a 96-well fluorometer at 535 nm with excitation wavelength of 485 nm. The florescence level of each sample was compared to a calibration curve. CFD levels were measured in nanogram per milliliter (ng/ml).
Cortisol
Plasma for cortisol was analyzed using an ELISA parameter assay kit (R&D systems, Abingdon, UK). Plasma frozen in liquid nitrogen was thawed at room temperature. This assay is based on the competitive binding technique in which cortisol present in a sample competes with a fixed amount of horseradish peroxidase (HRP)-labeled cortisol for sites on a mouse monoclonal antibody.
During the incubation, the monoclonal antibody becomes bound to the goat anti-mouse antibody coated onto the micro plate. Following a wash to remove excess conjugate and unbound sample, a substrate solution is added to the wells to determine the bound enzyme activity. The color development is stopped and the absorbance is read at 450 nm with a correction wave length of 540 nm. The intensity of the color is inversely proportional to the concentration of cortisol in the sample. Plasma samples were prepared per instructions in a 20-fold dilution of 20 μl sample + 380 μl Calibrator Diluent RD5-43. A standard curve was created by plotting the mean absorbance for each standard on a linear y-axis against the concentration on a logarithmic x-axis and drawing the best fit curve through the points on the graph. The zero standards were not included in the standard curve. Cortisol was measured in nanogram per milliliter (ng/ml).
Telomere length
Frozen blood was shipped on dry ice to Telomere Diagnostics, Inc. (Menlo Park, CA), for analysis using a quantitative polymerase chain reaction (PCR) measurement assay. The method is described in more detail in Hassett [29]. Telomere length in lymphocytes was measured as telomere-to-single copy gene (T/S) ratio, which is compatible to the telomere length in a cell. To obtain a normalizing factor, the same DNA was run 10 times and averaged. The relative telomere to single copy gene (T/S) ratio of each control DNA was divided by the normalized factor obtained. This was conducted for all samples and the average normalizing factor was used to acquire the final T/S ratio. The T/S ratio for each sample was measured twice. When the duplicate T/S value and the initial value varied by more than 7 %, samples were run a third time and the two closest values were reported.
Statistical analysis
Data analysis was performed using the Statistical Package for Social Sciences (SPSS 17.0, Chicago, IL, USA). The level of significance for all tests was 0.05. Pearson correlations were conducted between telomere length, CFD, and cortisol at all three time points. We decided to examine all time points because the time frame for the apoptosis-CFD-telomere shortening has not been examined in reproductive settings, and there is very little data regarding these time frames in other populations as well. A partial correlation analysis controlling for cortisol at each time point was then conducted. The comparison between the healthy controls and the IVF patients was conducted using a t test with bootstrapping (4000 replications) due to differences in the sample sizes of the healthy and IVF groups and to ensure the correctness of the tests.
Results
Women undergoing IVF had a variety of diagnoses; however, 70 % were undergoing IVF due to male factor infertility, 20 % had female mechanical factor, mainly, and 10 % had both male and female factor diagnoses. Table 1 describes the differences between the demographic and health behaviors of the IVF patients and healthy controls. Compared to healthy controls, more IVF patients were married and reported of high levels of exercise. The t test with bootstrapping with 4000 repetitions yielded statistically significant differences in mean CFD and telomere lengths between IVF patients and healthy controls. Women in the IVF group had shorter telomeres 1.15 (+0.18) t/s ratio compared with 1.53 (+0.126) t/s ratio: t(4) = 3.37, p = 0.029. Women in the IVF group also had higher concentrations of CFD 605.2 (+153) ng/ml compared with 342 (+155) ng/ml in the healthy controls: t(4) = −3.099, p = 0.031. There were not statistically significant differences in mean cortisol levels of women undergoing IVF and the healthy controls. For mean T/S ratio, CFD, and cortisol levels, see Table 2. For the correlation matrix, see Table 3. Pearson correlation yielded an inverse statistically significant relationship between CFD at time 1 and telomere lengths at time 2 (r = −0.817, p < 0.001). At time 2, CFD and telomere length were associated at a level that approached statistical significance (r = −0.630, p = 0.09). Telomere length at time 2 and CFD at time 3 were statistically significantly related (r = −0.539, p = 0.05). The correlations remained statistically significant after controlling for cortisol at both time points. At time 3, telomere lengths were inversely related with cortisol levels at T3 (r = −0.546, p < 0.05) and CFD was positively related to cortisol (r = 0.417, p = 0.05).
Table 2.
