Abstract
Purpose
We have investigated the potential of metabolomics to discover blood-based biomarkers relevant to lung cancer screening and early detection. An untargeted metabolomics approach was applied to identify biomarker candidates using prediagnostic sera from the Beta-Carotene and Retinol Efficacy Trial (CARET) study.
Patients and Methods
A liquid chromatography/mass spectrometry hydrophilic interaction method designed to profile a wide range of metabolites was applied to prediagnostic serum samples from CARET participants (current or former heavy smokers), consisting of 100 patients who subsequently developed non–small-cell lung cancer (NSCLC) and 199 matched controls. A separate aliquot was used to quantify levels of pro-surfactant protein B (pro-SFTPB), a previously established protein biomarker for NSCLC. On the basis of the results from the discovery set, blinded validation of a metabolite, identified as N1,N12-diacetylspermine (DAS), and pro-SFTPB was performed using an independent set of CARET prediagnostic sera from 108 patients with NSCLC and 216 matched controls.
Results
Serum DAS was elevated by 1.9-fold, demonstrating significant specificity and sensitivity in the discovery set for samples collected up to 6 months before diagnosis of NSCLC. In addition, DAS significantly complemented performance of pro-SFTPB in both the discovery and validations sets, with a combined area under the curve in the validation set of 0.808 (P < .001 v pro-SFTPB).
Conclusion
DAS is a novel serum metabolite with significant performance in prediagnostic NSCLC and has additive performance with pro-SFTPB.
INTRODUCTION
Recently, major efforts have been directed toward early detection of lung cancer through low-dose computed tomography (LDCT) scanning. Data from the National Lung Screening Trial (NLST) suggest that yearly screening with thoracic LDCT scanning for high-risk current and former smokers reduces lung cancer mortality by 20% and total mortality by 7%.1 However, issues including indeterminate nodules detected by LDCT and radiation exposure impact the practicality of LDCT-based screening on a national and global basis. A blood-based biomarker or multiplexed marker panel that could complement LDCT would represent a major advance in implementing lung cancer screening.
Efforts to develop blood-based biomarkers for lung cancer early detection using a variety of methodologies are currently ongoing.2 Proteomic studies have led to the identification of several candidate markers including pro-surfactant protein B (pro-SFTPB), a target of a lineage-survival oncogene in lung cancer, NKX2-1.3 Validation studies using blood samples collected at the time of LDCT screening for lung cancer substantiated the performance of pro-SFTPB. Multivariable logistic regression models were used to evaluate the predictive ability of pro-SFTPB. The area under the curve (AUC) values of the full model with and without pro-SFTPB were 0.741 (95% CI, 0.696 to 0.783) and 0.669 (95% CI, 0.620 to 0.717), respectively (difference in AUC, P < .001).4
Single markers are unlikely to have sufficient performance for implementation in a screening setting, hence the need to explore several discovery platforms to identify markers that provide complementary performance. Metabolomics represents a global unbiased approach to the profiling of small molecules and has been established as a platform for biomarker discovery for a variety of human biofluids and tissues.5–7 Here we used an untargeted liquid chromatography/mass spectrometry (MS) metabolomics approach to identify metabolites that distinguish human sera collected before the diagnosis of lung cancer from matched control sera in a prospective cohort of high-risk patients from the Beta-Carotene and Retinol Efficacy Trial (CARET).8,9 We describe for the first time, to our knowledge, increased serum levels of N1,N12-diacetylspermine (DAS) preceding a diagnosis of lung cancer using matched case-control specimens. We further provide evidence in blinded validation studies in a second independent patient cohort of its significant additive performance compared with pro-SFTPB alone.
PATIENTS AND METHODS
Study Population
Participants in this nested case-control study were selected from the CARET study (Data Supplement).9 In total, 208 patients with lung cancer were selected from 222 patients with non–small-cell lung cancer (NSCLC) with serum available in the CARET repository from blood draws that occurred up to 12 months before diagnosis. Fourteen patients with insufficient sample available for the study were excluded. Two control individuals who were free of lung cancer were matched to each patient case on age at baseline (5-year groups), sex, baseline smoking status (current v former), and study enrollment phase (pilot [1985 to 1988] or full-scale trial [1989 to 1994], thus accounting for sample storage time). For one patient, only a single control could be matched, resulting in a total of 208 patients and 415 controls included in the study. The samples were divided into two sets; samples from 100 matched sets were used for biomarker discovery (the discovery set), and the remaining 108 matched sets were reserved for validation of markers identified by the discovery efforts (the validation set). All CARET participants provided informed consent at recruitment and throughout follow-up, and the institutional review boards at each of the six study centers approved all study procedures.
Sample Preparation and MS Analysis of Metabolites
Detailed description of the methodology is provided in the Data Supplement. Briefly, serum samples (laboratory personnel were blinded to study information) were treated with ice cold methanol and prepared for untargeted metabolomics analysis using hydrophilic interaction chromatography with an acetonitrile:water gradient. MS analysis and data acquisition were performed using an Agilent quadrupole time-of-flight 6530 mass spectrometer (Agilent, Santa Clara, CA) with positive and negative ionization and in full scan mode with data-dependent MS/MS for compound identification. DAS (CAS No. 61345-83-3) was synthesized according to a previously published method.10
Pro-SFTPB Assay
The pro-SFTPB assay was performed as previously described by laboratory personnel blinded to the characteristics of the study participants.4,11 Pro-SFTPB concentrations were determined using anti–pro-SFTPB mouse monoclonal antibodies developed against the N-terminal propeptide of human SFTPB. Serum samples with 1:100 dilution and various amounts of N-terminal propeptide of SFTPB as standards were added to the wells. Anti– pro-SFTPB mouse monoclonal antibody was biotinylated with EZ-Link Sulfo-NHS-LC-Biotin (Thermo Fisher Scientific, Waltham, MA) and used for incubation at 0.5 μg/mL. After washing, each well was incubated with Streptavidin-HRP followed by incubation of color reagents (eBioscience, San Diego, CA) to which a stop solution was added. The absorbance was measured at 450 nm and 620 nm as the reference wavelength with a Versamax microplate reader (Molecular Devices, Sunnyvale, CA). All samples were assayed in duplicate.
