Abstract
Objective
The goal of this study was to assess the effect of high-dose simvastatin on cerebral vasospasm and its clinical outcome after aneurysmal subarachnoid hemorrhage (SAH) in Korean patients.
Methods
This study was designed as a prospective observational cohort study. Its subjects were aneurysmal SAH patients who had undergone aneurysm clipping or coiling. They were assigned to 1 of 3 groups : the 20 mg, 40 mg, and 80 mg simvastatin groups. The primary end-point was the occurrence of symptomatic vasospasm. The clinical outcome was assessed with the modified Rankin Scale (mRS) score after 1 month and 3 months. The risk factors of the development of vasospasm were assessed by logistic regression analysis.
Results
Ninety nine patients with aneurysmal SAH were treated and screened. They were sequentially assigned to the 20 mg (n=22), 40 mg (n=34), and 80 mg (n=31) simvastatin groups. Symptomatic vasospasm occurred in 36.4% of the 20 mg group, 8.8% of the 40 mg group, and 3.2% of the 80 mg group (p=0.003). The multiple logistic regression analysis showed that poor Hunt-Hess grades (OR=5.4 and 95% CI=1.09-26.62) and high-dose (80 mg) simvastatin (OR=0.09 and 95% CI=0.1-0.85) were independent factors of symptomatic vasospasm. The clinical outcomes did not show a significant difference among the three groups.
Conclusion
This study demonstrated that 80 mg simvastatin treatment was effective in preventing cerebral vasospasm after aneurysmal SAH, but did not improve the clinical outcome in Korean patients.
Keywords: Outcome, Statin, Subarachnoid hemorrhage, Vasospasm
INTRODUCTION
Aneurysmal subarachnoid hemorrhage (SAH) is a disease that affects the patient's prognosis with various complications even after clipping or coiling. The unique complication, known as vasospasm, is usually reported as occurring between 4 and 14 days after the surgery. The method of preventing such problem has been widely investigated5,10,13). Statins are known to influence various pathogenesis involving the maintenance of endothelial integrity and inhibition of vascular smooth muscle proliferation in experimental studies14). However, the studies in which statin was used for prophylaxis of cerebral vasospasm after aneurysmal SAH had different results1,6,8,11,15,16,17). A randomized clinical trial demonstrated that 80 mg simvastatin treatment significantly reduced the development of vasospasm, but recent multicenter randomized trial to assess the effects of 80 mg simvastatin failed to demonstrate a reduction in cerebral vasospasm compared with 40 mg simvastatin8,17). The aim of this study was to determine whether higher dose of simvastatin is superior to a lower dose with regard to cerebral vasospasm and clinical outcome after aneurysmal SAH in Korean cohort.
MATERIALS AND METHODS
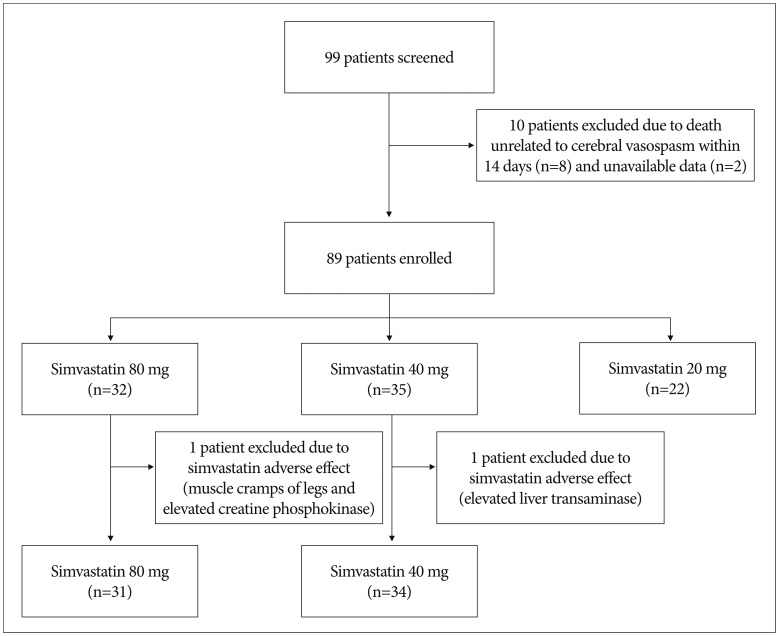
This study was designed as a prospective observational cohort study. Ninety-nine patients with aneurysmal SAH were treated through clipping or coiling, and were recruited over the 36-month period from January 2010 to December 2012. This study was approved by the institutional review board. The informed consent of either the patient or his or her next of kin was obtained. Previous studies showed that the short-term use of high-dose statin did not compromise safety7,8,17). Thus, we treated aneurysmal SAH patients with 80 mg, 40 mg, or 20 mg simvastatin (Simvastatin®; Yuhan Corporation, Seoul, Korea) for preventing cerebral vasospasm and retrospectively reviewed prospectively collected database. The patients were sequentially assigned to the 20 mg (n=22), 40 mg (n=34), or 80 mg (n=31) simvastatin groups. Twelve patients were excluded due to death within 14 days, unavailable data, and simvastatin side effects (Fig. 1). The causes of death in 8 patients were pneumonia (n=3), heart failure (n=1), and increased intracranial pressure (n=4) regardless of cerebral infarction or vasospasm on radiologic study.
