Abstract
Purpose
Chronic lung inflammation commonly induces a multitude of structural and functional adaptations within the lung tissue and airspaces. Yet the impact of a persistent inflammatory environment on alveolar macrophages is still incompletely understood. Here, we examined morphology and function of alveolar macrophages in a transgenic mouse model of chronic lung disease.
Methods
Imaging flow cytometry, flow cytometry, and microscopic evaluation of alveolar macrophages isolated from healthy and inflamed lungs were performed. Gene expression of polarization markers was compared by quantitative real-time RT-PCR. The pro-inflammatory immune response of alveolar macrophages toward bacterial ligands was assessed in in vivo clodronate-liposome depletion studies.
Results
Chronic lung inflammation is associated with a substantially altered, activated alveolar macrophage morphology, and blunted TNF-α response by these cells following stimulation with ligands derived from the respiratory pathogen Streptococcus pneumoniae.
Conclusions
These results demonstrate pleiotropic effects of pulmonary inflammation on alveolar macrophage phenotype and function and suggest a functional impairment of these cells during infection with airborne pathogens.
Keywords: Chronic lung disease, Inflammation, Alveolar macrophage, Macrophage polarization, Infection
Introduction
Chronic lung diseases (CLDs) are multi-factorial inflammatory disorders and frequently involve alterations of structural as well as hematopoietic cells in the airways. In line with this, substantial molecular alterations of alveolar macrophages (AMs) were described in asthma [1], chronic obstructive pulmonary disease (COPD) [2], and fibrosis [3] disease settings. Beyond their classical scavenger function, AMs are dedicated pulmonary sentinels and shape host defense via the initiation of a cytokine immune response following recognition of pathogen-associated molecular patterns (PAMPs). Notably, AMs (and other macrophage populations) have a unique propensity to dynamically adapt to the local microenvironment via polarization into M1 or M2 phenotypes, which are associated with unique physiological functions during inflammatory processes [4].
In recent years, it was shown by others that macrophage polarization states are intimately linked to a unique morphology [5] and that vice versa patterning of macrophages into a distinct cell shape induces their polarization [6]. However, these observations were either made in in vitro approaches and/or in a non-lung environment and a link between morphology and function for macrophages is still missing in respiratory inflammatory disorders.
In the present study, we aimed to elucidate the impact of a persistent inflammatory environment on alveolar macrophage phenotype and function in vivo. To this end, we utilized the SPC-HAxTCR-HA transgenic mouse model previously established and extensively characterized in our lab [7–9]. In these mice, the concomitant expression of a neo-self antigen (influenza A/PR8 hemagglutinin, HA) by alveolar epithelial cells and the generation of self-antigen specific (HA-specific) CD4+ T cells evokes the spontaneous development of chronic pulmonary inflammation. We have previously demonstrated that chronic lung inflammation in SPC-HAxTCR-HA mice is associated with multifocal interstitial, perivascular, and peribronchial lymphocytic infiltrations as well as the development of alveolar emphysema and hemosiderosis [7]. Here, we provide comparative morphological and functional analyses between alveolar macrophages of single transgenic SPC-HA (only pulmonary self-antigen expression; healthy control group) and double-transgenic SPC-HAxTCR-HA (pulmonary self-antigen expression and self-antigen specific T cells; chronic lung disease) mice.
Materials and Methods
Mice
TCR-HA [10], SPC-HA [7], and SPC-HAxTCR-HA [7] mice have been previously described. Mice were bred in the animal facility at the Helmholtz Centre for Infection Research in specific-pathogen-free (SPF) conditions and were used at 12–24 weeks of age. All animal experiments were performed according to national guidelines and were approved by government institutions (Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit, file no. 33.14-42505-04-14/1715).
Flow Cytometry and Imaging Flow Cytometry
Bronchoalveolar lavage (BAL) was performed by flushing the lungs once with 1 mL PBS and BAL cells were obtained by centrifugation. BAL cells were erythrocyte-depleted by treatment with ACK buffer (0.15 mM NH4Cl, 1 mM KHCO3, 0.1 mM EDTA, pH 7.2–7.4), Fcγ-receptors were blocked with anti-CD16/CD32 (clone: 93); cells were stained with anti-F4/80 (clone: BM8) and anti-CD11c (clone: N418, all BioLegend). In flow cytometry, single cells were analyzed using a LSR Fortessa flow cytometer (BD Biosciences). Alveolar macrophages (AMs) were identified as F4/80+ and—where indicated—CD11c+, autofluorescencehigh, forward scatterhigh, and side scatterhigh cells. Autofluorescence signals were detected in FITC channel (500–550 nm). In imaging flow cytometry, alveolar macrophages were identified as F4/80+ and CD11c+. To exclude T cells from morphological analyses in imaging flow cytometry, cell suspensions were additionally stained with antibody against CD3 (clone: 145-2C11, eBioscience). Alveolar macrophages were analyzed by applying the image-based features intensity, area, and bright detail intensity on the SSC-channel. Analyses were conducted using a FlowSight System (×20 magnification, Amnis, part of EMD Millipore) Inspire acquisition software and IDEAS software version 6.0.