Statistical Analysis
The performance of 2,161 individual metabolomic mass/retention time features was assessed using receiver operating characteristic (ROC) curve analysis in the discovery set. For each metabolomic feature, a total area under the ROC curve (AUC) was calculated to evaluate overall performance, and a partial-area-under ROC curve (pAUC) was calculated to evaluate classification performance focusing on high specificity. Permutation data sets (N = 1,000) were generated by randomly permuting case-control status from the original data set. Total AUCs and pAUCs for specificity ≥ 90%, 95%, and 98% were then calculated on the permuted data sets to obtain a distribution of AUCs/pAUCs under the null hypothesis that the markers have no association with cancer. The study set AUCs/pAUCs were evaluated against the distribution of values from the permuted data sets to calculate false discovery rates (FDRs) and adjusted P values.12
Potential confounders and effect modifiers on the DAS and lung cancer association were examined by the Mann-Whitney U test or Kruskal-Wallis test, stratified by case-control status, to assess association between DAS and several potential confounders, including sex, age, fasting time (hours since last meal), body mass index, smoking status (current v former), pack-years, and CARET exposure population (asbestos-exposed v heavy smoker cohort), as well as histology, stage of disease, and time of blood draw with respect to diagnosis (0 to 6 months v > 6 to 12 months prior) among patients with lung cancer only, as presented in the Data Supplement. DAS and pro-SFTPB were log2-transformed for development of the combined model. The P values for significance of the combination of DAS and pro-SFTPB were based on the likelihood ratio test in a logistic regression analysis. All statistical analyses were performed using Prism 6 (version 6.01; GraphPad, La Jolla, CA) and R version 3.0.2 (http://www.r-project.org/).
RESULTS
Increased Levels of DAS in Serum From Prediagnostic Patients With Lung Cancer in the Discovery Set
The untargeted metabolomics approach allowed profiling of a broad array of blood compounds, ranging from highly polar compounds, such as creatinine, amino acids, and acylcarnitines, to large lipids (Data Supplement). Blinded analysis of sera from 100 prediagnostic patients and 199 matched controls (the discovery set; Table 1) yielded 1.9-fold elevated levels of an unknown compound with a P < 10−7 and an adjusted P < .001 after applying a Benjimani-Hochberg FDR correction for multiple testing (Fig 1A). This compound achieved statistical significance for total AUC of 0.657, (P = .01 and FDR-adjusted P = .025). The pAUCs for ≥ 90% and 95% specificity were also statistically significant for this compound (pAUC = 0.02, P < .001, and FDR P < .001 for ≥ 90% specificity; and pAUC = 0.007, P = .006, and FDR P = .007 for ≥ 95% specificity). The coefficient of variation was 7.8% for within run and 13.4% for between run. None of the other compounds achieved significance after FDR correction for multiple testing.
Table 1.
Characteristics of Patients and Controls in the Discovery and Validation Sets
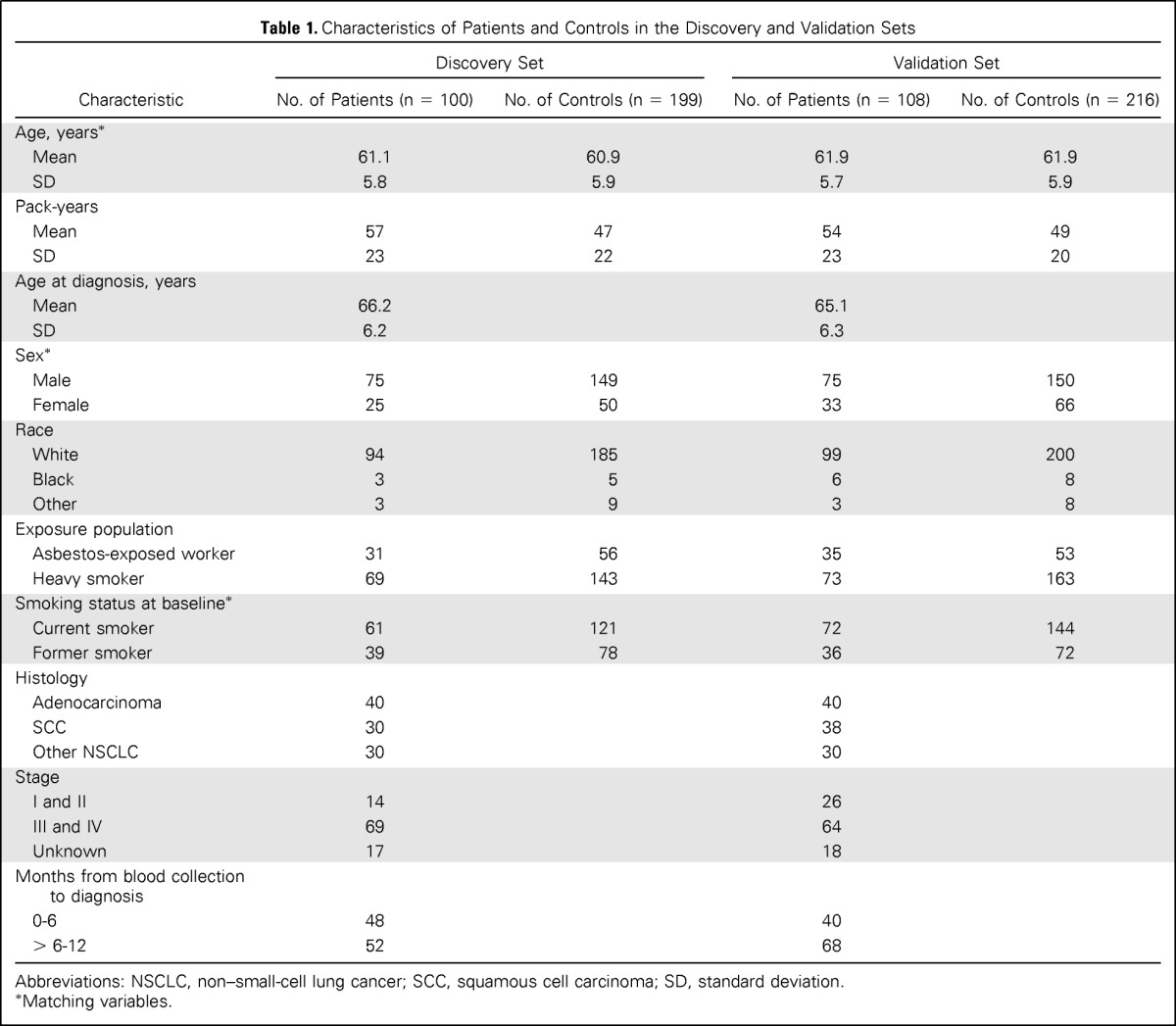
| Characteristic | Discovery Set |
Validation Set |
||
|---|---|---|---|---|
| No. of Patients (n = 100) | No. of Controls (n = 199) | No. of Patients (n = 108) | No. of Controls (n = 216) | |
| Age, years* | ||||
| Mean | 61.1 | 60.9 | 61.9 | 61.9 |
| SD | 5.8 | 5.9 | 5.7 | 5.9 |
| Pack-years | ||||
| Mean | 57 | 47 | 54 | 49 |
| SD | 23 | 22 | 23 | 20 |
| Age at diagnosis, years | ||||
| Mean | 66.2 | 65.1 | ||
| SD | 6.2 | 6.3 | ||
| Sex* | ||||
| Male | 75 | 149 | 75 | 150 |
| Female | 25 | 50 | 33 | 66 |
| Race | ||||
| White | 94 | 185 | 99 | 200 |
| Black | 3 | 5 | 6 | 8 |
| Other | 3 | 9 | 3 | 8 |
| Exposure population | ||||
| Asbestos-exposed worker | 31 | 56 | 35 | 53 |
| Heavy smoker | 69 | 143 | 73 | 163 |
| Smoking status at baseline* | ||||
| Current smoker | 61 | 121 | 72 | 144 |
| Former smoker | 39 | 78 | 36 | 72 |
| Histology | ||||
| Adenocarcinoma | 40 | 40 | ||
| SCC | 30 | 38 | ||
| Other NSCLC | 30 | 30 | ||
| Stage | ||||
| I and II | 14 | 26 | ||
| III and IV | 69 | 64 | ||
| Unknown | 17 | 18 | ||
| Months from blood collection to diagnosis | ||||
| 0-6 | 48 | 40 | ||
| > 6-12 | 52 | 68 | ||
Abbreviations: NSCLC, non–small-cell lung cancer; SCC, squamous cell carcinoma; SD, standard deviation.