Fig. 1. Diagram showing study design. The reasons for exclusion are as follows : 1) Ten patients died within 14 days after ictus. 2) Two patients were transferred to pulmonary medicine due to lung diseases, thus had insufficient data about the occurrence of vasospasm. 3) Two patients had statin side effects. One patient (40 mg group) showed elevated liver transaminase up to 200 U/L. Another patient (80 mg group) complained muscle cramp of legs and creatine phosphokinase elevated to 1200 U/L. After cessation of statin, laboratory values were normalized within 3 days.
The pre-existing medical conditions (history of hypertension, diabetes mellitus, and hyperlipidemia) and the clinical characteristics, including the Hunt-Hess grade, Fisher's grade, location of the aneurysm, treatment methods (clipping or coiling), and drainage of cerebrospinal fluid were investigated.
The inclusion criteria were radiological evidence of saccular-type aneurysmal SAH through computed tomographic (CT) angiography or distal subtraction angiography, and its presentation at less than 48 hours from ictus. The patients received simvastatin medication for 14 days after their surgery. Vasospasm was defined based in the following symptomatic or TDC criteria. Symptomatic vasospasm was defined as a decrease of at least 2 points on the Glasgow Coma Scale and clinical deterioration of the patient's condition (i.e., insidious onset of confusion, disorientation, altered consciousness, and focal neurological deficits) with no evidence of surgical complications, metabolic disturbances, infections, or hydrocephalus. Transcranial Doppler ultrasonography (TCD) was performed every other day after the surgery until the 14th day after the SAH. A patient was defined as in a state of TCD vasospasm when the highest mean velocity of the middle cerebral artery was above 150 cm/s in a single test, when the change in the highest mean velocity of the middle cerebral artery was above 50 cm/s in a serial test, or when the Lindegaard ratio (middle cerebral artery mean blood flow velocity/extracranial internal carotid artery mean blood flow velocity) was above 3. Digital subtraction angiography was not routinely performed in the presence of vasospasm, and thus angiographic criteria were not used to determine vasospasm.
The SAH patients were clinically managed according to the standardized guidelines9). The CT scans were routinely performed on the day of the operation and, 2 days, 7 days, and 14 days after the operation. Newly developed lesions on the CT scans within 2 days after the operation were regarded as procedure-related damages.
Calcium channel blockers were routinely used. When post-surgical or post-interventional neurological deficits developed, triple H therapies (volume expansion with albumin, increase in the blood pressure with dopamine, and maintenance of the hematocrit level at 30-35%) were initiated until the vasospasm was resolved. If the symptoms were not resolved, the patients underwent catheter angiography with the administration of intra-arterial nicardipine.