Panoptic Staining with Pappenheim Method
For analyses of alveolar macrophage phenotype, BAL cells from SPC-HA and SPC-HAxTCR-HA mice were isolated and erythrocyte-depleted as described above. Cells were spun (800 rpm, 15 min, RT) onto SuperFrost® glass slides (Thermo Scientific), air dried, and stained with May-Grünwald and Giemsa reagent (both Merck). Slides were washed and air dried, and samples were covered with Neo-Mount (Merck) reagent and sealed by glass cover slips. Images were captured at 63× oil immersion objective using an Axio Imager Z2 microscope equipped with AxioCam MRc5 and ZEN software (both Carl Zeiss Microscopy GmbH).
In Vivo Experiments
For in vivo bacterial stimulation experiments, a pneumococcal serotype 4 strain (S.pneumoniae TIGR4, ATCC BAA-334 [11] ) was used. Pneumococci were grown overnight on Columbia blood agar plates (BD) at 37 °C and 5 % CO2, single colonies were resuspended and cultured in Todd-Hewitt broth & yeast extract to mid-logarithmic phase (OD600nm 0.3–0.5), washed, and diluted in PBS to the desired concentration. CFUs were determined by growing serial dilutions of the inoculums on blood agar plates. Bacterial suspension was spun down (17,900×g), the supernatant was removed, and the bacterial pellet was resuspended in ice-cold ethanol (70 % v/v) and incubated at 4 °C for 1 h. The bacterial suspension was again spun down, and the pellet was resuspended in 1 mL sterile PBS/Glycerol solution (25 % v/v). Plating after ethanol treatment was carried out to verify effective bacterial killing. Ethanol-killed bacterial suspensions were stored at −70 °C until further use. Mice were anesthetized by intraperitoneal injection of ketamine/xylazine, and 20 µL of bacterial suspension—equivalent to 106 CFU—was oropharyngeally administered. Mice were sacrificed at 4 h post-administration, and BAL was performed by flushing the lungs with 1 mL PBS. In order to deplete alveolar macrophages, 50 µL of clodronate-liposomes (=250 µg encapsulated clodronate, purchased from ClodLip BV) was oropharyngeally administered to mice 3 days before treatment with ethanol-killed pneumococcal suspensions.
Purification of Alveolar Macrophages
BAL cells were erythrocyte-depleted and incubated in DMEM (Gibco, supplemented with 10 % fetal bovine serum and antibiotics) for 90 min at 37 °C, 5 % CO2 in bacteriological plastic petri dishes. Non-adherent cells were then removed by extensively rinsing the surface with PBS. To verify effective purification, adherent cells were detached by treatment with 0.05 % Trypsin/EDTA (Gibco), stained with antibodies against F4-80 and CD11c and analyzed by flow cytometry. For RNA analysis, adherent cells were directly lysed on the plastic surface by treatment with RLT buffer (Qiagen, supplemented with 1 % mercaptoethanol), and RNA extraction protocol was applied. RNA was extracted and purified using RNeasy Mini and RNase-free DNase kit (both Qiagen) according to the manufacturer’s instructions.
Quantitative Real-Time RT-PCR
For gene expression analyses of purified alveolar macrophages, RNA was analyzed using the SensiFASTTM SYBR® No-ROX One-Step Kit (Bioline) according to the manufacturer’s instructions. Transcript levels were normalized to the housekeeping gene Actb. Following primer sequences were used: Actb, 5′-CTTCTTTGCAGCTCCTTCGT-3′ (forward) and 5′-TCCTTCTGACCCATTCCCAC-3′ (reverse); Msr1, 5′-GGAATAAGAGGTATTCCAGGTG-3′ (forward) and 5′-TTTGTCCTTTAGGTCCAGGAG-3′ (reverse); Fizz1, 5′-ACGAGTAAGCACAGGCAGTT-3′ (forward) and 5′-TGCCAATCCAGCTAACTATCCC-3′ (reverse).