Matching variables.
Fig 1.
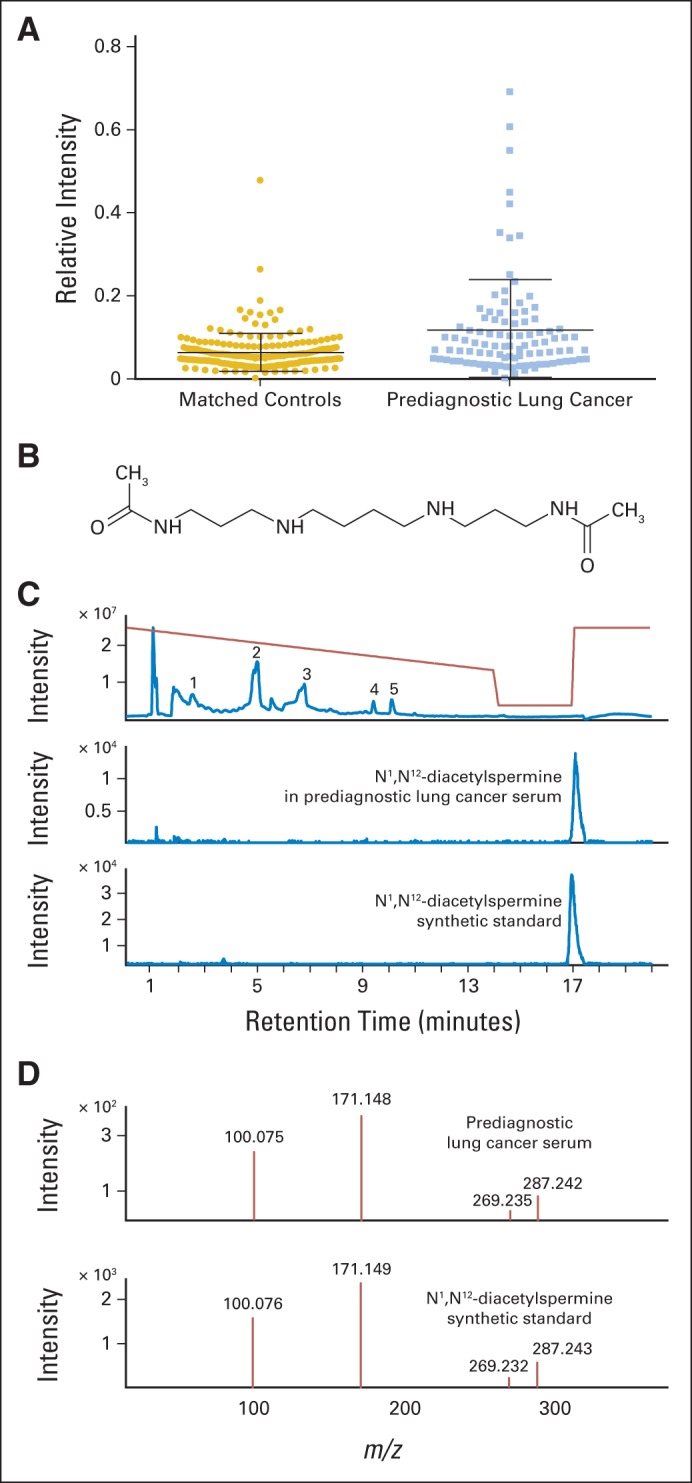
Identification of a compound with 1.9-fold elevated levels in prediagnostic lung cancer sera as N1,N12-diacetylspermine (DAS). (A) Relative intensity of DAS (originally an unknown with mass-to-charge ratio [m/z] of 287.2437) in metabolomics data in prediagnostic lung cancer versus matched controls. Bars indicate mean and standard deviation. (B) Chemical structure of DAS. (C) Top panel shows total ion chromatogram of serum from a participant later diagnosed with lung cancer, showing peaks for (1) creatinine, (2) diacyl lipids, (3) lysophosphatidylcholine lipids, (4) acetylcarnitine, and (5) carnitine. Gradient profile is indicated in red. Extracted ion chromatogram for DAS (m/z, 287.24) in serum from a participant later diagnosed with lung cancer (middle panel) and for synthetic DAS (bottom panel). (D) Quadruple time-of-flight (QTOF) collision-induced dissociation (tandem mass spectrometry [MS/MS]) at 20 V of the unknown ion (m/z, 287.24) isolated from prediagnostic lung cancer serum (top panel) and QTOF MS/MS at 20 V of synthetic DAS (bottom panel).
