Abstract
Polysulfonated macromolecules are known to bind selectins, adhesion membrane proteins which are broadly implicated in inflammation. Commercially available branched polyethyleneimine (PEI) was reacted with chlorosulfonic acid to generate sulfonated PEI with varying degrees of sulfonation. Remaining unreacted amine groups were then used for straightforward conjugation with pyropheophoribide-a, a near infrared photosensitizer. Photosensitizer-labeled sulfonated PEI conjugates inhibited blood coagulation and were demonstrated to specifically bind to cells genetically programmed to overexpress L-selectin (CD62L) or P-selectin (CD62P). In vitro, following targeting, selectin-expressing cells could be destroyed via photodynamic therapy.
Introduction
Inflammation plays a central role in numerous chronic conditions that adversely affect health including heart disease1, cancer2 and metabolic disorders3. One of the key steps in inflammatory responses involves the migration and extravasation of leukocytes from blood vessels to the site of insult. This process is mediated by selectins, a family of cell-surface glycoproteins that include endothelial (E-), platelet (P-) and leukocyte (L-) selectin.4 Selectins contain characteristic extracellular domains that include a 1) calcium-dependent, carbohydrate-binding lectin domain, 2) an epidermal growth factor domain, and 3) a domain consisting of two to nine short consensus repeat units involved in protein binding.5 Following damage, tissues release cytokines that induce endothelial cells to express E- and P-selectin that in turn bind to circulating leukocytes to induce adhesion to the endothelium. Inflammatory activation by molecules like IL-1β and TNFα increases E-selectin expression over a period of hours, whereas other mediators including thrombin, histamine and peroxides induce P-selectin expression over a period of minutes.6
The binding partners of selectins and their associated biological significance are still being elucidated, but are numeous.7 One selectin-binding surface protein of interest that is expressed on circulating leukocytes is P-selectin glycoprotein ligand (PSGL-1).8, 9 PSGL-1 contains the tetrasaccharide sialyl-Lewis X (sLeX) as well as O-linked tyrosine-sulfate residues and these two components together regulate selectin-binding. The unbranched sulfonated heparin glycosaminoglycan, a broadly used anticoagulant, is known to inhibit acute inflammation by reducing L- and P- selectin binding function.10 Several other sulfonated macromolecules have been reported to interact with selectins to some degree, including fucoidan, dextran sulfate, chondroitin sulfate, as well as other sulfonated lipids and sugars.11
Given the importance of selectins in disease, they have become a target in molecular imaging research. P-selectin antibodies have been used to functionalize microbubbles for ultrasound imaging of renal tissue injury12 and ischaemia13 in mice. Other antibody-based approaches have been used to target E-14 and L-15, 16 selectins. Theranostic selectin targeting has also been described using selectin-binding peptides,17, 18 aptamers,19 and sLeX analogs20. One noteworthy synthetic approach involves the use of dendritic polyglycerol sulfate (dPGS) as a platform for L- and P- selectin binding.21, 22 dPGS, which like heparin is a polysulfonated macromolecule, has also been used as a scaffold for fluorescence imaging of inflammation using near infrared dyes,23, 24 optoacoustic imaging via conjugation to gold nanorods25 and has capacity for radiolabeling with synthesis at the kilo scale.26 However, synthesis of dendrimers can be an intensive process and dPGS itself contains only terminal hydroxyl groups which require further functionalization prior to bioconjugation. Therefore, alternate selectin-binding platforms that are more readily accessible or that are more easily chemically modified could potentially be useful. One candidate includes a derivative of polyethyleneimine (PEI), which is abundantly available, contains a large amount of readily modifiable amine groups, and is easily modified to generate sulfonated PEI (s-PEI). s-PEI has been explored in diverse applications related to anticoagulants27, gene delivery28, environmental detoxification29, 30 and membrane processes31.
In this study, we report the synthesis and characterization of s-PEI and subsequent conjugation to the photosensitizer pyropheophorbide-a (pyro). Photosensitizers are used in combination with light delivery to target tissues in photodynamic therapy, which is a clinical procedure used to combat various diseases including cancer.32 Molecular targeting of photosensitizers aims to increase the amount of photosensitizer in the target tissue, thereby reducing harm to non-target tissues.33–35 Numerous methods have been explored for photosensitizer targeting including conjugation to antibodies36, 37, sugars38, aptamers39 and small molecules40. Photosensitizer targeting strategies for cancer typically involves active targeting cell surface receptors expressed on cancer cells themselves or vascular targeting the tumor blood vessels, either actively or passively.41 To our knowledge, the development of photosensitizers targeted to selectins has not yet been explored. Since E-selectin is overexpressed in cancers including breast 42 and prostate43, selectin-targeted photosensitizers could possibly offer improved tumor selectivity for PDT treatments. Alternatively, as selectin expression has been reported to increase shortly following PDT,44, 45 it might be possible to use PDT to strategically induce selectin expression. This would induce a positive feedback effect in attracting more selectin-targeted photosensitizers to the irradiated tissue. Such an approach could be effective in lowering the total amount of injected photosensitizer, thereby reducing systemic side-effects to the patient such as sunlight skin toxicity.