Enzyme-Linked Immunosorbent Assay (ELISA)
For detection of TNF-α in BAL fluid, the Mouse TNF-α ELISA MAX™ Standard kit (BioLegend) was used according to the manufacturer’s instructions.
Statistical Analyses and Artwork
Statistical evaluation and figure design was performed using Graph Pad Prism Software. Unpaired, two-tailed Mann–Whitney-test was chosen to compare two groups. For comparison of multiple groups, one-way or two-way ANOVA followed by Bonferroni post-test was applied.
Results
Altered Fluorescence Characteristics and Cellular Texture of Alveolar Macrophages in CLD
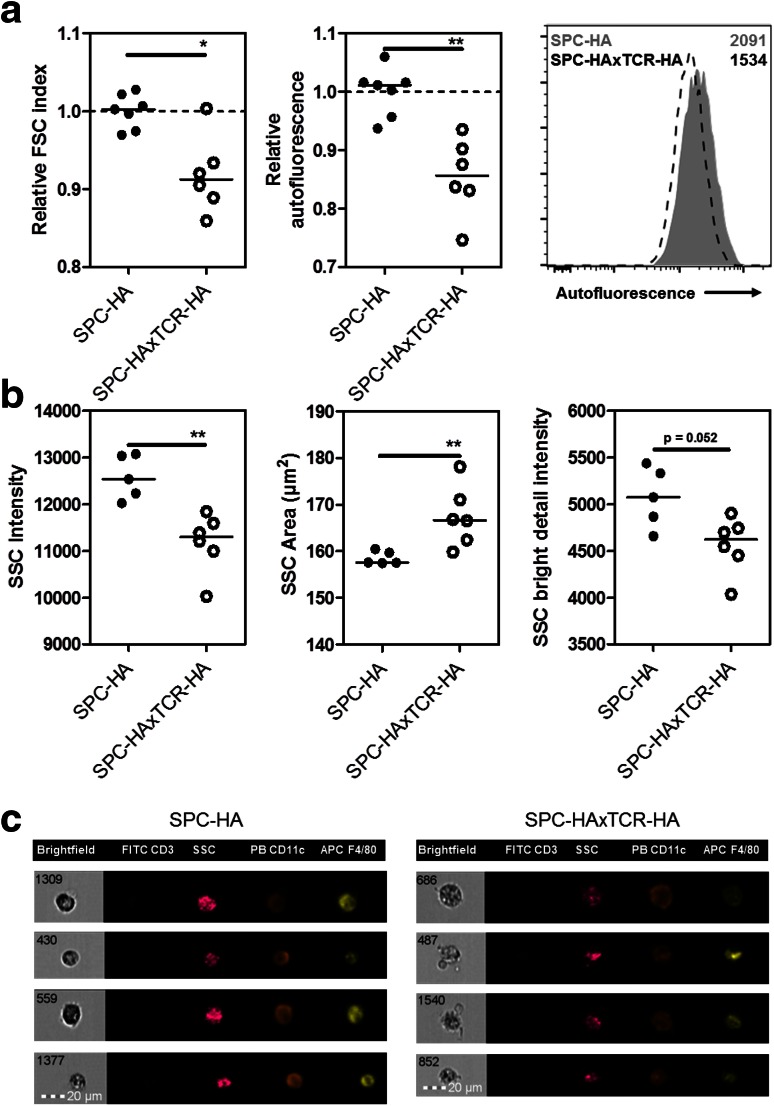
For a primary characterization of the AM phenotype in chronic lung inflammation, these cells were recovered from SPC-HA and SPC-HAxTCR-HA mice by BAL and were subjected to morphologic analyses by conventional flow cytometry (FACS) as well as imaging flow cytometry. FACS analyses revealed AMs from diseased SPC-HAxTCR-HA mice to have significantly smaller size compared to AMs from healthy SPC-HA control mice—indicated by reduced forward scatter (FSC) properties. Moreover, quantification of autofluorescence—an intrinsic characteristic of alveolar macrophages—yielded significantly reduced indices for AMs from SPC-HAxTCR-HA mice (Fig. 1a). In imaging flow cytometry, these cells displayed altered cytoplasmic signals reflected by alterations of side scatter (SSC) characteristics (Fig. 1b, c), indicating a markedly changed cellular texture of alveolar macrophages in a persistent inflammatory pulmonary environment.
Fig. 1.