The unknown compound was characterized by an accurate mass-to-charge ratio of 287.2437, and identification was initiated by using the accurate mass to generate molecular formulas. This yielded a single likely molecular formula, C14H30N4O2 [M+H]+, which is within 1 ppm of the measured mass-to-charge ratio. Using the Chemspider database (www.chemspider.com),13 37 possible compounds were found corresponding to this single molecular formula. Of these, one compound, DAS, was judged to be biologically and biochemically the most likely of the candidate compounds (Fig 1B). To confirm the identity of this compound, DAS was synthesized and analyzed using MS.10 The unknown compound exhibited a match to the synthetic DAS based on the following four criteria: column retention time, isotope pattern, MS fragmentation (collision-induced dissociation MS/MS), and accurate mass (Figs 1C and 1D). Thus, the unknown compound was unequivocally identified as DAS based on four orthogonal criteria. The synthesized standard allowed determination of DAS concentration in serum, which was estimated at an average of 3.75 nmol/L among controls.
Diagnostic Performance of DAS in the Discovery Set
An ROC analysis yielded an AUC of 0.657 (95% CI, 0.586 to 0.727) for all patients combined (Table 2). DAS levels were significantly elevated in all subgroups. Of note, the AUC was 0.610 (95% CI, 0.510 to 710) for serum samples collected 6 to 12 months before diagnosis and 0.710 (95% CI, 0.613 to 0.808) for sera collected 0 to 6 months before diagnosis. In addition, DAS levels were significantly higher in samples collected 0 to 6 months before diagnosis than samples collected 6 to 12 months before diagnosis (P = .0110, Mann-Whitney U test; Data Supplement), suggesting the association of increased serum DAS levels with tumor development and progression.
Table 2.
Performance of DAS in the Discovery Set
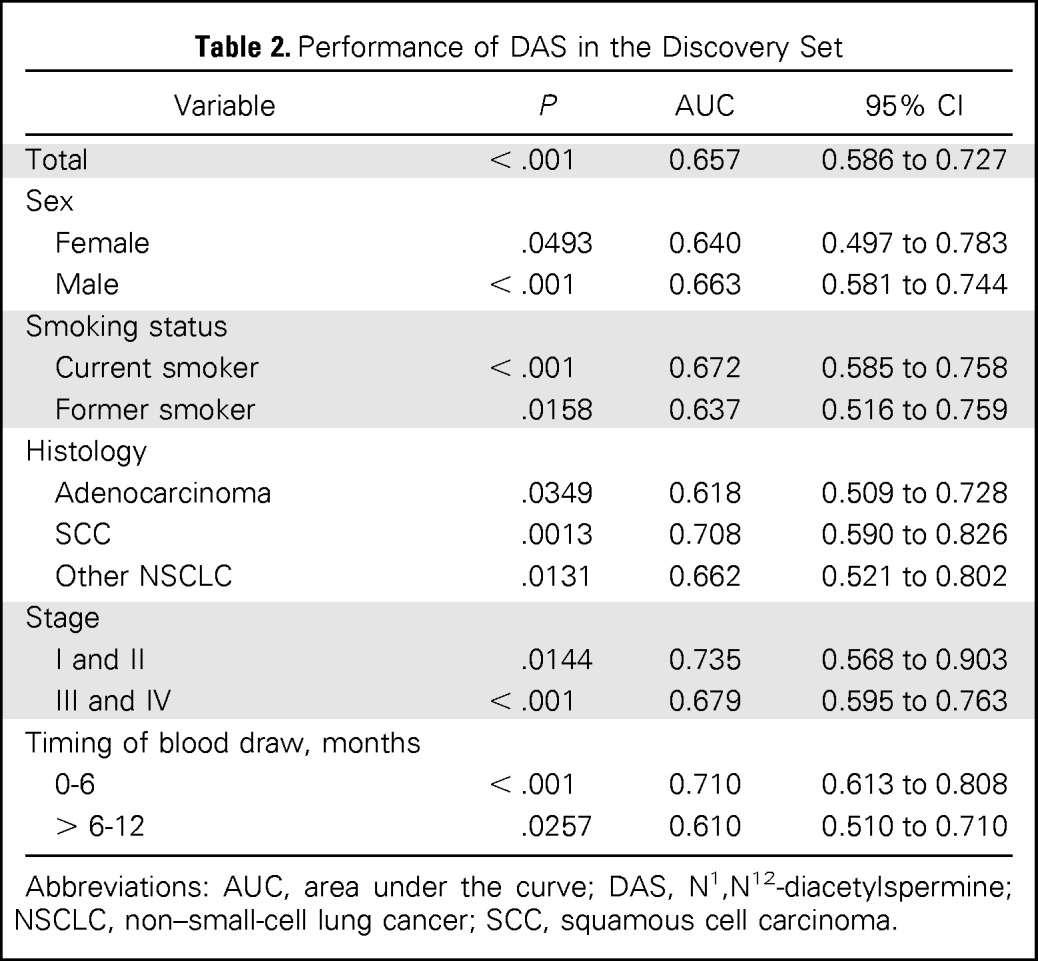
| Variable | P | AUC | 95% CI |
|---|---|---|---|
| Total | < .001 | 0.657 | 0.586 to 0.727 |
| Sex | |||
| Female | .0493 | 0.640 | 0.497 to 0.783 |
| Male | < .001 | 0.663 | 0.581 to 0.744 |
| Smoking status | |||
| Current smoker | < .001 | 0.672 | 0.585 to 0.758 |
| Former smoker | .0158 | 0.637 | 0.516 to 0.759 |
| Histology | |||
| Adenocarcinoma | .0349 | 0.618 | 0.509 to 0.728 |
| SCC | .0013 | 0.708 | 0.590 to 0.826 |
| Other NSCLC | .0131 | 0.662 | 0.521 to 0.802 |
| Stage | |||
| I and II | .0144 | 0.735 | 0.568 to 0.903 |
| III and IV | < .001 | 0.679 | 0.595 to 0.763 |
| Timing of blood draw, months | |||
| 0-6 | < .001 | 0.710 | 0.613 to 0.808 |
| > 6-12 | .0257 | 0.610 | 0.510 to 0.710 |
Abbreviations: AUC, area under the curve; DAS, N1,N12-diacetylspermine; NSCLC, non–small-cell lung cancer; SCC, squamous cell carcinoma.
