Abstract
Purpose
Macrozoospermia is a rare condition of male infertility characterized by the presence of close to 100 % large-headed multiflagellar spermatozoa. The homozygous mutation (c.144delC) in aurora kinase C gene (AURKC) has been identified as the most frequent mutation causing macrozoospermia in North African patients. The aim of this study was to evaluate the prevalence of this condition in Tunisia and estimate the frequency of c.144delC mutation among infertile and control populations.
Methods
Sequencing c.144delC mutation was carried out in 33 macrozoospermic patients among 6652 infertile men. Minisequencing of exon3 was performed in 250 unrelated control individuals to estimate the frequency of c.144delC heterozygosity.
Results
More than 80 % of macrozoospermic patients were c.144delC homozygous. The prevalence of homozygous c.144delC was 0.4 % among infertile men (27/6652). The frequency of heterozygosity was 0.4 % among controls (1/250). Surprisingly, it is five times less common than established in the general population of North Africa (2 %) or in the Moroccan population (1.7 %).
Conclusions
We show that this mutation is relatively less frequent in the Tunisian population than in other Maghrebian populations. The occurrence of homozygous mutation among infertile men can be attributed to the high rate of consanguinity and its impact on the expression of this autosomal recessive male infertility disorder rather than a high frequency of heterozygous carriers among the general population. This highlights the importance of the molecular analysis of AURKC mutations for infertile men with high percentage of large-headed multiflagellar spermatozoa in order to limit unnecessary in vitro fertilization attempts for them.
Keywords: Male infertility, Macrozoospermia, AURKC mutations, Tunisia
Introduction
Infertility concerns at least 70 million couples worldwide [1]. In about half of cases, infertility is due to the inability of the male partner to produce spermatozoa of sufficient number (oligozoospermia), adequate motility (asthenozoospermia), or normal morphology (teratozoospermia) or combinations of these defects. Most of these cases are believed to have a genetic component since many familial cases were described. The implementation of whole-genome approaches, such as linkage analysis involving a genome-wide microsatellite scan, SNP arrays, whole-genome, or exome analysis through next generation sequencing, has recently allowed identifying few causal genes directly implicated in and linked to different and rare forms of male infertility [2–5]. AURKC is one of the first identified genes associated to a particular form of monomorphic teratozoospermia referred to as “macrocephalic multiflagellar spermatozoa syndrome” or “macrozoospermia” [2]. This rare condition (frequency <1 % [6]) is commonly associated with severe oligozoospermia (<2 millions/ml) and characterized in its typical profile by ejaculates consistently showing total teratozoospermia with close to 100 % large-headed and multiflagellar spermatozoa. However, it has been reported that macrozoospermia may sometimes appear in milder forms with the coexistence of various percentages of large-headed spermatozoa and morphologically normal spermatozoa [7]. Among these milder forms, different aspects were described depending on the morphology of these abnormal large-headed spermatozoa; regular, irregular, duplicated [8], or monoflagellar [9, 10]. So far, four AURKC mutations severely affecting the protein function have been identified and associated to the typical phenotype form [2, 11–13]. The homozygous frameshift mutation (c.144delC) has been identified as the most frequent mutation causing macrozoospermia in North African patients [12], and a carrier frequency of 1/50 was established in individuals from the Maghrebian general population [12]. A further study led to the identification of a second recurrent nonsense mutation p.Y248* in both European and North African infertile men [13].
AURKC protein is the third member of the Aurora subfamily of serine/threonine protein kinases and is preferentially expressed in the testis [14–16]. It was demonstrated that AURKC participates in the control of microtubule-kinetochore attachment, verifying the bi-orientation of the tensions preceding chromosome segregation, thus ensuring the production of euploid gametes. In addition, AURKC is involved in the spindle assembly checkpoint (SAC) which is essential in mitosis to limit the production of aneuploid cells with misaligned chromosomes or abnormal microtubule tension that would result in incorrect segregation and in meiosis to limit the conception of chromosomally abnormal gametes [13, 17]. Macrozoospermia typical phenotype caused by the absence of AURKC confirm therefore its role in the SAC since in the absence of AURKC meiotic cytokinesis is blocked, but spermiogenesis is not stopped leading to the production of large-headed multiflagellar tetraploid spermatozoa [12, 13, 17]. Consequently, a positive molecular diagnosis for infertile men presenting with typical macrozoospermia indicates a systematic contraindication for in vitro fertilization by intracytoplasmic sperm injection (ICSI). Moreover, the extent of chromosome segregation defects and division failure can therefore be variable, and abnormal aneuploid large-headed spermatozoa with different aspects (duplicated, monoflagellar…) associated to morphologically normal spermatozoa are produced and not fully incompatible with fertility [9, 18].