Flow cytometric analyses of AM morphology in healthy and diseased lungs. a BAL cells from SPC-HA (n = 6) and SPC-HAxTCR-HA (n = 6) mice were analyzed by FACS; AMs were identified as FSChighSSChighF4-80+autofluorescencehigh cells. Relative FSC and autofluorescence were calculated by median fluorescence intensity (MFI) of individual sample/mean MFI of the SPC-HA (healthy) control group. Representative histogram depicts autofluorescent signal intensities of AMs from SPC-HA (gray shaded) versus SPC-HAxTCR-HA (dashed line) mice and their respective MFI (see numbers). b BAL cells from SPC-HA (n = 5) and SPC-HAxTCR-HA (n = 6) mice were analyzed by imaging flow cytometry (analyzed SSC-parameters: intensity, area, bright detail intensity); AMs were identified as F4-80+CD11c+CD3− cells. Statistical analyses were performed by using unpaired, two-tailed Mann–Whitney-test. *p < 0.05, **p < 0.01 c Representative images from imaging flow cytometer FlowSight® of alveolar macrophages from SPC-HA and SPC-HAxTCR-HA mice, PB pacific blue
Activated Phenotype of Alveolar Macrophages in Murine CLD
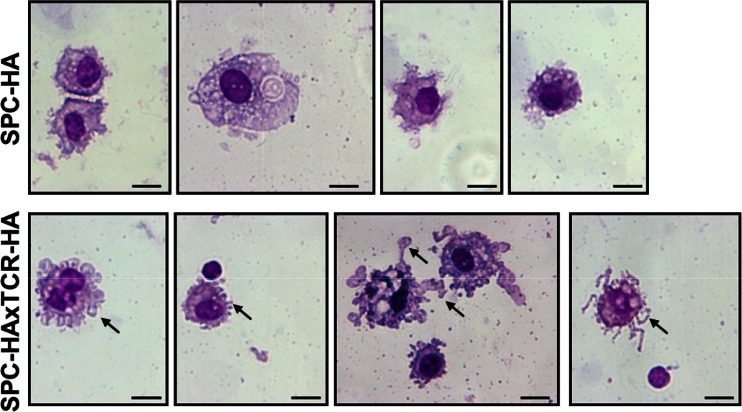
Further comparative morphologic analyses of alveolar macrophages were conducted using Pappenheim-stained cytospin samples of bronchoalveolar lavage fluid cells from SPC-HA and SPC-HAxTCR-HA mice. Microscopic evaluation demonstrated AMs from diseased SPC-HAxTCR-HA mice to display an activated phenotype, specified by increased formations of membrane protrusions (Fig. 2, see black arrows).
Fig. 2.
Analyses of AM morphology in healthy and diseased lungs by light microscopy. Cytospins from BAL samples from SPC-HA and SPC-HAxTCR-HA were stained with May-Grünwald and Giemsa reagent (Pappenheim method), and cell morphology was analyzed by light microscopy. Representative images of alveolar macrophages from n = 4 mice/group at ×63 magnification are depicted. Scale bar 10 µm. Black arrows indicate membrane protrusions
Together, the observed morphologic alterations provide first evidence for an impact of chronic pulmonary inflammation on alveolar macrophages and establish a basis for further investigations regarding functional alterations of AMs in murine lung disease.