The levels of pro-SFTPB were significantly elevated in prediagnostic sera from patients with lung cancer compared with controls (P < .001, Mann-Whitney U test; AUC = 0.635) in the initial discovery set used for metabolomic profiling (Data Supplement). Levels of pro-SFTPB increased significantly in both samples collected closer to diagnosis (0 to 6 months; P = .0142, Mann-Whitney U test; AUC = 0.626) and samples collected farther from diagnosis (> 6 to 12 months; P = .0031, Mann-Whitney U test; AUC = 0.645). Serum pro-SFTPB levels were also significantly elevated in current smokers (P < .001, Mann-Whitney U test; AUC = 0.682), patients with adenocarcinoma (P = .0015, Mann-Whitney U test; AUC = 0.677), and patients with advanced stage (stage III and IV; P < .001, Mann-Whitney U test; AUC = 0.663).
Validation of DAS and Pro-SFTPB As Single and Combined Markers in an Independent CARET Test Set
An independent validation set was used that consisted of 108 patients and 216 controls (Table 1), with patients and controls matched by age, sex, and smoking status at baseline, as with the discovery set. DAS was assayed using the same MS approach as for the discovery set. DAS levels were significantly elevated among patients versus controls, with an AUC of 0.650 (95% CI, 0.585 to 0.716; Table 3). As was observed in the discovery study, higher DAS levels were observed in samples from patients with lung cancer collected 0 to 6 months versus 6 to 12 months before diagnosis (P = .0318, Mann-Whitney U test; Data Supplement). In addition, ROC analysis yielded an AUC for DAS of 0.740 (95% CI, 0.635 to 0.845) in samples collected 0 to 6 months before diagnosis and 0.588 (95% CI, 0.505 to 0.671) in samples collected 6 to 12 months before diagnosis. Pro-SFTPB levels were also significantly elevated in prediagnostic sera from patients with lung cancer compared with controls (P < .001, Mann-Whitney U test; AUC = 0.699; Data Supplement). Pro-SFTPB levels were significantly elevated in all subgroup analyses in the validation set.
Table 3.
Performance of DAS in the Validation Set
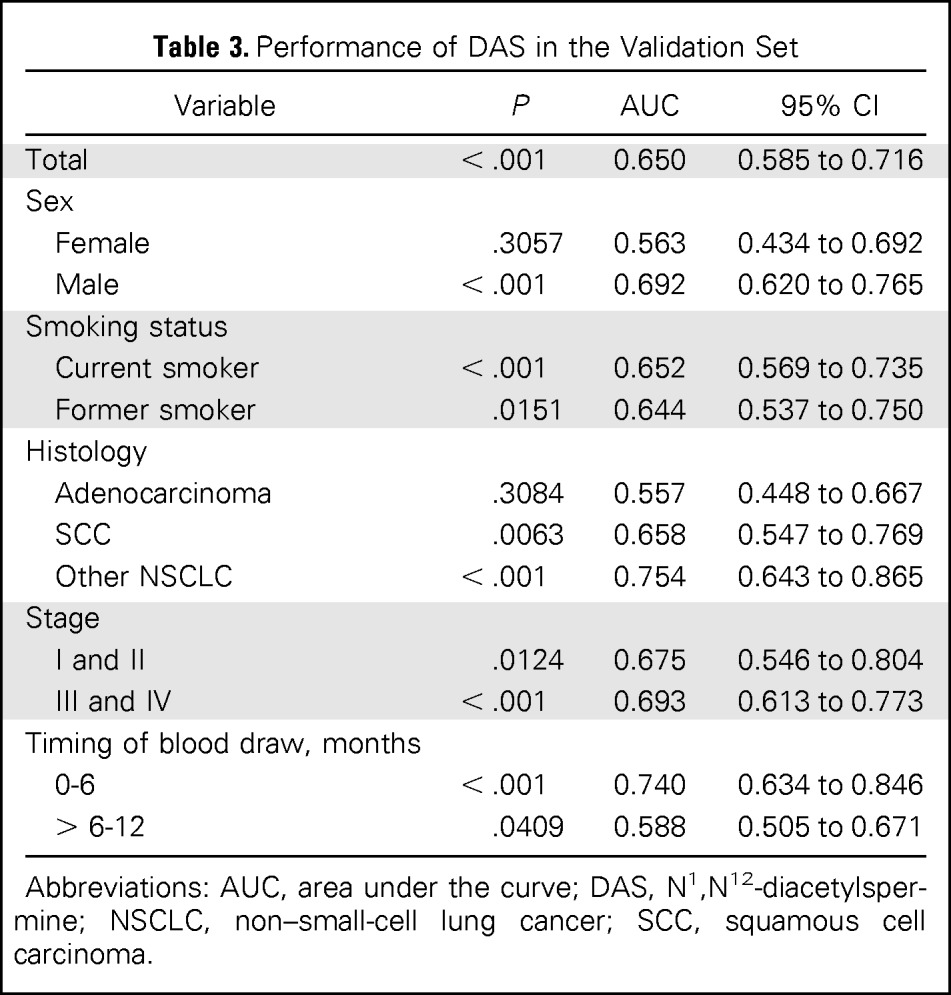
| Variable | P | AUC | 95% CI |
|---|---|---|---|
| Total | < .001 | 0.650 | 0.585 to 0.716 |
| Sex | |||
| Female | .3057 | 0.563 | 0.434 to 0.692 |
| Male | < .001 | 0.692 | 0.620 to 0.765 |
| Smoking status | |||
| Current smoker | < .001 | 0.652 | 0.569 to 0.735 |
| Former smoker | .0151 | 0.644 | 0.537 to 0.750 |
| Histology | |||
| Adenocarcinoma | .3084 | 0.557 | 0.448 to 0.667 |
| SCC | .0063 | 0.658 | 0.547 to 0.769 |
| Other NSCLC | < .001 | 0.754 | 0.643 to 0.865 |
| Stage | |||
| I and II | .0124 | 0.675 | 0.546 to 0.804 |
| III and IV | < .001 | 0.693 | 0.613 to 0.773 |
| Timing of blood draw, months | |||
| 0-6 | < .001 | 0.740 | 0.634 to 0.846 |
| > 6-12 | .0409 | 0.588 | 0.505 to 0.671 |
Abbreviations: AUC, area under the curve; DAS, N1,N12-diacetylspermine; NSCLC, non–small-cell lung cancer; SCC, squamous cell carcinoma.