Here, we estimate the prevalence of macrozoospermia among infertile Tunisian men. As far as we know, this is the first report about screening results of AURKC gene in a large cohort of macrozoospermic Tunisian patients and determination of the heterozygosity frequency of the recurrent c.144delC mutation in a control Tunisian population.
Materials and methods
Patients and control population
We included, in this study, a total number of 33 patients referred to our laboratory over a period of 6 years (2009–2014) for routine semen analysis. All patients were described to have an absolute teratozoospermia (100 % abnormal morphology), and most of them (28 out of 33) presented the typical profile of macrozoospermia with close to 100 % macrocephalic multiflagellar spermatozoa. Hormone concentrations of FSH, LH, testosterone, and the seminal levels of biochemical markers of accessory sex glands were in the normal range.
To estimate the frequency of AURKC c.144delC mutation in a Tunisian control population, a total of 250 unrelated individuals were genotyped; (i) 80 were fertile men that fathered at least one child, (ii) 70 for whom we controlled their semen parameters and they were normospermic men, but we have no clear data if they have children or not, and (iii) 100 individuals (males and females) from different Tunisian regions about whom no fertility status data are available.
All patients, controls and anonymous donors, gave their written informed consent. The study was approved by the ethics committee of Farhat Hached University Hospital of Sousse (Tunisia).
Sperm analysis
The basic semen parameters were evaluated according to the World Health Organization guidelines [19]. All sperm analyses were performed at least twice within a period of 3 months. We determined the percentage of morphologically abnormal spermatozoa (irregular, large-headed, duplicated heads, multiflagellar spermatozoa) according to the David’s classification [20] by analyzing 100 spermatozoa for each patient.
Molecular analysis
DNA extraction and DNA sequencing
Genomic DNA was extracted from peripheral blood leukocytes using the FlexiGene DNA Kit (Qiagen) according to the manufacturer’s instructions. The seven AURKC exons and their intronic boundaries were amplified using primers described previously by Dieterich et al. [2]. Sequencing analyses were carried out using the Big Dye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems) and an ABI Prism Genetic Analyzer 310 (Applied Biosystems).
Multiplex minisequencing assay (SNaPshot)
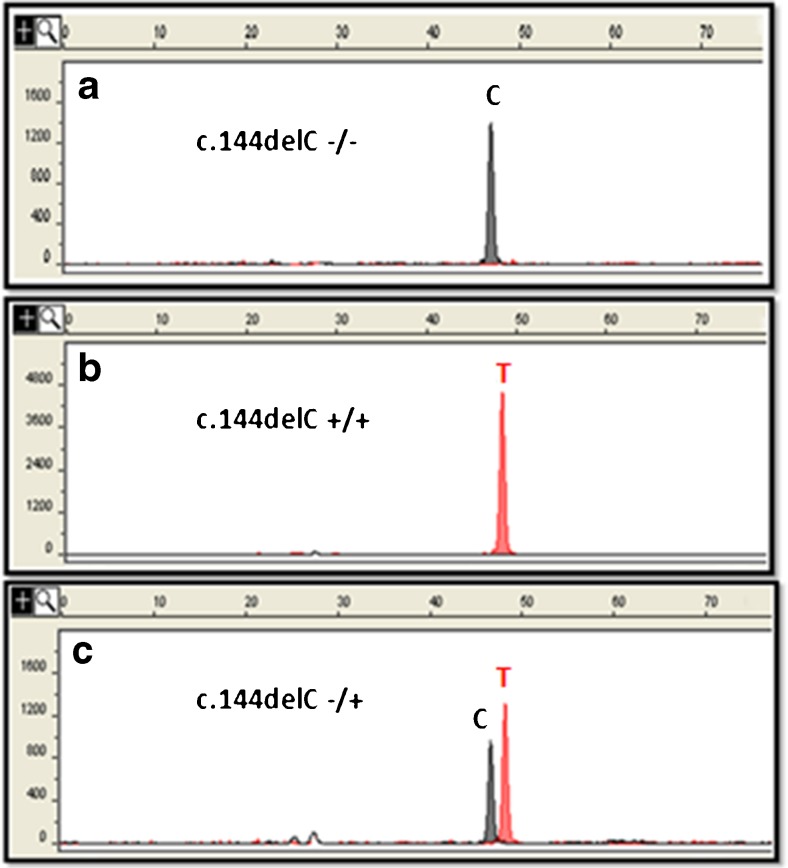
Screening for the c.144delC deletion in the 250 control subjects was performed by Single Nucleotide Extension technique using the SNaPshot Multiplex Kit (Applied Biosystems). Briefly, AURKC exon 3 was amplified and PCR products were purified with the QIAquick PCR Purification Kit (Qiagen). The minisequencing reaction was carried out in a total volume of 6 μl containing 2.5 μl of SNaPshot Multiplex Ready Reaction reagent (Applied Biosystems), 2.5 μl of a specific primer (5 pmol/μl) complementary to the analyzed region plus a poly (T) tail, which was added to ensure spatial resolution of the extension product during capillary electrophoresis (5′-T(21) ATGACTTTGAAATCGGGCGTCCC- 3′), and 1 μl of purified PCR product. The SNaPshot Multiplex Ready Reaction reagent set contains fluorescently labeled dideoxynucleotide triphosphates (ddNTPs), reaction buffer, and AmpliTaq DNA polymerase which extend the primer by one nucleotide, adding a single ddNTP to its 3′end. The minisequencing reaction consisted of 25 cycles at 96 °C for 10 s, 50 °C for 5 s, and 60 °C for 30 s. After extension, the SNaPshot products were treated with 1 unit of calf intestinal alkaline phosphatase (CIP) (Invitrogen) for 15 min at 37 °C, followed by 15 min at 65 °C and 7 min at 4 °C to remove excess fluorescent dye terminators. The purified products (1 μl) were mixed with 17 μl of formamide and 0.8 μl of GeneScan-120 LIZ Size Standard (Applied Biosystems) and incubated at 95 °C for 2 min, followed by 2 min on ice. The reaction products were separated by capillary electrophoresis using an ABI PRISM 310 Genetic Analyzer (Applied Biosystems). To reveal the electrophoresis data, the peak signal was analyzed with GeneScan Analysis Software (Applied Biosystems); the dye color of the fragment was used to identify the nucleotide of interest. For the minisequencing technique, color was assigned to individual ddNTP as follows: green/A, black/C, blue/G, red/T. The minisequencing reaction produces one (homozygote) or two (heterozygote) peaks depending on the genotype at this locus (Fig. 3). Heterozygous cases were then confirmed by Sanger Sequencing.
Fig. 3.
Electropherogram of SNaPshot peaks showing the single extension base products a) homozygous wild type (c.144delC −/−), b) homozygous for the mutation (c.144delC +/+), and c) heterozygous for the mutation (c.144delC −/+)
Results
Characteristics of the patients
The majority of patients (93.9 %) consulted for primary infertility with a mean duration of 5.5 years. Their ages ranged from 25 to 56 (mean 37.16). Only two patients consulted for a secondary infertility with duration of 2 years, and they were aged 39 and 34 years old. All patients were unrelated apart three pairs of siblings. Twenty patients were issued from a consanguineous marriage (60.6 %) and including the sibling pairs, eight other patients had the notion of infertility in the family either brothers, cousins, or uncles who do not consult or consulting at another place. Analyzed macrozoospermic patients were from all the regions of Tunisia but about 75 % of them were from the central region. All patients had normal somatic karyotype and none of them was positive for Y microdeletions.
Sperm morphology
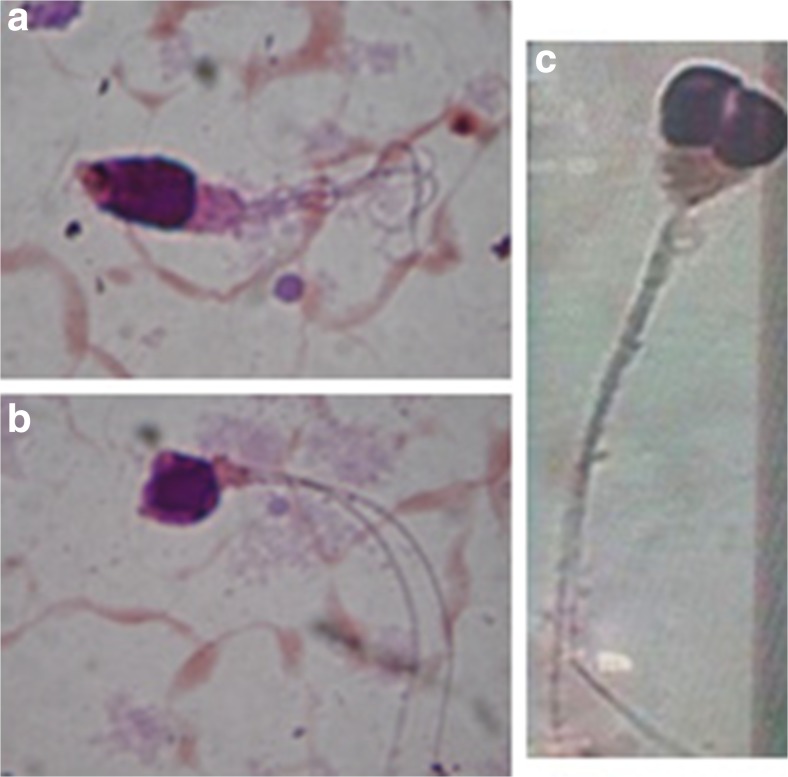
The conventional semen parameters are summarized in Table 1. Semen analysis showed a “classical” macrozoospermia phenotype for the majority of patients (28/33) consisting of 100 % teratozoospermia with an average of 80 % large-headed, 86 % irregular head, 45 % multiflagellar spermatozoa, and 95 % acrosomal abnormalities (Fig. 1a, b). Fifteen out of the 28 patients had a low sperm count (<10 million/ml). Sperm concentration ranged from 0.62 to 48 million/ml, with a mean value of 12.5 million/ml. The average viability was 45 % and the sperm progressive mobility was 0 % for almost all patients, apart for P3 and P4 (5 % and 12 % respectively).