M2 Signature of Alveolar Macrophages in Murine CLD
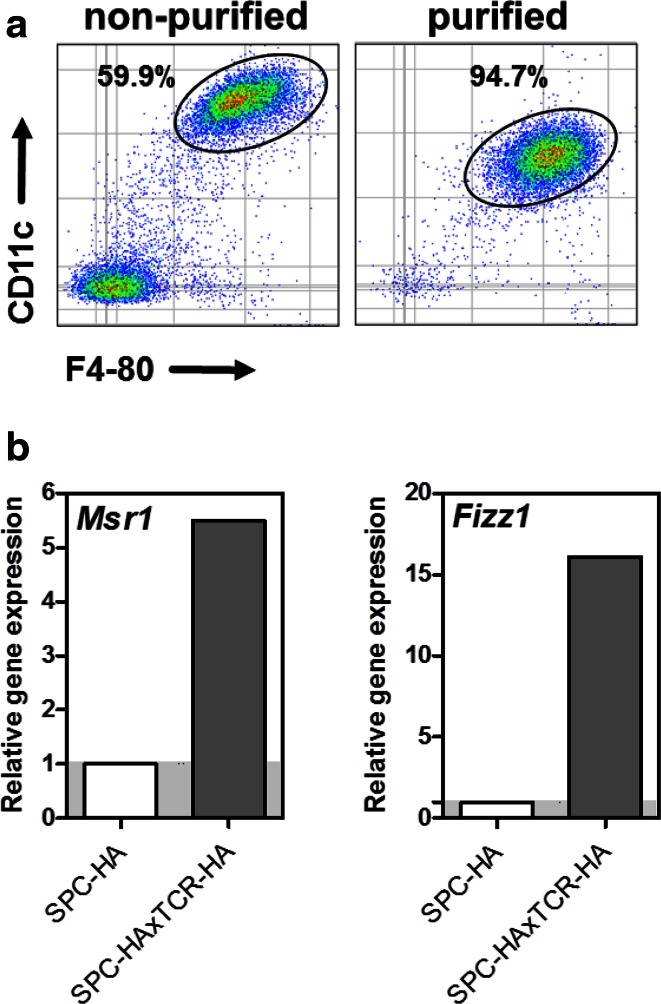
Inflammatory conditions in multiple infectious as well as non-infectious disease settings are intimately linked to macrophage polarization into classically activated M1 or alternatively activated M2 phenotypes [12, 13]. In order to elucidate the impact of CLD on AM polarization status, BAL cells from SPC-HA and SPC-HAxTCR-HA mice were isolated and AMs were purified by adhesion. This method allowed for the fast and efficient isolation of highly pure (~95 %) alveolar macrophages (Fig. 3a). Comparative gene expression analyses of macrophage polarization markers were conducted by quantitative real-time RT-PCR. Here, up-regulation of the gene encoding for macrophage scavenger receptor 1 (Msr1, fold change 5.5) and Resistin-like molecule alpha1 (Fizz1, fold change 16.1), both molecules are known to be overrepresented in M2 macrophages, was observed (Fig. 3b).
Fig. 3.
Analyses of purified AMs from healthy versus diseased lungs by quantitative real-time RT-PCR. a AMs from erythrocyte-depleted BAL cell suspensions were purified by adhesion (90 min, 37 °C) on bacteriological plastic, detached by trypsin–EDTA treatment, and purity was analyzed by FACS. Left square exemplarily depicts percentages of AMs (CD11chigh, F4-80high) of analyzed singlets in non-purified BAL samples from SPC-HAxTCR-HA mice. Right square depicts AM percentages of BAL cells from SPC-HAxTCR-HA mice after purification. Note the decreased abundance of the surface molecule CD11c on AMs after trypsin–EDTA treatment. b Purified alveolar macrophages from SPC-HA and SPC-HA xTCR-HA mice were pooled (n = 3–5 mice/group), RNA was extracted, and relative expression of indicated transcripts was determined. Gene expression was normalized to the SPC-HA control group
Alveolar Macrophages in Murine CLD are Hyporesponsive to Stimulation with Bacterial Ligands
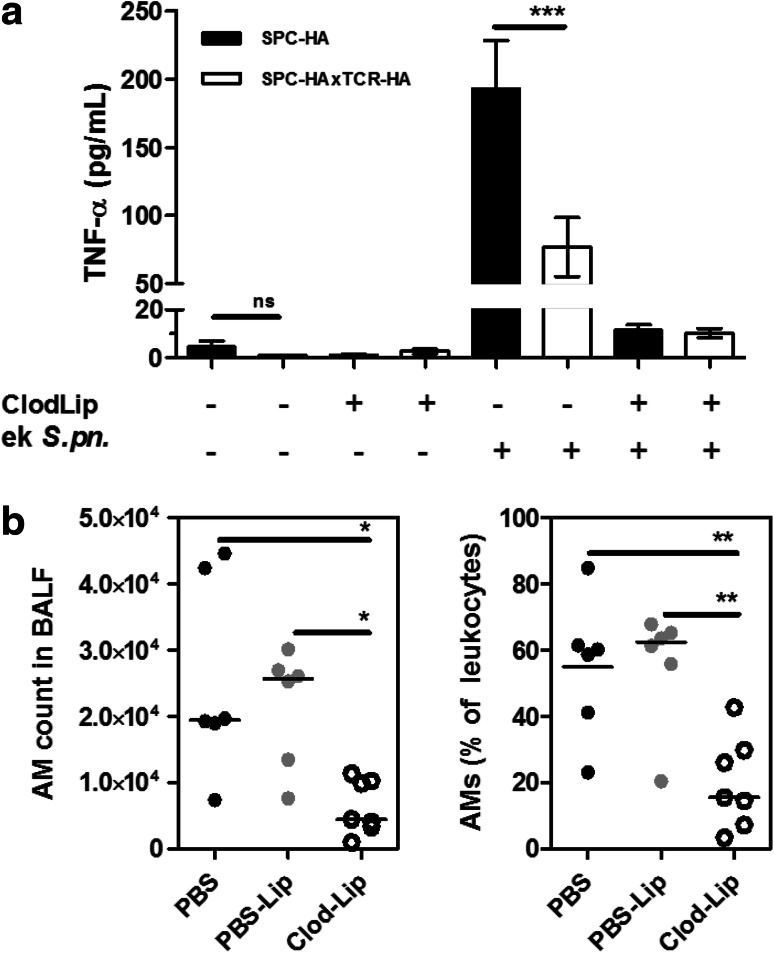
Following recognition of respiratory pathogens, alveolar macrophages mount a pro-inflammatory immune response by secreting (among other mediators) TNF-α. This step is critical for the rapid elimination of microbial threats but, on the other hand, involves the risk to develop immune pathology at the expense of lung tissue integrity. In order to test a possible influence of chronic lung inflammation on alveolar macrophage functionality, SPC-HAxTCR-HA mice and healthy controls were oropharyngeally inoculated with ethanol-killed Streptococcus pneumoniae TIGR4, and the early airway inflammatory response was quantified by assessing TNF-α levels in BAL fluid (BALF) at 4 h following administration. As expected, bacterial stimulation led to an