Results and Discussion
Synthesis and labeling of sulfonated polyethyleneimine
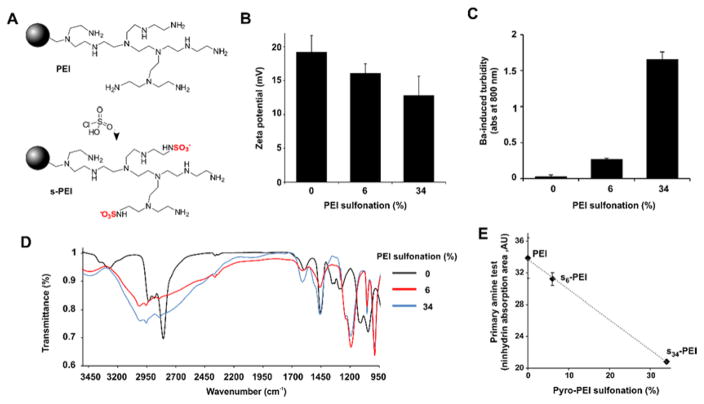
Commercially available branched PEI was modified according to published procedures to produce s-PEI with 6% (s6-PEI) and 34% (s34-PEI) sulfonation.27 PEI was stirred in methanol at 60 °C with varying amounts of chlorosulfonic acid to generate the s-PEI. Figure 1A shows the chemical reaction, with the bulk of the polymer represented by a sphere and an exemplary segment branch shown. Following the reaction, the product was dissolved in water, was then precipitated and washed with methanol, and was then dried under vacuum to obtain s-PEI. The zeta potential of the s-PEI remained positive showing that numerous free amine groups remained on the polymer, outweighing the sulfate contribution (Figure 1B). The decrease in zeta potential from +19 mV for the unconjugated PEI to +16 mV for s6-PEI and +13 mV for s34-PEI was due to the decrease in net positive charge induced by the replacement of cationic amine groups with anionic sulfate residues. A simple and standard analytical test for the presence of sulfate ions involves incubation with barium. This results in an insoluble barium-sulfate complex that can be readily detected by an optical turbidity measurement. We applied this approach to equal concentrations of PEI or s-PEI (10 mg/mL) to confirm the presence of sulfate in s-PEI. As shown in Figure 1C, barium chloride did not induce significant precipitation when added to a solution of standard PEI. However, barium rapidly complexed with s6-PEI to induce visible aggregation and turbidity in the solution. s34-PEI generated a greater amount of precipitation relative to s6-PEI. Fourier transform infrared spectroscopy (FTIR) was used to further validate the sulfate group linkages with PEI. Absorption bands at 1190 cm−1 and 990 cm−1 were observed in the s-PEI, but absent in the PEI samples (Figure 1D). These correspond to S=O (asymmetric) and S=O (symmetric) bonds, and the observed bands occurred at wavenumbers close to those previously reported for s-PEI by others.30 The prominent band appearing close to 2800 cm−1 in the PEI sample is attributed to N-H stretching30 and is weakened in the s-PEI samples. To further confirm the decrease in number of amine groups due to their conversion to sulfate, we used the ninhydrin assay, which is a common and simple colorimetric method to determine the presence of amines. When ninhydrin was added to solutions of PEI and s-PEI, absorption peaks at 570 nm emerged, which are generated due to the reaction of primary amines with ninhydrin. The peaks were integrated and these values are shown in Figure 1E, as a function of the expected sulfonation degree. An inverse linear relationship was observed, suggesting that PEI and s-PEI contained the expected loss of amine groups during their conversion to sulfates. Although the achieved degree of sulfonation was assumed to be consistent with published patent literature27, based on the ninhydrin assay to detect a loss in primary amines, the degree of sulfonation was similar to what was expected (7.8% observed vs 6% expected for s6-PEI and 38.5% observed vs 34% expected for s34-PEI). Additional analysis would be required to more accurately confirm the degree of sulfonation of the samples. Therefore, based on various analytical characterization methods, s-PEI was successfully synthesized and contained available amine groups for further modification.
Figure 1.
Synthesis and characterization of s-PEI. A) Reaction of PEI sulfonation. B) Zeta potential of PEI and s-PEI. C) Barium chloride turbidity assay for sulfate detection. D) FTIR spectra of PEI and s-PEI. E) Ninhydrin test for free amines.