Table 1.
Sperm parameters of analyzed patients (n = 33) according to AURKC genotype
| Sperm characteristics | AURKC mutation (n = 28) | No AURKC mutation (n = 5) | |||||
|---|---|---|---|---|---|---|---|
| Average | Range | P1a | P2 | P3 | P4 | P5 | |
| Sperm volume (ml) | 2.51 | 0.5–5.2 | 2.2 | 2.5 | 4 | 5 | 2 |
| Sperm concentration (×106 /ml) | 12.52 | 0.62–48 | <0.1 | 6.2 | 11.2 | 8 | 0.7 |
| Progressive Mobility (after 1 h) (%) | 0 | 0 | 0 | 0 | 5 | 12 | 0 |
| Viability (%) | 45.06 | 10–88 | (24/50) | 47 | 67 | 37 | 44 |
| Large-headed spz (%) | 80.42 | 30–100 | (38/50) | 8 | 26 | 45 | 41 |
| Irregular head (%) | 86.48 | 53–100 | (35/50) | 57 | 54 | 79 | 88 |
| Duplicated head (%) | 0.68 | 0–6 | (4/50) | 45 | 38 | 0 | 0 |
| Multiflagellar spz (%) | 44.82 | 15–65 | (14/50) | 28 | 26 | 24 | 5 |
| Abnormal acrosome (%) | 94.85 | 57–100 | (42/50) | 100 | 97 | 95 | 100 |
| Multiple Anomalies Index (%) | 3.52 | 2.80–4.13 | 3.52 | 3.48 | 3.68 | 3.04 | 3.49 |
Spz spermatozoa
aDue to the very low sperm concentration for this patient we analyzed only 50 spz instead of 100
Fig. 1.
Observation at light microscopy of large-headed multiflagellar spermatozoa (a and b) and spermatozoa with a duplicated head (c)
Despite a total teratozoospermia, the remaining five patients presented “milder” phenotypes of the syndrome. For patient P1, extreme oligozoospermia was observed (<0.1 million spermatozoa/ml) and sperm morphology was analyzed only for 50 spermatozoa instead of 100. For P2 and P3, spermocytogram showed an important presence of duplicated head (38 and 45 % respectively) with a lower frequency of large headed (8 and 26 % respectively) and multiflagellar (26 and 28 % respectively) (Fig. 1c). For P4 and P5, 45 and 41 % of large headed and 24 and 5 % multiflagellar were respectively noted (Table 1).
Over the last 6 years (2009 to 2014), among 6652 infertile men consulting from all regions of Tunisia, only 33 patients presented with macrozoospermia. Then, in our study, we estimated the frequency of this rare syndrome to be 0.5 % among infertile Tunisian patients.
Molecular analysis of the AURKC gene in patients
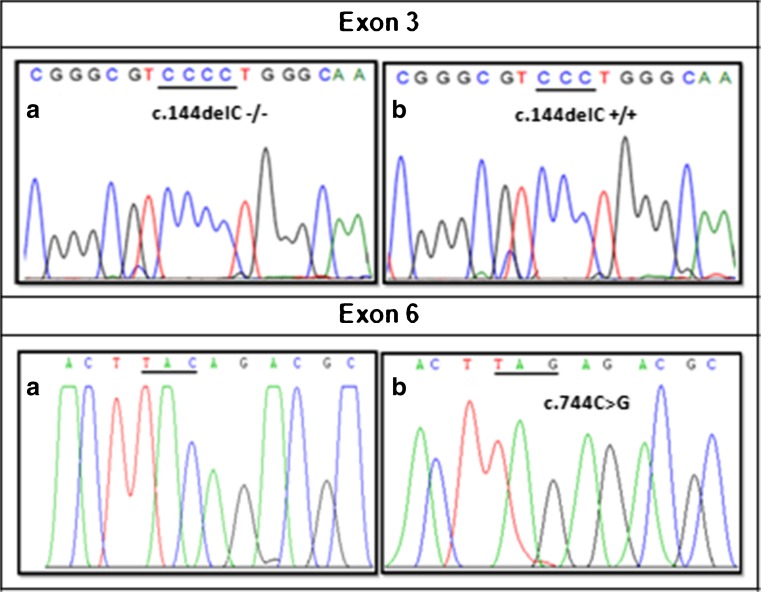
Direct sequencing of AURKC exon 3 revealed the presence of the c.144delC recurrent mutation at a homozygous state in 27 patients out of 33. The remaining six AURKC exons and their boundaries were then sequenced for the other six patients. In one patient the c.744C > G (p.Y248*) mutation in exon 6 was identified and no AURKC mutations were detected in the other five patients (Fig. 2). Overall, in our cohort of macrozoospermic patients, a molecular defect of AURKC was identified in 84.8 % (28/33) or in 83.3 % (25/30) considering only one proband in familial cases (30 genetically independent analyzed patients). Therefore, the frequency of the homozygous c.144delC deletion was 80 % (24/30) while p.Y248* accounted only for 3.3 % (1/30) and in 16.7 % (5/30) of cases no AURKC mutations were identified.