early and robust cytokine response in the airways of healthy SPC-HA mice (Fig. 4a). Strikingly, TNF-α levels were ~60 % decreased in the diseased SPC-HAxTCR-HA group. In order to verify that this blunted inflammatory response was mediated by alveolar macrophages, these cells were depleted by oropharyngeal administration of clodronate-liposomes (ClodLip) 3 days prior bacterial stimulation. Clodronate treatment led to a 75 % reduction of viable AMs (Mean ± SEM AM counts PBS-group vs. ClodLip group: 25383 ± 6040 vs. 6330 ± 1536) within 3 days, whereas the single administration of control liposomes (PBS-Lip) or carrier solution (PBS) had no effect on alveolar macrophage numbers (Fig. 4b). Notably, TNF-α levels following bacterial stimulation in AM-depleted mice were substantially reduced and close to detection limit in both mouse groups. This validates AMs to be the main early airway TNF-α source following pneumococcal stimulation and, more importantly, suggests a functional impairment of AMs in chronic lung disease.
Fig. 4.
Quantification of the AM-dependent TNF-α response toward bacterial ligands in vivo. a Mice were oropharyngeally inoculated with 50 µL of clodronate-liposomes (ClodLip) on d0; control mice received PBS. At d3, mice were oropharyngeally administered with 106 CFU ethanol-killed S. pneumoniae TIGR4 (ek S.pn.) or PBS, respectively. 4 h after bacterial inoculation, mice were sacrificed and TNF-α levels in BAL fluid were determined by ELISA. Depicted are mean results ± SEM of pooled samples from 2 independent experiments. (n = 6–10/group). Statistical significance was calculated using two-way ANOVA followed by Bonferroni post-test. ***p < 0.001. b SPC-HA mice were oropharyngeally inoculated with 50 µL Clodronate-Liposomes (Clod-Lip), PBS-Liposomes (PBS-Lip) or PBS only. At d3 post administration mice were sacrificed, and alveolar macrophages (CD11chighF4-80+autofluorescencehigh) in BAL fluid were quantified by flow cytometry. Data were pooled from two independent experiments with similar outcome. Statistical significance was calculated using one-way ANOVA followed by Bonferroni post-test. *p < 0.05; **p < 0.01
Discussion
In the present study, we aimed to dissect the impact of chronic lung disease on the alveolar macrophage phenotype. To this end, we utilized the SPC-HAxTCR-HA transgenic mouse model for T cell-mediated lung inflammation. Despite different etiologies, a participation of lymphocyte immune responses has been suggested for the pathogenesis of several lung disorders such as COPD [14] or sarcoidosis [15] and SPC-HAxTCR-HA lungs phenocopy [7] histopathological manifestations of human CLD, e.g., airway remodeling and lymphocytic infiltrations.