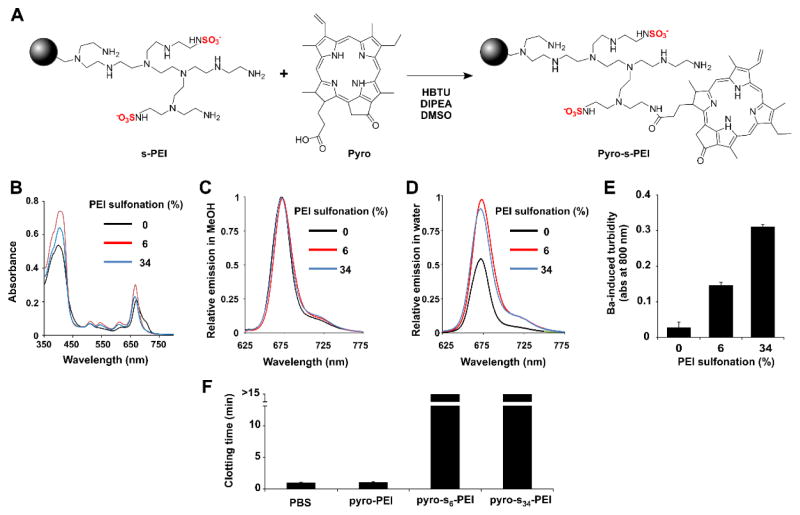
The photosensitizer pyro, which has a single carboxylic acid group and has been used previously to make targeted photosensitizers38 was next conjugated. Pyro was reacted with PEI or s-PEI using a condensation reaction in dimethylsulfoxide (DMSO) with HBTU as an acid activator and diisopropylethylamine (DIPEA) as a base (Figure 2A). As pyro contains a carboxylic acid group, it could easily react with the amines of PEI and s-PEI to generate pyro-PEI and pyro-s-PEI, respectively. Following the reaction, free pyro was removed by repeated aqueous extraction and then remaining small molecule reactants were removed with dialysis. The resulting conjugates were investigated with spectroscopy. As shown in Figure 2B, there was no shift in the peaks of the absorption profile of the conjugated pyro, pyro effectively labeled both the PEI and s-PEI samples, based on the observed absorption intensities. When the absorption of the samples was adjusted to be equal, all the samples exhibited similar fluorescence when measured in methanol (Figure 2C). However, in water, pyro-PEI exhibited self-quenching compared to pyro-s6-PEI and pyro-s34-PEI, both of whose brightness was only slightly attenuated compared to free pyro in methanol (Figure 2D). It is possible that the sulfonation inhibited self-quenching in the pyro-PEI samples, which may have been caused by structurally-induced pyro dimerization. To verify that pyro-s-PEI retained its sulfate groups during the course of pyro conjugation, the barium turbidity assay was carried out. As expected, with increasing degree of sulfonation, an increased generation in the turbidity was observed, confirming the intactness of the sulfate groups (Figure 2E). When freshly drawn mouse blood was mixed with pyro-s-PEI, but not pyro-PEI, coagulation was effectively inhibited (Figure 2F). Thus, pyro-s-PEI retained the anti-coagulatory properties of sulfonated macromolecules.
Figure 2.
Synthesis and characterization of pyro-s-PEI. A) Reaction of s-PEI with pyro. B) Absorption spectra of labeled PEI and s-PEI samples in water following labeling and purification. C) Fluorescence emission of pyro-labeled samples in methanol, normalized to free pyro in methanol. All samples had the equal absorption values at the excitation wavelength of 410 nm. D) Fluorescence emission of pyro-labeled PEI and s-PEI samples in water, normalized to the maximum emission intensity of free pyro (which was measured in methanol since it has limited water solubility). E) Barium chloride turbidity assay for sulfate detection. F) Blood clotting time of freshly drawn mouse blood immediately incubated with indicated samples (for PEI samples, the final concentration was 100 μg/mL). Mean +/− std. dev. for triplicate measurements.
Cellular activity of pyro-PEI and pyro-s-PEI
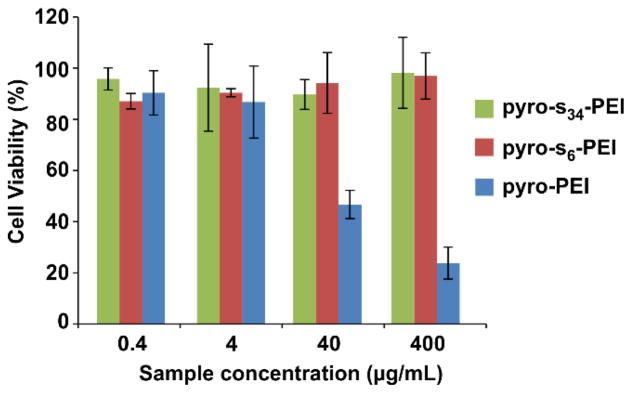
Like many cationic polymers, toxicity concerns are associated with PEI, due to the interactions of large amounts of positive amine groups with cellular structures.46 The in vitro toxicity of pyro-PEI and pyro-s-PEI was assessed. Cell viability was examined by incubating Chinese hamster ovary (CHO) cells with different concentrations of pyro-conjugated samples (0.4 – 400 μg/mL) for 90 minutes and then assessing the viability 24 hours later. As shown in Figure 3, following incubation, pyro-s-PEI induced no significant decrease in cellular viability at any of the concentrations examined. However, pyro-PEI significantly inhibited the cellular viability by 50% and 75% at incubation concentrations of 40 and 400 μg/ml, respectively. Therefore, pyro-s-PEI appeared to be less toxic compared to pyro-PEI. These results are consistent with literature examples which have shown that anionic modification of PEI can reduce its cellular toxicity.47
Figure 3.
Cell viability of CHO cells incubated with varying concentrations of PEI and s-PEI. Following 90 minutes incubation, media was replaced and 24 hours later viability was assessed using the XTT assay. Mean +/− std. dev. for n=3.