Fig. 2.
Sequence chromatograms of part of AURKC exon 3 showing a) homozygous for the wild-type sequence (c.144delC −/−) and b) homozygous for the mutation (c.144delC +/+) and part of exon 6 showing a) homozygous for the wild-type sequence and b) homozygous for the mutation c.744C > G (p.Y248*)
AURKC c.144delC mutation analysis in the control population
A minisequencing assay (SNaPshot) was developed to genotype the c.144delC mutation on 250 unrelated individuals from the general population. Only one heterozygous female case was detected for this deletion from the third subgroup; (i) 0/80 fertile men, (ii) 0/70 normospermic men, and (iii) 1/100 individuals (males and females) from different Tunisian regions (Fig. 3). Thus, we estimate a frequency of AURKC c.144delC mutation heterozygous carriers to be 0.4 % (1/250) in a Tunisian control population.
Discussion
Today’s knowledge about the genetic causes of male infertility should be integrated in reproductive care when confronted with an infertile couple, especially in case of some particular infertility phenotypes. Macrocephalic multiflagellar spermatozoa syndrome is an autosomal recessive type of absolute teratozoospermia resulting in infertility or rather in sterility associated with a negative reproductive prognosis and a very poor outcome of infertility treatment. It was reported that among the infertile population, the frequency of this condition accounts for <1 %, and it was estimated by a French team to be 0.27 % [21, 22]. In our, study we found a frequency of macrozoospermia among infertile patients to be 0.5 % which is double than that previously described. We assume that this high frequency is due to the high rate of consanguineous marriage in our country that increases the expression of this autosomal recessive male infertility form. Different mutations in AURKC gene were related to this sperm defect and two recurrent mutations (c.144delC and p.Y248*) were the most described. The encoded protein AURKC is a member of the cell cycle regulatory serine/threonine kinases (AURK A, B and C) which are essential during the mitotic cell division. AURKC is expressed specifically in the testis [14, 23] where it is involved in chromatin condensation and proper attachment of homologous chromosomes during the first meiotic division [24, 16]. The two recurrent truncating mutations, frameshift c.144delC mutation and p.Y248*, are expected to have a severe impact on the protein function. Transcript analysis showed that AURKC RNA is not present in mutated patients, suggesting that these two AURKC mutations were subjected to nonsense-mediated mRNA decay resulting in the complete absence of the protein [13, 11] leading to a defective meiosis with a blockage of spermatogenesis before the first meiotic divisions and the production of tetraploid large-headed multiflagellar spermatozoa [13, 11, 12].
Molecular analysis studies of AURKC gene demonstrated that most affected men were of North African origin. This was confirmed in Algerian and Moroccan populations [25, 26, 17]. We report here the first results of AURKC gene screening in a large cohort of Tunisian patients. It was found that, among 30 genetically independent patients, 80 % of them carried the most common mutation c.144delC and only 3.3 % carried the p.Y248* mutation. Our findings confirm again that the recurrent deletion c.144delC is the most frequent mutation causing macrozoospermia in Maghrebian patients and especially in Tunisian ones. Ben Khelifa et al., analyzed 83 probands from Maghrebian and European origin and identified the c.144delC deletion with a frequency of 85.5 % while the p.Y248* mutation accounted for 13 % [13]. El Kerch et al. found that among 18 Moroccan patients, 11 (61.1 %) carried the c.144delC mutation whereas no mutations were found for the remaining patients (38.9 %) [25]. By screening c.144delC mutation in 326 infertile Moroccan patients, Eloualid et al., found this mutation with an allelic frequency of 2.14 % (4 homozygous and 6 heterozygous out of 326) [26]. Ounis et al., analyzed 14 Algerian patients with macrozoospermia and found that c.144delC variant accounted for 71.4 % and p.Y248* accounting for 7.1 % [17].