By using fluorescence-based cytometric assays, we detected several important morphological changes of alveolar macrophages in our in vivo model. In detail, we show that their size, structural, and intrinsic fluorescence properties are significantly affected within an inflammatory environment. Interestingly, alterations in airway macrophage SSC and autofluorescence have also been reported in another study in SHIP1 (an inositol polyphosphate-5-phosphatase)-deficient mice, that spontaneously develop severe lung inflammation associated with a pathologic M2-macrophage phenotype [16]. These findings are of general interest for two reasons. First, FACS technology provides a valuable and commonly utilized tool to characterize inflammatory processes on airway mucosal surfaces. Yet the massive autofluorescence signals of airway macrophages within, e.g., FITC, PE, and PerCp detectors require well-considered panel design and the utilization of additional control stainings to correctly separate large intrinsic (false) from antibody-derived (true) fluorescence signals. Since we and others have proven fundamental differences of AM fluorescence properties in healthy versus pathologic conditions, it is likely to observe similar effects in other non-infectious and infectious respiratory disease settings. In practice, this would adversely affect accuracy of, e.g., cell surface molecule quantitation. Thus, for similar studies using comparative flow cytometric investigations on airway macrophages, the aforementioned effects should definitely be considered. Second, information on the mentioned parameters offers a simple additive tool to characterize the alveolar macrophage phenotype in more detail and to detect morphologic alterations that possibly hint to molecular and functional cellular adaptations. Accordingly, we could provide a link between affected fluorescent properties and activation, polarization, and functional alterations in alveolar macrophages in CLD. Here, the blunted AM-dependent TNF-α response toward Gram-positive bacterial ligands in SPC-HAxTCR-HA airways suggests a functional impairment of these cells during infection with airborne pathogens. Of note, TNF-α was shown to exert protective efficacy in several models of respiratory infection [17–20]. In detail, this cytokine acts on macrophages as well as neutrophils by enhancing phagocytosis of opsonized bacteria and antibody-dependent cytotoxicity [21, 22]. On the other hand, it was previously demonstrated that lung-specific overexpression of TNF-α in mice was linked to a disease phenotype mirroring hallmark features of emphysema and pulmonary fibrosis [23]. In this context, macrophages from pre-diseased human and murine lungs were found to mount blunted cytokine responses upon stimulation with bacterial ligands [24, 25]. It is speculated that this airway hyporesponsiveness provides an advantage to the host by evading PAMP-induced immunopathology that would further compromise tissue integrity in the pre-inflamed lung. However, given the two faces of TNF-α (and other pro-inflammatory molecules) in alveolar macrophage-mediated immune responses, further studies will be needed to explore their role on the shape of antibacterial host defense in the pre-diseased lung.
Conclusion
Using a model of chronic lung disease, we provide a link between phenotypical changes in alveolar macrophages to distinct functional alterations. We further plan to characterize the alveolar macrophage phenotype in more detail and aim to elucidate the functional role of these adaptations on pulmonary immunity.
Acknowledgments
This research project has been supported by the President’s Initiative and Networking Funds of the Helmholtz Association of German Research Centres (HGF) under contract number VH-GS-202 and by a Grant from the German Research Foundation (SFB 587 to DB). DB is supported by the President´s Initiative and Networking Fund of the Helmholtz Association of German Research Centers (HGF) under contract number W2/W3-029.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Footnotes
Andreas Jeron and Dunja Bruder have contributed equally to this work.
References
- 1.Borish L, Mascali JJ, Dishuck J, Beam WR, Martin RJ, Rosenwasser LJ. Detection of alveolar macrophage-derived IL-1 beta in asthma. Inhibition with corticosteroids. J Immunol. 1992;149(9):3078–3082. [PubMed] [Google Scholar]
- 2.Barnes PJ. Alveolar macrophages as orchestrators of COPD. Copd. 2004;1(1):59–70. doi: 10.1081/COPD-120028701. [DOI] [PubMed] [Google Scholar]
- 3.Karo-Atar D, Moshkovits I, Eickelberg O, Konigshoff M, Munitz A. Paired immunoglobulin-like receptor-B inhibits pulmonary fibrosis by suppressing profibrogenic properties of alveolar macrophages. Am J Respir Cell Mol Biol. 2013;48(4):456–464. doi: 10.1165/rcmb.2012-0329OC. [DOI] [PubMed] [Google Scholar]