Liver transaminases and creatine phosphokinase were monitored for early signs of hepatitis or myositis every three days or based on clinical suspicion. The use of statin was stopped if the level of liver transaminases was greater than three-times the normal level (>180 U/L) or if the creatine phosphokinase level was >1000 U/L.
The attending neurosurgeon assessed the clinical outcomes using the modified Rankin Scale (mRS) score after one month and three months. The mRS scores were classified as follows : 0, no symptom; 1, no significant disability; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability; and 6, dead. The scores were dichotomized into favorable (mRS score=0-2) and unfavorable (mRS score=3-6).
All continuous variables were presented as the mean±standard deviation. We used ANOVA or Kruskal-Wallis test for continuous variable and a chi-square test or Fisher's exact test for categorical variable. The results with two-tailed values of less than p=0.05 were considered statistically significant. Multivariate logistic regression analysis was performed using the possible factors of vasospasm. They were selected from the published risk factors or the variables that differed among three groups in the univariate analysis (p<0.2). The data were reported with 95% CIs. All the statistical analyses were performed using SPSS 20.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
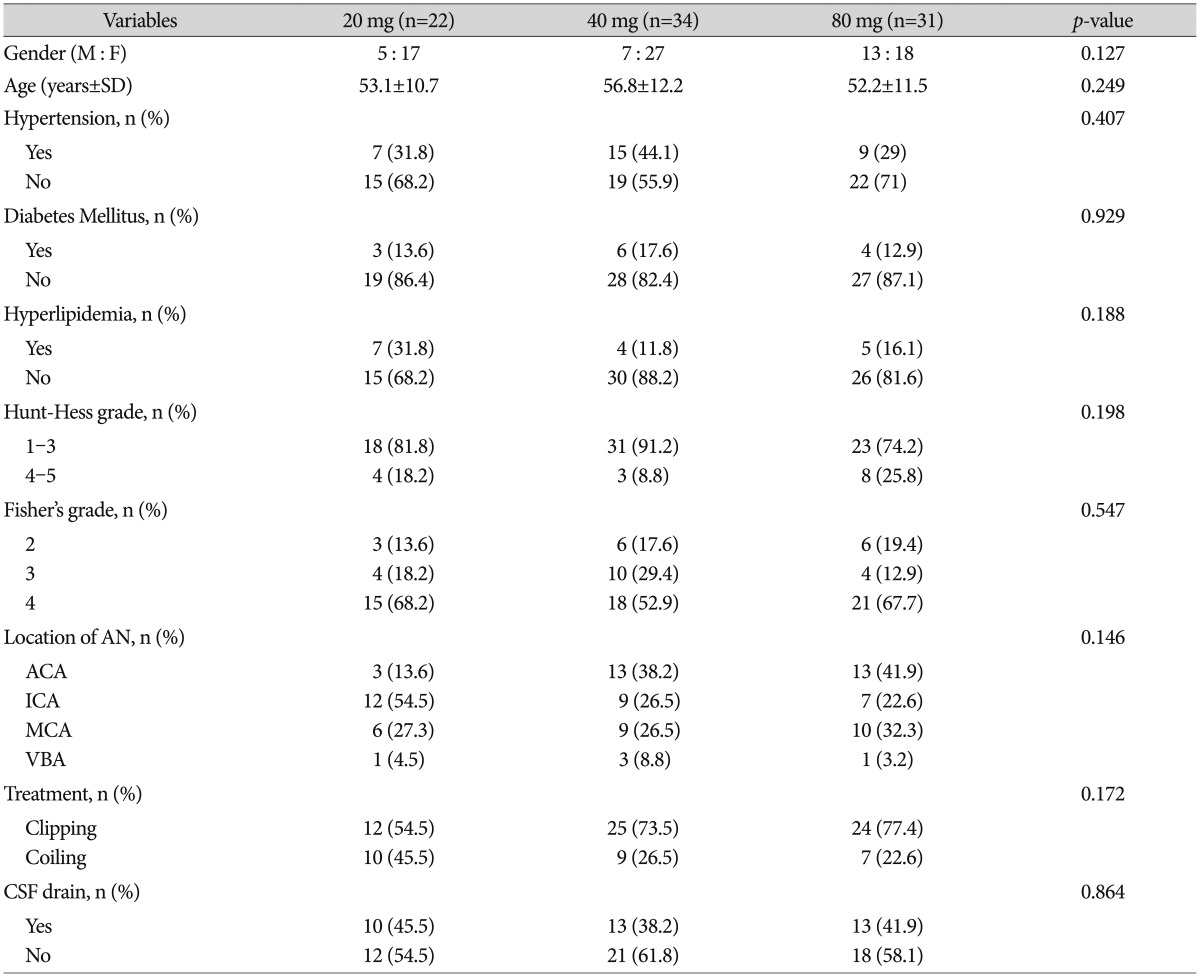
The mean age of the 87 patients was 54.8±11.6 years. Sixty-two (71.3%) of the subjects were women, and 25 (28.7%) were men. Patient's age, sex, Hunt-Hess grade upon admission, Fisher's grade, aneurysm location, and the treatment method of aneurysm are presented in Table 1. There were no significant differences among 3 groups.
Table 1. Clinical and radiographic data among statin 20 mg, 40 mg, and 80 mg group.
M : male, F : female, SD : standard deviation, AN : aneurysm, ACA : anterior cerebral artery, ICA : internal carotid artery, MCA : middle cerebral artery, VBA : vertebrobasilar artery, CSF : cerebrospinal fluid
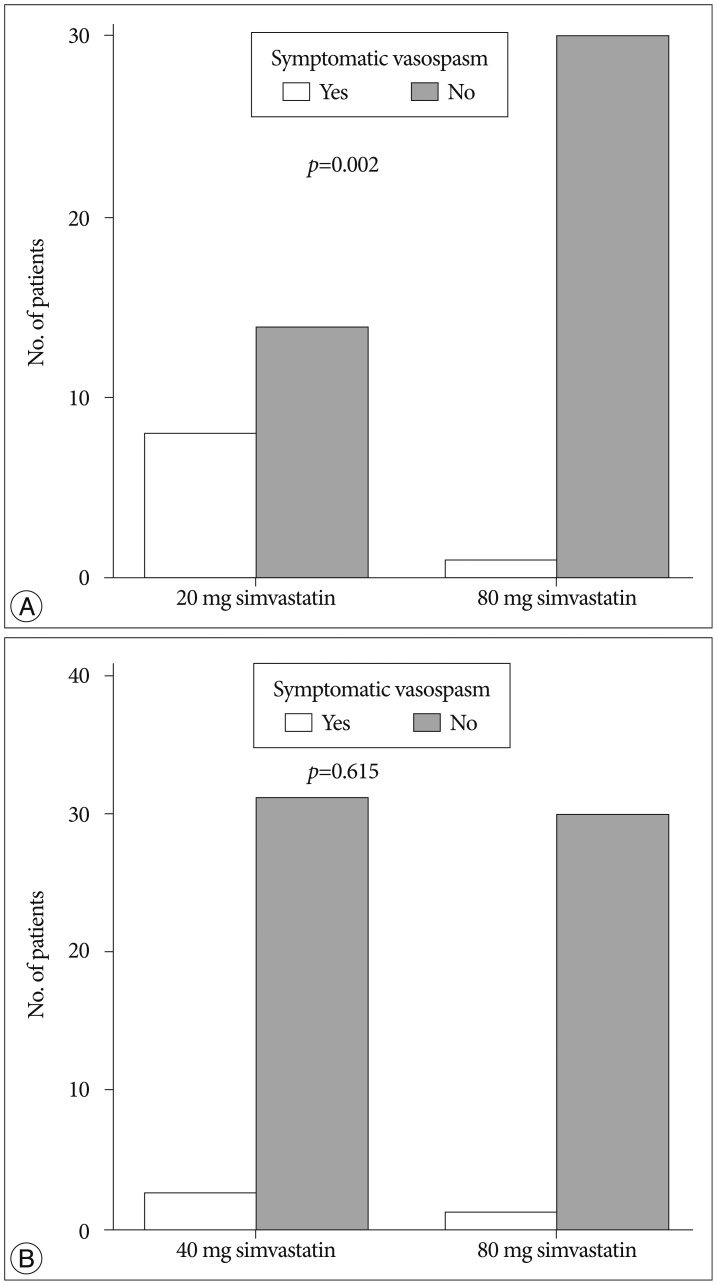
Symptomatic vasospasm occurred in 8 subjects (36.4%) in the 20 mg group, 3 subjects (8.8%) in the 40 mg group, and in 1 subject (3.2%) in the 80 mg group. One patent in the 20 mg group and 1 patient in the 40 mg group underwent intra-arterial nicardipine injection. There were significant differences among 3 groups (p=0.003). In the subgroup analysis, the incidence of symptomatic vasospasm was significantly different between 20 mg and 40 mg simvastatin group, but not significantly different between 40 mg and 80 mg simvastatin group (Fig. 2).