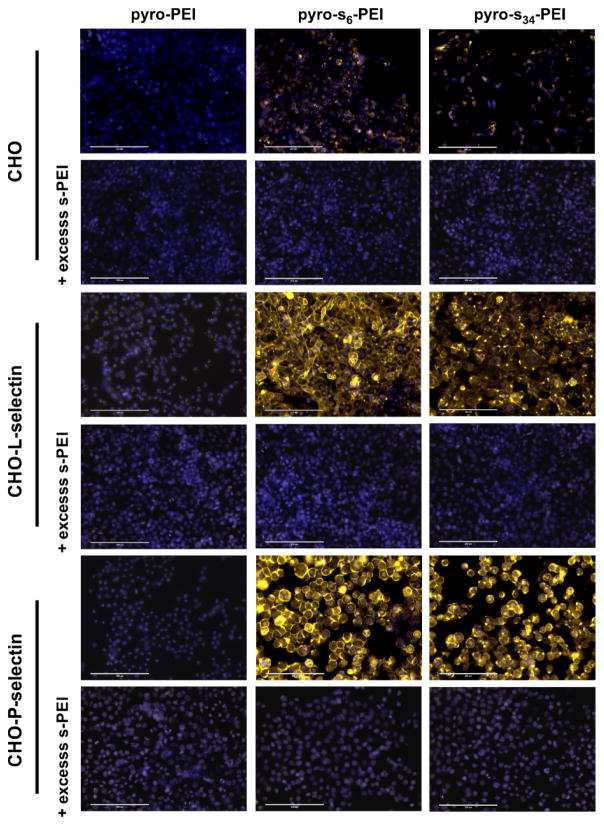
Macromolecule sulfonation is important for L- and P-selectin binding11, and the vast majority of these sulfate ligands are O-linked groups. However, N-linked sulfates are found (along with O-linked sulfates) on some selectin-binding macromolecules such as heparin. We examined the binding of pyro-s-PEI, which contains N-linked sulfates, to cells overexpressing these selectins. CHO cells were stably transfected with P- and L- selectin as previously described, to generate CHO-P and CHO-L cells, respectively.48 Pyro-PEI and pyro-s-PEI samples were incubated with all three types of cells for just 3 minutes at 37 °C, at 100 nM pyro concentration. Following incubation, the cell nuclei were stained with Hoechst dye and the cells were microscopically imaged by examining both the pyro signal and the Hoechst signal (Figure 4). CHO cells exhibited only a relatively weak pyro fluorescence (shown in yellow) when incubated with either pyro-PEI or pyro-s-PEI. However, CHO-P and CHO-L both displayed a prominent pyro signal when incubated with both pyro-s6-PEI and pyro-s34-PEI, but not non-sulfonated pyro-PEI. This suggests that the pyro-s-PEI samples could rapidly bind to P-selectins and L-selectins expressed on the surface of CHO-P and CHO-L cells, respectively. To further probe the specificity of binding, 50 folds of excess unlabeled s-PEI was co-incubated with the cells. When excess s-PEI was present, pyro-s-PEI binding was inhibited, suggesting that sulfate interaction to the cells was responsible for the binding.
Figure 4.
Binding of pyro-s-PEI to selectin-expressing cells. CHO cells expressing the indicated selectins were incubated with pyro-PEI and pyro-s-PEI for three minutes, then were washed and cells visualized with fluorescence microscopy. Pyro is shown in yellow and the nuclear Hoechst stain is shown in blue. Representative results of 4 separate experiments. 200 μm scale bars are indicated.
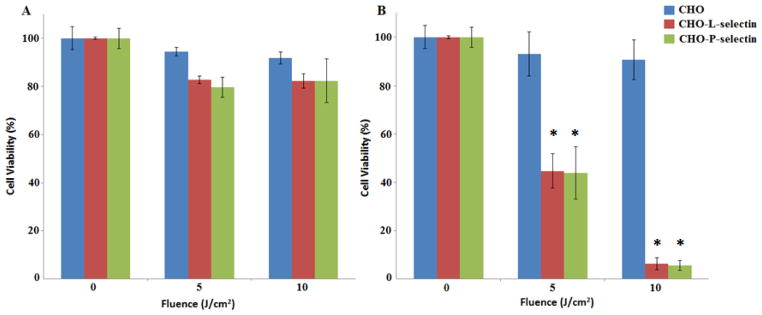
Given the binding of pyro-s-PEI to cells expressing L- and P- selectin, and that pyro is a photosensitizer, we investigated cell-targeting based for photodynamic therapy (PDT) applications. As a control, non-sulfonated pyro-PEI was incubated with CHO, CHO-P, and CHO-L cells, but following irradiation did not induce dramatic PDT cell killing (Figure 5A). Pyro-s34-PEI was incubated with the same cells and then the cells were treated with the same 665 nm irradiation. When pyro-s34-PEI was used, CHO-P and CHO-L were significantly more impacted by the treatment, with 15-fold less viability at a 10 J/cm2 light dose, compared to CHO cells not expressing selectins (Figure 5B). This demonstrates the efficacy of pyro-s-PEI as a targeted PDT agent.
Figure 5.