- 4.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinetti-Gbaguidi G, Baron M, Bouhlel MA, Vanhoutte J, Copin C, Sebti Y, et al. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARgamma and LXRalpha pathways. Circ Res. 2011;108(8):985–995. doi: 10.1161/CIRCRESAHA.110.233775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci USA. 2013;110(43):17253–17258. doi: 10.1073/pnas.1308887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruder D, Westendorf AM, Geffers R, Gruber AD, Gereke M, Enelow RI, et al. CD4 T Lymphocyte-mediated lung disease: steady state between pathological and tolerogenic immune reactions. Am J Respir Crit Care Med. 2004;170(11):1145–1152. doi: 10.1164/rccm.200404-464OC. [DOI] [PubMed] [Google Scholar]
- 8.Gereke M, Grobe L, Prettin S, Kasper M, Deppenmeier S, Gruber AD, et al. Phenotypic alterations in type II alveolar epithelial cells in CD4 + T cell mediated lung inflammation. Respir Res. 2007;8:47. doi: 10.1186/1465-9921-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gereke M, Jung S, Buer J, Bruder D. Alveolar type II epithelial cells present antigen to CD4(+) T cells and induce Foxp3(+) regulatory T cells. Am J Respir Crit Care Med. 2009;179(5):344–355. doi: 10.1164/rccm.200804-592OC. [DOI] [PubMed] [Google Scholar]
- 10.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180(1):25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, Peterson S, et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293(5529):498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- 12.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181(6):3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 13.Boorsma CE, Draijer C, Melgert BN. Macrophage heterogeneity in respiratory diseases. Mediat Inflamm. 2013;2013:769214. doi: 10.1155/2013/769214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360(23):2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 15.Statement on sarcoidosis Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160(2):736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 16.Duan M, Li WC, Vlahos R, Maxwell MJ, Anderson GP, Hibbs ML. Distinct macrophage subpopulations characterize acute infection and chronic inflammatory lung disease. J Immunol. 2012;189(2):946–955. doi: 10.4049/jimmunol.1200660. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Havell EA, Harmsen AG. Importance of endogenous tumor necrosis factor alpha and gamma interferon in host resistance against Pneumocystis carinii infection. Infect Immun. 1992;60(4):1279–1284. doi: 10.1128/iai.60.4.1279-1284.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gosselin D, DeSanctis J, Boule M, Skamene E, Matouk C, Radzioch D. Role of tumor necrosis factor alpha in innate resistance to mouse pulmonary infection with Pseudomonas aeruginosa. Infect Immun. 1995;63(9):3272–3278. doi: 10.1128/iai.63.9.3272-3278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanchard DK, Djeu JY, Klein TW, Friedman H, Stewart WE., 2nd Protective effects of tumor necrosis factor in experimental Legionella pneumophila infections of mice via activation of PMN function. J Leukoc Biol. 1988;43(5):429–435. doi: 10.1002/jlb.43.5.429. [DOI] [PubMed] [Google Scholar]
- 20.Buret A, Dunkley ML, Pang G, Clancy RL, Cripps AW. Pulmonary immunity to Pseudomonas aeruginosa in intestinally immunized rats roles of alveolar macrophages, tumor necrosis factor alpha, and interleukin-1 alpha. Infect Immun. 1994;62(12):5335–5343. doi: 10.1128/iai.62.12.5335-5343.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierangeli SS, Sonnenfeld G. Treatment of murine macrophages with murine interferon-gamma and tumour necrosis factor-alpha enhances uptake and intracellular killing of Pseudomonas aeruginosa. Clin Exp Immunol. 1993;93(2):165–171. doi: 10.1111/j.1365-2249.1993.tb07960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan S, Fehr HG, Adams D. Activation of macrophages for ADCC in vitro: effects of IL-4, TNF, interferons-alpha/beta, interferon-gamma, and GM-CSF. Cell Immunol. 1991;135(1):78–87. doi: 10.1016/0008-8749(91)90255-A. [DOI] [PubMed] [Google Scholar]
- 23.Lundblad LK, Thompson-Figueroa J, Leclair T, Sullivan MJ, Poynter ME, Irvin CG, et al. Tumor necrosis factor-alpha overexpression in lung disease: a single cause behind a complex phenotype. Am J Respir Crit Care Med. 2005;171(12):1363–1370. doi: 10.1164/rccm.200410-1349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berenson CS, Wrona CT, Grove LJ, Maloney J, Garlipp MA, Wallace PK, et al. Impaired alveolar macrophage response to Haemophilus antigens in chronic obstructive lung disease. Am J Respir Crit Care Med. 2006;174(1):31–40. doi: 10.1164/rccm.200509-1461OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Didierlaurent A, Goulding J, Patel S, Snelgrove R, Low L, Bebien M, et al. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J Exp Med. 2008;205(2):323–329. doi: 10.1084/jem.20070891. [DOI] [PMC free article] [PubMed] [Google Scholar]