Fig. 2. Bar graph showing the incidence of symptomatic vasospasm between 20 mg and 80 mg simvastatin group (A) and between 40 mg and 80 mg simvastatin group (B).
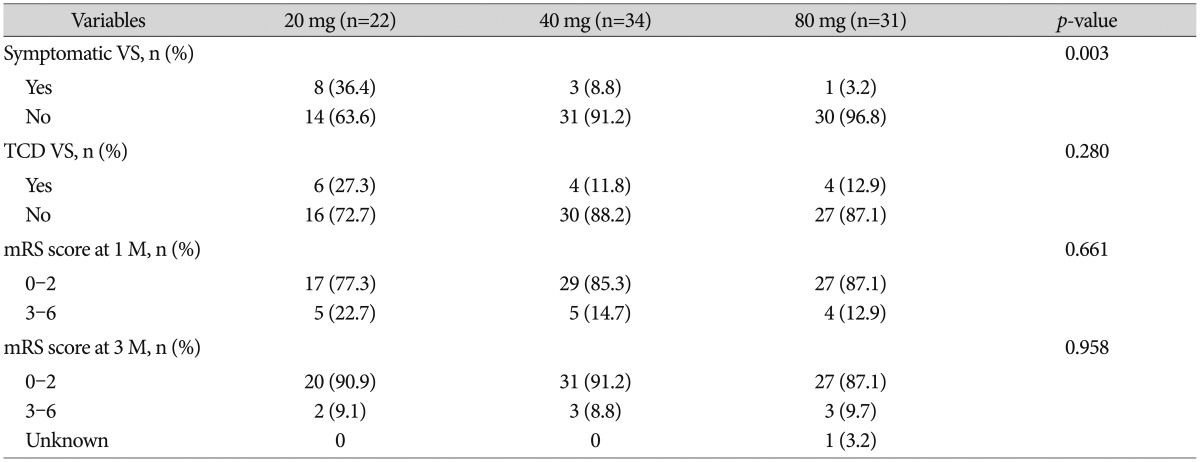
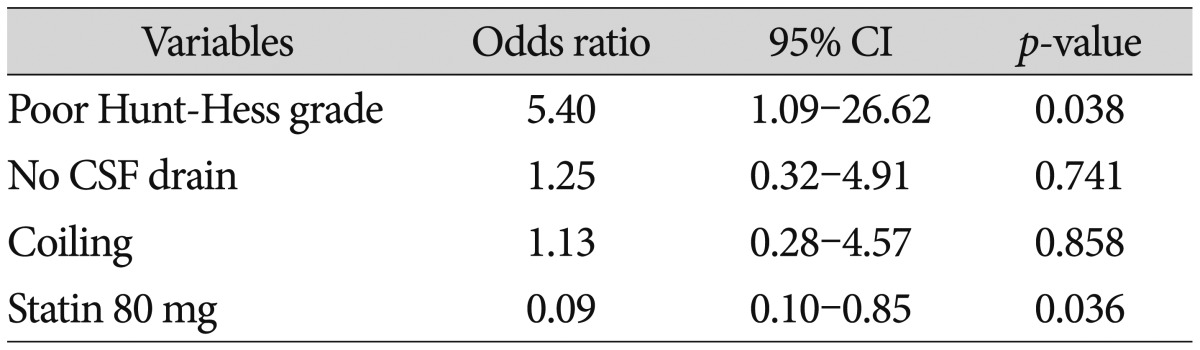
The clinical outcomes at 1 and 3 months after the SAH did not significantly differ among 3 groups (Table 2). New cerebral infarction on basal ganglia occurred in 1 patient. One patient died due to pneumonia on postoperative 25 days. Multiple logistic regression analysis showed that a poor Hunt-Hess grade (OR=5.4 and 95% CI=1.09-26.62) and high-dose (80 mg) simvastatin (OR=0.09 and 95% CI=0.1-0.85) were independent factors of symptomatic vasospasm (Table 3).