Telomere length, CFD, and cortisol levels
| T1 Mean (SD) Min-Max |
T2 Mean (SD) Min-Max |
T3 Mean (SD) Min-Max |
|
|---|---|---|---|
| Telomere length, T/S ratio | 1.2 (0.25) | 1.11 (0.13) | 1.13 (0.19) |
| 0.86–1.57 | 1.00–1.46 | 0.87–1.49 | |
| CFD, ng/ml | 620 (208) | 556 (126) | 640 (308) |
| 149–1073 | 306–840 | 345–1746 | |
| Cortisol, ng/ml | 75 (40) | 54 (31) | 93 (44) |
| 32–160 | 11–133 | 37–181 |
Table 3.
Correlation matrix: telomere length and CFD levels controlling for cortisol levels
| T1 telomere length, T/S ratio | T2 telomere length, T/S ratio | T3 telomere length, T/S ratio | |
|---|---|---|---|
| T1 CFD, ng/ml | 0.59 | −0.817** | −0.106 |
| T2 CFD, ng/ml | 0.742 | −0.630 | −0.539* |
| T3 CFD, ng/ml | 0.153 | 0.991 | 0.581 |
*p = 0.05; **p < 0.01
Discussion
To the best of our knowledge, this is the first study to explore the relationship between CFD and telomere length, not only in women undergoing IVF but in humans at all. Our small exploratory study found a statistically significant relationship between elevated cell-free DNA and telomere length.
As hypothesized, we found differences in telomere length and CFD levels between women undergoing IVF and women without fertility problems. We did not find differences in cortisol levels, which may be related to the fact that many of our healthy control samples were medical students and may have been exposed to psychological stress related to their studies. This also may indicate that there are additional factors other than psychological stress and its effect on HPA axis that result in shortened telomeres and elevated CFD. We would suggest in future studies to look at pro-inflammatory cytokines as well.
We proposed a model in which chronic and acute psychological stress factors result in increased apoptosis, elevated CFD, and telomere shortening. We proposed that the activation of the stress response via the HPA axis may enhance apoptotic processes and would result in elevated CFD and telomere shortening. Indeed, we found relationships between telomere length and CFD levels at different time points, which may point to the fact that there is a different time frame through which the biological process that result in CFD and telomere shortening. Due to the small number of participants, it is difficult to make a firm conclusion. However, these results do indicate that further investigation is worthwhile.
CFD appears in the bloodstream as a result of apoptotic processes and has been shown to be related to pathology in several illnesses, including infertility [2]. Other studies have shown a relationship between apoptosis and rapid telomere shortening [30, 31]. Kalmbach and colleagues have suggested that shortened telomere length may be related to female infertility [3]. In the current small pilot study, we have shown a relationship between increased cell-free DNA and telomere length during the IVF cycle. This relationship remains after controlling for cortisol levels. Cortisol levels were related to both telomere length and CFD at T3, which is the time of the βhCG test. Thus, it seems worthwhile to further explore the relationships between telomere length, CFD, and hypothalamic-pituitary-adrenal axis functioning in women undergoing IVF. Furthermore, it may be worthwhile to examine how some women undergoing IVF may show increases in apoptotic processes in lymphocytes and possibly in other cells in the reproductive tract that affect implantation and successful IVF outcome.
Our pilot study demonstrated a relationship between CFD and telomere length at different time points. Further investigation is needed in order to examine the time frame in which apoptotic processes result in elevated CFD and shortened telomere length. We did not find studies examining the time frame for cell-free DNA to manifest in plasma, but one study indicated that telomere shortening following apoptosis is rather rapid [30]. Furthermore, a recent review article delineates and supports a theory coined the CFD/telomere hypothesis, attempting to explain the mechanism that leads to premature parturition. According to this theory, telomere shortening in the placenta triggers apoptosis which leads to increases in CFD levels. In turn, pro-inflammatory cytokines are released which results in premature parturition [32]. While this hypothesis relates to local membranes surrounding the fetus and our data is from an earlier phase in reproduction, it could possibly be translational to peripheral cells as well as in regard to the implantation phase.
We found statistically significant differences in CFD levels and telomere length between women undergoing IVF and healthy controls. While our sample size is small, this may indicate that there are specific mechanisms at play related to infertility or IVF treatment that are not seen in women without infertility problems. Previous literature found higher than norm levels of CFD in women who did not become pregnant after IVF treatment. We did not find differences in the cortisol levels of both populations, which may be related to the sensitivity of this test to the time of day collection and its relationship with the time of awakening of the subject. While we did collect at similar times of day, we did not control for awakening time.