PDT cytotoxicity caused by A) pyro-PEI and B) pyro-s34-PEI in CHO cells expressing P- or L- selectin. Pyro-PEI or pyro-s34-PEI was incubated with indicated cells at a concentration of 100 nM pyro in a 96-well plate and then PDT was performed using 665 nm light at the indicated fluences. Cell viability was assessed 24 hours later using the XTT assay. Mean +/− std. dev. for n=3. * denotes statistically significant difference (P < 0.05) between CHO and CHO-L-selectin or CHO-P-selectin cells based on one-way analysis of variance with post hoc Tukey’s test.
Conclusion
In summary, s-PEI can readily be generated from commercially available PEI, with varying degrees of sulfonation. s-PEI can then be used for further bioconjugation with photosensitizers to generate materials such a pyro-s-PEI. Pyro-s-PEI was able to specifically bind to cells overexpressing selectins and be used as a selective PDT agent against those cells. Future work includes the application of these chemical compounds in in vivo disease models.
Experimental
Materials
Unless otherwise stated, materials were obtained from Sigma.
Sulfonated-PEI synthesis
Branched polyethyleneimine with a molecular weight of 10 kDa as determined by gel permeation chromatography was obtained from Sigma (# 408727). s-PEI was synthesized as previously reported.27 In brief, 5 g of PEI was dissolved in 50 mL of methanol and was stirred for 30 minutes in a three-necked round bottom flask until PEI was completely dissolved in methanol. Then the solution containing methanol was constantly stirred mechanically and chlorosulfonic acid was added (8.5 mL for 6 % and 31 mL for 34 %) to the solution. After addition, the solution was heated to 60 °C for 30 minutes. A thick yellow paste formed which was then dissolved in 5 mL of water. A precipitate was obtained by adding the aqueous mixture in methanol drop wise. The precipitate at the bottom was washed with methanol twice. This procedure was repeated thrice in order to get a purified product and then it was placed under vacuum for 24 hours to obtain s-PEI in powder form. The degree of sulfonation was assumed to be as reported per the patent literature.27
Pyro conjugation
Pyropheophorbide-a (pyro) was synthesized as previously reported.49 s-PEI was dissolved in 1 mL dimethyl sulfoxide (DMSO) via sonication. Subsequently, O-benzotriazol-1-yl-tetramethyluronium hexafluorophosphate (HBTU, VWR # 101116-588) was added along with diisopropylethylamine (DIPEA) and pyro, which was also dissolved in DMSO. This mixture was magnetically stirred for 24 hours after which it was added to water and dichloromethane (DCM) in a 1:1 ratio. The aqueous phase was extracted and this process was repeated thrice to obtain a purified product. Excess solvent and reagents were further removed via membrane-based dialysis with tubing with a molecular weight cutoff of 3.5 kDa (Spectra/Por # S632720) and water was replaced thrice over 12 hours.
Polymer characterization
Fourier transform infrared spectroscopy (FTIR) spectra were collected on Bruker Ram II spectrometer using sulfonated-PEI pellets. Zeta potential was measured with Brookhaven 90Plus PALS instrument in a 10 mM phosphate buffer (pH 7.4). Absorption measurements were recorded on a Perkin-Elmer Lambda 35 UV/VIS spectrometer. Fluorescence measurements were recorded in a Photon Technology International fluorometer.
Primary amines in sulfonated-PEI were quantified by the ninhydrin assay. In short, 5.4 g of sodium acetate was dissolved in 6 mL of deionized water to make a sodium acetate buffer. pH was adjusted to 5.2 using approximately 1 mL of acetic acid and the flask was filled to 10 mL with deionized water. Minutes before the assay, ninhydrin solution was prepared by adding 200 μg of ninhydrin to 7.5 mL of DMSO. Subsequently, 2.5 mL of 4 M acetate buffer was added and then the solution was analyzed. 0.75 mg of PEI/ sulfonated-PEI was dissolved in water and 400 μL of this solution was mixed with 300 μL of ninhydrin test solution and heated to 80°C. The solution was cooled and 400 μL of ethanol was added to the cooled solution. Absorbance of the sample, which obtained a peak at 570 nm was measured and integrated. For the barium chloride assay, 100 mg barium chloride was dissolved in 10 mL deionized water and sonicated until the salt was completely dissolved. 10 mg of PEI or s-PEI was dissolved in 0.5 mL deionized water. 0.5 mL of barium chloride solution was added to 0.5 mL of PEI or s-PEI solution and following 5 minutes of incubation, the turbidity was measured at 800 nm using a UV/ Vis spectrometer.
Animal experiments were carried out in accordance with the Institutional Care and Use Committee of University at Buffalo. Clotting activity of blood was measured by incubating the polymer samples with freshly drawn blood of ICR mice. 100 μL of blood was incubated (within few seconds after drawing) with 2 μL of polymer samples for a final polymer concentration of 100 μg/mL and coagulation was observed at 1 minute intervals for 15 minutes.