Table 2. Incidence of vasospasm and the clinical outcomes among statin 20 mg, 40 mg, and 80 mg group.
VS : vasospasm, TCD : transcranial Doppler sonography, mRS : modified Rankin scale, M : month
Table 3. Multivariate logistic regression analysis of risk factors related to symptomatic vasospasm.
CI : confidence interval, CSF : cerebrospinal fluid
DISCUSSION
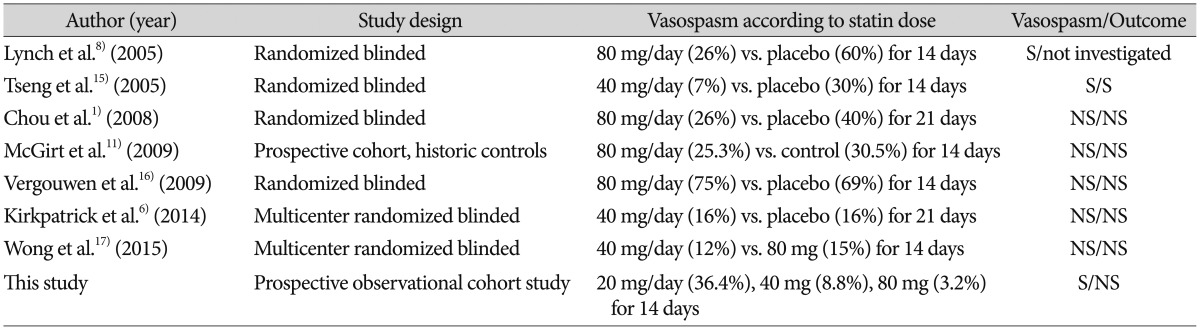
This study showed that high doses of statin are effective in preventing cerebral vasospasm in aneurysmal SAH patients. Statin, a competitive inhibitor of HMG CoA reductase, has been proven to have neuroprotective effects by modulating inflammation and augmenting the cerebral blood flow after intracerebral hemorrhage or SAH2,3,4,12). The results of the published prospective studies on the effectiveness of statin are summarized in Table 4. The effects of statin in the prevention of cerebral vasospasm after SAH are still controversial. Some randomized trials showed a decrease in the incidence of vasospasm in the statin groups unlike in the control group, but other studies did not show a significant difference1,6,8,11,15,16,17). The result of a recent multicenter prospective study that compared patients treated with 40 mg of statin for 14 days with the control group showed no difference in the incidence of vasospasm and the clinical outcome6). The reason for this discrepancy is unclear. A possible reason may be the difference in the definitions of cerebral vasospasm. Catheter angiography is well-known as the most accurate tool for confirming cerebral vasospasm. In this study, however, not all the patients could be tested routinely with catheter angiography. Catheter angiography was performed onlyfor intra-arterial nicardipine delivery or with an inflating angioplasty balloon. This discrepancy might have influenced the incidence of cerebral vasospasm. In addition, the incidence of symptomatic vasospasm was low in 80 mg simvastatin group compared with recent multicenter study17). This finding might be explained by the fact that 46% of 80 mg statin group in that study had poor clinical grade on admission compared with our result (25.8%).