This study is too small in order to make conclusive statements regarding the relationship between CFD and telomere length in women undergoing IVF, it suggests that further research is warranted. Furthermore, it seems that plasma cortisol may play a role in the complex relationship between DNA integrity and telomere length and that there may be a unique mechanism at play that cause elevated plasma CFD levels and lymphocyte telomere shortening in women with infertility or undergoing IVF treatment. Identifying the mechanism at play driving apoptosis, increased CFD and shortened telomeres would enable physicians to tailor interventions that could potentially increase fertility in women undergoing IVF with elevated CFD and shorter telomeres and those who are not conceiving.
Acknowledgments
This study was completed with the generous help of the Warshawsky family, in memory of their daughter, Dr. Lora Warshawsky-Livne, z’l. While submitting this paper, Johanna Czamanski-Cohen was supported by a NIH R25T Cancer Prevention and Control Translational Research postdoctoral fellowship, grant number: R25 (CA078447-14), Alberts (PI).
Compliance with ethical standards
The protocol was approved by the hospital’s ethics review board.
Footnotes
Capsule Women undergoing IVF had shorter telomeres and higher concentrations of cell free DNA (CFD) compared to healthy controls. An inverse relationship between telomere length and CFD was found among women undergoing IVF treatment. Further research is indicated to identify potential mechanisms that cause shortened telomere length and elevated CFD in women undergoing IVF.
References
- 1.Scalici E, Traver S, Molinari N, Mullet T, Monforte M, Vintejoux E, et al. Cell-free DNA in human follicular fluid as a biomarker of embryo quality. Hum Reprod. 2014;29(12):2661–9. doi: 10.1093/humrep/deu238. [DOI] [PubMed] [Google Scholar]
- 2.Czamanski-Cohen J, Sarid O, Cwikel J, Lunenfeld E, Douvdevani A, Levitas E, et al. Increased plasma cell-free DNA is associated with low pregnancy rates among women undergoing IVF-embryo transfer. Reprod Biomed Online. 2013;26(1):36–41. doi: 10.1016/j.rbmo.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Kalmbach KH, Antunes DMF, Dracxler RC, Knier TW, Seth-Smith ML, Wang F, et al. Telomeres and human reproduction. Fertil Steril. 2013;99(1):23–9. doi: 10.1016/j.fertnstert.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Destouni A, Vrettou C, Antonatos D, Chouliaras G, Traeger-Synodinos J, Patsilinakos S, et al. Cell-free DNA levels in acute myocardial infarction patients during hospitalization. Acta Cardiol. 2009;64(1):51–7. doi: 10.2143/AC.64.1.2034362. [DOI] [PubMed] [Google Scholar]
- 5.Shimony A, Zahger D, Gilutz H, Goldstein H, Orlov G, Merkin M, et al. Cell free DNA detected by a novel method in acute ST-elevation myocardial infarction patients. Acute Card Care. 2010;12(3):109–11. doi: 10.3109/17482941.2010.513732. [DOI] [PubMed] [Google Scholar]
- 6.Iida M, Iizuka N, Sakaida I, Moribe T, Fujita N, Miura T, et al. Relation between serum levels of cell-free DNA and inflammation status in hepatitis C virus-related hepatocellular carcinoma. Oncol Rep. 2008;20(4):761–5. [PubMed] [Google Scholar]
- 7.Zhang R, Shao F, Wu X, Ying K. Value of quantitative analysis of circulating cell free DNA as a screening tool for lung cancer: a meta-analysis. Lung Cancer. 2010;69(2):225–31. doi: 10.1016/j.lungcan.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 8.García Moreira V, Prieto García B, Baltar Martín JM, Ortega Suárez F, Alvarez FV. Cell-free DNA as a noninvasive acute rejection marker in renal transplantation. Clin Chem. 2009;55(11):1958–66. doi: 10.1373/clinchem.2009.129072. [DOI] [PubMed] [Google Scholar]
- 9.Hahn S, Zhong XY, Bürk MR, Troeger C, Kang A, Holzgreve W. Both maternal and fetal cell-free DNA in plasma fluctuate. Ann N Y Acad Sci. 2001;945:141–4. doi: 10.1111/j.1749-6632.2001.tb03875.x. [DOI] [PubMed] [Google Scholar]
- 10.Hahn S, Zimmermann BG. Cell-free DNA in maternal plasma: has the size-distribution puzzle been solved? Clin Chem. 2010;56(8):1210–1. doi: 10.1373/clinchem.2010.149609. [DOI] [PubMed] [Google Scholar]
- 11.Zhong X, Holzgreve W, Hahn S. The Levels of circulatory fetal DNA in maternal plasma are elevated prior to the onset of preeclampsia. Hypertens Pregnancy. 2002;21(1):1. doi: 10.1081/PRG-120002911. [DOI] [PubMed] [Google Scholar]