Cell Viability and PDT
Chinese Hamster Ovary (CHO) cells were cultured at 37 °C (5% CO2) in Dulbecco’s modified eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% streptomycin/penicillin. CHO cells were seeded in a 96-well plate with 10,000 cells per well. After 24 hours, wells were rinsed with PBS (twice) in order to remove the floating cells. Growth medium was replaced with polymer samples of varying concentrations diluted in growth medium followed by a 90 minute incubation at 37 °C (5% CO2). Cells were rinsed with PBS (twice) after incubation and were incubated with 100 μL of fresh media containing serum for 24 hours.
For PDT, cell media was replaced with PBS containing pyro-conjugated polymers. The cells were incubated with pyro-PEI and pyro-s34-PEI (pyro concentration: 100 nM) for 3 minutes and the medium was replaced with 100 μL of fresh media and subsequently treated with 665 nm irradiation at different fluences (0, 5 and 10 J/cm2). A custom built 665 nm LED-based light box was used for irradiation at a constant fluence rate of 15.3 mW/cm2. Viability was assessed with XTT after the cells were incubated for another 24 hours at 37 °C (5% CO2).
100 μL of PBS containing 50 μg/mL of XTT (2, 3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)-2H-Tetrazolium-5-Carboxanilide) and 30 μg/mL of PMS (N-methyl dibenzopyrazine methyl sulfate) was added to each well and incubated for 3 hours. Absorbance values were measured at 450 nm and background values at 630 nm were subtracted from the values at 450 nm at 3 hours. To quantify cell viability, cell viability was predefined as the ratio of absorbance of samples with polymer to samples without polymer (control group). Blank XTT values were subtracted from both samples. XTT viability assay was performed in triplicates and standard deviation was calculated based on the triplicate values.
Microscopy
CHO cells (1x105) were plated on a 96-well plate in DMEM supplemented with 10% FBS and 1% antibiotics at 37 °C (5% CO2) and allowed to adhere for 24 hours. After washing with PBS twice, the cells were incubated with polymer samples of pyro-PEI or pyro-s-PEI with pyro concentration of 100 nM for 3 minutes. After washing the samples with PBS twice, nuclei were stained with Hoechst 33342 Fluorescent Stain (100 μL of 7.8μg/mL) and incubated for 15 minutes at 37 °C (5% CO2). The wells were washed with PBS thrice and cells were imaged with an EVOS FL cell imaging system.
Acknowledgments
This work was supported by research funds from the National Institutes of Health (DP5OD017898, HL103411). C.Y.L. was supported by a T32 NIH Ruth L. Kirschstein Postdoctoral Research Training Grant.
Footnotes
Notes:
The authors declare no competing financial interest.
References
- 1.Libby P, Ridker PM, Maseri A. Inflammation and Atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 4.Kansas G. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 5.Tedder TF, Steeber DA, Chen A, Engel P. The selectins: vascular adhesion molecules. FASEB J. 1995;9:866–73. [PubMed] [Google Scholar]
- 6.Lasky L. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992;258:964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- 7.Varki A. Selectin ligands: will the real ones please stand up? J Clin Invest. 1997;99:158–162. doi: 10.1172/JCI119142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simanek EE, McGarvey GJ, Jablonowski JA, Wong CH. Selectin–Carbohydrate Interactions: From Natural Ligands to Designed Mimics. Chem Rev. 1998;98:833–862. doi: 10.1021/cr940226i. [DOI] [PubMed] [Google Scholar]
- 9.Lo CY, Antonopoulos A, Gupta R, Qu J, Dell A, Haslam SM, Neelamegham S. Competition between core-2 GlcNAc-transferase and ST6GalNAc-transferase regulates the synthesis of the leukocyte selectin ligand on human P-selectin glycoprotein ligand-1. J Biol Chem. 2013;288:13974–13987. doi: 10.1074/jbc.M113.463653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson RM, Cecconi O, Roberts WG, Aruffo A, Linhardt RJ, Bevilacqua MP. Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood. 1993;82:3253–3258. [PubMed] [Google Scholar]
- 11.Varki A. Selectin ligands. Proc Natl Acad Sci USA. 1994;91:7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindner JR, Song J, Christiansen J, Klibanov AL, Xu F, Ley K. Ultrasound Assessment of Inflammation and Renal Tissue Injury With Microbubbles Targeted to P-Selectin. Circulation. 2001;104:2107–2112. doi: 10.1161/hc4201.097061. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann BA, Lewis C, Xie A, Mirza-Mohd A, Lindner JR. Detection of recent myocardial ischaemia by molecular imaging of P-selectin with targeted contrast echocardiography. Eur Heart J. 2007;28:2011–2017. doi: 10.1093/eurheartj/ehm176. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds PR, Larkman DJ, Haskard DO, Hajnal JV, Kennea NL, George AJT, Edwards AD. Detection of Vascular Expression of E-selectin in Vivo with MR Imaging. Radiology. 2006;241:469–476. doi: 10.1148/radiol.2412050490. [DOI] [PubMed] [Google Scholar]