Table 4. Prospective studies investigating the effectiveness of statin to prevent cerebral vasospasm after aneurysmal subarachnoid hemorrhage.
S : significant, NS : not significant
Although the higher-dose group tended to have better outcomes, this study did not show a significant difference in the clinical outcomes (mRS scores) after one month in relation to the statin dosage after aneurysmal SAH (77.3%, 85.3%, and 87.1%, respectively, p=0.661) (Table 2). This finding is consistent with the results of recent studies, and implies that various factors (the Hunt-Hess grade upon admission, previous medical conditions, the age, surgical complications, etc.), besides vasospasm may have contributed to the clinical outcome of the aneurysmal SAH patients6,17).
In predicting which patients are more likely to present symptomatic vasospasm, our data suggests that the predictive factors are high-dose statin use (OR=0.09 and CI=0.1-0.85) and poor Hunt-Hess grades upon admission (OR=5.4 and CI=1.09-26.62). A multivariate analysis of the risks of vasospasm, statin therapy, Fisher grade 3 SAH, and rupture of the anterior cerebral or internal carotid artery aneurysm were independent risk factors9).
This study had some limitations. First, this study was a single-center, observational study. Although the ideal study design is a multicenter prospective randomized trial, single-institution studies have limitations in recruiting enough patients to collect ideal statistics. Therefore, participants were sequentially rather than randomly allocated. Second, although the dose-dependent effect of statin on vasospasm was analyzed, this study has a limitation by its small sample size and absence of a control group. Third, cerebral vasospasm defined by symptomatic and TDC criteria. Compared with the incidence of angiographic vasospasm, the incidence of symptomatic vasospasm may be underestimated. However, this study could be considered significant, to the author's knowledge, because it is the first prospective observational study of the prophylactic use of statin in South Korea. Further multicenter prospective trials with larger sample sizes will be needed to determine the effectiveness of statin therapy in lowering the incidence of vasospasm after aneurysmal SAH.
CONCLUSION
This study showed that preventive 80 mg simvastatin is an effective treatment for cerebral vasospasm after aneurysmal SAH in Korean patients, but does not necessarily improve the clinical outcomes of the patients.
References
- 1.Chou SH, Smith EE, Badjatia N, Nogueira RG, Sims JR, 2nd, Ogilvy CS, et al. A randomized, double-blind, placebo-controlled pilot study of simvastatin in aneurysmal subarachnoid hemorrhage. Stroke. 2008;39:2891–2893. doi: 10.1161/STROKEAHA.107.505875. [DOI] [PubMed] [Google Scholar]
- 2.Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, et al. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1998;95:8880–8885. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jwa CS, Yi HJ, Oh SJ, Hwang SJ. Prior use of 3-hydroxy-3-methyl-glutaryl-coenzyme a reductase inhibitor, simvastatin fails to improve outcome after experimental intracerebral hemorrhage. J Korean Neurosurg Soc. 2011;50:403–408. doi: 10.3340/jkns.2011.50.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karki K, Knight RA, Han Y, Yang D, Zhang J, Ledbetter KA, et al. Simvastatin and atorvastatin improve neurological outcome after experimental intracerebral hemorrhage. Stroke. 2009;40:3384–3389. doi: 10.1161/STROKEAHA.108.544395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kassell NF, Torner JC, Haley EC, Jr, Jane JA, Adams HP, Kongable GL. The International cooperative study on the timing of aneurysm surgery. Part 1 : overall management results. J Neurosurg. 1990;73:18–36. doi: 10.3171/jns.1990.73.1.0018. [DOI] [PubMed] [Google Scholar]