Considering data from 24 (out of 27) homozygous patients for the common c.144delC mutation, 14 were issued from a consanguineous marriage (58.3 %). This confirms the very strong impact of consanguinity on increasing the rate of homozygous genotype and therefore on the expression of this autosomal recessive male infertility disorder. In Tunisia, as in many Arab countries, there is a high preference for unions between relatives (first cousin marriages are the most represented). The rate of consanguinity is estimated to be more than 32 % and may reach 60 % in rural areas, which highly increases the risk of recessively inherited disorders [27]. Otherwise, 10 patients (41.7 %) were issued from non-consanguineous parents suggesting the relative frequency of this mutation in the general population.
To determine the c.144delC heterozygous frequency in a control Tunisian population, we applied a new method of genetic analysis called “minisequencing” or “SnapShot.” It is a relatively simple and affordable method that allows more efficient and quick results when it is compared with Sanger Sequencing. In total, 250 individuals were genotyped and unexpectedly, only 1 heterozygous case was found (0.4 %). It seems surprising that we found such a low frequency compared to that established in the general North African population found to be 2 % (8 heterozygous out of 385 analyzed individuals) [12] or to the frequency in the Moroccan population estimated to be 1.7 % (8 out of 459) [26]. The frequency of homozygous for the c.144delC mutation among infertile population in our study was estimated to be 0.4 % (27 homozygous out of 6652 infertile men) which is consistent with the frequency of heterozygous carriers in the control population that we found (0.4 %). Therefore, we can suggest that this mutation is less frequent in the Tunisian population than in the other Maghrebian population. We can assume that the higher prevalence of macrozoospermia observed in our study (0.5 %) among infertile population is, therefore probably, not due to a higher allelic frequency of c.144delC mutation in the general population but to the higher rate of consanguineous marriages which increase the rate of mutated homozygous c.144delC. Nevertheless, we can expect that many patients did not consult because of the taboo still frequently associated with male infertility in our society.
All patients with an AURKC mutation had a typical phenotype of macrozoospermia with 0 % normal spermatozoa and more than 80 % (on average) large-headed spermatozoa. No AURKC mutation was identified for five patients showing milder phenotype which is consistent with previous studies [2, 12, 25, 17]. In fact, semen analysis of these patients showed that one of them (P1) presented an extreme oligozoospermia; it is supposed then that, despite the total teratozoospermia he presents, he more likely suffered from extreme oligo-asthenozoospermia rather than macrozoospermia. For P2 and P3, both of them showed a particular milder form characterized by the presence of duplicated heads (respectively, 45 and 38 %), large-headed spermatozoa accounting only for 8 and 26 %, respectively. Duplicated heads can have different morphological aspects; either tight nuclei that may result from an absence of meiotic division and spermatozoa with this abnormality are considered as macrocephalic spermatozoa or two heads perfectly individualized that may result from an abnormal spermiogenesis [28]. The absence of AURKC mutations in these two patients with this particular milder phenotype of macrozoospermia and in the three other patients indicates that it is clear that besides AURKC, other genes involved in cell cycle regulation and cell division could be responsible for the different milder forms of described macrozoospermia.
It was shown that large-headed spermatozoa from patients carrying c.144delC AURKC mutation are tetraploid [12]. This indicates that in case of typical form of macrozoospermia, unnecessary intracytoplasmic sperm injection (ICSI) cycles should be avoided. However, a few successful deliveries with birth of healthy babies after in vitro fertilization [28, 18, 17] or a pregnancy bound to miscarriage [9] were reported in patients suffering from milder forms of macrozoospermia. Two patients, in our cohort, presented with duplicated headed milder phenotype. One couple (patient P2) reported having suffered several spontaneous abortions and patient P3 fathered a healthy child after a spontaneous pregnancy proving that for this form of macrozoospermia fertilization and implantation could be possible, but the fetus could or not be viable.
In conclusion, our findings confirm the recurrent character of c.144delC AURKC mutation in macrozoospermic Tunisian patients. Contrary to what is expected, the frequency of c.144delC heterozygosity in our control population was found to be 1/250 (0.4 %). We noticed that this observed frequency is five times less than the frequency estimated in the general North African population (2 %) and approximately four times less than reported in the Moroccan population (1.7 %). Although we cannot demonstrate if this difference is statistically significant, we can suppose according to our funding that this mutation is less frequent in the Tunisian population. Among infertile Tunisian population, macrozoospermia represents 0.5 % which is more frequent that what was reported by others [21, 22]. This could be explained by the high rate of consanguinity (58.3 %) observed among analyzed patients which increase the rate of homozygous c.144delC mutation allowing the occurrence of this form of infertility. In 83.3 % of the cases, a molecular defect of AURKC was identified. This highlights the importance of AURKC molecular analysis for macrozoospermic patients for limiting unnecessary ICSI attempts. In fact, the chance of achieving pregnancy for a patient homozygous for an AURKC mutation is null, and in our country, adoption remains the best and only option for them. Nevertheless, in milder forms of macrozoospermia with an absence of AURKC mutations, fertility is not fully compromised. We recommend for these men fluorescence in situ hybridization (FISH) analysis of chromosomal aneuploidies and sperm DNA fragmentation in order to predict the chances of success in assisted reproductive technology. Furthermore, preimplantation genetic diagnosis (PGD) as mentioned by others [29] could also be proposed after FISH analysis, but in our country, it is not yet allowed.