- 12.Traver S, Assou S, Scalici E, Haouzi D, Al-Edani T, Belloc S, et al. Cell-free nucleic acids as non-invasive biomarkers of gynecological cancers, ovarian, endometrial and obstetric disorders and fetal aneuploidy. Hum Reprod Update. 2014;20(6):905–23. doi: 10.1093/humupd/dmu031. [DOI] [PubMed] [Google Scholar]
- 13.Atamaniuk J, Kopecky C, Skoupy S, Säemann MD, Weichhart T. Apoptotic cell-free DNA promotes inflammation in haemodialysis patients. Nephrol Dial Transplant. 2012;27(3):902–5. doi: 10.1093/ndt/gfr695. [DOI] [PubMed] [Google Scholar]
- 14.Carp HJ, Selmi C, Shoenfeld Y. The autoimmune bases of infertility and pregnancy loss. J Autoimmun. 2012;38(2):J266–J74. doi: 10.1016/j.jaut.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Hart EA, Patton WC, Jacobson JD, King A, Corselli J, Chan PJ. Luteal phase serum cell-free DNA as a marker of failed pregnancy after assisted reproductive technology. J Assist Reprod Genet. 2005;22(5):213–7. doi: 10.1007/s10815-005-4924-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czamanski-Cohen J, Sarid O, Cwikel J, Levitas E, Lunenfeld E, Douvdevani A, et al. Decrease in cell free DNA levels following participation in stress reduction techniques among women undergoing infertility treatment. Arch Womens Ment Health. 2014;17(3):251–3. doi: 10.1007/s00737-013-0407-2. [DOI] [PubMed] [Google Scholar]
- 17.Hayflick L, Moorhead P. The human fibroblast and a new model for cellular senescence. Exp Cell Res. 1961;25:585–8. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 18.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–60. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 19.O’Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, et al. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS One. 2011;6(5) doi: 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarthy A. Taking stock of telomere length at telome health. Chemistry & biology. 2011;18(8):937–938. doi: 10.1016/j.chembiol.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Wikgren M, Maripuu M, Karlsson T, Nordfjäll K, Bergdahl J, Hultdin J, et al. Short telomeres in depression and the general population are associated with a hypocortisolemic state. Biol Psychiatry. 2012;71(4):294–300. doi: 10.1016/j.biopsych.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Tomiyama AJ, O’Donovan A, Lin J, Puterman E, Lazaro A, Chan J, et al. Does cellular aging relate to patterns of allostasis? An examination of basal and stress reactive HPA axis activity and telomere length. Physiol Behav. 2012;106(1):40–5. doi: 10.1016/j.physbeh.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Donovan A, Tomiyama AJ, Lin J, Puterman E, Adler NE, Kemeny M, et al. Stress appraisals and cellular aging: a key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain Behav Immun. 2012;26(4):573–9. doi: 10.1016/j.bbi.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price LH, Kao H-T, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biol Psychiatry. 2013;73(1):15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smeenk JM, Verhaak CM, Vingerhoets AJ, Sweep CG, Merkus JM, Willemsen SJ, et al. Stress and outcome success in IVF: the role of self-reports and endocrine variables. Hum Reprod. 2005;20(4):991–6. doi: 10.1093/humrep/deh739. [DOI] [PubMed] [Google Scholar]
- 26.Verhaak CM, Smeenk JM, Evers AW, Kremer JA, Kraaimaat FW, Braat DD. Women’s emotional adjustment to IVF: a systematic review of 25 years of research. Hum Reprod Update. 2007;13(1):27–36. doi: 10.1093/humupd/dml040. [DOI] [PubMed] [Google Scholar]
- 27.Kudielka BM, Wüst S. Human models in acute and chronic stress: assessing determinants of individual hypothalamus–pituitary–adrenal axis activity and reactivity. Stress. 2010;13(1):1–14. doi: 10.3109/10253890902874913. [DOI] [PubMed] [Google Scholar]
- 28.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896(1):30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 29.Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79(1):1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Ramírez R, Carracedo J, Jiménez R, Canela A, Herrera E, Aljama P, et al. Massive telomere loss is an early event of DNA damage-induced apoptosis. J Biol Chem. 2003;278(2):836–42. doi: 10.1074/jbc.M206818200. [DOI] [PubMed] [Google Scholar]
- 31.Mondello C, Scovassi AI. Telomeres, telomerase, and apoptosis. Biochem Cell Biol. 2004;82(4):498–507. doi: 10.1139/o04-048. [DOI] [PubMed] [Google Scholar]
- 32.Phillippe M. Cell-Free Fetal DNA, Telomeres, and the Spontaneous Onset of Parturition. Reproductive Sciences; 2015:1933719115592714. [DOI] [PubMed]