- 6.Kirkpatrick PJ, Turner CL, Smith C, Hutchinson PJ, Murray GD STASH Collaborators. Simvastatin in aneurysmal subarachnoid haemorrhage (STASH) : a multicentre randomised phase 3 trial. Lancet Neurol. 2014;13:666–675. doi: 10.1016/S1474-4422(14)70084-5. [DOI] [PubMed] [Google Scholar]
- 7.Kramer AH, Gurka MJ, Nathan B, Dumont AS, Kassell NF, Bleck TP. Statin use was not associated with less vasospasm or improved outcome after subarachnoid hemorrhage. Neurosurgery. 2008;62:422–427. discussion 427-430. doi: 10.1227/01.neu.0000316009.19012.e3. [DOI] [PubMed] [Google Scholar]
- 8.Lynch JR, Wang H, McGirt MJ, Floyd J, Friedman AH, Coon AL, et al. Simvastatin reduces vasospasm after aneurysmal subarachnoid hemorrhage : results of a pilot randomized clinical trial. Stroke. 2005;36:2024–2026. doi: 10.1161/01.STR.0000177879.11607.10. [DOI] [PubMed] [Google Scholar]
- 9.Mayberg MR, Batjer HH, Dacey R, Diringer M, Haley EC, Heros RC, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Circulation. 1994;90:2592–2605. doi: 10.1161/01.cir.90.5.2592. [DOI] [PubMed] [Google Scholar]
- 10.McGirt MJ, Blessing R, Alexander MJ, Nimjee SM, Woodworth GF, Friedman AH, et al. Risk of cerebral vasopasm after subarachnoid hemorrhage reduced by statin therapy : a multivariate analysis of an institutional experience. J Neurosurg. 2006;105:671–674. doi: 10.3171/jns.2006.105.5.671. [DOI] [PubMed] [Google Scholar]
- 11.McGirt MJ, Garces Ambrossi GL, Huang J, Tamargo RJ. Simvastatin for the prevention of symptomatic cerebral vasospasm following aneurysmal subarachnoid hemorrhage : a single-institution prospective cohort study. J Neurosurg. 2009;110:968–974. doi: 10.3171/2008.10.JNS08901. [DOI] [PubMed] [Google Scholar]
- 12.McGirt MJ, Lynch JR, Parra A, Sheng H, Pearlstein RD, Laskowitz DT, et al. Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke. 2002;33:2950–2956. doi: 10.1161/01.str.0000038986.68044.39. [DOI] [PubMed] [Google Scholar]
- 13.Sabri M, Macdonald RL. Statins : a potential therapeutic addition to treatment for aneurysmal subarachnoid hemorrhage? World Neurosurg. 2010;73:646–653. doi: 10.1016/j.wneu.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 14.Sugawara T, Ayer R, Zhang JH. Role of statins in cerebral vasospasm. Acta Neurochir Suppl. 2008;104:287–290. doi: 10.1007/978-3-211-75718-5_59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng MY, Czosnyka M, Richards H, Pickard JD, Kirkpatrick PJ. Effects of acute treatment with pravastatin on cerebral vasospasm, autoregulation, and delayed ischemic deficits after aneurysmal subarachnoid hemorrhage : a phase II randomized placebo-controlled trial. Stroke. 2005;36:1627–1632. doi: 10.1161/01.STR.0000176743.67564.5d. [DOI] [PubMed] [Google Scholar]
- 16.Vergouwen MD, Meijers JC, Geskus RB, Coert BA, Horn J, Stroes ES, et al. Biologic effects of simvastatin in patients with aneurysmal subarachnoid hemorrhage : a double-blind, placebo-controlled randomized trial. J Cereb Blood Flow Metab. 2009;29:1444–1453. doi: 10.1038/jcbfm.2009.59. [DOI] [PubMed] [Google Scholar]
- 17.Wong GK, Chan DY, Siu DY, Zee BC, Poon WS, Chan MT, et al. High-dose simvastatin for aneurysmal subarachnoid hemorrhage : multicenter randomized controlled double-blinded clinical trial. Stroke. 2015;46:382–388. doi: 10.1161/STROKEAHA.114.007006. [DOI] [PubMed] [Google Scholar]