Acknowledgments
The authors appreciate the cooperation and generosity of all patients and control individuals. We would like to thank Pr. Khaled Khadim-Allah, English Professor at the Faculty of Medicine Ibn Eljazzar (Sousse) for his revisions of the manuscript. We also appreciate the expert technical assistance of Hajer Farroukh, Lamia Hammami, Khaled Chabbah, Ahlem Msakni, Sihem Sassi, and Safa Bouker.
Funding
Not applicable.
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Capsule The c.144delC mutation in AURKC gene was found to be recurrent in Tunisian infertile male suffering from macrozoospermia with a relative low frequency of c.144delC heterozygosity detected in the control population.
References
- 1.Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22(6):1506–12. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 2.Dieterich K, Soto Rifo R, Faure AK, Hennebicq S, Ben Amar B, Zahi M, et al. Homozygous mutation of AURKC yields large-headed polyploid spermatozoa and causes male infertility. Nat Genet. 2007;39(5):661–5. doi: 10.1038/ng2027. [DOI] [PubMed] [Google Scholar]
- 3.Dam AH, Koscinski I, Kremer JA, Moutou C, Jaeger AS, Oudakker AR, et al. Homozygous mutation in SPATA16 is associated with male infertility in human globozoospermia. Am J Hum Genet. 2007;81(4):813–20. doi: 10.1086/521314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koscinski I, Elinati E, Fossard C, Redin C, Muller J, Velez de la Calle J, et al. DPY19L2 deletion as a major cause of globozoospermia. Am J Hum Genet. 2011;88(3):344–50. doi: 10.1016/j.ajhg.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben Khelifa M, Coutton C, Zouari R, Karaouzene T, Rendu J, Bidart M, et al. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am J Hum Genet. 2014;94(1):95–104. doi: 10.1016/j.ajhg.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrin A, Morel F, Moy L, Colleu D, Amice V, DeBraekeleer M. Study of aneuploidy in large-headed, multiple-tailed spermatozoa: case report and review of the literature. Fertil Steril. 2008;90(4):1201 e13–7. doi: 10.1016/j.fertnstert.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Achard V, Paulmyer-Lacroix O, Mercier G, Porcu G, Saias-Magnan J, Metzler-Guillemain C, et al. Reproductive failure in patients with various percentages of macronuclear spermatozoa: High level of aneuploid and polyploid spermatozoa. J Androl. 2007;28(4):600–6. doi: 10.2164/jandrol.106.001933. [DOI] [PubMed] [Google Scholar]
- 8.Guichaoua MR, Geoffroy-Siraudin C, Mercier G, Achard V, Paulmyer-Lacroix O, Metzler-Guillemain C. Genetic aspects of the teratozoospermia. Gynecol Obstet Fertil. 2009;37(6):540–5. doi: 10.1016/j.gyobfe.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Molinari E, Mirabelli M, Raimondo S, Brussino A, Gennarelli G, Bongioanni F, et al. Sperm macrocephaly syndrome in a patient without AURKC mutations and with a history of recurrent miscarriage. Reprod Biomed Online. 2013;26(2):148–56. doi: 10.1016/j.rbmo.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Guthauser B, Albert M, Ferfouri F, Ray PF, Rabiey G, Selva J, et al. Inverse correlation between chromatin condensation and sperm head size in a case of enlarged sperm heads. Reprod Biomed Online. 2011;23(6):711–6. doi: 10.1016/j.rbmo.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Ben Khelifa M, Zouari R, Harbuz R, Halouani L, Arnoult C, Lunardi J, et al. A new AURKC mutation causing macrozoospermia: implications for human spermatogenesis and clinical diagnosis. Mol Hum Reprod. 2011;17(12):762–8. doi: 10.1093/molehr/gar050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dieterich K, Zouari R, Harbuz R, Vialard F, Martinez D, Bellayou H, et al. The aurora kinase C c.144delC mutation causes meiosis I arrest in men and is frequent in the North African population. Hum Mol Genet. 2009;18(7):1301–9. doi: 10.1093/hmg/ddp029. [DOI] [PubMed] [Google Scholar]
- 13.Ben Khelifa M, Coutton C, Blum MG, Abada F, Harbuz R, Zouari R, et al. Identification of a new recurrent aurora kinase C mutation in both European and African men with macrozoospermia. Hum Reprod. 2012;27(11):3337–46. doi: 10.1093/humrep/des296. [DOI] [PubMed] [Google Scholar]