- 15.Kang HW, Josephson L, Petrovsky A, Weissleder R, Bogdanov A. Magnetic Resonance Imaging of Inducible E-Selectin Expression in Human Endothelial Cell Culture. Bioconjugate Chem. 2002;13:122–127. doi: 10.1021/bc0155521. [DOI] [PubMed] [Google Scholar]
- 16.Hauff P, Reinhardt M, Briel A, Debus N, Schirner M. Molecular Targeting of Lymph Nodes with L-Selectin Ligand-specific US Contrast Agent: A Feasibility Study in Mice and Dogs. Radiology. 2004;231:667–673. doi: 10.1148/radiol.2313030425. [DOI] [PubMed] [Google Scholar]
- 17.Funovics M, Montet X, Reynolds F, Weissleder R, Josephson L. Nanoparticles for the Optical Imaging of Tumor selectin. Neoplasia. 2005;7:904–911. doi: 10.1593/neo.05352. [DOI] [PubMed] [Google Scholar]
- 18.Jin AY, Tuor UI, Rushforth D, Filfil R, Kaur J, Ni F, Tomanek B, Barber PA. Magnetic resonance molecular imaging of post-stroke neuroinflammation with a P-selectin targeted iron oxide nanoparticle. Contrast Media Mol Imaging. 2009;4:305–311. doi: 10.1002/cmmi.292. [DOI] [PubMed] [Google Scholar]
- 19.Mann AP, Tanaka T, Somasunderam A, Liu X, Gorenstein DG, Ferrari M. E-Selectin-Targeted Porous Silicon Particle for Nanoparticle Delivery to the Bone Marrow. Adv Mater. 2011;23:H278–H282. doi: 10.1002/adma.201101541. [DOI] [PubMed] [Google Scholar]
- 20.Jubeli E, Moine L, Nicolas V, Barratt G. Preparation of E-selectin-targeting nanoparticles and preliminary in vitro evaluation. Int J Pharm. 2012;426:291–301. doi: 10.1016/j.ijpharm.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 21.Dernedde J, Rausch A, Weinhart M, Enders S, Tauber R, Licha K, Schirner M, Zügel U, von Bonin A, Haag R. Dendritic polyglycerol sulfates as multivalent inhibitors of inflammation. Proc Natl Acad Sci. 2010;107:19679–19684. doi: 10.1073/pnas.1003103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Türk H, Haag R, Alban S. Dendritic Polyglycerol Sulfates as New Heparin Analogues and Potent Inhibitors of the Complement System. Bioconjugate Chem. 2004;15:162–167. doi: 10.1021/bc034044j. [DOI] [PubMed] [Google Scholar]
- 23.Licha K, Welker P, Weinhart M, Wegner N, Kern S, Reichert S, Gemeinhardt I, Weissbach C, Ebert B, Haag R, et al. Fluorescence Imaging with Multifunctional Polyglycerol Sulfates: Novel Polymeric near-IR Probes Targeting Inflammation. Bioconjugate Chem. 2011;22:2453–2460. doi: 10.1021/bc2002727. [DOI] [PubMed] [Google Scholar]
- 24.Biffi S, Dal Monego S, Dullin C, Garrovo C, Bosnjak B, Licha K, Welker P, Epstein MM, Alves F. Dendritic Polyglycerolsulfate Near Infrared Fluorescent (NIRF) Dye Conjugate for Non-Invasively Monitoring of Inflammation in an Allergic Asthma Mouse Model. PLoS One. 2013;8:e57150. doi: 10.1371/journal.pone.0057150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vonnemann J, Beziere N, Böttcher C, Riese SB, Kuehne C, Dernedde J, Licha K, von Schacky C, Kosanke Y, Kimm, et al. Polyglycerolsulfate Functionalized Gold Nanorods as Optoacoustic Signal Nanoamplifiers for In Vivo Bioimaging of Rheumatoid Arthritis. Theranostics. 2014;4:629–641. doi: 10.7150/thno.8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gröger D, Paulus F, Licha K, Welker P, Weinhart M, Holzhausen C, Mundhenk L, Gruber AD, Abram U, Haag R. Synthesis and Biological Evaluation of Radio and Dye Labeled Amino Functionalized Dendritic Polyglycerol Sulfates as Multivalent Anti-Inflammatory Compounds. Bioconjugate Chem. 2013;24:1507–1514. doi: 10.1021/bc400047f. [DOI] [PubMed] [Google Scholar]
- 27.Murashige Y, Yanagase A, Kawachi Y, Soga J. Sulfonated polyethyleneimine useful as blood anticoagulant. 4639339. US Patent. 1987 Jan 27;
- 28.Sun J, Zeng F, Jian H, Wu S. Conjugation with Betaine: A Facile and Effective Approach to Significant Improvement of Gene Delivery Properties of PEI. Biomacromolecules. 2013;14:728–736. doi: 10.1021/bm301826m. [DOI] [PubMed] [Google Scholar]
- 29.Leroy D, Martinot L, Mignonsin P, Strivay D, Weber G, Jérôme C, Jérôme R. Complexation of uranyl ions by polypyrrole doped by sulfonated and phosphonated polyethyleneimine. J Appl Polym Sci. 2003;88:352–359. [Google Scholar]