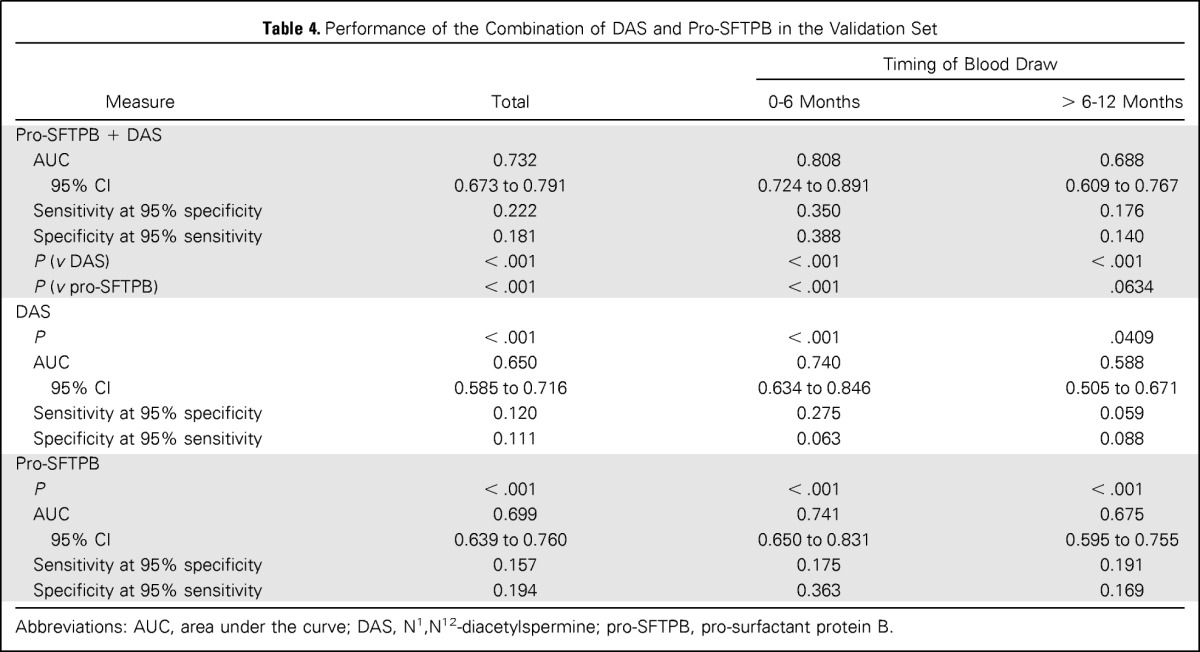
Because pro-SFTPB is an established lung cancer biomarker, we assessed the added contribution of DAS to the performance of pro-SFTPB alone in the validation set. The performance of the combination of pro-SFTPB and DAS using logistic regression was significantly improved compared with pro-SFTPB alone (P < .001, likelihood ratio test) and DAS alone (P < .001, likelihood ratio test; Table 4 and Fig 2). The AUC for combination of DAS and pro-SFTPB was 0.808 (95% CI, 0.724 to 0.891) with a sensitivity of 0.350 at 95% specificity in the comparison of lung cancer samples collected 0 to 6 months before diagnosis compared with healthy controls and an AUC of 0.732 overall.
Table 4.
Performance of the Combination of DAS and Pro-SFTPB in the Validation Set
| Measure | Total | Timing of Blood Draw |
|
|---|---|---|---|
| 0-6 Months | > 6-12 Months | ||
| Pro-SFTPB + DAS | |||
| AUC | 0.732 | 0.808 | 0.688 |
| 95% CI | 0.673 to 0.791 | 0.724 to 0.891 | 0.609 to 0.767 |
| Sensitivity at 95% specificity | 0.222 | 0.350 | 0.176 |
| Specificity at 95% sensitivity | 0.181 | 0.388 | 0.140 |
| P (v DAS) | < .001 | < .001 | < .001 |
| P (v pro-SFTPB) | < .001 | < .001 | .0634 |
| DAS | |||
| P | < .001 | < .001 | .0409 |
| AUC | 0.650 | 0.740 | 0.588 |
| 95% CI | 0.585 to 0.716 | 0.634 to 0.846 | 0.505 to 0.671 |
| Sensitivity at 95% specificity | 0.120 | 0.275 | 0.059 |
| Specificity at 95% sensitivity | 0.111 | 0.063 | 0.088 |
| Pro-SFTPB | |||
| P | < .001 | < .001 | < .001 |
| AUC | 0.699 | 0.741 | 0.675 |
| 95% CI | 0.639 to 0.760 | 0.650 to 0.831 | 0.595 to 0.755 |
| Sensitivity at 95% specificity | 0.157 | 0.175 | 0.191 |
| Specificity at 95% sensitivity | 0.194 | 0.363 | 0.169 |
Abbreviations: AUC, area under the curve; DAS, N1,N12-diacetylspermine; pro-SFTPB, pro-surfactant protein B.
Fig 2.
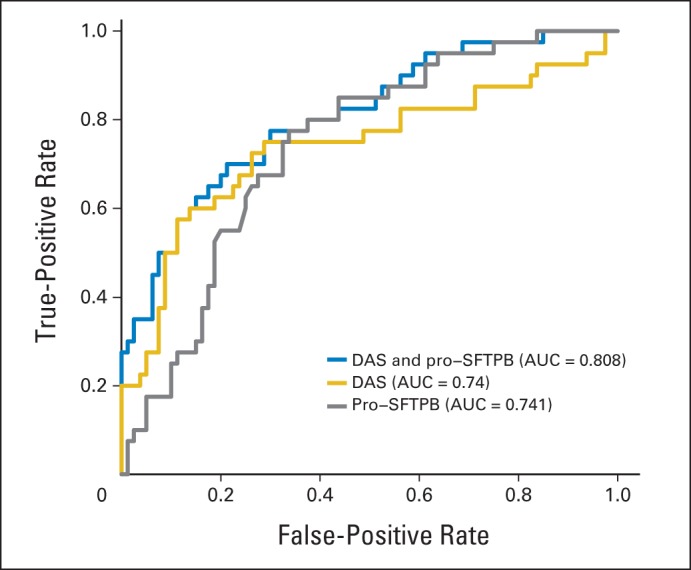
Receiver operative characteristic curves of pro-surfactant protein B (pro-SFTPB), N1,N12-diacetylspermine (DAS), and combination of pro-SFTPB and DAS in lung cancer samples collected 0 to 6 months before diagnosis compared with matched controls in the validation set. AUC, area under the curve.