- 14.Kimura M, Matsuda Y, Yoshioka T, Okano Y. Cell cycle-dependent expression and centrosome localization of a third human aurora/Ipl1-related protein kinase, AIK3. J Biol Chem. 1999;274(11):7334–40. doi: 10.1074/jbc.274.11.7334. [DOI] [PubMed] [Google Scholar]
- 15.Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4(11):842–54. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- 16.Yan XM, Cao LH, Li Q, Wu YH, Zhang HX, Saiyin H, et al. Aurora C is directly associated with Survivin and required for cytokinesis. Genes Cells. 2005;10(6):617–26. doi: 10.1111/j.1365-2443.2005.00863.x. [DOI] [PubMed] [Google Scholar]
- 17.Ounis L, Zoghmar A, Coutton C, Rouabah L, Hachemi M, Martinez D, et al. Mutations of the aurora kinase C gene causing macrozoospermia are the most frequent genetic cause of male infertility in Algerian men. Asian J Androl. 2015;17(1):68–73. doi: 10.4103/1008-682X.136441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimizu Y, Kiumura F, Kaku S, Izuno M, Tomita K, Thumkeo D, et al. Successful delivery following ICSI with macrocephalic sperm head syndrome: a case report. Reprod Biomed Online. 2012;24(6):603–5. doi: 10.1016/j.rbmo.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 19.WHO. (World Health Organization) Laboratory manual for the examination and processing of human semen. 5. New York: Cambridge University Press; 2010. [Google Scholar]
- 20.Auger J, Eustache F, David G. Standardisation de la classification morphologique des spermatozoïdes humains selon la méthode de David modifiée. Andrologie. 2000;10(4):358–73. doi: 10.1007/BF03034491. [DOI] [Google Scholar]
- 21.Guthauser B, Vialard F, Dakouane M, Izard V, Albert M, Selva J. Chromosomal analysis of spermatozoa with normal-sized heads in two infertile patients with macrocephalic sperm head syndrome. Fertil Steril. 2006;85(3):750 e5–e7. doi: 10.1016/j.fertnstert.2005.07.1334. [DOI] [PubMed] [Google Scholar]
- 22.Achard V, Guichaoua MR. Syndrome des spermatozoides macrocephales polyflagelles et assistance médicale à la procreation [French] Andrologie. 2005;15:185–8. doi: 10.1007/BF03035152. [DOI] [Google Scholar]
- 23.Tang CJ, Chuang CK, Hu HM, Tang TK. The zinc finger domain of Tzfp binds to the tbs motif located at the upstream flanking region of the Aie1 (aurora-C) kinase gene. J Biol Chem. 2001;276(22):19631–9. doi: 10.1074/jbc.M100170200. [DOI] [PubMed] [Google Scholar]
- 24.Tang CJ, Lin CY, Tang TK. Dynamic localization and functional implications of aurora-C kinase during male mouse meiosis. Dev Biol. 2006;290(2):398–410. doi: 10.1016/j.ydbio.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 25.El Kerch F, Lamzouri A, Laarabi FZ, Zahi M, Ben Amar B, Sefiani A. Confirmation of the high prevalence in Morocco of the homozygous mutation c.144delC in the aurora kinase C gene (AURKC) in the teratozoospermia with large-headed spermatozoa. J Gynecol Obstet Biol Reprod. 2011;40(4):329–33. doi: 10.1016/j.jgyn.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Eloualid A, Rouba H, Rhaissi H, Barakat A, Louanjli N, Bashamboo A, et al. Prevalence of the aurora kinase C c.144delC mutation in infertile Moroccan men. Fertil Steril. 2014;101(4):1086–90. doi: 10.1016/j.fertnstert.2013.12.040. [DOI] [PubMed] [Google Scholar]
- 27.Romdhane L, Abdelhak S. Research unit on molecular investigation of genetic orphan, collaborators. Genetic diseases in the Tunisian population. Am J Med Genet A. 2011;155A(1):238–67. doi: 10.1002/ajmg.a.33771. [DOI] [PubMed] [Google Scholar]
- 28.Guichaoua MR, Mercier G, Geoffroy-Siraudin C, Paulmyer-Lacroix O, Lanteaume A, Metzler-Guillemin C, et al. Macrocephalic spermatozoa. What would be the impact on reproduction? Gynecol Obstet Fertil. 2009;37(9):703–11. doi: 10.1016/j.gyobfe.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Kahraman S, Sertyel S, Findikli N, Kumtepe Y, Oncu N, Melil S, et al. Effect of PGD on implantation and ongoing pregnancy rates in cases with predominantly macrocephalic spermatozoa. Reprod Biomed Online. 2004;9(1):79–85. doi: 10.1016/S1472-6483(10)62114-1. [DOI] [PubMed] [Google Scholar]