- 30.Saad DMG, Cukrowska EM, Tutu H. Sulfonated cross-linked polyethylenimine for selective removal of mercury from aqueous solutions. Toxicol Environ Chem. 2012;94:1916–1929. [Google Scholar]
- 31.Shen LQ, Xu ZK, Yang Q, Sun HL, Wang SY, Xu YY. Preparation and characterization of sulfonated polyetherimide/polyetherimide blend membranes. J Appl Polym Sci. 2004;92:1709–1715. [Google Scholar]
- 32.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. Photodynamic therapy of cancer: An update. CA: Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one—photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodynamic Therapy. 2004;1:279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solban N, Rizvi I, Hasan T. Targeted photodynamic therapy. Lasers Surg Med. 2006;38:522–531. doi: 10.1002/lsm.20345. [DOI] [PubMed] [Google Scholar]
- 35.Verma S, Watt GM, Mai Z, Hasan T. Strategies for Enhanced Photodynamic Therapy Effects. Photochem Photobiol. 2007;83:996–1005. doi: 10.1111/j.1751-1097.2007.00166.x. [DOI] [PubMed] [Google Scholar]
- 36.Hudson R, Carcenac M, Smith K, Madden L, Clarke OJ, Pelegrin A, Greenman J, Boyle RW. The development and characterisation of porphyrin isothiocyanate-monoclonal antibody conjugates for photoimmunotherapy. Br J Cancer. 2005;92:1442–1449. doi: 10.1038/sj.bjc.6602517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Governatore M, Hamblin MR, Shea CR, Rizvi I, Molpus KG, Tanabe KK, Hasan T. Experimental Photoimmunotherapy of Hepatic Metastases of Colorectal Cancer with a 17.1A Chlorine6 Immunoconjugate. Cancer Res. 2000;60:4200–4205. [PubMed] [Google Scholar]
- 38.Zhang M, Zhang Z, Blessington D, Li H, Busch TM, Madrak V, Miles J, Chance B, Glickson JD, Zheng G. Pyropheophorbide 2-Deoxyglucosamide: A New Photosensitizer Targeting Glucose Transporters. Bioconjugate Chem. 2003;14:709–714. doi: 10.1021/bc034038n. [DOI] [PubMed] [Google Scholar]
- 39.Mallikaratchy P, Tang Z, Tan W. Cell Specific Aptamer–Photosensitizer Conjugates as a Molecular Tool in Photodynamic Therapy. ChemMedChem. 2008;3:425–428. doi: 10.1002/cmdc.200700260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider R, Schmitt F, Frochot C, Fort Y, Lourette N, Guillemin F, Müller JF, Barberi-Heyob M. Design, synthesis, and biological evaluation of folic acid targeted tetraphenylporphyrin as novel photosensitizers for selective photodynamic therapy. Bioorg Med Chem. 2005;13:2799–2808. doi: 10.1016/j.bmc.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 41.Chen B, Pogue BW, Hoopes PJ, Hasan T. Vascular and Cellular Targeting for Photodynamic Therapy. Crit Rev Eukaryot Gene Expr. 2006;16:279–306. doi: 10.1615/critreveukargeneexpr.v16.i4.10. [DOI] [PubMed] [Google Scholar]
- 42.Cazet A, Julien S, Bobowski M, Burchell J, Delannoy P. Tumour-associated carbohydrate antigens in breast cancer. Breast Cancer Res. 2010;12:204–204. doi: 10.1186/bcr2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai Y, Wang J, Li R, Ayala G, Ittmann M, Liu M. GGAP2/PIKE-A Directly Activates Both the Akt and Nuclear Factor-κB Pathways and Promotes Prostate Cancer Progression. Cancer Res. 2009;69:819–827. doi: 10.1158/0008-5472.CAN-08-2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evangelou G, Farrar MD, White RD, Sorefan NB, Wright KP, McLean K, Andrew S, Watson RE, Rhodes LE. Topical aminolaevulinic acid-photodynamic therapy produces an inflammatory infiltrate but reduces Langerhans cells in healthy human skin in vivo. Br J Dermatol. 2011;165:513–9. doi: 10.1111/j.1365-2133.2011.10433.x. [DOI] [PubMed] [Google Scholar]
- 45.Gollnick SO, Evans SS, Baumann H, Owczarczak B, Maier P, Vaughan L, Wang WC, Unger E, Henderson BW. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br J Cancer. 2003;88:1772–9. doi: 10.1038/sj.bjc.6600864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Controlled Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Zintchenko A, Philipp A, Dehshahri A, Wagner E. Simple Modifications of Branched PEI Lead to Highly Efficient siRNA Carriers with Low Toxicity. Bioconjugate Chem. 2008;19:1448–1455. doi: 10.1021/bc800065f. [DOI] [PubMed] [Google Scholar]
- 48.Buffone A, Mondal N, Gupta R, McHugh KP, Lau JTY, Neelamegham S. Silencing α1,3-Fucosyltransferases in Human Leukocytes Reveals a Role for FUT9 Enzyme during E-selectin-mediated Cell Adhesion. J Biol Chem. 2013;288:1620–1633. doi: 10.1074/jbc.M112.400929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pallenberg AJ, Dobhal MP, Pandey RK. Efficient Synthesis of Pyropheophorbide-a and Its Derivatives. Org Process Res Dev. 2004;8:287–290. [Google Scholar]