DISCUSSION
This study identified, for the first time to our knowledge, increased serum levels of DAS, a doubly acetylated polyamine compound, preceding a diagnosis of lung cancer. Prior studies identified DAS in urine from patients with colorectal, breast, and bladder cancers14–16; in tissues from colorectal cancer; and in liver metastasis.17
The predictive value of DAS was greater in samples collected 0 to 6 months compared with 6 to 12 months before diagnosis, suggesting that increased levels of DAS tracked with the development and progression of lung cancer up to the time of diagnosis. Our case-control study establishes the potential contribution of DAS to early detection of lung cancer. In this study, two control individuals who were free of lung cancer were matched to each patient on age at baseline, sex, baseline smoking status, and study enrollment phase. Matching risk factors (age and smoking) potentially impacts the performance of DAS on sensitivity and specificity if DAS levels are associated with smoking or age.18 DAS was significantly associated with age of the control individuals in the discovery set (Data Supplement) but not in the validation set (data not shown). Therefore, DAS levels are likely independent from these risk factors, and the potential bias will be minimal. Most relevant to potential clinical applications is whether DAS and pro-SFTPB could identify asymptomatic patients with lung cancer in a screening setting. Assuming a lung cancer annual incidence rate of 0.0625%, as observed in the NLST study,1 and based on the sensitivity of 35% at 95% specificity for the model combining pro-SFTPB and DAS in prediagnostic sera collected within 6 months before diagnosis of lung cancer, the positive predictive value is 4.2%. This compares favorably to the positive predictive value of 3.6% observed in NLST for nodules suggestive of lung cancer by LDCT. Likewise, DAS levels in benign lung disease, such as chronic obstructive pulmonary disease, will need to be assessed, as a recent study has indicated the association of higher plasma pro-SFTPB levels with increased severity of airflow limitation and rapid progression of chronic obstructive pulmonary disease over 2 years.19
DAS is an acetylated derivative of spermine, a well-known polyamine. Polyamines are small cationic molecules that have long been implicated in regulating key metabolic enzymes in normal and cancer cells.20 Polyamines are essential in normal mammalian cells with levels that are controlled through a complex network that regulates their synthesis, degradation, and transport. Ornithine decarboxylase and S-adenosylmethionine decarboxylase are two important enzymes in the polyamine biosynthetic pathway, which are themselves tightly regulated through various transcriptional, translational, and post-translational mechanisms.21 Polyamines are also regulated by oncogenes and tumor-suppressor genes in epithelial tumors and have even been tested as targets of chemopreventative agents and pharmaceutical inhibitors in cancer treatment.20,22
The biosynthetic pathway of DAS has not been fully elucidated. DAS is likely produced from the substrate acetylspermine via the enzyme spermidine/spermine N1-acetyltransferase.23 Overexpression of spermidine/spermine N1-acetyltransferase results in high levels of both acetylspermine and diacetylspermine.24 Polyamine export from mammalian cells occurs primarily via acetylated polyamines, including DAS, by a diamine transporter that has been identified as SLC3A2, a component of CD9825 more commonly known as the amino acid transporter LAT1.26 Therefore, SLC3A2 may affect the blood DAS levels as observed in our study. The transporter coprecipitates with the spermine acetylase SAT1, thus supporting its role in polyamine metabolism.27 SLC3A2 is also colocalized with SLC7A5, and the complex, designated CD98, is known to be highly expressed in several human cancers,28 including lung cancer.29,30 Inhibition of CD98 has antitumor activity in NSCLC.31 CD98 has prognostic significance in NSCLC29,30,32 and in neuroendocrine tumors.33 Moreover, an increased supply of spermine in the context of glucose restriction in the tumor microenvironment has been found to increase DAS secretion by macrophages.34
Unbiased metabolomics profiling has identified 1.9-fold elevated levels of DAS in a discovery set of prediagnostic lung cancer sera related to matched controls, which was validated in an independent blinded set of samples. We further demonstrated significant additive performance of the combination of DAS and pro-SFTPB compared with either biomarker alone, suggesting the utility of this metabolite for lung cancer early detection as part of a marker panel.
Supplementary Material
Footnotes
See accompanying editorial on page 3847
Supported by Department of Defense Congressionally Directed Medical Research Program Grant No. W81XWH-10-1-0635, National Institutes of Health Grants No. UM1 CA167462 and U24 DK097154, the Canary Foundation, the Rubenstein Family Foundation, and the Lyda Hill Foundation.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: William R. Wikoff, Samir Hanash, Ziding Feng, David Gandara, Oliver Fiehn, Ayumu Taguchi
Financial support: David Gandara, Oliver Fiehn
Administrative support: David Gandara
Provision of study materials or patients: Gary Goodman, David Gandara
Collection and assembly of data: William R. Wikoff, Brian DeFelice, Suzanne Miyamoto, Gary Goodman, David Gandara, Oliver Fiehn, Ayumu Taguchi
Data analysis and interpretation: William R. Wikoff, Samir Hanash, Matt Barnett, Yang Zhao, Ziding Feng, David Gandara, Oliver Fiehn, Ayumu Taguchi
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Diacetylspermine Is a Novel Prediagnostic Serum Biomarker for Non–Small-Cell Lung Cancer and Has Additive Performance With Pro-Surfactant Protein B
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
William R. Wikoff
No relationship to disclose
Samir Hanash
No relationship to disclose
Brian DeFelice
No relationship to disclose
Suzanne Miyamoto
No relationship to disclose
Matt Barnett
No relationship to disclose
Yang Zhao
No relationship to disclose
Gary Goodman
No relationship to disclose
Ziding Feng
No relationship to disclose
David Gandara
No relationship to disclose
Oliver Fiehn
No relationship to disclose
Ayumu Taguchi
No relationship to disclose
REFERENCES
- 1.National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hassanein M, Callison JC, Callaway-Lane C, et al. The state of molecular biomarkers for the early detection of lung cancer. Cancer Prev Res (Phila) 2012;5:992–1006. doi: 10.1158/1940-6207.CAPR-11-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taguchi A, Politi K, Pitteri SJ, et al. Lung cancer signatures in plasma based on proteome profiling of mouse tumor models. Cancer Cell. 2011;20:289–299. doi: 10.1016/j.ccr.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sin DD, Tammemagi CM, Lam S, et al. Pro-surfactant protein B as a biomarker for lung cancer prediction. J Clin Oncol. 2013;31:4536–4543. doi: 10.1200/JCO.2013.50.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wikoff WR, Gangoiti JA, Barshop BA, et al. Metabolomics identifies perturbations in human disorders of propionate metabolism. Clin Chem. 2007;53:2169–2176. doi: 10.1373/clinchem.2007.089011. [DOI] [PubMed] [Google Scholar]
- 6.Sreekumar A, Poisson LM, Rajendiran TM, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 7.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 9.Goodman GE, Thornquist MD, Balmes J, et al. The Beta-Carotene and Retinol Efficacy Trial: Incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. 2004;96:1743–1750. doi: 10.1093/jnci/djh320. [DOI] [PubMed] [Google Scholar]
- 10.Samejima K, Hiramatsu K, Takahashi K, et al. Identification and determination of urinary acetylpolyamines in cancer patients by electrospray ionization and time-of-flight mass spectrometry. Anal Biochem. 2010;401:22–29. doi: 10.1016/j.ab.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Taguchi A, Hanash S, Rundle A, et al. Circulating pro-surfactant protein B as a risk biomarker for lung cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:1756–1761. doi: 10.1158/1055-9965.EPI-13-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodd LE, Pepe MS. Partial AUC estimation and regression. Biometrics. 2003;59:614–623. doi: 10.1111/1541-0420.00071. [DOI] [PubMed] [Google Scholar]
- 13.Williams AJ. Public chemical compound databases. Curr Opin Drug Discov Devel. 2008;11:393–404. [PubMed] [Google Scholar]
- 14.Hiramatsu K, Takahashi K, Yamaguchi T, et al. N(1),N(12)-Diacetylspermine as a sensitive and specific novel marker for early- and late-stage colorectal and breast cancers. Clin Cancer Res. 2005;11:2986–2990. doi: 10.1158/1078-0432.CCR-04-2275. [DOI] [PubMed] [Google Scholar]
- 15.Kawakita M, Hiramatsu K, Yanagiya M, et al. Determination of N(1),N(1)(2)-diacetylspermine in urine: A novel tumor marker. Methods Mol Biol. 2011;720:367–378. doi: 10.1007/978-1-61779-034-8_23. [DOI] [PubMed] [Google Scholar]
- 16.Nakayama Y, Torigoe T, Minagawa N, et al. The clinical usefulness of urinary N(1),N(12)-diacetylspermine (DiAcSpm) levels as a tumor marker in patients with colorectal cancer. Oncol Lett. 2012;3:970–974. doi: 10.3892/ol.2012.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuwata G, Hiramatsu K, Samejima K, et al. Increase of N1, N12-diacetylspermine in tissues from colorectal cancer and its liver metastasis. J Cancer Res Clin Oncol. 2013;139:925–932. doi: 10.1007/s00432-013-1405-5. [DOI] [PubMed] [Google Scholar]
- 18.Pepe MS, Fan J, Seymour CW, et al. Biases introduced by choosing controls to match risk factors of cases in biomarker research. Clin Chem. 2012;58:1242–1251. doi: 10.1373/clinchem.2012.186007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung JM, Mayo J, Tan W, et al. Plasma pro-surfactant protein B and lung function decline in smokers. Eur Respir J. 2015;45:1037–1045. doi: 10.1183/09031936.00184214. [DOI] [PubMed] [Google Scholar]
- 20.Paz EA, Garcia-Huidobro J, Ignatenko NA. Polyamines in cancer. Adv Clin Chem. 2011;54:45–70. doi: 10.1016/b978-0-12-387025-4.00002-9. [DOI] [PubMed] [Google Scholar]
- 21.Persson L. Polyamine homoeostasis. Essays Biochem. 2009;46:11–24. doi: 10.1042/bse0460002. [DOI] [PubMed] [Google Scholar]
- 22.Nowotarski SL, Woster PM, Casero RA., Jr Polyamines and cancer: Implications for chemotherapy and chemoprevention. Expert Rev Mol Med. 2013;15:e3. doi: 10.1017/erm.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casero RA, Jr, Pegg AE. Spermidine/spermine N1-acetyltransferase: The turning point in polyamine metabolism. FASEB J. 1993;7:653–661. [PubMed] [Google Scholar]
- 24.Vujcic S, Halmekyto M, Diegelman P, et al. Effects of conditional overexpression of spermidine/spermine N1-acetyltransferase on polyamine pool dynamics, cell growth, and sensitivity to polyamine analogs. J Biol Chem. 2000;275:38319–38328. doi: 10.1074/jbc.M003270200. [DOI] [PubMed] [Google Scholar]
- 25.Xie X, Gillies RJ, Gerner EW. Characterization of a diamine exporter in Chinese hamster ovary cells and identification of specific polyamine substrates. J Biol Chem. 1997;272:20484–20489. doi: 10.1074/jbc.272.33.20484. [DOI] [PubMed] [Google Scholar]
- 26.Uemura T, Stringer DE, Blohm-Mangone KA, et al. Polyamine transport is mediated by both endocytic and solute carrier transport mechanisms in the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2010;299:G517–G522. doi: 10.1152/ajpgi.00169.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uemura T, Yerushalmi HF, Tsaprailis G, et al. Identification and characterization of a diamine exporter in colon epithelial cells. J Biol Chem. 2008;283:26428–26435. doi: 10.1074/jbc.M804714200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantor JM, Ginsberg MH. CD98 at the crossroads of adaptive immunity and cancer. J Cell Sci. 2012;125:1373–1382. doi: 10.1242/jcs.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao T, Ying W, Li L, et al. An approach to studying lung cancer-related proteins in human blood. Mol Cell Proteomics. 2005;4:1480–1486. doi: 10.1074/mcp.M500055-MCP200. [DOI] [PubMed] [Google Scholar]
- 30.Kaira K, Oriuchi N, Imai H, et al. CD98 expression is associated with poor prognosis in resected non-small-cell lung cancer with lymph node metastases. Ann Surg Oncol. 2009;16:3473–3481. doi: 10.1245/s10434-009-0685-0. [DOI] [PubMed] [Google Scholar]
- 31.Imai H, Kaira K, Oriuchi N, et al. Inhibition of L-type amino acid transporter 1 has antitumor activity in non-small cell lung cancer. Anticancer Res. 2010;30:4819–4828. [PubMed] [Google Scholar]
- 32.Kaira K, Oriuchi N, Imai H, et al. Prognostic significance of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (CD98) expression in surgically resectable stage III non-small cell lung cancer. Exp Ther Med. 2010;1:799–808. doi: 10.3892/etm.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaira K, Ohde Y, Endo M, et al. Expression of 4F2hc (CD98) in pulmonary neuroendocrine tumors. Oncol Rep. 2011;26:931–937. doi: 10.3892/or.2011.1384. [DOI] [PubMed] [Google Scholar]
- 34.Hamaoki M, Nagata A. Host macrophages produce diacetylspermine related with tumorigenesis. Cancer Lett. 2006;243:128–134. doi: 10.1016/j.canlet.2005.11.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


